Abstract
Background:
The Dyspnoea, Obstruction, Smoking, Exacerbation (DOSE) index was designed to assess disease severity and for the clinical management of chronic obstructive pulmonary disease (COPD), but has not been evaluated as a prognostic instrument for mortality in a population including primary care patients.
Aims:
The aim of this study was to investigate the associations of the DOSE index with mortality in primary and secondary care COPD patients.
Methods:
Information was collected from 1,111 COPD patients aged 34–75 years randomly selected from 70 Swedish primary and secondary care centres. Data were obtained using patient questionnaires and record review and the Swedish Board of Health and Welfare provided mortality data. The study population included 562 patients with data on all DOSE index components. The DOSE index was calculated using the MRC dyspnoea scale, forced expiratory volume in 1 second (FEV1) as percentage of predicted (FEV1%pred), smoking status, and exacerbation rate. The exacerbation rate over 6 months prior to record review was used to estimate the annual rate. Cox regression analyses estimated survival with adjustment for age, sex, and heart disease.
Results:
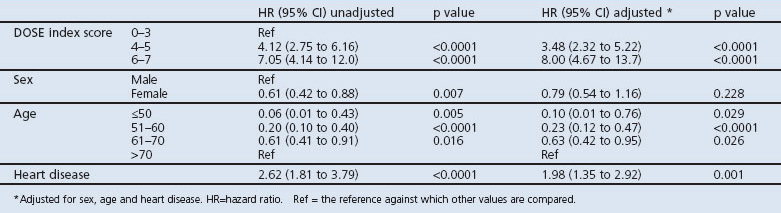
Over 5 years, 116 patients (20.6%) died. Mortality was higher in patients with DOSE index ≥4 (42.4%) than for lower scores (11.0%) (p<0.0001). Compared with a DOSE index score of 0–3, the hazard ratio for mortality was 3.48 (95% CI 2.32 to 5.22) for a score of 4–5, and was 8.00 (95% CI 4.67 to 13.7) for a score of 6–7.
Conclusions:
The DOSE index is associated with mortality in COPD patients in primary and secondary care and can be used to assess prognosis in addition to other clinically relevant issues.
Keywords: COPD, DOSE index, mortality, prognosis
Introduction
COPD is a common serious disease with an overall global prevalence of 9–10%,1 and is predicted to increase from sixth to third place in the international ranking of diseases causing mortality between 1990 and 2020.2,3 In studies of patients discharged from hospital, 1-year mortality rates of 22%4 and 23%5, and 2-year mortality rates of 29.3%6 and 35.6%4, have been reported.
The Dyspnoea, Obstruction, Smoking, Exacerbation (DOSE) index combines the modified 5-point version of the Medical Research Council (MRC) dyspnoea scale7,8 with obstruction (forced expiratory volume in 1 second as percentage of predicted value, FEV1%pred),9 smoking status, and exacerbation rate during the previous year.10 By combining measures relevant to important clinical issues in COPD as well as providing a convenient measure of disease severity for use in routine clinical settings, the DOSE index appears to be a useful complement to other composite measures such as the body mass index, airflow obstruction, dyspnoea, and exercise capacity (BODE) index and the age, dyspnoea and airflow obstruction (ADO) index.11,12 The DOSE index predicts hospital admission, respiratory failure and exacerbation risk.10 To our knowledge, only one study has evaluated the DOSE index as a prognostic instrument in a population of male hospital outpatients.13
This study examined associations of the DOSE index with mortality in male and female COPD patients receiving primary and secondary care in a multicentre Swedish population.
Methods
Procedure
The participants were from a larger cohort of COPD patients in seven Swedish counties.14,15 A total of 1,548 patients aged 34–75 years with a COPD diagnosis (ICD code J44) recorded in medical records between 2000 and 2003 were randomly selected, 1,084 from primary care and 464 from hospital clinics.
