Zhang and Balachandran preview new findings from Sai et al. revealing that necroptosis signaling mediators can directly inhibit Listeria replication in gut epithelium.
Abstract
RIPK3 induces necroptosis by phosphorylating MLKL, which then induces plasma membrane rupture and necrotic cell death. In this issue, Sai et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201810014) show that RIPK3-MLKL signaling in epithelial cells promotes Listeria clearance by directly suppressing cytosolic bacterial replication, without activating cell death.
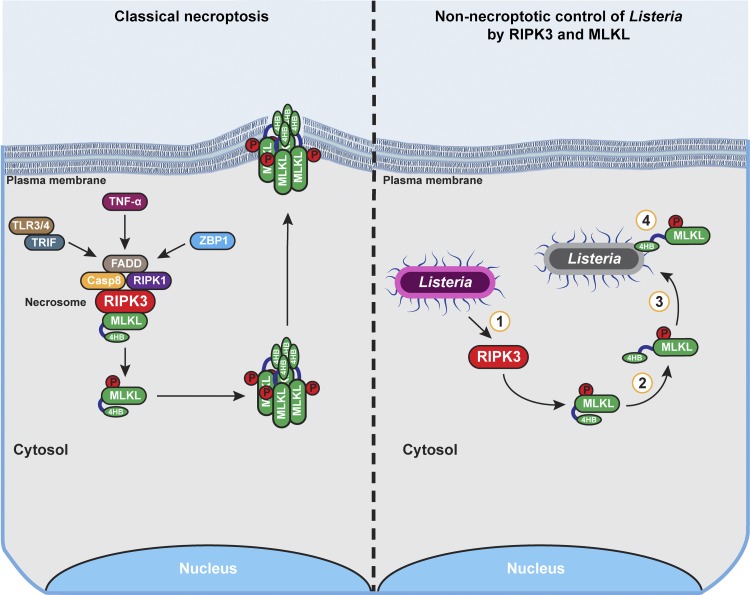
Necroptosis is a form of programmed necrotic cell death defined by its reliance on the kinase RIPK3. Initial insight into the process of necroptosis, and the discovery that RIPK3 is central to this form of death, came from studies with the cytokine TNF-α. TNF-α initiates necroptosis by engaging its receptor TNFR1 and promoting assembly of a cytosolic RIPK3-containing protein complex called the necrosome. The necrosome contains numerous proteins, incuding RIPK1, FADD, caspase-8, and MLKL, and appears to serve a scaffolding function necessary for RIPK3 clustering and activation. From within the necrosome, active RIPK3 phosphorylates its primary target, MLKL, setting into motion the effector phase of necroptotic cell death. MLKL comprises an N-terminal four-helix bundle (4HB) domain and a C-terminal pseudokinase domain. Phosphorylation by RIPK3 induces conformational changes in MLKL that promote both its oligomerization as well as release of its 4HB domain into an active, lipid-binding module with affinity for multiple phospholipids, incuding select phosphatidylinositol phosphates (e.g., PI(5)P and PI(4,5)P2) and cardiolipin. Oligomerized MLKL with its newly acquired lipid-interacting capacity then homes to phospholipids in the plasma membrane and perforates this membrane, resulting in eventual necrotic lysis of the cell (1; Fig. 1).
Figure 1.
The necroptosis machinery promotes noncytolytic clearance of Listeria from epithelial cells. In classical necroptosis signaling (left), activation of RIPK3 by cytokines (e.g., TNF-α) or pattern recognition recptors (e.g., TLRs3/4 and ZBP1) results in formation of a RIPK3-containing necrosome in which MLKL is phosphorylated and activated. MLKL subsequently oligomerizes and undergoes conformational changes that expose its phospholipid binding 4HB domain. Active MLKL oligomers traffic to the plama membrane, where they form pores that eventually promote necrotic lysis of the cell. In contrast, during Listeria infection of epithelial cells (right), RIPK3 is activated and phosphorylates MLKL, but MLKL does not oligomerize and target the plasma membrane for disruption. Instead, phosphorylated MLKL directly associates with cytosolic Listeria and inhibits its replication. The key steps in this process that merit further investigation are depicted by numbers within circles: (1) the mechanism by which Listeria activates RIPK3, (2) the process governing activation of MLKL without inducing its oligomerization, (3) the means by which MLKL localizes to cytosolic Listeria, and (4) the mechanism by which MLKL limits Listeria replication in the cytosol.
