Abstract
Background:
An increasing prevalence of obesity in the UK has also seen a rise in the diagnosis of co-morbidities. Obstructive sleep apnoea (OSA) has previously been associated with body mass index (BMI) but has not been fully explored in a UK population.
Aims:
To quantify the association between BMI and a recorded diagnosis of OSA in primary care for people aged 50 years or over in the UK.
Methods:
A descriptive analysis is given of men and women aged 50 or over in the UK from The Health Improvement Network (THIN) database with regard to diagnosis of OSA, snoring, and BMI. Logistic regression was performed for the likelihood of OSA depending on BMI classification recorded after adjusting for gender, age, region, and socioeconomic status (Townsend quintile). The analyses were repeated for snoring.
Results:
After adjusting for confounders, those with a BMI recorded of 40+ kg/m2 were 27.39 times (95% CI 24.64 to 30.46) more likely to have OSA (p<0.0001). There was a lower prevalence of OSA with increasing age and levels of deprivation.
Conclusions:
Obesity and snoring were both strongly associated with a diagnosis of OSA. The decreasing prevalence of OSA with increasing age and levels of deprivation needs further study to ensure that these groups are not being systematically under-investigated.
Keywords: body mass index, epidemiology, primary care, sleep apnoea
Introduction
The prevalence of obesity is increasing in western societies. It was recently estimated that nearly 25% of the population in England were obese,1 with predictions that this figure would rise substantially over the coming years. Obesity is associated with many different co-morbidities including obstructive sleep apnoea (OSA).2–5 Worldwide, studies have found a high level of clinically undiagnosed OSA in obese patients, particularly those who are morbidly obese.3,4,6 However, no studies have sought to establish the size of this association in clinical practice in the UK. This needs to be addressed as OSA is associated with premature death and reduced health-related quality of life; for example, an Israeli study7 found that the combination of obesity and severe OSA conferred a 44% higher risk of death than OSA alone (p=0.03).
Snoring has traditionally been used in primary care as a predictor of OSA and is frequently cited as a symptom during OSA diagnosis. Romero et al.8 found a positive predictive value for snoring and OSA of 84.7%, and Resta et al.9 found snoring to be a symptom in 100% of the patients in their study who had apnoeas during sleep. However, the usefulness of snoring to predict the presence of OSA in UK primary care is not known.
The aim of this cross-sectional study was to quantify the prevalence of a recorded diagnosis of OSA in general practice in the UK and to determine how this prevalence varies with body mass index (BMI), gender, and deprivation. We also repeated these analyses using snoring as the outcome and, in addition, quantified the extent to which a history of snoring predicts a diagnosis of OSA.
Methods
Data for this cross-sectional study were derived from The Health Improvement Network (THIN) database, a longitudinal computerised database containing data collected as part of routine primary care. The protocol was reviewed and approved by the THIN Scientific and Review Committee.
Our initial aims were to determine the prevalence of a recorded diagnosis of OSA in primary care in the UK on 1 July 2005 and then to quantify the demographic predictors of this diagnosis. Data were identified for individuals with a date of birth before 30 June 1945 to ensure capture of all those aged 50 years or over at 1 July 2005.
We extracted the following demographic information for this population: sex, age (recorded in 5-year age bands), BMI (recoded into World Health Organization groups10), and social class (quintiles of Townsend score derived from the 2001 UK Census and linked by post code to super output areas). Diagnoses of OSA and the presence of self-recorded snoring were extracted from THIN using Read Codes (codes used available on request).
In our initial analysis we used univariate logistic regression to quantify the association between a recorded diagnosis of OSA and each of our covariates. We then used multivariate logistic regression to model the effects of age group, sex, and social class simultaneously. We then repeated this strategy using snoring rather than OSA as the outcome and finally quantified the association between snoring and a recorded diagnosis of OSA, both before and after adjusting for our covariates.
Stata (V.10.3) was used for all of the statistical analyses and likelihood ratio tests were used for the hypothesis tests.
Results
In our initial descriptive analysis we identified 1,073,116 individuals in the dataset aged 50 years or over on 1 July 2005 in the UK. This comprised 507,553 men (47%) and 565,563 women (53%). The average age of the individuals in the dataset was 66 years. A total of 6,527 (0.6%) of the total population had a diagnosis of OSA, with a higher proportion among men (79% of the total diagnosed, Table 1). The average age of individuals with OSA was 62 years, with the 60–64 age group having the highest proportion of OSA diagnoses (23%). A total of 12,499 individuals were recorded as snorers (1.2%), of which 8,968 (72%) were men.