Data collection
Data were collected in 2005 by self-completed questionnaire and record review for 2000–2003. The questionnaire response rate was 75%, and 98% (1,111 patients) consented to medical record review. The Swedish Board of Health and Welfare provided mortality data for 2005 to 2010, including underlying cause of death from death certificates. Two research nurses entered the data.
Measures
Age (<50 years, 51–60 years, 61–70 years and >70 years), sex, level of education, self-reported height and weight, dyspnoea (MRC range 0–4), smoking status (current or non-smoking), exacerbation rate, current treatment, and vaccination status were taken from patient questionnaires. The dichotomous education variable identified those who continued in full-time education for at least 2 years beyond the compulsory period of 9 years and those with shorter education. Obesity was defined as body mass index (BMI) ≥30, overweight as BMI <30 and ≥25, and underweight as BMI <20. An exacerbation was defined as an unscheduled or emergency visit or a course of oral steroids due to worsening of COPD. The questionnaire identified the number of exacerbations during the 6 months prior to questionnaire completion. Current treatment was categorised as: no maintenance therapy besides short-acting beta-agonists (SABA) or ipratropium; maintenance therapy with tiotropium or long-acting beta-agonists (LABA); inhaled corticosteroids (ICS) alone; and ICS combined with LABA or tiotropium therapy. Influenza vaccination in the last year and pneumococcal vaccination in the last 5 years were identified.
Information about co-morbid diagnoses and lung function was taken from patients' records. Heart disease was defined as ischaemic heart disease or heart failure, and types 1 or 2 diabetes mellitus were identified. Depression was defined as a diagnosis combined with antidepressant drug treatment. Lung function data were available for 594 (53%) patients. FEV1 was expressed as percentage of predicted using the European Community for Steel and Coal reference values.16 Mortality was categorised as death by respiratory disease, cardiovascular disease, cancer and other causes of death.
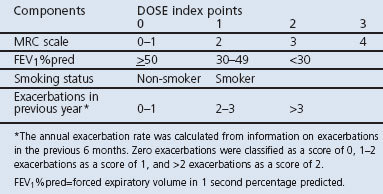
The DOSE index
The DOSE index was calculated using the MRC dyspnoea scale, FEV1%pred, smoking status, and the exacerbation rate in the previous year (Table 1).10 The DOSE index uses the sum of its components and the total score ranges from 0 to 8; the higher the score, the more severe the disease. Current smoking was defined as daily smoking. The exacerbation rate over the previous 6 months was used to estimate the annual rate (Table 1). Complete information was available for 319 patients from primary health care centres (PHCCs) and 243 from hospital clinics.
Table 1. DOSE index scoring system.
Statistical analysis
The analyses were performed using PASW Version 18.0 (SPSS Inc, Chicago, Illinois, USA). Kaplan-Meier curves and Cox regression assessed survival. Assessment using Kaplan-Meier curves indicated that the proportional hazards assumption was justified. The DOSE index was examined as a single score (categorised as scores of 6–8, 4–5 and <4) and other models examined its components separately. The analyses were adjusted for age, sex, and heart disease. Stepwise regression was used to identify the most predictive measure for mortality risk. The analyses were repeated with further adjustment for treatment and vaccination status. Cause-specific mortality associations with the DOSE index were adjusted for age and sex. The study period was from when patients received their questionnaires until death or September 2010. Stratification and interaction analyses investigated potential effect modification by sex, level of care, and age.
The smoking variable was modified to include categories for daily smokers, ex-smokers, never-smokers and occasional smokers, with adjustment for age, sex, and heart disease. Cox regression was used to analyse mortality risk by whether patients had (n=594) or did not have (n=517) spirometry data. A reduced ‘DSE index’ without lung function was created and its association with mortality analysed among excluded patients (n=549) and patients included in the main analysis (n=562).
Ethics
The study was approved by the Regional Ethical Review Board of Uppsala University (Dnr 2010/090). Written consent to use the information for future analysis was obtained for all participating patients.
