Abstract
Background: This study was initially designed to examine whether oxaliplatin-based regimen was superior to cisplatin-based regimen in tumour remission as first-line chemotherapy for advanced gastric cancer (GC).
Methods: Literature in EMBASE, PUBMED, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) was searched. Only phase II or III randomized controlled trials (RCTs) comparing the effectiveness and safety between oxaliplatin-based and cisplatin-based regimens as first-line treatment for advanced GC were selected. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported. The primary endpoints were complete remission rate (CRR), partial remission rate (PRR), objective response rate (ORR), and disease control rate (DCR). The second endpoint was the toxicity response.
Results: 2,140 patients from six phase II or III RCTs were included. Compared to cisplatin-based therapy, subjects who received oxaliplatin-based treatment had significantly higher PRR (OR: 1.25, 95%CI: 1.05-1.48, P=0.01, I2=0%), ORR (OR: 1.21, 95%CI: 1.02-1.44, P=0.03, I2=0%) and DCR (OR: 1.76, 95%CI: 1.31-2.38, P=0.0002, I2=25%), but not CRR (OR: 0.70, 95%CI: 0.37-1.31, P=0.27, I2=0%). In addition, oxaliplatin-based therapy significantly decreased all grades of leukopenia, neutropenia, anemia, febrile neutropenia, nausea, stomatitis, creatinine elevation and thromboembolism, as well as grades 3-4 of leukopenia, neutropenia, anemia and febrile neutropenia than cisplatin-based regimen. However, oxaliplatin-based treatment strikingly increased the risk of thrombocytopenia, sensory neuropathy, diarrhea, fatigue and liver dysfunction.
Conclusions: Oxaliplatin-based regimen is superior to cisplatin-based regimen in tumour remission as first-line chemotherapy for advanced GC, and is associated with less toxicity and better tolerability.
Keywords: oxaliplatin, cisplatin, tumour remission, chemotherapy, gastric cancer
Introduction
Gastric cancer (GC) remains the fifth most common cancer and the third leading cause of cancer death worldwide 1-2. Although great progress has been made in early screening and detection, only about 25% of all subjects with GC are presented with resectable disease. The majority of the patients are diagnosed with locally advanced or metastatic GC needing systemic palliative chemotherapy 3-4. Accordingly, a platinum-based two- or three-drug combination regimen has been identified as one of the standard first-line options for patients with advanced GC, with an approximately objective response rate (ORR) of 20 to 30%, and median overall survival (OS) of 6 to 10 months 4-7.
Cisplatin is the first platinum compound to be discovered and shows strong anti-tumour activity in various malignancies. However, its application in clinical practice is extremely limited due to its severe nephrotoxicity, high-frequency of vomiting, obvious peripheral neuropathy, as well as the requirement of intravenous hydration, especially for old patients 6-7. Oxaliplatin, a third-generation platinum with a 1,2-diaminocyclohexane (DACH) carrier ligand, has been confirmed to have comparable efficacy and less nephrotoxicity and gastrointestinal toxicity compared to cisplatin 8-9. Thus, it is now widely used in patients with colorectal cancer, GC, and relapsed or refractory lymphoma 10.
As both oxaliplatin and cisplatin showed significant anti-tumour activity in advanced GC, researchers began to pay attention to the difference between oxaliplatin-based and cisplatin-based regimens 11-16. Al-Batran et al. found that fluorouracil plus leucovorin and oxaliplatin (FLO) reduced toxicity as compared with fluorouracil plus leucovorin and cisplatin (FLP) in patients with advanced GC. In addition, FLO was also associated with improved efficacy in older adult cases 11. Moreover, researchers from Japan performed a phase III study comparing oxaliplatin plus S-1 (SOX) with cisplatin plus S-1 (CS) in chemotherapy-naive patients with advanced GC, and the results showed that SOX was as effective as CS in terms of progression-free survival (PFS) and OS, and with favorable safety profile 15. Furthermore, a recent meta-analysis showed that there were no significant difference in ORR, PFS and OS between oxaliplatin-based therapy and cisplatin- based therapy. And the oxaliplatin-based regimen could generally decrease the risk of adverse events except neurosensory toxicity and thrombocytopenia 17. However, there is still lacking efficient data evaluating the difference in tumour remission between oxaliplatin-based and cisplatin-based regimens for advanced GC.