Necroptosis research has since expanded beyond the classical TNF-α paradigm, with the demonstration over the past few years that numerous innate-immune stimuli besides TNF-α can also activate RIPK3. For example, herpesvirus and influenza virus infections are sensed by the protein ZBP1, which can directly engage RIPK3 and induce necroptosis (2). Similarly, TLR3 and TLR4 detect viral and microbial ligands (double-stranded RNA and lipopolysaccharide, respectively) and activate RIPK3 via the adaptor protein TRIF (3; Fig.1).
Results from RIPK3-deficient mice implicate this kinase as an important weapon in the vertebrate host defense arsenal and suggest that necroptosis contributes to pathogen clearance in at least two ways. First, and most directly, it limits pathogen spread by killing the host cell and preventing it from becoming a pathogen factory. Second, by triggering cell destruction and release of debris into the extracellular space, it functions to galvanize host adaptive immune responses (2).
In this latter regard, necroptosis may, in fact, represent a more immunogenic form of death than its more established counterpart, apoptosis. Whereas apoptosis results in orderly, caspase-driven disassembly of the cell into discrete membrane-bound vesicles, necroptosis culminates in a cellular “explosion” that spills the cell’s contents into the extracellular mileu. Many of these contents (including genomic DNA itself, for example) are potent immunogenic cues that provoke robust adaptive immune responses (4). They are, of course, also highly inflammatory, and inopportune activation of necroptosis can have severe pathological consequences that may well trump the potential pathogen clearance benefts afforded by this mode of death (4). Indeed, the necroptosis machinery is only seen in vertebrates, and then too is remarkably poorly conserved among members of this subphylum. For example, birds do not appear to express either Ripk3 and Zbp1, and carnivores lack Mlkl (5).
Moderating necroptosis to ensure that necrotic lysis of the cell is restricted to scenarios essential for host defense, and not in settings where such death can be deleterious to the host, is therefore of paramount importance. Effective control of necroptosis becomes particularly relevant in tissues (such as the gut epithelium) where regular contact with microbes is unavoidable. How do these tissues regulate necroptosis signaling to ensure that pathogen clearance is maintained but potentially dangerous inflammation is prevented? In this issue, Sai et al. outline a mechanism by which this might be achieved (6). They present evidence that RIPK3-MLKL signaling in epithelial cells can limit intracellular replication of the Gram-positive bacterium Listera monocytogenes without triggering cell death. They show that RIPK3 is activated during Listeria infection and phosphorylates MLKL. Phosphorylated MLKL, however, does not traffic to the plasma membrane to effect cell lysis; instead, MLKL directly impinges on the bacterium, preventing its replication (Fig. 1).
Sai et al. began their studies with the observation that cells and tissues of the gastrointestinal (GI) tract expressed RIPK3 at levels that were significantly higher than those seen in other tissues (such as the heart, pancreas, liver, and brain) and comparable only to RIPK3 levels in immune cells (6). As an aside, most adherent human cell lines commonly used in biomedical research do not express RIPK3 and/or other necroptosis components, perhaps explaining why this mode of cell death went undiscovered for as long as it did. Interestingly, some cell lines derived from the intestinal epithelium (e.g., Caco2 and HT-29) retain RIPK3 expression. Elevated RIPK3 expression in immune cells may be explained by its importance to host defense, and high levels of this kinase in intestinal epithelial cells could stem from its role in regulating autophagy in the gut (7). But whether RIPK3 in the intestinal epithelium also functions in immunity and, perhaps more importantly, how epithelial cells of the GI tract can handle high levels of RIPK3 despite their constant exposure to necroptosis-activating microbial ligands remains enigmatic.
Sai et al. hypothesized that RIPK3 and MLKL, which is also abundantly expressed in the small intestine, might function in the GI tract to guard against enteropathogenic bacteria (6). Using Listeria—a foodborne Gram-positive bacterium and causative agent of listeriosis—as a model enteropathogen, they discovered that RIPK3- and MLKL-deficient mice infected orally with Listeria manifested significantly elevated bacterial loads in their livers. Notably, almost no bacterial colonization was seen in control mice, demonstrating that RIPK3 and MLKL are required to prevent dissemination of Listera to organs beyond the GI tract.
To examine how RIPK3 and MLKL functioned to restrict Listeria spread, the authors switched to cell cuture models, in which they found that infection of epithelial cells in cellulo with Listeria resulted in RIPK3-dependent phosphorylation of MLKL. Importantly, if RIPK3 was absent or phosphorylation of MLKL was prevented, Listeria replication was significantly increased. These results demonstrate that the heightened spread of Listeria seen in vivo when RIPK3 and MLKL are absent can be directly attributable to cell intrinsic antibacterial activity of the necroptosis machinery in epithelial cells.