Results
Patient characteristics
During the average study period of 5 years, 116 patients (20.6%) died. Information on cause of death was available for 113 patients (97.4%). No patient reached the maximum DOSE index score of 8. The number of deaths was higher in COPD stage 3 and 4,9 in secondary care, among men, older patients and those with co-morbid heart disease or those who were underweight. Influenza/pneumococcal vaccinations and maintenance therapy with the combination of ICS and LABA or tiotropium were more common among patients who died, and mortality was lower in the group treated with only SABA and ipratropium (Table 2).
Table 2. Patient characteristics.
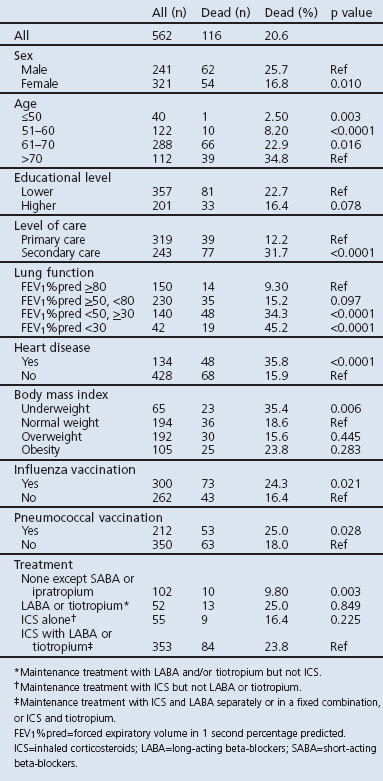
In univariate analyses, male sex, older age, lower FEV1%pred, MRC dyspnoea scale values of 3 and 4, two or more exacerbations in the previous year, underweight, and heart disease were associated with raised mortality. Age, heart disease, smoking status, FEV1%pred, and MRC dyspnoea scale remained statistically significantly associated with mortality when all variables were included in the model (data not shown).
DOSE index
Mortality was higher in patients with a DOSE index score ≥4 (42.4%) than for lower scores (11.0%, p<0.0001; Table 3). The association between the DOSE index and mortality is shown by Kaplan-Meier curves and Cox regression analyses in Figures 1 and 2. DOSE index scores of 6–7 and 4–5 compared with 0-3 were statistically significantly and independently associated with higher mortality in Cox regression analyses before and after adjustment for age, sex, and heart disease (Table 4).
Table 3. DOSE index characteristics.
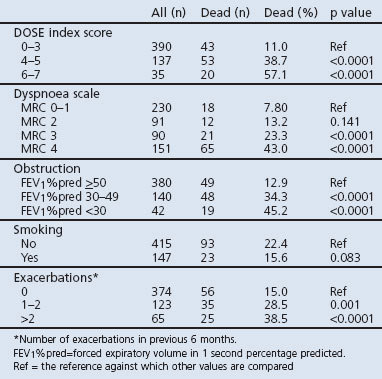
Figure 1. DOSE index and mortality.
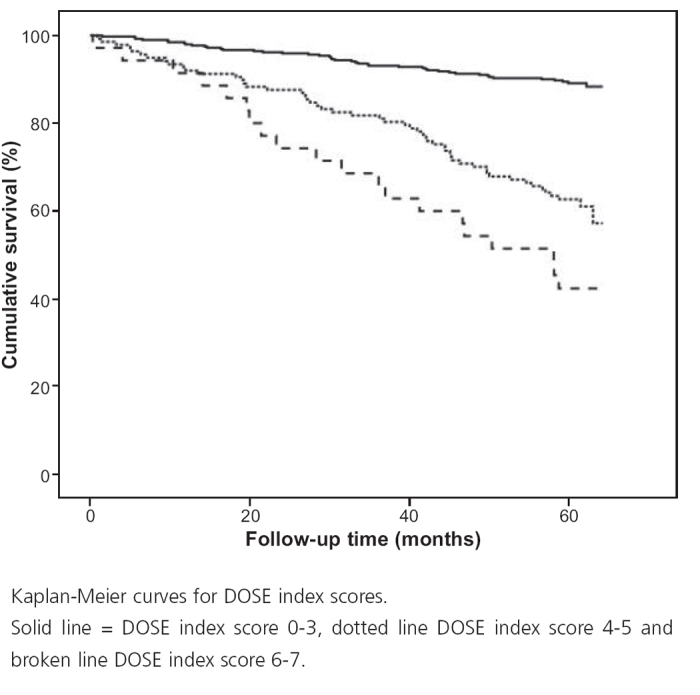
Figure 2. DOSE index score and mortality.