Therefore, this study was initially designed to examine whether oxaliplatin-based regimen was superior to cisplatin-based regimen in tumour remission as first-line chemotherapy for patients with advanced GC.
Materials and methods
Literature-search strategy
EMBASE, PUBMED, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, as well as American Society of Clinical Oncology (ASCO) and European Society of Medical Oncology (ESMO) (up to June 2018) were systematically searched using the following combination terms: (“platinum” or “cisplatin” or “oxaliplatin”), (“gastric” or “stomach” or “gastro-oesophageal” or “oesophago-gastric”), (“cancer” or “carcinoma” or “tumor” or malignancy” or “neoplasia”), (“metastatic” or “locally advanced” or “unresectable” or “recurrent” or “stage IV”), and (“clinical trial” or “prospective trials” or “randomized controlled trial”). The search was limited to English language, and relevant references in the primary publications were also checked to find additional studies. All studies were selected and systemically reviewed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Inclusion criteria
Relevant studies that met the following criteria were included: (1) patients were histopathologically or pathologically diagnosed with advanced or recurrent GC; (2) patients received cisplatin-based or oxaliplatin-based combination treatment as first-line chemotherapy in the same study; (3) prospective phase II or III randomized controlled trials (RCTs); (4) trials with reports on response and toxicity profiles; (5) there was enough data for extraction. Review articles, irrelevant topics, non-comparative studies, case reports, and animal experimental studies were excluded.
Data extraction and study endpoints
The publications and data were reviewed and extracted by two independent investigators (Z.Y.Y. and Z.F.). The relevant information of each study including: (1) article or publication information, such as first author's name, year of publication, etc.; (2) patient characteristics, such as diagnosis, age, gender, etc.; (3) study designation information, such as phase, total study population, subjects enrolled per arm; (4) information about treatment, such as treatment strategy, dose and cycle used; (5) response and toxicity profile and so on were carefully extracted, and they were recorded and entered into an electronic database.
And any discrepancy was resolved by discussion and consensus with a third reviewer (J.Z.Y.). Response was determined in accordance with Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) by the investigators. While adverse events were assessed and recorded according to the National Cancer Institute's Common Toxicity Criteria for Adverse Events (NCI-CTCAE) (version 3.0 or 4.0), which has been widely used in cancer clinical trials. The primary endpoints were complete remission rate (CRR), partial remission rate (PRR), ORR, and disease control rate (DCR). The second endpoint was the toxicity response.
Statistical analysis
Review Manager (RevMan) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) software was applied to conduct the statistical analyses of the odds ratios (ORs) for CRR, PRR, ORR and DCR, as well as treatment-related adverse events. Heterogeneity between selected studies was determined by the χ2-based Q statistic, and it was considered with statistical significance when Pheterogeneity < 0.05 or I2 > 50%. When heterogeneity existed, data was analyzed with a random-effects model; otherwise, a fixed-effects model was performed. OR > 1 indicated more deaths or progressions with the cisplatin-based regimen, and OR > 1 indicated more toxicities and ORR with the cisplatin-based regimen. A two-sided p value < 0.05 was considered statistically significant. All confidence intervals (CIs) had a two-sided probability and coverage of 95%. A funnel plot was used to assess potential publication bias.
