Van Den Bosch highlights the discovery by Kalinski et al. of Miro1 as an HDAC6 substrate that regulates mitochondrial trafficking in neurons and axon regeneration.
Abstract
Myelin-associated glycoprotein and chondroitin sulfate proteoglycans in the extracellular matrix can prevent regeneration of injured axons. In this issue, Kalinski et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201702187) report that inhibition of HDAC6 prevents the deacetylation of Miro1, increases mitochondrial axonal transport, and restores the size of axonal growth cones.
Axonal damage of neurons is a common problem that occurs during many different types of central nervous system (CNS) injury. Unfortunately, regeneration of these axons in the CNS is prevented by extracellular factors that inhibit the outgrowth of the axon and this often leads to persistent deficits (1). These extracellular factors include, but are not limited to, myelin-associated glycoprotein (MAG) and chondroitin sulfate proteoglycans (CSPGs).
The inhibition of neurite outgrowth induced by MAG or CSPGs can be overcome by inhibition of histone deacetylase 6 (HDAC6) using small molecule inhibitors or by down-regulation of HDAC6 using siRNAs (2). In contrast to what the name suggests, HDAC6 has many targets but histones are far from the most important ones because HDAC6 is mainly localized in the cytoplasm. α-Tubulin, one of the building blocks of microtubules, is considered to be the most important HDAC6 substrate, and inhibition of HDAC6 results in increased tubulin acetylation in MAG- or CSPG-stimulated neurons (3). The acetylation of α-tubulin is regulated by the opposing activities of HDAC6 and tubulin acetyltransferase-1 (α-TAT1). In MAG- or CSPG-stimulated neurons, α-TAT1 was found to be down-regulated, but overexpression of α-TAT1 by lentiviral infection increased α-tubulin acetylation and could overcome neurite growth inhibition (4). Taken together, these data indicate that the balance between tubulin acetylation and deacetylation could contribute to axon growth on nonpermissive substrates. In this issue, Kalinski et al. (5) discovered that HDAC6 has another important substrate in addition to α-tubulin, namely, Miro1, which contributes to the positive effect of HDAC6 inhibition on the size of the axonal growth cone after MAG or CSPG stimulation.
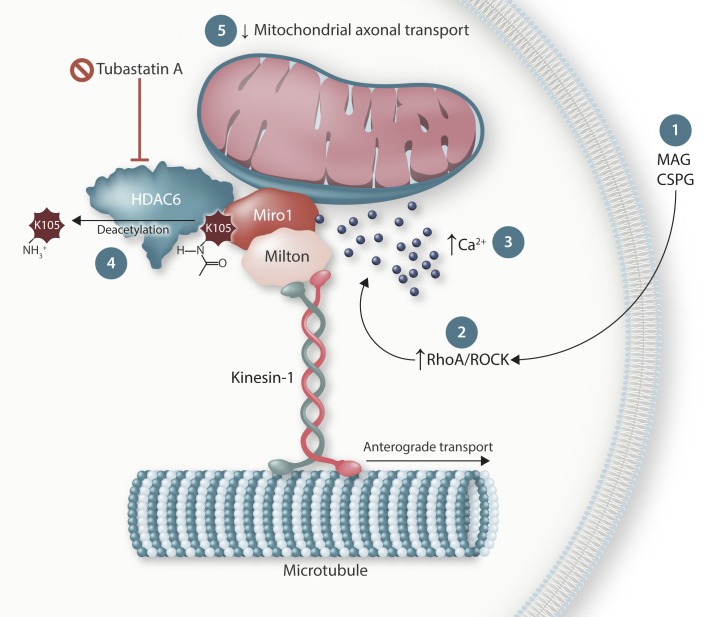
Miro1 is a well-known partner in the Miro/Milton complex that links mitochondria to motor proteins. While Milton binds to the kinesin-1 motor involved in anterograde transport, or to the dynein/dynactin complex responsible for retrograde transport, Miro1 is a calcium-binding outer mitochondrial membrane protein. Deacetylation of Miro1 decreased mitochondrial transport in axons and attenuated the axonal growth cone. Changing the lysine 105 acetylation site in Miro1 into an acetylation-mimetic, thus preventing any negative effect of HDAC6, was sufficient to counteract the decrease in mitochondrial transport and growth cone size induced by CSPG. Moreover, HDAC6 and Miro1 interact as both proteins could be coimmunoprecipitated. These data strongly indicate that HDAC6 plays an important role in mitochondrial transport through its deacetylating effect on Miro1 (Fig. 1).
Figure 1.
