Abstract
Background:
Most patients with asthma are managed exclusively in primary care. Little is known about the patterns of airway dysfunction in these patients and how these relate to other aspects of the disease.
Aims:
We set out to assess this in a cross-sectional study of 262 patients.
Methods:
Symptoms, spirometry, airway responsiveness, reversibility, and airway inflammation were all assessed. Exacerbations requiring oral corticosteroids in the preceding year were enumerated.
Results:
Patients had heterogeneous patterns of airway dysfunction. Those with a post-bronchodilator forced expiratory volume in 1 sec/ forced vital capacity ratio of <0.7 had more exacerbations in the previous year (2.2 vs. 0.8; mean difference 1.4; 95% CI 0.4 to 2.4; p=0.007). Patients with normal results had less inflammation (proportion with a sputum eosinophil count of >1.9%, 20% vs. 48%, χ2=14.8, df=3; p<0.001) and fewer exacerbations (0.5 vs. 1.4; mean difference −0.9; 95% CI −1.4 to −0.4; p=0.001) but similar symptom scores (6.2 vs. 6.9; p=0.2) compared with patients with any abnormality.
Conclusions:
Patients with a diagnosis of asthma have mixed patterns of physiological impairment; many have no airflow obstruction or airway hyper-responsiveness. The physiological characterisation of asthma is not related to symptoms and is of little value in predicting exacerbations or eosinophilic airway inflammation.
Keywords: asthma, COPD, diagnosis, primary care
Introduction
Asthma is a common and treatable condition affecting eight million people in the UK. Of these, 5.1 million people receive treatment, with the total cost to the UK National Health Service (NHS) being approximately £850 million/year.1 Four main features comprise the current description of asthma: symptoms, airway inflammation, airway hyper-responsiveness, and variable airflow obstruction.2 Airway abnormalities in asthma can be functionally assessed by demonstrating reversible airflow obstruction and/or an abnormal bronchoconstrictor response to an inhaled stimulus. International guidelines recommend the use of these tests to support a diagnosis of asthma, assess asthma control, and quantify the risk of an exacerbation.2 These guidelines assume that patients with suspected asthma who have positive test results are more likely to have asthma-related morbidity and pathology. However, there remains uncertainty about how airway dysfunction relates to other important features of asthma such as symptoms, exacerbations, and eosinophilic airway inflammation. This is particularly the case in primary care where the majority of patients with asthma are managed.1
We set out to evaluate patterns of airway dysfunction and their relation to other features of the disease in a population of 262 patients with asthma managed exclusively in primary care.
Methods
Patients
Patients were identified from 12 general practice registers around Leicester between July 2004 and July 2006. All patients were aged >18 years and had a diagnosis of asthma in their GP notes. Patients were eligible if they had received at least one asthma medication prescription in the last 12 months. The study was restricted to non-smokers with a smoking history of <10 pack-years. Ethical approval for the study was given by the ethics committees of both the University Hospitals of Leicester and the Leicester Primary Research Care Alliance (University Hospitals of Leicester 9141: Leicester Primary Research Care Alliance 0274, Clinical trial registered with www.controlled-trials.com ISRCTN08067387). Patients were initially recruited to participate in two different studies, one assessing the use of exhaled nitric oxide in the management of asthma3 and the other the role of breathing training.4
Test methods
Clinical
A structured respiratory history and examination was performed, spirometry was measured using a Vitalograph® spirometer, and airway hyper-responsiveness was assessed using increasing concentrations of methacholine to provoke a 20% fall in forced expiratory volume in 1 s (FEV1) (PC20).5 Patients with an FEV1 <80% predicted and FEV1/forced vital capacity (FVC) ratio of <0.7 had spirometry repeated 15 mins after inhaling 400μg salbutamol instead of a PC20. Induced sputum analysis using a Medix ultrasonic nebuliser to assess airway inflammation was performed as previously described.6
Questionnaires
To establish the number of previous asthma exacerbations, patients were asked to recall the number of steroid courses administered on account of their asthma over the previous year. This information was cross-checked against both primary and secondary care records wherever possible. Asthma control was assessed using the modified Juniper Asthma Control Questionnaire (ACQ) with removal of spirometric values and bronchodilator use. This questionnaire consists of five questions which assess daytime and night-time symptoms.7 Questions are scored on a scale of 0–6 with lower numbers representing better control of symptoms. The presence or absence of other co-morbidities commonly found in asthma were assessed by validated questionnaires: the Nijmegen questionnaire was used to quantify levels of hyperventilation with a value of >23 suggestive of hyperventilation8 and a gastro-oesophageal reflux score was used to assess levels of reflux experienced by the patients, again with higher scores indicating higher levels of reflux.9
Patterns of airflow limitation
Cut-off values to delineate the groups were established a priori on the basis of the current Global Initiative for Asthma (GINA)2 and Global Initiative for Chronic Obstructive Lung Disease (GOLD)10 guidelines. Patients with an improvement in FEV1 after bronchodilator of >12% were considered to have reversible airflow obstruction. Patients with a post-bronchodilator FEV1/FVC ratio of <0.7 were assumed to have fixed airflow obstruction. Airway hyper-responsiveness was defined as a methacholine PC20 of <8mg/ml and eosinophilic airway inflammation was defined as a differential sputum eosinophil count >1.9%.11
The percentage eosinophil count and methacholine PC20 were log-normally distributed and were therefore log-transformed for statistical methods which assume normal distribution. An independent t-test with Tukey's post hoc analysis was used for multiple comparisons between the numerical factors in the groups. χ2 analysis was used for comparison of categorical data. Study size reflected recruitment into the two original studies.