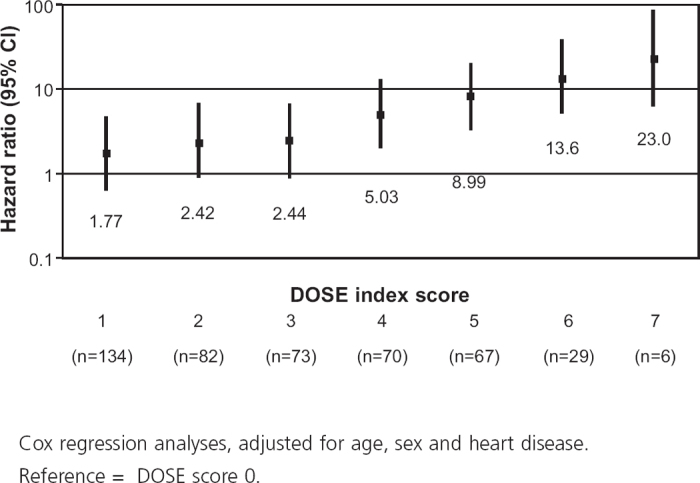
Table 4. Results of Cox regression analysis of DOSE index.
Components of the DOSE index
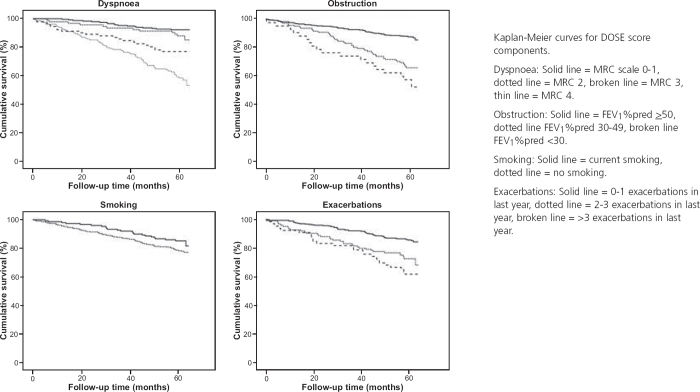
When the components were included in the same model, all were statistically significantly associated with mortality, although the association with smoking was not statistically significant in univariate analysis (Table 5, Figure 3). When the DOSE index and its components were combined in a stepwise elimination regression model, the combined index remained, as it explained more variance than the individual components. Chi-square values (with adjustment for age, sex, and heart disease) were 146.4 for combined index, 116.0 for dyspnoea, 91.2 for obstruction, 54.0 for smoking, and 75.5 for exacerbations.
Table 5. Results of Cox regression analysis of DOSE index components.
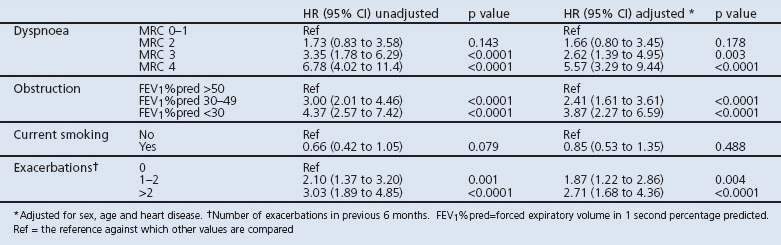
Figure 3. DOSE index components and mortality.