Results
Literature search
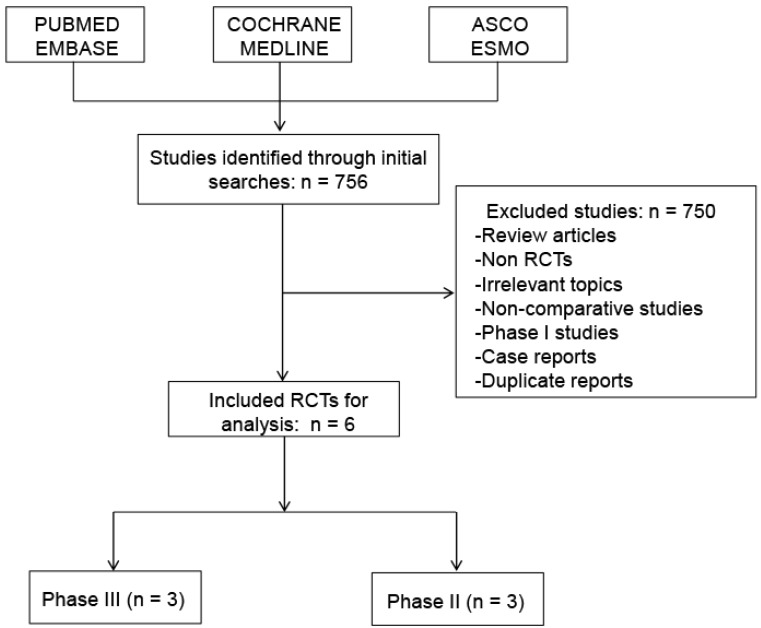
A total of 2,140 patients from six phase II or III RCTs met the inclusion criteria and were selected in the final analysis (Figure 1). The evaluation of references in the primary publications did not yield any additional studies for analysis. Review articles, non-RCTs, irrelevant topics, non-comparative studies, phase I studies and case reports were excluded.
Figure 1.
Flow diagram of studies identified, included, and excluded. RCTs, randomized controlled trials.
Characteristics of eligible studies
756 initial publications were obtained through a systematic database search. Six RCTs with 2140 subjects diagnosed with advanced GC were included in the present analysis. The characteristics of the selected studies are listed in Table 1. All of the enrolled patients received a cisplatin-based or oxaliplatin-based regimen as first-line treatment. And all six studies were reported in full text and had sufficient data for data extraction.
Table 1.
Characteristics of the included studies.
| Study | Year | Phase | Patients | Patients per arm | Treatment | Jadad score |
|---|---|---|---|---|---|---|
| (Total) | (ITT population) | |||||
| Al-Batran et al. | 2008 | III | 220 | 112 | FLO: 5-FU 2600 mg/m2 24h-c.i.v. day 1, leucovorin 200 mg/m2 | 2 |
| 11 | 200 mg/m2 day 1, oxaliplatin 85 mg/m2 day 1, every 2 weeks | |||||
| 108 | FLP: 5-FU 2000 mg/m2 24h-c.i.v. day 1, leucovorin 200 mg/m2 | |||||
| day 1, weekly, cisplatin 50 mg/m2 day 1, every 2 weeks | ||||||
| Cunningham et al. | 2008 | III | 508 | 245 | EOF: epirubicin 60 mg/m2 day 1, oxaliplatin 85 mg/m2 | 3 |
| 12 | day 1, 5-FU c.i.v. 200 mg/m2 daily, every 3 weeks | |||||
| 263 | ECF: epirubicin 60 mg/m2 day 1, cisplatin 50 mg/m2 | |||||
| day 1, 5-FU c.i.v. 200 mg/m2 daily, every 3 weeks | ||||||
| Cunningham et al. | 2008 | III | 494 | 244 | EOX: epirubicin 60 mg/m2 day 1, oxaliplatin 85 mg/m2 | 3 |
| 12 | day 1, capecitabine 625 mg/m2 ×2 daily, every 3 weeks | |||||
| 250 | ECX: epirubicin 60 mg/m2 day 1, cisplatin 50 mg/m2 | |||||
| day 1, capecitabine 625 mg/m2 ×2 daily, every 3 weeks | ||||||
| Hironaka et al. | 2016 | II | 96 | 47 | SLO: S-1 40-60 mg plus leucovorin 25 mg twice a day for 1 week, | 2 |
| 16 | oxaliplatin 85 mg/m2 day 1, every 2 weeks | |||||
| 49 | SC: S-1 40-60 mg twice a day for 3 weeks, | |||||
| cisplatin 60 mg/m2 day 8, every 5 weeks | ||||||
| Kim et al. 14 | 2014 | II | 77 | 39 | DO: docetaxel 35 mg/m2 day 1 and 8, | 2 |
| oxaliplatin 120 mg/m2 day 1, every 3 weeks | ||||||
| 38 | DC: docetaxel 35 mg/m2 day 1 and 8, | |||||
| cisplatin 60 mg/m2 day 1, every 3 weeks | ||||||
| Popov et al. 13 | 2008 | II | 72 | 36 | FOL: 5-FU 400 mg/m2 i.v. bolus, 600 mg/m2 22h c.i.v. days 1-2, | 2 |
| oxaliplatin 85 mg/m2 day 1, FA 200 mg/m2 , days 1-2, every 2 weeks | ||||||
| 36 | FCL: 5-FU 400 mg/m2 i.v. bolus, 600 mg/m2 22h c.i.v. days 1-2, | |||||
| cisplatin 50 mg/m2 day1, FA 200 mg/m2 , days 1-2, every 2 weeks | ||||||
| Yamada et al. | 2015 | III | 673 | 338 | SOX: S1 80-120 mg/day for 2 weeks, | 3 |
| 15 | oxaliplatin 100 mg/m2 day 1, every 3 weeks | |||||
| 335 | SC: S1 80-120 mg/day for 3 weeks, | |||||
| cisplatin 60 mg/m2 day 8, every 5 weeks |
ITT, intention-to-treat, FA, folinic acid.