A major surprise was in store when Sai et al. examined the viability of Listeria-infected cells (6). None of the cells harboring replicating Listeria appeared to die, despite activation of RIPK3 and MLKL. The same cells succumbed readily by necroposis to TNF-α; what could account for this disparity? Sai et al. offer preliminary molecular insight into this apparent paradox by demonstrating that active MLKL, although phosphorylated to the same extent in cells exposed to either TNF-α or Listeria, fails to oligomerize in Listeria-infected cells (6). Intriguingly, MLKL in Listeria-infected cells colocalizes with cytosolic bacteria and does not traffic to the plasma membrane. The authors further demonstrate that the 4HB phospholipid-binding domain of MLKL may directly associate with Listeria, and that cell lysates containing active MLKL-4HB prevent Listeria replication when added to cultures of this bacterium. Of note, replication of other intracellular enteropathogens, such as the Gram-negative bacterium Salmonella enterica, are not detectably affected by MLKL activity, and the MLKL-4HB domain does not associate with either Salmonella or another Gram-negative bacterium, Escherichia coli. Integrating these findings, the authors propose a model in which Listeria infection of gut epithelial cells results in activation of RIPK3 and phosphorylation of MLKL, but phosphorylated MLKL does not oligomerize, and hence does not traffic to the plasma membrane and trigger necroptotic demise of infected cells. Instead, it acts directly on the bacterium, perhaps drawn in by cardiolipin, a phospholipid and known MLKL attractant, present on the Listeria cell membrane (Fig. 1). Cardiolipin is less abundant on the outer membranes of Gram-negative bacteria (compared with their inner membranes) perhaps explaining why MLKL selectively associates with Listeria and not other intracellular bacteria. Although Listeria is a Gram-positive bacterium with a peptidoglycan cell wall cloaking its cardiolipin-containing membrane, the authors note that the cell wall of Gram-positive bacteria can be penetrated by molecules with molecular masses up to 57 kD, which is approximately the size of MLKL.
As with all interesting findings, the study by Sai et al. raises many fascinating questions. How is RIPK3 activated by Listeria infection? Why does phosphorylated MLKL in Listeria-infected cells not oligomerize and traffic to the plasma membrane? (In this regard, Dovey et al. [8] have found a role for inositol phosphate kinases in mediating MLKL oligomerization at a step after its phosphorylation; whether Listeria infection may blunt the activity of these kinases will be interesting to test.) How does nonoligomerized MLKL localize to Listeria? And, finally, how does MLKL limit Listeria growth (Fig. 1)?
While the observations by Sai et al. strongly warrant further study, they already expand our understanding of the necroptosis machinery in two important ways. First, they place MLKL alongside the pyroptosis effector Gasdermin D as a pore-forming host cell protein capable of directly restricting intracellular bacterial replication (9). Second, they demonstrate that necroptosis effectors have evolved noncytolytic, and likely cell and pathogen type-specific, modes of viral and bacterial clearance, echoing the findings of Daniels et al. (10), who showed RIPK3 in neurons restricts West Nile virus pathogenesis without triggering necroptosis. Sai et al. extend cell death–independent functions of RIPK3 to include clearance of Listeria from epithelial cells (6). More generally, these studies suggest that necroptosis may be disfavored as a host defense strategy under conditions when the costs of necrotic death—either by eliciting hyper-inflammatory responses (such as in the gut) or by eliminating nonrenewable cell types like neurons—outweigh its benefits.
Acknowledgments
We thank James Murphy and Glenn Rall for comments on this article.
Work in the Balachandran laboratory is supported by National Institutes of Health grants AG058642, CA168621, CA190542, and AI135025 and by National Institutes of Health Cancer Center Support Grant P30CA006927.
The authors declare no competing financial interests.
References
- 1.Grootjans S., et al. Cell Death Differ. 2017 doi: 10.1038/cdd.2017.65. [DOI] [Google Scholar]
- 2.Upton J.W., et al. Immunol. Rev. 2017 doi: 10.1111/imr.12539. [DOI] [Google Scholar]
- 3.Kaiser W.J.,et al. J. Biol. Chem. 2013 doi: 10.1074/jbc.M113.462341. [DOI] [Google Scholar]
- 4.Kaczmarek A., et al. Immunity. 2013 doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Dondelinger Y., et al. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Sai K., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201810014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris K.G., et al. Cell Host Microbe. 2015 doi: 10.1016/j.chom.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dovey C.M., et al. Mol. Cell. 2018 doi: 10.1016/j.molcel.2018.05.010. [DOI] [Google Scholar]
- 9.Liu X., et al. Nature. 2016 doi: 10.1038/nature18629. [DOI] [Google Scholar]
- 10.Daniels B.P., et al. Cell. 2017 doi: 10.1016/j.cell.2017.03.011. [DOI] [Google Scholar]