Sex, age and level of care
The relationship between a DOSE index score of 6–7 and mortality was of higher magnitude in men (hazard ratio (HR) 13.9, 95% CI 6.84 to 28.2) than in women (HR 4.00, 95% CI 1.62 to 9.88), with a p for interaction of 0.018. There was no effect modification by age or level of care (data not shown).
Treatment
Additional adjustment for treatment and vaccination did not alter the main results significantly (data not shown).
DOSE index and cause-specific mortality
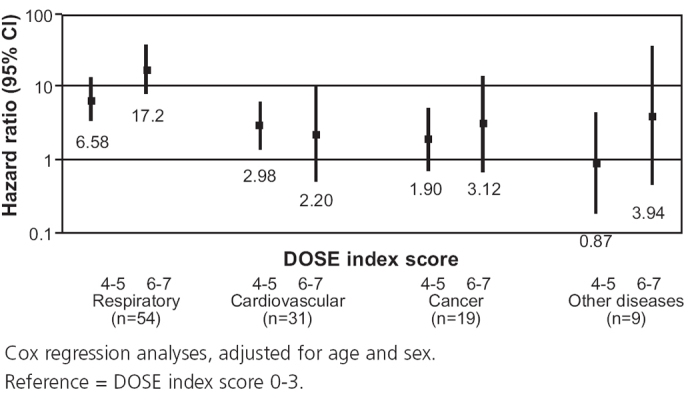
Cox regression analysis of cause-specific mortality showed that a higher DOSE index score was statistically significantly and independently associated with an increased risk of respiratory disease-specific mortality. A DOSE index score of 4–5 was also independently and statistically significantly associated with an increased risk of cardiovascular mortality. No associations were found for DOSE index with death due to cancer or other diseases (Figure 4).
Figure 4. DOSE index and cause-specific mortality.
Smoking definition and lung function
No statistically significant differences in mortality were found between ex-smokers and current smokers (data not shown). Patients with spirometry data (n=594) were compared with those without (n=517). Among patients with missing spirometry data (n=517), 25.0% died and, among patients with available spirometry data (n=594), 21.2% died (p=0.139). In the entire group no statistical significance was found for mortality associated with missing spirometry data (HR 1.10, 95% CI 0.86 to 1.41). The HR for mortality associated with missing spirometry data was statistically significantly raised when the analysis was restricted to primary care patients (HR 1.68, 95% CI 1.16 to 2.41), but not among secondary care patients (HR 1.20, 95% CI 0.79 to 1.81). In the group included in the main analysis (n=562), 20.6% died and, among patients excluded because of any missing data (n=549), 25.3% died (p=0.064). Excluding FEV1%pred from the DOSE index among the participants included in the main analysis produced a HR for the 4–5 group compared with the 0–3 group of 3.43 (95% CI 2.37 to 4.95) after adjustment for age, sex, and heart disease (no patients achieved a score of 6). Among the patients excluded from the main analysis due to missing data, the adjusted HR for mortality associated with a DOSE index score was 2.08 (95% CI 1.38 to 3.13) for a score of 4–5, and was 6.19 (95% CI 2.65 to 14.5) for a score of 6.
Discussion
Main findings
The primary finding of this multicentre study of COPD patients from primary and secondary care is that a higher DOSE index score is associated with increased mortality risk independent of sex, age, and level of care.
Interpretation of findings in relation to previously published work
A previous study has shown that the DOSE index can predict hospital admission, respiratory failure, and future exacerbations10, and another study has found an association with mortality in male hospital outpatients.13 To our knowledge, this is the first study where an association with mortality is shown for both sexes and in a population including patients from both primary and secondary care. Thus, this index could be a convenient clinical tool for estimating mortality risk among COPD patients in primary care.