Table 1. Descriptive analysis of study population by OSA sufferer and snoring broken down by gender, age, and socioeconomic status.
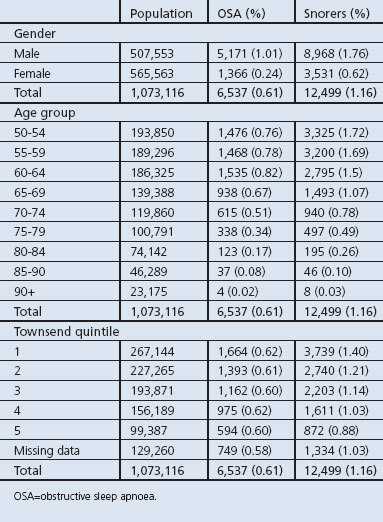
In general, the prevalence of both a recorded diagnosis of OSA and self-reported snoring were much more common in men and decreased with increasing age. There was no apparent social class gradient on the prevalence of recorded diagnoses of OSA but the prevalence of snoring was higher in less-deprived people (Table 1).
BMI was calculated for 945,435 people (88%) in the dataset. In general, the prevalence of obesity (BMI >30 kg/m2) and morbid obesity (BMI >40 kg/m2) was higher in females, increased with increasing levels of deprivation, and was lowest in the oldest age groups (Table 2).
Table 2. Descriptive analysis of study population by WHO BMI classification broken down by gender, age and socioeconomic status.
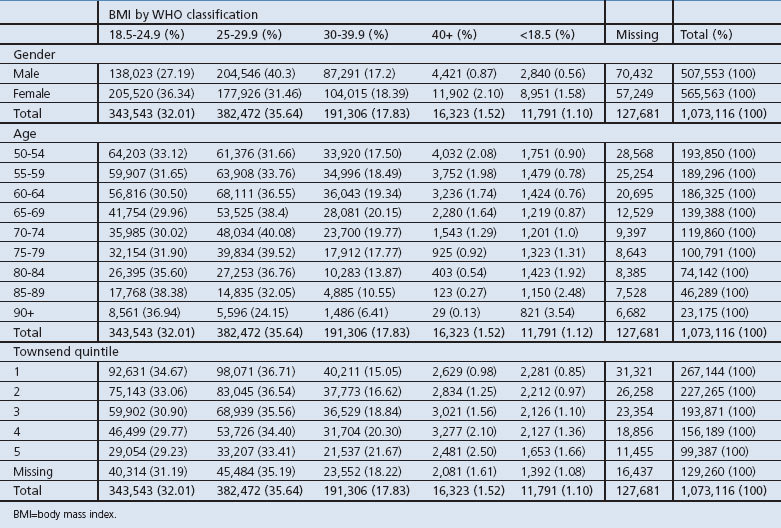
The total number of individuals in the dataset who had a recorded BMI and also had a diagnosis of OSA was 6,266 (0.6%). Although this is a very small proportion of the overall numbers, it represents a large proportion (96%) of those with a diagnosis of OSA, demonstrating that BMI was highly recorded for individuals with OSA. The prevalence of OSA increased progressively with increasing BMI and, in those with a BMI recorded in the 40+ category, the prevalence was 4.7%.