Meta-analysis of CRR and PRR
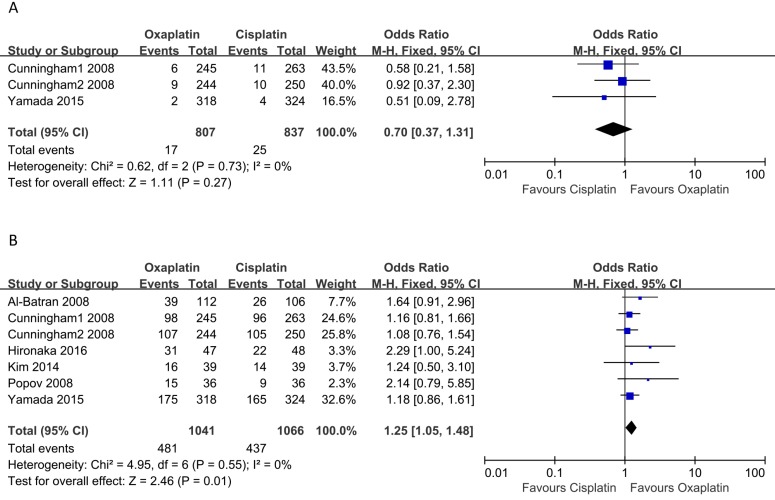
Three studies reported data on CR. The pooled analysis of CRR using a fixed-effects model did not demonstrate a significant difference between cisplatin-based and oxaliplatin-based regimens (OR: 0.70, 95% CI: 0.37-1.31, p = 0.37, Figure 2A), and there was no significant heterogeneity among the studies (I2 = 0, p = 0.73). As for PRR, all six included studies covered the PR. As shown in Figure 2B, the results showed that oxaliplatin-based therapy was significantly associated with higher PRR compared to cisplatin-based therapy (OR: 1.25, 95% CI: 1.05-1.48, p = 0.01). Moreover, no heterogeneity among the studies was observed (I2 = 0, p = 0.55).
Figure 2.
Meta-analysis of complete remission rate (A) and partial remission rate (B).
Meta-analysis of ORR and DCR
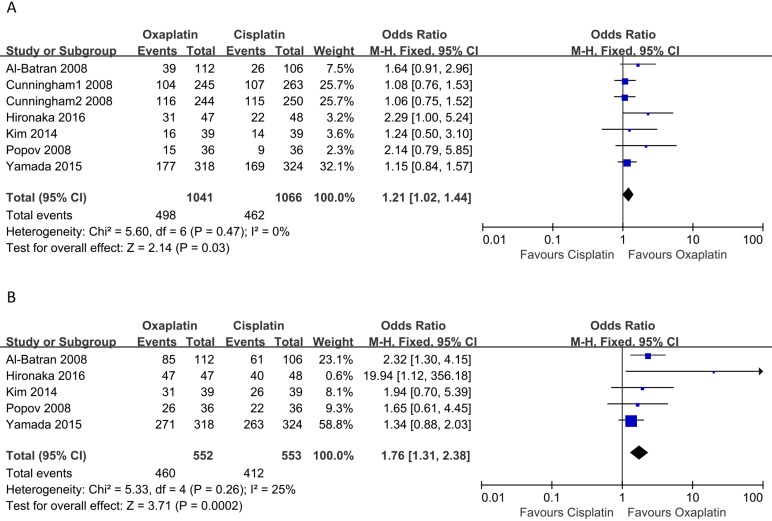
There were six and five of the selected studies reporting PRR and DCR, respectively. And the meta-analysis performed with a fixed-effects model suggested that patients who received oxaliplatin- based treatment tended to have higher ORR (OR: 1.21, 95% CI: 1.02-1.44, p = 0.03, Figure 3A) and DCR (OR: 1.76, 95% CI: 1.31-2.38, p = 0.0002, Figure 3B) than cisplatin-based treatment. In addition, no heterogeneity among the studies was indicated (I2 = 0, p = 0.47) and (I2 = 25%, p = 0.26), respectively.
