Abstract
Early and accurate diagnosis and rigorous clinical and microbiological monitoring of multidrug-resistant tuberculosis (MDR-TB) treatment can curb morbidity and mortality. While others are still under evaluation, the World Health Organization has recommended few novel molecular methods for MDR-TB diagnosis only. We present current molecular methods for diagnosis and monitoring of MDR-TB treatment in TB-endemic settings. A systematic meta-narrative review was conducted according to the RAMESES recommendations. Electronic databases were searched for relevant articles published in English language from January 2013 to June 2018. Based on predefined criteria, two independent reviewers extracted the key messages from relevant articles. Disagreement between them was resolved through discussion and the involvement of a third reviewer, if needed. Key messages were synthesized to create the meta-narratives for method’s accuracy, drug-susceptibility capability, and laboratory infrastructure required. We included 33 articles out of 1213 records retrieved, of which 16 (48%) and 12 (36%) were conducted in high- and low-TB-endemic settings, respectively. Xpert® MTB/RIF, GenoType MTBDRplus, GenoType MTBDRsl, FlouroType™ MTBDR, TB TaqMan® array card, and DNA sequencers can accurately guide effective treatment regimens. Molecular bacterial load assay quantifies mycobactericidal impact of these regimens. Although they present inherent advantages compared to the current standard of care, they carry important limitations to implementation and/or scale-up. Therefore, considerable effort must now be directed to implementation and health systems research to maximize these forecasted benefits for individual patient’s health outcomes.
Keywords: Anti-tuberculosis therapy, diagnosis, drug-resistant tuberculosis, molecular methods, monitoring
Introduction
Treatment of multidrug-resistant tuberculosis (MDR-TB) defined as TB disease caused by Mycobacterium tuberculosis complex (MTBC) strains with resistance to rifampicin (RIF) and isoniazid is complex.[1] There are now multiple treatment regimens and duration options for MDR-TB, depending on patient characteristics and MTBC drug-susceptibility testing (DST) results.[1,2] These regimens contain at least five effective drugs consisting of one fluoroquinolone (FQ: levofloxacin, moxifloxacin, and gatifloxacin), one second-line injectable drug (SLID: kanamycin, amikacin, and capreomycin), two other core drugs (e.g., ethionamide/prothionamide, cycloserine/terizidone, linezolid, and clofazimine), and add-on drugs (high-dose isoniazid, pyrazinamide, and ethambutol). Pre-extensively drug-resistant TB (pre-XDRTB) and XDR-TB are defined as either resistance to FQs or SLIDs for pre-XDR TB or resistance to both FQs and SLIDs for XDR-TB. Both require individualized regimens by substituting the offending drugs, preferably with a drug such as bedaquiline.[2,3] Evidence from South Africa TB program shows that bedaquiline-containing regimens for treating MDR-TB and XDR-TB patients considerably reduced mortality as compared to other regimens. The recent WHO rapid communication prefers an all oral bedaquiline-containing second-line regimen, should DST results otherwise allow.[4] The new World Health Organization (WHO) guidance has not only emphasized the importance of DST but also generally ushered in a new era of more personalized focus on bedaquiline-containing treatment regimens.[5] Regardless of regimen used, all patients with MDR-TB require routine monthly microbiological monitoring to track their progress and identify treatment failures or reversions.[6,7]
Despite advances in diagnosis and treatment of MDR-TB in the past decade, incomplete DST and inability to rigorously monitor microbiological response to anti-TB therapy with the current technologies make it difficult to effectively treat patients. Consequently, approximately 50% of the patients receiving MDR-TB treatment have unfavorable outcomes. In many TB-endemic settings, DST to all drugs in the regimen is not performed. This exposes patients to fewer active drugs, increasing their risk of acquiring drug-resistance, and undue toxicity if treated with a potentially harmful medication with little or no in vivo benefit.[8,9] The End TB Strategy of the WHO aims to decrease TB incidence and mortality by 90% and 95%, respectively, by 2035. This will only be possible if a comprehensive series of interventions is utilized including field-tested methods for rapid diagnosis, more complete DST, and thorough monitoring anti-TB therapy.[10,11] Until now, only Xpert® MTB/RIF (Cepheid, Sunnyvale, California, USA) and the line-probe assays GenoType® MTBDRplus and GenoType® MTBDRsI (both Hain LifeScience GmbH, Nehren, Germany) have received the WHO approval and all have important positive and negative performance characteristics and drug-susceptibility capabilities.