Logistic regression
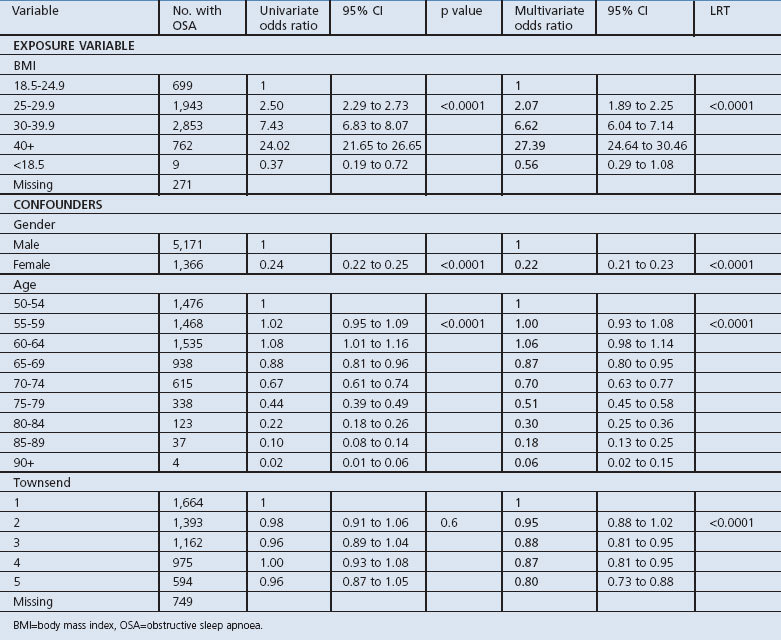
In our univariate analysis there was a strong dose-response relationship between BMI and the odds of having a recorded diagnosis of OSA (Table 3). This relationship was not greatly altered by adjusting for the effects of age, sex, and social class. After adjusting for confounders, those in the 30+ BMI category had a 6.5 times greater likelihood of having OSA than people with a normal BMI (OR 6.62, 95% CI 6.04 to 7.14), and this rose considerably in the 40+ group to a greater likelihood of nearly 27.5 times (OR 27.39, 95% CI 24.64 to 30.46). The risk of having OSA was much lower in women and decreased with increasing age. In our univariate analysis there was little evidence of an association between social class and OSA but, after adjusting for age and sex, there was a trend such that recording the diagnoses was higher in the more affluent groups (Table 3).
Table 3. Univariate and multivariate analysis of BMI and OSA after adjusting for gender, age, and socioeconomic status.
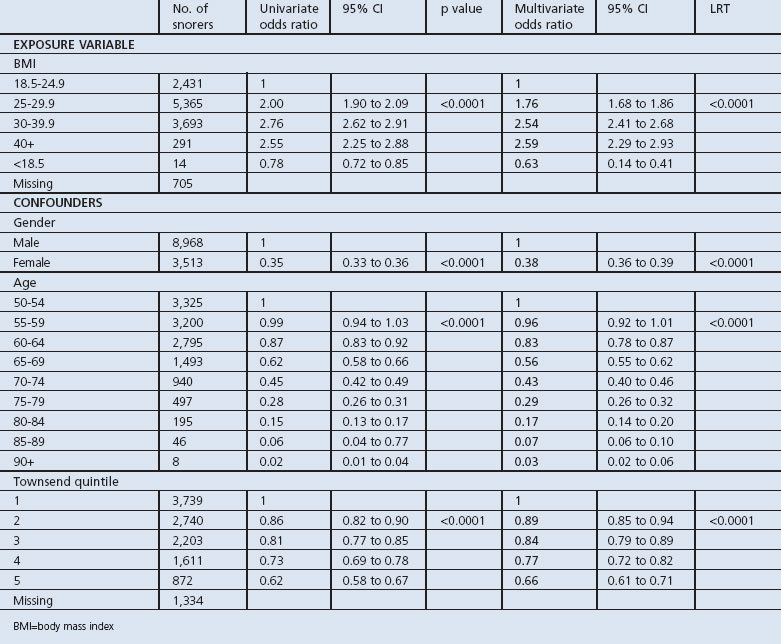
Our analysis for a recorded symptom of snoring also showed an association between increasing odds of snoring and increasing BMI, both before and after adjusting for confounding variables. However, the strength of this association in terms of odds ratios was much smaller than that for recorded diagnoses of OSA. Again the odds of snoring were lower in older people and women and decreased with increasing levels of socioeconomic disadvantage (Table 4).
Table 4. Univariate and multivariate analysis of BMI and snoring after adjusting for sex, age, and socioeconomic status.
A total of 1,750 (26.8%) of individuals with a recorded diagnosis of OSA also had a record of being a snorer. This was equivalent to an odds ratio of 35.9 (95% CI 33.9 to 38.1) in our univariate analysis and 25.9 (95% CI 24.4 to 27.5) in our multivariate analysis.
Discussion
Main findings
The results of our study show that, in the UK, among people over the age of 50 years the majority (88%) of the population had a recorded BMI and 0.6% of them had a diagnosis of OSA recorded in their primary care records. This diagnosis of OSA was more common in men (1.01%) and much more common in people with a BMI of >30 kg/m2 and, after adjusting for confounders, those with a BMI recorded of 40+ kg/m2 were more than 27 times more likely to have OSA. The presence of a record of snoring was also strongly associated with a diagnosis of OSA. There was a lower prevalence of OSA with increasing age and levels of deprivation.