The total DOSE index score was robustly associated with mortality. The majority of the separate components that constitute the index were also associated with mortality. The most established predictor of mortality in COPD is FEV1%pred,9,17 although results from one study suggested that dyspnoea measured by the MRC dyspnoea scale is a more reliable predictor.18 Our study demonstrates that the DOSE index — which includes both of these measures — is an even better predictor of mortality. Our analysis also found that the composite DOSE index was a more effective predictor of mortality than its separate components.
The univariate association between smoking status and mortality was not statistically significant. However, in analyses adjusted for the other DOSE index components, smoking was statistically significantly associated with mortality, thus possibly adding a useful contribution to the combined index. A dichotomous measure of current smoking status is potentially limited in precision since previous smoking is not taken into account. An alternative measure of smoking using pack-years has been shown to predict mortality in stable COPD,19 but a study of COPD patients with severe obstruction failed to show an independent association with mortality for number of pack-years.20 Other authors have found current smoking in COPD patients to be associated with increased mortality compared with never or ex-smokers, independent of pack-years.21–23
Although the DOSE index was associated with mortality in both men and women, the interaction analysis showed that the associations were not identical. An HR of higher magnitude was found for a specific DOSE index score in men compared with women. In contrast, a study of the BODE index24 found an HR of higher magnitude in women than in men. In another study,25 BMI was shown to be a more significant component of the total BODE index score in women than in men. Additional adjustment for BMI and depression26,27 in our study indicated that these factors did not explain sex differences in the associations with mortality.
The analyses of cause-specific mortality showed that the DOSE index is more predictive of death by respiratory disease than other causes. The association of the DOSE index with cardiovascular disease mortality was only statistically significant for a DOSE index score of 4–5, possibly due to a small number of individuals in the 6–7 score group. Determination of cause of death from death certificates can be problematic as certificates may have been incorrectly completed. Several studies have reported that COPD is predominantly under-reported as the underlying cause of death,28,29 especially when the primary cause is not pulmonary.30 This suggests that the association of the DOSE index with cardiovascular mortality might be overestimated due to over-recording of cardiovascular disease instead of COPD. However, the important association between the DOSE index and mortality by respiratory disease should not have been overestimated.
The BODE index combines BMI, obstruction (FEV1%pred), dyspnoea (MRC scale), and exercise capacity measured by the 6-minute walking distance (6MWD), and has also been demonstrated to predict mortality.11,12,31,32 Exercise capacity cannot be examined easily, which makes it unsuitable for most clinical settings. Another composite prognostic instrument is the ADO index, including age, dyspnoea (MRC scale) and obstruction (FEV1%pred).12 Both the ADO and the DOSE index are convenient to administer in clinical settings. An additional advantage of the DOSE index is that it includes measures of health and behaviour such as exacerbations and smoking that can be modified by intervention. Exacerbations have been associated with lung function decline,33 lower quality of life,34 and mortality5 and can be modified by treatment.35,36
Strengths and limitations
The strengths of this study are that it is longitudinal and that the study population is sampled from multiple centres representing both primary and secondary care. The data from medical records were initially recorded prospectively and should be reliable and not subject to recall bias. A possible limitation is that a proportion of the original study population was not included in the main analysis, mainly due to lack of spirometry data.
The fact that many COPD diagnoses are based on clinical findings rather than spirometry has been previously demonstrated.14 The exclusion of patients without this information may result in selection bias, which could potentially influence the results as there was higher mortality among patients with missing data in both primary and secondary care. Possible explanations might be that the patients with most severe disease have been unable or unwilling to perform a lung function test or that they had a diagnosis confirmed by spirometry before study entry. However, when we analysed the entire population after removal of the lung function component from the index, the association with mortality remained in patients both with and without spirometry data. Thus, an index excluding lung function data could be an alternative measure that can be used to predict mortality when spirometry is not available.