Figure 3.
Meta-analysis of objective response rate (A) and disease control rate (B).
Meta-analysis of adverse events
The results of the pooled analysis of all grades and grades 3-4 adverse events were listed in Table 2. In comparison with cisplatin-based regimen, oxaliplatin-based regimen therapy significantly decreased the the risk of all grades of leukopenia (OR: 0.63, 95% CI: 0.49-0.81, p < 0.0001), neutropenia (OR: 0.58, 95% CI: 0.47-0.70, p < 0.0001), anemia (OR: 0.49, 95% CI: 0.41-0.60, p < 0.0001), febrile neutropenia (OR: 0.58, 95% CI: 0.40-0.82, p < 0.01), nausea (OR: 0.63, 95% CI: 0.49-0.82, p < 0.0001), stomatitis (OR: 0.82, 95% CI: 0.68-0.98, p = 0.03), creatinine elevation (OR: 0.16, 95% CI: 0.11-0.24, p < 0.0001) and thromboembolism (OR: 0.42, 95% CI: 0.28-0.64, p < 0.0001). Unfortunately, oxaliplatin-based therapy markedly increased the risk of of thrombocytopenia (OR: 1.35, 95% CI: 1.09-1.68, p < 0.01), diarrhea (OR: 1.33, 95% CI: 1.11-1.58, p < 0.01), sensory neuropathy (OR: 11.04, 95% CI: 8.91-13.67, p < 0.0001), total bilirubin elevation (OR: 1.91, 95% CI: 1.38-2.63, p < 0.0001), aspartate aminotransferase (OR: 5.29, 95% CI: 3.82-7.32, p < 0.0001) and alanine aminotransferase (OR: 2.22, 95% CI: 1.62-3.04, p < 0.0001) elevations at all grades. There was no statistically significant difference in vomiting or fatigue between the two groups.
Table 2.
Treatment-related adverse events.
| Adverse events | All grades | Grades 3-4 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | I2 | OR (95% CI) | p value | I2 | |
| Hematological | ||||||
| Leukopenia | 0.63 (0.49-0.81) | < 0.0001* | 40% | 0.28 (0.18-0.43) | < 0.0001* | 48% |
| Neutropenia | 0.58 (0.47-0.70) | < 0.0001* | 70% | 0.46 (0.38-0.56) | < 0.0001* | 55% |
| Anemia | 0.49 (0.41-0.60) | < 0.0001* | 34% | 0.47 (0.36-0.61) | < 0.0001* | 20% |
| Thrombocytopenia | 1.35 (1.09-1.68) | < 0.01* | 1% | 1.01 (0.71-1.44) | 0.97 | 0% |
| Febrile neutropenia | 0.58 (0.40-0.82) | < 0.01* | 65% | 0.58 (0.39-0.86) | < 0.01* | 67% |
| Non-hematological | ||||||
| Nausea | 0.63 (0.49-0.82) | < 0.0001* | 0% | 0.97 (0.55-1.71) | 0.92 | 15% |
| Vomiting | 0.78 (0.61-1.00) | 0.05 | 70% | 0.73 (0.31-1.71) | 0.47 | 11% |
| Stomatitis | 0.82 (0.68-0.98) | 0.03* | 1% | 1.86 (1.02-3.40) | 0.04* | 10% |
| Diarrhea | 1.33 (1.11-1.58) | < 0.01* | 89% | 1.94 (1.36-2.77) | <0.001* | 72% |
| Fatigue | 1.14 (0.88-1.49) | 0.31 | 76% | 1.64 (1.10-2.45) | 0.02* | 83% |
| Sensory neuropathy | 11.04 (8.91-13.67) | < 0.0001* | 70% | 9.16 (4.48-18.74) | < 0.0001* | 25% |
| Total bilirubin | 1.91 (1.38-2.63) | < 0.0001* | 40% | 1.74 (0.60-5.03) | 0.3 | 15% |
| AST | 5.29 (3.82-7.32) | < 0.0001* | 54% | 1.86 (0.68-5.09) | 0.23 | 26% |
| ALT | 2.22 (1.62-3.04) | < 0.0001* | 0% | 2.24 (0.77-6.52) | 0.14 | 44% |
| Creatinine | 0.16 (0.11-0.24) | < 0.0001* | 38% | 0.17 (0.03-0.99) | 0.05 | 0% |
| Thromboembolism | 0.42 (0.28-0.64) | < 0.0001* | 7% | NA | NA | NA |
OR, odds ratio; CI, confidence interval; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NA, not assessable. *p < 0.05, the difference is statistically significant.