Results
A total of 4,039 patients were invited to participate, 322 of whom met the entry criteria for the initial studies; 20 patients declined consent and 40 patients were excluded due to insufficient data, so 262 patients (152 female) were therefore included in the study. Of these, 18% were at step 1 of the BTS asthma guidelines, 43% at step 2, 28% at step 3, 10% at step 4, and 1% at step 5. Patient details are shown in Table 1.
Table 1. Patient demographics (N=262).
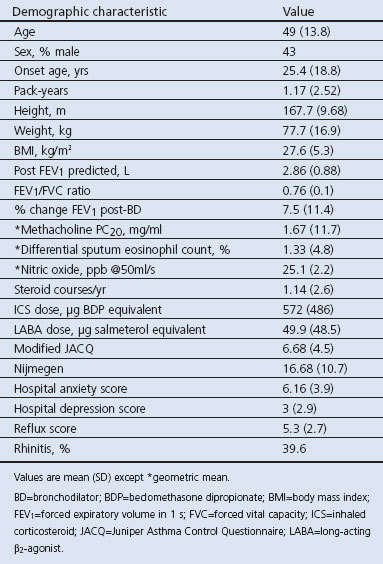
Patients had heterogeneous patterns of airway dysfunction. The largest group had no evidence of airflow obstruction or airway reversibility (n=169, 65%); of these, 87 (51%) had a methacholine PC20 of <8mg/ml. Reversible airflow obstruction was seen in 57 patients (22%); only 31 had fully reversible airflow obstruction (i.e. improvement in FEV1 after bronchodilator of >12% and a post-bronchodilator FEV1/FVC ratio of >0.7). A degree of fixed airflow obstruction (post-bronchodilator FEV1/FVC ratio <0.7) was present in 62 patients (24%), of whom 41% had an increase in post-bronchodilator FEV1 of >12%.
Patients with normal tests had similar symptom scores to those with airway dysfunction (ACQ 6.2 vs. 6.9, p=0.2) despite a lower prevalence of asthma-related inflammation (proportion with sputum eosinophil count of >1.9%, 20% vs. 48%, χ2=14.8; p<0.001; df=3) and fewer exacerbations (0.5 vs. 1.4; mean difference −0.9; 95% CI −1.4 to −0.4; p=0.001). Markers of depression, dysfunctional breathing, and gastro-oesophageal reflux and the proportion of patients reporting rhinitis were similar in all groups (Table 2).
Table 2. Parameters of airway dysfunction.
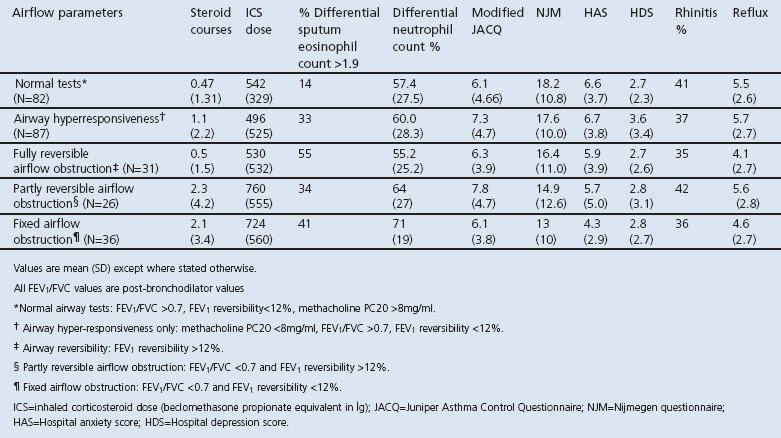
Patients with a post-bronchodilator FEV1/FVC <0.7 had both increased numbers of exacerbations in the previous year (2.2 vs. 0.8, mean difference 1.4, 95% CI 0.4 to 2.4, p=0.007) and higher total sputum neutrophil numbers (68 vs 58, mean difference 9.7, 95% CI 2.1 to 17.3, p=0.013) whether or not they had a significant bronchodilator response.