Implications for future research, policy and practice
In our opinion, the DOSE index appears to be very suitable for use in primary care since it combines information relevant to both clinical management and assessment of prognosis. Thus, it could be a useful instrument for clinicians in both primary and secondary care to identify mortality risk in COPD patients, enabling optimised management and treatment.
Conclusions
We conclude that the DOSE index can be used as a prognostic instrument for mortality in COPD. The combined DOSE index was a better predictor of mortality than any of its components alone. Our data also indicate that a composite index with dyspnoea, smoking and exacerbations without spirometry data can be clinically useful for assessing prognosis.
Acknowledgments
Handling editor Niels Chavannes
Statistical review Gopal Netuveli
Funding The study was supported by grants from the county councils of the Uppsala-Örebro Health Care region, the Swedish Heart and Lung Association, the Swedish Asthma and Allergy Association, the Bror Hjerpstedts Foundation, and the Örebro Society of Medicine.
The authors thank Ulrike Spetz-Nyström and Eva Manell for reviewing the patient records and all the participating centres.
Footnotes
The authors declare they have no conflicts of interest in relation to this article. BS is an Associate Editor of the PCRJ, but was not involved in the editorial review of, nor the decision to publish, this article.
References
- Rabe KF, Beghe B, Luppi F, Fabbri LM. Update in chronic obstructive pulmonary disease 2006. Am J Respir Crit Care Med 2007;175(12):1222–32. http://dx.doi.org/10.1164/rccm.200704-586UP [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997;349(9064):1498–504. http://dx.doi.org/10.1016/S0140-6736(96)07492-2 [DOI] [PubMed] [Google Scholar]
- Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest 2000;117(2 Suppl):1S–4S. http://dx.doi.org/10.1378/chest.117.2_suppl.1S [DOI] [PubMed] [Google Scholar]
- Almagro P, Calbo E, Ochoa de Echaguen A, et al. Mortality after hospitalization for COPD. Chest 2002;121(5):1441–8. http://dx.doi.org/10.1378/chest.121.5.1441 [DOI] [PubMed] [Google Scholar]
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest 2003;124(2):459–67. http://dx.doi.org/10.1378/chest.124.2.459 [DOI] [PubMed] [Google Scholar]
- Gudmundsson G, Gislason T, Lindberg E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res 2006;7:109. http://dx.doi.org/10.1186/1465-9921-7-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. BMJ 1959;2(5147):257–66. http://dx.doi.org/10.1136/bmj.2.5147.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93(3):580–6. http://dx.doi.org/10.1378/chest.93.3.580 [DOI] [PubMed] [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for diagnosis, management, and prevention of COPD 2001 [updated 2009; cited 2012 20th April]; Available from: http://www.goldcopd.com.
- Jones RC, Donaldson GC, Chavannes NH, et al. Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease: the DOSE index. Am J Respir Crit Care Med 2009;180(12):1189–95. http://dx.doi.org/10.1164/rccm.200902-0271OC [DOI] [PubMed] [Google Scholar]
- Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350(10):1005–12. http://dx.doi.org/10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- Puhan MA, Garcia-Aymerich J, Frey M, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009;374(9691):704–11. http://dx.doi.org/10.1016/S0140-6736(09)61301-5 [DOI] [PubMed] [Google Scholar]
- Oga T, Tsukino M, Hajiro T, Ikeda A, Nishimura K. Predictive properties of different multidimensional staging systems in patients with chronic obstructive pulmonary disease. Int J COPD 2011;6:521–6. http://dx.doi.org/10.2147/COPD.S24420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arne M, Lisspers K, Stallberg B, et al. How often is diagnosis of COPD confirmed with spirometry? Respir Med 2010;104(4):550–6. http://dx.doi.org/10.1016/j.rmed.2009.10.023 [DOI] [PubMed] [Google Scholar]