As for grades 3-4 adverse events, oxaliplatin- based therapy significantly decreased the the risk of leukopenia (OR: 0.28, 95% CI: 0.18-0.43, p < 0.0001), neutropenia (OR: 0.46, 95% CI: 0.38-0.56, p < 0.0001), anemia (OR: 0.47, 95% CI: 0.36-0.61, p < 0.0001) and febrile neutropenia (OR: 0.58, 95% CI: 0.39-0.86, p < 0.01) compared to cisplatin-based treatment. However, it increased the risk of stomatitis (OR: 1.86, 95% CI: 1.02-3.40, p = 0.04), diarrhea (OR: 1.94, 95% CI: 1.36-2.77, p < 0.001), fatigue (OR: 1.64, 95% CI: 1.10-2.45, p = 0.02) and sensory neuropathy (OR: 9.16, 95% CI: 4.48-18.74, p < 0.0001). No significant difference was observed in thrombocytopenia, nausea, vomiting, total bilirubin, creatinine, aspartate aminotransferase or alanine aminotransferase elevation between the two groups.
Publication bias
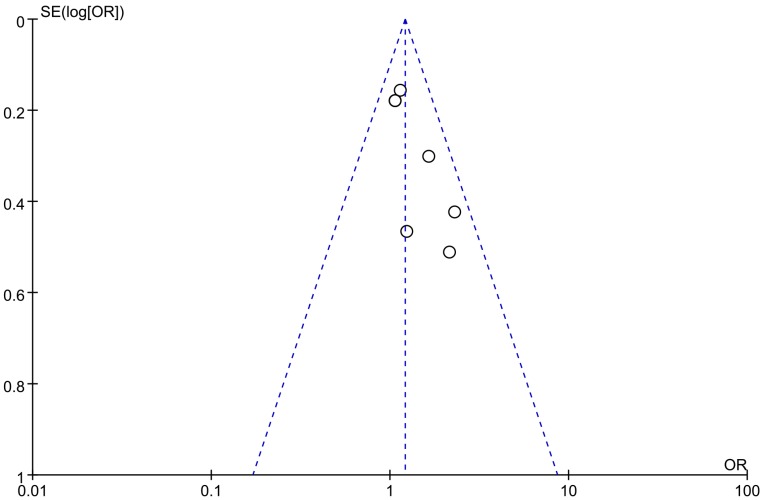
The potential publication bias was determined by performing a funnel plot analysis, and there was no evidence of obvious asymmetry or publication bias among the included studies (Figure 4).
Figure 4.
Funnel plot of publication bias in the meta-analysis.
Discussion
Accordingly, first-line systematic chemotherapy with platinum, fluorouracil and/or taxane could significantly improve the efficacy in patients with advanced GC, leading to an approximately ORR of 20 to 30% and median OS of 6 to 10 months 3-7. Moreover, a recent meta-analysis demonstrated that the platinum-based treatment was associated with more favorable therapeutic effect compared to non-platinum-containing therapy in such cases 19. Therefore, oxaliplatin and cisplatin have become the most commonly used agents in the care of advanced GC. On one hand, Lu et al. suggested that the addition of oxaliplatin to S1 could significantly prolong the median PFS (6.5 versus 4.0 months, p = 0.02), OS (14.0 versus 11.0 months, p = 0.03) and increase 1-year survival rate (63.8% versus 48.9%) compared to S1 monotherapy 20. On the other hand, in combination with S1, cisplatin remarkably increased the median OS (13.0 versus 11.0 months, p = 0.04) and PFS (6.0 versus 4.0 months, p < 0.0001) than S1 alone in a Japanese study 21.