Discussion
Main findings
This study is the first to investigate systematically the patterns of airflow limitation in a population of patients with a primary care diagnosis of asthma and to relate these to symptoms and exacerbations. We found that patients with a primary care diagnosis of asthma who are currently receiving treatment have mixed patterns of physiological impairment. One-third of patients had no evidence of airway dysfunction. This population had low rates of asthma exacerbation and a low prevalence of eosinophilic airway inflammation. A significant population met current diagnostic criteria for chronic obstructive pulmonary disease (COPD); this group had a high frequency of exacerbations.
Strengths and limitations of this study
Our findings are important in that they show that abnormalities of airway function traditionally associated with asthma were not associated with differences in other features such as symptoms and eosinophilic airway inflammation, implying that a symptom-based assessment would only provide a limited perspective. Moreover, our findings suggest that abnormalities of airway function traditionally associated with asthma are often not seen in treated asthma patients in the community. The highest rate of exacerbation in the preceding 12 months was seen in patients with spirometric results normally associated with COPD.10 This group (a quarter of the study population) had other features of the disease (they were older and had a sputum neutrophilia). Subjects were current non-smokers with a smoking history of <10 pack-years, so smoking is unlikely to have been an aetiological factor. Other factors including duration of asthma, recurrent exacerbations,12 environmental factors, and genetic susceptibility are more likely explanations for this pattern of lung function abnormality. The important clinical point is that subjects in this group had a high exacerbation frequency in the previous year and may therefore represent a high-risk group requiring closer monitoring and more intensive treatment. Corticosteroid-responsive eosinophilic airway inflammation was as prevalent in this group as in the others, suggesting that the current management paradigm which advocates late corticosteroid treatment in patients labelled with COPD might lead to under-treatment. The existence of this population suggests that more intensive assessment which moves beyond simple spirometry and reversibility testing and evaluates all aspects of airway disease may be required for optimum management.
There are two important limitations to our study; first, as our study is cross-sectional, we have no information on what basis the original diagnosis of asthma was made. We cannot exclude the possibility that treatment or time obscured the relationship between reversible airflow obstruction, airway hyper-responsiveness, and the other markers of asthma. We could not assess whether assessment of peak expiratory flow variability added any value to the tests assessed as these data were not collected. However, abnormal peak flow variability is an insensitive test in patients with symptomatic asthma,13 suggesting that it would not. Second, there may be differences in symptoms and treatment levels between patients who replied to our study invitation and those who did not; however, the rate of airway abnormality seen in our study is similar to that observed in others.14,15
Interpretation of findings in relation to previously published work
Our finding that around one-third of the patients assessed had normal spirometry and airway responsiveness is consistent with previous cross-sectional and epidemiological studies.14,15 It is possible that these normal findings reflect a particularly good response to treatment, although intervention studies have shown that it is unusual for methacholine responsiveness to return to normal following treatment in adults with asthma16 and symptom scores were not appreciably lower than in patients with airway dysfunction. A more likely possibility is that some of the group had alternative explanations for their asthma-like symptoms. We found no evidence that markers of conditions that could be an alternative cause for asthma-type symptoms such as rhinitis, gastro-oesophageal reflux, and dysfunctional breathing were associated particularly with this group, arguing against this possibility. However, a more rigorous evaluation is required; further longitudinal studies are also needed to determine whether this group can be treated and assessed less intensively.
Implications for future research, policy and practice
This study suggests that asthma seen in primary care is a heterogeneous condition — as is asthma seen in secondary care17 — and that one-third of patients have no evidence of airway dysfunction despite having symptoms. Future research should focus on identifying patients with symptoms and no evidence of airway dysfunction to assess long-term outcomes, need for asthma treatment, and the risk of over-diagnosis in primary care. As evidence grows that asthma diagnosis is difficult in primary care,18,19 the research focus should shift to understanding the heterogeneity and interplay between physiology and symptoms in mild to moderate asthma rather than refractory asthma alone. This may help to identify new treatments — both pharmacological and supportive — that reduce the patient and economic burden of this common presentation.
Our results suggest that more attention should be paid to getting the diagnosis correct before treatment is started, especially as a diagnosis of asthma has the potential for long-term treatment and also because a diagnosis can be harder to confirm or refute once treatment has commenced.