Schematic illustration of the interaction between HDAC6 and Miro1. Nonpermissive substrates like MAG and CSPGs activate RhoA/ROCK, increase intracellular calcium, and induce the deacetylation of Miro1 at lysine 105 (K105). This decreases mitochondrial axonal transport and inhibits the outgrowth of the axonal growth cone. Selective inhibition of the deacetylase activity of HDAC6 using tubastatin A restores axonal transport, as well as all the other phenotypes. While the interaction with kinesin-1 is responsible for anterograde transport of the mitochondria, a similar mechanism might also be present for the retrograde transport machinery.
However, an important question remains: how does HDAC6 become activated? To find out, Kalinski et al. investigated the role of the classical signaling pathways activated by MAG or CSPG stimulation. HDAC6 inhibition using the selective HDAC6 inhibitor tubastatin A counteracted one of the well-known downstream mediators of the growth inhibitory effects of MAG or CSPG, the RhoA/ROCK pathway (5). Not unexpectedly, a calcium chelator also prevented the phenotypes induced by the CNS growth inhibitors. No additive effects were observed when the ROCK inhibitor and/or the calcium chelator were combined with tubastatin A, which proves that HDAC6 inhibition is downstream of the RhoA/ROCK pathway and of the increase in intracellular calcium (Fig. 1). As far we know, HDAC6 expression, activity, and/or localization are not regulated by the RhoA/ROCK pathway. It is also not a calcium-dependent process. This is in contrast to Miro1, which interacts with the motor domain of kinesin-1 and prevents association of this motor protein with the microtubules after binding calcium to the EF-hand motifs within the protein (6). This results in a calcium-dependent arrest of mitochondrial movement, which is also observed in Kalinski et al. (5; Fig. 1). Moreover, the sensitivity of Miro1 to calcium was essential for the inhibition of axon regeneration by CSPG. Further work will be necessary to understand if or how HDAC6 activity is specifically regulated in this context.
What are the functional consequences of HDAC6 inhibition when neurons are treated with MAG or CSPG? In the presence of tubastatin A, Kalinski et al. find that mitochondria significantly increased anterograde versus retrograde transport along the axons (5). This resulted in the accumulation of mitochondria not only in growth cones of cultured dorsal root ganglion neurons in vitro but also in the sciatic nerve of adult rats in vivo. Exposure of this nerve to tubastatin A resulted in higher mitochondrial numbers, and also in vivo imaging showed that the anterograde movement of mitochondria increased significantly. Moreover, HDAC6 inhibition compensated for defective mitochondria, either induced artificially or by exposure to CSPG by increasing their transport toward those regions containing the defective mitochondria. Altogether, a clear correlation was found between growth cone retraction and the absence of functional mitochondria, strongly indicating that both processes are interconnected.
Why have HDAC6 and Miro1 evolved the ability to interact since this does not seem to have positive consequences? A possibility is that the deacetylation of Miro1 occurs while HDAC6 is close by linking ubiquitinated proteins with motor proteins (7). A similar hypothesis has been proposed to explain the therapeutic effect of HDAC6 inhibitors in models of neurodegeneration (8). While HDAC6 is removing ubiquitinated proteins from the axon, it may also deacetylate α-tubulin as an unwanted side effect. Deacetylation of α-tubulin reduces the efficiency with which motor proteins bind to microtubules (9) and this could lead to axonal transport defects. Therefore, the therapeutic effect of HDAC6 inhibition is linked to the increased acetylation of microtubules, leading to improved binding of the motor proteins and more efficient clearance of misfolded proteins. A major difference with the current study by Kalinski et al. (5) is that this leads to improved axonal transport of different cargoes, and not only mitochondria (10). While a similar mechanism could be at play in the presence of CNS growth inhibitors, more research is needed to understand the exact relationship with the newly discovered HDAC6/Miro1 interaction.
Acknowledgments
The author declares no competing financial interests.
References
- 1.Yiu G., et al. Nat. Rev. Neurosci. 2006 doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivieccio M.A., et al. Proc. Natl. Acad. Sci. USA. 2009 doi: 10.1073/pnas.0907935106. [DOI] [Google Scholar]
- 3.Wong V.S.C., et al. eNeuro. 2018 doi: 10.1523/ENEURO.0240-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita Y., et al. Front. Neurosci. 2014 doi: 10.3389/fnins.2014.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalinski A.L., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201702187. [DOI] [Google Scholar]
- 6.Wang X., et al. Cell. 2009 doi: 10.1016/j.cell.2008.11.046. [DOI] [Google Scholar]
- 7.Kawaguchi Y., et al. Cell. 2003 doi: 10.1016/S0092-8674(03)00939-5. [DOI] [Google Scholar]
- 8.d’Ydewalle C., et al. Traffic. 2012 doi: 10.1111/j.1600-0854.2012.01347.x. [DOI] [PubMed] [Google Scholar]
- 9.Reed N.A., et al. Curr. Biol. 2006 doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Guo W., et al. Nat. Commun. 2017 doi: 10.1038/s41467-017-00911-y. [DOI] [Google Scholar]