As both of them have showed pronounced anti-tumour activity in advanced GC, plenty of efforts have been made to evaluate the difference of efficacy and safety between oxaliplatin-based and cisplatin-based regimens in certain subjects 11-16. A previous meta-analysis suggested that compared to cisplatin-based therapy, oxaliplatin-based therapy could significantly improve PFS and OS 22. However, another randomized phase III study from Japan indicated that oxaliplatin-based treatment was non-inferior to cisplatin treatment in terms of PFS (5.5 versus 5.4 months; HR: 1.004, 95% CI: 0.840-1.199) and OS (14.1 versus 13.1 months; HR: 0.958, 95% CI: 0.803-1.142) 15. Furthermore, a most recent meta-analysis performed by Huang J et al. demonstrated that there was no significant difference in PFS (HR: 0.92, 95% CI: 0.84-1.01, p = 0.09, I2 = 0%) and OS (HR: 0.91, 95% CI: 0.82-1.01, p = 0.07, I2 = 0%) between oxaliplatin-based and cisplatin-based therapies 17. However, there is still lacking efficient data evaluating the difference in tumour remission between the two different strategies.
To the best of our knowledge, this meta-analysis was the first one showing that oxaliplatin-based therapy was significantly associated with higher PRR (OR: 1.25, 95% CI: 1.05-1.48, p = 0.01), ORR (OR: 1.21, 95% CI: 1.02-1.44, p = 0.03) and DCR (OR: 1.76, 95% CI: 1.31-2.38, p = 0.0002) compared to cisplatin-based therapy as first-line chemotherapy for advanced GC. The results were consistent with reports by Al-Batran et al. They found that oxaliplatin-based regimen could improve the ORR in comparison with cisplatin-based regimen 11. However, no significant difference between oxaliplatin-based and cisplatin-based therapies for advanced GC in terms of ORR was observed in a randomized phase II study 14. In addition, another recent meta-analysis including five RCTs demonstrated that there was also no significant difference in ORR between the two different approaches 17. It was noteworthy that one more phase II RCT conducted by Hironaka et al. was enrolled in our pooled-analysis, and supported the results that oxaliplatin-based therapy was significantly associated with higher PRR (66% versus 46%), ORR (66% versus 46%) and DCR (100% versus 84%) compared to cisplatin-based therapy 16. This might explain the distinction between the present and previous studies.
As for the adverse events, similar to the results reported by Huang J et al., oxaliplatin-based regimen therapy significantly decreased the risk of all grades of neutropenia, anemia, febrile neutropenia, nausea, stomatitis, creatinine elevation and thromboembolism, as well as leukopenia, neutropenia, anemia and febrile neutropenia at grades of 3-4 compared to cisplatin-based regimen. Unfortunately, oxaliplatin- based therapy markedly increased the risk of all grades of thrombocytopenia, diarrhea, sensory neuropathy, total bilirubin, aspartate aminotransferase and alanine aminotransferase elevations, and grades 3-4 of diarrhea, fatigue and sensory neuropathy 17. The results were in accordance with previous studies 17-18.
However, it was noteworthy that several limitations should be taken into consideration when interpreting the results of this meta-analysis. The main limitation was the lack of adequate random sequence generation and blinding procedure of the RCTs, which might increase the risk of bias of this pooled-analysis. In addition, the present meta- analysis was based on published results, but not individual patient data. Finally, although no efforts were spared to search the literature comprehensively, there still existed the possibility that few relevant publications were not identified.