Strengths and weaknesses of this study
The main strengths of our study are the large size of the population afforded by using primary care records and the fact that this represents the ‘real-life’ state of clinical care in the UK. The main weaknesses relate to the validity of the diagnoses of OSA, the ascertainment of cases of OSA, error measurement in the calculation of BMI, and the restricted age range of our study population.
Most previous epidemiological studies have focused on small cohorts of people studied in detail with sleep studies.5,11 In studies such as these we would expect the prevalence of OSA to be much higher as the population is screened for the diagnosis. To have a diagnosis recorded in the THIN database, a patient needs to initiate a trip to their general practitioner (GP) and the GP needs to make a diagnosis or refer the patient to secondary care to have the diagnosis confirmed and the disease treated. This means that levels of case ascertainment will have a strong influence on the prevalence of the condition. Although we have not accessed the secondary care records for this study, it seems unlikely to us that a GP would record a diagnosis of OSA without confirmation from secondary care review, in part because of the implications for driving in this population. We suspect therefore that the recorded diagnoses of OSA are likely to be correct, in keeping with other diagnoses in the same dataset,12–16 but that these cases represent the ‘tip of the iceberg’ and may represent the severe end of the spectrum of OSA with many other cases being currently undiagnosed. There may also be a selection bias in our data. GPs are often told to suspect OSA in people who are obese or who snore loudly, so these may be the very cases that GPs diagnose. It is possible then that the proportion of people with OSA and a normal BMI are under-represented in our study. There is no set method for measuring height and weight in our dataset, so there is likely to be random error in the BMI calculations which will in turn mean that the strong dose-response relationship observed between BMI and OSA is an underestimate of the true relationship. Finally, we restricted our study to people over the age of 50 mainly to keep the size of the dataset manageable, but also to be able to go on and study the incidence of heart disease in this population. Given the finding that the prevalence of OSA decreases with age, it would be worth expanding our study population to all adults and possibly children.
Interpretation of findings in relation to previously published work
The prevalence amongst those with a BMI measurement ever taken and OSA was approximately 0.6%, which is lower than national figures17 and those found in the literature. This may be partly because previous studies have focused on high-risk populations such as obese people and middle-aged men5,6,18 rather than the general population. It also seems likely that under-ascertainment of cases in general practice is an important problem, and it is possible that an analysis of a wider range of computer Read morbidity codes for symptoms which manifest prior to diagnosis may have revealed a higher prevalence, but it would not have been possible to verify as OSA.
The findings of this study broadly fit with those of previous studies, with an increased risk of OSA diagnosis with increased rise in obesity19 and a lessening of symptoms with weight loss in those diagnosed. Busetto et al.11 found that a 15% reduction in baseline body weight substantially increased the mean pharyngeal cross-sectional area of the throat and decreased the severity of OSA.
When looking at the association between snoring and BMI, there was also a clear increase in the risk of snoring with higher BMI classifications, although the magnitude of the effect was smaller. This concurs with previous studies.20 The association between snoring and OSA has already been well documented.8,9
Implications for future research, policy and practice
Our work on the association between OSA, BMI and level of deprivation is new and shows that, after adjusting for age, sex and BMI, people in more deprived areas (Townsend quintiles) were less likely to have OSA despite having a higher BMI. This finding seems counterintuitive and may reflect a greater awareness of the condition and hence higher levels of diagnosis in more affluent people and the ‘worried well’. More work is required to determine whether this condition is particularly under-recognised in the poorest sectors of society and to see if more can be done to raise the profile of the disease in relation to symptoms and treatment among this cohort to encourage presentation for diagnosis in primary care.
The decrease in OSA with increased age could also be erroneous. There may be a level of discrimination against elderly individuals with regard to specialist referral for OSA and more ‘age-related’ co-morbidities may take precedence for treatment
Conclusions
This study found that 1% of men and 0.24% of women aged >50 years in the UK have a recorded diagnosis of OSA and that being obese or a snorer are strongly predictive of this diagnosis. The finding of a decreasing prevalence in socioeconomically disadvantaged people and older people warrants further study to ensure that these groups are presenting with symptoms and are not being systematically under-investigated and under-diagnosed.
Acknowledgments
Handling editor Gopal Netuveli
Statistical review Gopal Netuveli
Funding None of the authors received funding to conduct this study.
Footnotes
The authors declare that they have no conflicts of interest in relation to this article.
References
- Association of Public Health Observatories. www.apho.org.uk (http://www.noo.org.uk/NOO_about_obesity/adult_obesity/epidemiology). 2011.