Conclusions
We have shown that patients with a primary care diagnosis of asthma who are receiving treatment have mixed patterns of airway dysfunction despite having similar symptom scores. We found no evidence that tests traditionally associated with asthma identified patients with more asthma-related morbidity. Normal test results were associated with less eosinophilic airway inflammation and a low exacerbation frequency, whereas a pattern more traditionally associated with COPD was associated with increased exacerbations. Consequently, patients with spirometric evidence of COPD may represent a high-risk group requiring closer monitoring and more intensive treatment.
Our findings suggest that the focus of asthma assessment in primary care should expand from the presence or absence of reversibility and that more attention should be paid to accuracy of diagnosis in a disease that is both diagnosed and categorised by treatment response.
Acknowledgments
Handling editor Maureen George
Statistical review Gopal Netuveli
Funding Funded by a grant from Asthma UK.
The authors acknowledge the expert input of the laboratory staff: Debbie Parker, Will Monterio, and Natalie Neale. The authors also thank the volunteers for their time, effort and commitment.
Footnotes
The authors declare they have no conflicts of interest in relation to this article.
References
- Asthma UK. Where do we stand? Asthma in the UK today. 2005.
- Bousquet J. Global Initiative for Asthma (GINA) and its objectives. Clin Exp Allergy 2000;30(Suppl 1):2–5. http://dx.doi.org/10.1046/j.1365-2222.2000.00088.x [DOI] [PubMed] [Google Scholar]
- Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med 2007;176(3):231–7. http://dx.doi.org/10.1164/rccm.200610-1427OC [DOI] [PubMed] [Google Scholar]
- Thomas M, McKinley RK, Mellor S, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax 2009;64(1):55–61. http://dx.doi.org/10.1136/thx.2008.100867 [DOI] [PubMed] [Google Scholar]
- Juniper EF, Frith PA, Dunnett C, Cockcroft DW, Hargreave FE. Reproducibility and comparison of responses to inhaled histamine and methacholine. Thorax 1978;33(6):705–10. http://dx.doi.org/10.1136/thx.33.6.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax 1997;52(6):498–501. http://dx.doi.org/10.1136/thx.52.6.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper EF, O'Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway calibre and rescue beta2-agonist use? Respir Med 2001;95(5):319–23. http://dx.doi.org/10.1053/rmed.2001.1034 [DOI] [PubMed] [Google Scholar]
- van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen Questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res 1985;29(2):199–206. http://dx.doi.org/10.1016/0022-3999(85)90042-X [DOI] [PubMed] [Google Scholar]
- Manterola C, Munoz S, Grande L, Bustos L. Initial validation of a questionnaire for detecting gastroesophageal reflux disease in epidemiological settings. J Clin Epidemiol 2002;55(10):1041–5. http://dx.doi.org/10.1016/S0895-4356(02)00454-7 [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163(5):1256–76. [DOI] [PubMed] [Google Scholar]
- Green RH, Brightling CE, Woltmann G, Parker D, Wardlaw AJ, Pavord ID. Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids Thorax 2002;57(10):875–9. http://dx.doi.org/10.1136/thorax.57.10.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest 2004;126(1):59–65. http://dx.doi.org/10.1378/chest.126.1.59 [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Brightling CE, Woltmann G, Wardlaw AJ, Pavord ID. A comparison of the validity of different diagnostic tests in adults with asthma. Chest 2002;121(4):1051–7. http://dx.doi.org/10.1378/chest.121.4.1051 [DOI] [PubMed] [Google Scholar]
- LindenSmith J, Morrison D, Deveau C, Hernandez P. Overdiagnosis of asthma in the community. Can Respir J 2004;11(2):111–16. [DOI] [PubMed] [Google Scholar]
- Marklund B, Tunsater A, Bengtsson C. How often is the diagnosis bronchial asthma correct? Family Pract 1999;16(2):112–16. http://dx.doi.org/10.1093/fampra/16.2.112 [DOI] [PubMed] [Google Scholar]
- Boulet LP. Physiopathology of airway hyperresponsiveness. Curr Allergy Asthma Rep 2003;3(2):166–71. http://dx.doi.org/10.1007/s11882-003-0030-9 [DOI] [PubMed] [Google Scholar]
- Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178(3):218–24. http://dx.doi.org/10.1164/rccm.200711-1754OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks VP, Vandemheen KL, Aaron SD. Confirmation of asthma in an era of overdiagnosis. Eur Respir J 2010;36(2):255–60. http://dx.doi.org/10.1183/09031936.00165109 [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ 2008;179(11):1121–31. http://dx.doi.org/10.1503/cmaj.081332 [DOI] [PMC free article] [PubMed] [Google Scholar]