In summary, oxaliplatin-based regimen was significantly superior to cisplatin-based regimen in terms of PRR, ORR and DCR as first-line chemotherapy for advanced GC, and it could reduce the occurrence of most adverse events, but with an increased risk of thrombocytopenia, sensory neuropathy, diarrhea, fatigue and liver dysfunction. Thus, on the one hand, for patients with bulky disease that may lead to the obstruction of digestive tract, the primary tumor should be shrunk as soon as possible to alleviate the related symptoms, the oxaliplatin- based chemotherapy regimen is preferred. On the other hand, the cisplatin-based therapy is preferred for patients with renal insufficiency or abnormal liver function, in order to prolong survival time and improve the quality of life for advanced GC.
Acknowledgments
This study was supported by grants from Anhui Natural Science Foundation (NO. 1808085MH306).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen WQ, Zheng RS, Baade PD, Cancer statistics in China, 2015. CA Cancer J Clin; 2016. Mar-Apr: 115-32. [DOI] [PubMed] [Google Scholar]
- 3.Waters JS, Norman A, Cunningham D. et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer. 1999;80:269–72. doi: 10.1038/sj.bjc.6690350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhoefer U, Rougier P, Wilke H. et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: A trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–57. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 5.Wagner AD, Grothe W, Haerting J. et al. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006;24:2903–9. doi: 10.1200/JCO.2005.05.0245. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Moiseyenko VM, Tjulandin S. et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–7. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 7.Kang YK, Kang WK, Shin DB. et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III non-inferiority trial. Ann Oncol. 2009;20:666–73. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 8.Raymond E, Faivre S, Woynarowski JM. et al. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25:4–12. [PubMed] [Google Scholar]
- 9.Raymond E, Chaney SC, Taamma A. et al. Oxaliplatin: a review of preclinical and clinical studies. Ann Oncol. 1998;9:1053–71. doi: 10.1023/a:1008213732429. [DOI] [PubMed] [Google Scholar]
- 10.Becouarn Y, Agostini C, Trufflander N. et al. Oxaliplatin: Available data in non-colorectal gastrointestinal malignancies. Crit Rev Oncol Hematol. 2001;40:265–72. doi: 10.1016/s1040-8428(01)00169-x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Batran SE, Hartmann JT, Probst S. et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–42. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 12.Cunningham D, Starling N, Rao S. et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 13.Popov I, Radosevic-Jelic L, Jezdic S, Biweekly oxaliplatin, fluorouracil and leucovorin versus cisplatin, fluorouracil and leucovorin in patients with advanced gastric cancer. Journal of BUON; 2008. Oct-Dec: 505-11. [PubMed] [Google Scholar]
- 14.Kim YS, Sym SJ, Park SH. et al. A randomized phase II study of weekly docetaxel/cisplatin versus weekly docetaxel/oxaliplatin as first-line therapy for patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2014;73:163–9. doi: 10.1007/s00280-013-2334-3. [DOI] [PubMed] [Google Scholar]
- 15.Yamada Y, Higuchi K, Nishikawa K. et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naive patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8. doi: 10.1093/annonc/mdu472. [DOI] [PubMed] [Google Scholar]
- 16.Hironaka S, Sugimoto N, Yamaguchi K. et al. S-1 plus leucovorin versus S-1 plus leucovorin and oxaliplatin versus S-1 plus cisplatin in patients with advanced gastric cancer: a randomised, multicentre, open-label, phase 2 trial. Lancet Oncology. 2016;17:99–108. doi: 10.1016/S1470-2045(15)00410-6. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Zhao Y, Xu Y. et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: A meta-analysis of randomized controlled trials. Oncotarget. 2016;7:34824–31. doi: 10.18632/oncotarget.9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner AD, Syn NLX, Moehler M. et al. Chemotherapy for advanced gastric cancer. Cochrane Database of Systematic Reviews. 2017 doi: 10.1002/14651858.CD004064.pub4. DOI: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen WW, Wang F, Xu RH. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: a meta-analysis. PLoS One. 2013;8:e68974. doi: 10.1371/journal.pone.0068974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Liu Z, Zhang J. S-1 plus oxaliplatin vs. S-1 as first-line treatment in patients with previously untreated advanced gastric cancer: a randomized phase II study. J Chemother. 2014;26:159–64. doi: 10.1179/1973947813Y.0000000128. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi W, Tanabe S, Saigenji K. et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–12. doi: 10.1038/sj.bjc.6601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montagnani F, Turrisi G, Marinozzi C. et al. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14:50–5. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]