- de Sousa AGP, Cercato C, Mancini MC, Halpern A. Complications of obesity: Obesity and obstructive sleep apnea-hypopnea syndrome. Obes Rev 2008;9:340–54. http://dx.doi.org/10.1111/j.1467-789X.2008.00478.x [DOI] [PubMed] [Google Scholar]
- Daltro C, Gregorio PB, Alves E, et al. Prevalence and severity of sleep apnoea in a group of morbidly obese patients. Obes Surg 2007;17:809–14. http://dx.doi.org/10.1007/s11695-007-9147-6 [DOI] [PubMed] [Google Scholar]
- Palla A, Digiorgio M, Carpenè N, et al. Sleep apnea in morbidly obese patients: prevalence and clinical predictivity. Respiration 2009;78:134–40. http://dx.doi.org/10.1159/000165371 [DOI] [PubMed] [Google Scholar]
- Valencia-Flores M, Orea A, Castaño VA, et al. Prevalence of sleep apnea and electrocardiographic disturbances in morbidly obese patients. Obes Res 2000;8:262–9. http://dx.doi.org/10.1038/oby.2000.31 [DOI] [PubMed] [Google Scholar]
- Sharma SK, Kurian S, Malik V, et al. A stepped approach for prediction of obstructive sleep apnea in overtly asymptomatic obese subjects: a hospital based study. Sleep Med 2004;5:351–7. http://dx.doi.org/10.1016/j.sleep.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res 2007;16:128–34. http://dx.doi.org/10.1111/j.1365-2869.2007.00578.x [DOI] [PubMed] [Google Scholar]
- Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: predictive symptoms for obstructive sleep apnea. Sleep Breath 2010;14:337–43. http://dx.doi.org/10.1007/s11325-009-0310-2 [DOI] [PubMed] [Google Scholar]
- Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obesity 2001;25:669–75. http://dx.doi.org/10.1038/sj.ijo.0801603 [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic. Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
- Busetto L, Enzi G, Inelmen EM, et al. Obstructive sleep apnoea syndrome in morbid obesity. Chest 2005;128:618–23. http://dx.doi.org/10.1378/chest.128.2.618 [DOI] [PubMed] [Google Scholar]
- Langley T, Szatkowski L, Gibson J, et al. Validation of the Health Improvement Network (THIN) primary care database for monitoring prescriptions for smoking cessation medications. Pharmacoepidemiol Drug Saf 2010;19:586–90. http://dx.doi.org/10.1002/pds.1960 [DOI] [PubMed] [Google Scholar]
- Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007;16:393–401. http://dx.doi.org/10.1002/pds.1335 [DOI] [PubMed] [Google Scholar]
- Meal A, Leonardi-Bee J, Smith C, Hubbard R, Bath-Hextall F. Validation of THIN data for non-melanoma cancer. Qual Primary Care 2008;16:49–52. [PubMed] [Google Scholar]
- Ruigómez A, Martin-Merino E, Rodriguez LA. Validation of ischemic cerebrovascular diagnoses in the Health Improvement Network. Pharmacoepidemiol Drug Saf 2010;19:579–85. http://dx.doi.org/10.1002/pds.1919 [DOI] [PubMed] [Google Scholar]
- Vincent LR, Haynes K, Forde K, Localio AR, Schinnar R, Lewis JD. Validity of the Health Improvement Network (THIN) for epidemiological studies of hepititis C virus infection. Pharmacoepidemiol Drug Saf 2009;18:807–14. http://dx.doi.org/10.1002/pds.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Choices. http://www.nhs.uk/conditions/Sleep-apnoea/Pages/Introduction.aspx. 2010.
- Flemons WW. Obstructive sleep apnea. N Engl J Med 2002;347:498–504. http://dx.doi.org/10.1056/NEJMcp012849 [DOI] [PubMed] [Google Scholar]
- Gibson GJ. Obstructive sleep apnoea syndrome: underestimated and undertreated. Br Med Bull 2004;72:49–64. http://dx.doi.org/10.1093/bmb/ldh044 [DOI] [PubMed] [Google Scholar]
- Li QY, Huang SG, Li M, Liu JL, Wan HY. BMI is an independent risk factor for snoring in Chinese women aged over 30 years. Sleep Breath 2009;13:289–93. http://dx.doi.org/10.1007/s11325-008-0236-0 [DOI] [PubMed] [Google Scholar]