- Sundh J, Stallberg B, Lisspers K, Montgomery SM, Janson C. Co-morbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD 2011;8(3):173–81. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- Siafakas NM, Vermeire P, Pride NB, et al. Optimal assessment and management of chronic obstructive pulmonary disease (COPD). The European Respiratory Society Task Force. Eur Respir J 1995;8(8):1398–420. http://dx.doi.org/10.1183/09031936.95.08081398 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121(5):1434–40. http://dx.doi.org/10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- Esteban C, Quintana JM, Aburto M, et al. Predictors of mortality in patients with stable COPD. J Gen Intern Med 2008;23(11):1829–34. http://dx.doi.org/10.1007/s11606-008-0783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FJ, Foster G, Curtis JL, et al. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006;173(12):1326–34. http://dx.doi.org/10.1164/rccm.200510-1677OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EF, Phanareth K, Laursen LC, Kok-Jensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159(4 Pt 1):1267–71. [DOI] [PubMed] [Google Scholar]
- Hersh CP, DeMeo DL, Al-Ansari E, et al. Predictors of survival in severe, early onset COPD. Chest 2004;126(5):1143–51. http://dx.doi.org/10.1378/chest.126.5.1443 [DOI] [PubMed] [Google Scholar]
- Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142(4):233–9. [DOI] [PubMed] [Google Scholar]
- de Torres JP, Cote CG, Lopez MV, et al. Sex differences in mortality in patients with COPD. Eur Respir J 2009;33(3):528–35. http://dx.doi.org/10.1183/09031936.00096108 [DOI] [PubMed] [Google Scholar]
- de Torres JP, Casanova C, de Garcini AM, Jaime AA, Celli BR. COPD heterogeneity: gender differences in the multidimensional BODE index. Int J COPD 2007;2(2):151–5. [PMC free article] [PubMed] [Google Scholar]
- Fan VS, Ramsey SD, Giardino ND, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med 2007;167(21):2345–53. http://dx.doi.org/10.1001/archinte.167.21.2345 [DOI] [PubMed] [Google Scholar]
- de Voogd JN, Wempe JB, Koeter GH, et al. Depressive symptoms as predictors of mortality in patients with COPD. Chest 2009;135(3):619–25. http://dx.doi.org/10.1378/chest.08-0078 [DOI] [PubMed] [Google Scholar]
- Hansell AL, Walk JA, Soriano JB. What do chronic obstructive pulmonary disease patients die from? A multiple cause coding analysis. Eur Respir J 2003;22(5):809–14. http://dx.doi.org/10.1183/09031936.03.00031403 [DOI] [PubMed] [Google Scholar]
- Jensen HH, Godtfredsen NS, Lange P, Vestbo J. Potential misclassification of causes of death from COPD. Eur Respir J 2006;28(4):781–5. http://dx.doi.org/10.1183/09031936.06.00152205 [DOI] [PubMed] [Google Scholar]
- Drummond MB, Wise RA, John M, Zvarich MT, McGarvey LP. Accuracy of death certificates in COPD: analysis from the TORCH trial. COPD 2010;7(3):179–85. http://dx.doi.org/10.3109/15412555.2010.481695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote CG, Pinto-Plata VM, Marin JM, Nekach H, Dordelly LJ, Celli BR. The modified BODE index: validation with mortality in COPD. Eur Respir J 2008;32(5):1269–74. http://dx.doi.org/10.1183/09031936.00138507 [DOI] [PubMed] [Google Scholar]
- Martinez FJ, Han MK, Andrei AC, et al. Longitudinal change in the BODE index predicts mortality in severe emphysema. Am J Respir Crit Care Med 2008;178(5):491–9. http://dx.doi.org/10.1164/rccm.200709-1383OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the Lung Health Study. Am J Respir Crit Care Med 2001;164(3):358–64. [DOI] [PubMed] [Google Scholar]
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157(5 Pt 1):1418–22. [DOI] [PubMed] [Google Scholar]
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356(8):775–89. http://dx.doi.org/10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359(15):1543–54. http://dx.doi.org/10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]