[12] There is no molecular method endorsed for monitoring treatment response at this time, necessitating the continued use of phenotypic methods.[12,13] However, while serving as the gold standard for both DST and monitoring of microbiological response to treatment, phenotypic methods suffer practical limitations.[13,14] First, the specimen must be processed to amplify the number of MTBC before DST and this must be performed in a Biosafety Level 3 Laboratory (BSL 3), which may not be easily found in a differentially resourced area. Furthermore, the results can be delayed for 4–12 weeks and up to 15% of the samples may be contaminated; this is a significant barrier for timely case management and can hinder infection prevention and control practices.[15–17] In addition, routine culture misses more dormant nonreplicating MTBC subpopulations which can cause reversion to culture positivity after negative results or lead to relapse after therapy is completed.[18,19]
Most reviews have evaluated a single molecular method or compared the performance of multiple tests in TB-endemic, usually focusing on methods endorsed by the WHO using narrative approach.[20,21] This approach does not give readers an opportunity to assess methodological process, and it suffers study selection bias.[22] This review summarizes the current publicly available molecular methods for MDR-TB diagnosis and monitoring of treatment response using a systematic meta-narrative approach and focusing on advantages and limitations and concluding with an informed assessment of future directions for the field.
Methods
Study design and inclusion criteria
This systematic review was conducted within meta-narrative format, which qualitatively discusses a diverse concepts of molecular methods by highlighting the contrasting and complementary ways from different researchers.[23] A protocol containing a set of eligibility criteria was developed and approved by authors according to the RAMESES meta-narrative review publication standards. Articles were included in the review if they met the following criteria: (i) original article published in English language from January 2013 to June 2018, (ii) cross-sectional or cohort studies that evaluated molecular method’s technical performance (sensitivity, specificity, and accuracy or concordance) for either MDR-TB diagnosis or monitoring anti-TB therapy using either sputa or isolates, and (iii) adult participants aged ≥18 years with presumptive pulmonary TB. Articles with the following features were excluded: (i) case reports; (ii) review articles, commentary articles, and short communications; (iii) epidemiological studies describing molecular epidemiology, drug resistance profile, case detection/notification rates, or lack of DST results; (iv) author evaluated multiple at once or an outmoded version of a method; (v) use in extra-pulmonary TB; and (vi) immunological or host biomarkers either for diagnosis of MDR-TB or monitoring anti-TB therapy.
Search strategies and changes in the review process
In this review, recent evidence on molecular methods for monitoring anti-TB therapy was sparse. Therefore, the article search was extended to articles published from January 2011. We first searched Medline/PubMed, and then additional articles were obtained from Google Scholar and through scanning citations. Searching for relevant articles was conducted using the following terms: (molecular OR genotyp* OR “polymerase chain reaction” OR “PCR”) AND (“drug resistan* tuberculosis”) AND diagnosis OR (molecular or genotyp* OR “polymerase chain reaction” OR “PCR”) AND (“multidrug resistan* tuberculosis”) AND monitor* AND (“tuberculosis treatment response” OR “anti-tuberculosis therapy”).
Selection and appraisal of articles
Two independent reviewers (PMM and SYM) screened the titles and abstracts of identified articles as per the eligibility criteria. An article was read in full if the abstract mentioned, in some capacity, performance of molecular method for DR-TB diagnosis or for monitoring anti-TB therapy. Duplicates were removed. A final consensus was discussed between the two reviewers. An opinion from a third reviewer (SGM) was sought for any disagreement between the two. Ultimately, eligible articles were archived in Mendeley-reference management Software (www.mendeley.com) referencing manager.
Data extraction
A standardized data extraction form was developed, piloted, and revised to improve clarity. Independently, the two reviewers extracted data such as author’s name, year of publication, country, name of molecular method, target biomarker, intended use, study population, type and number of specimens tested, and the method’s technical performance measured against either phenotypic culture or genotypic-based DST from the relevant articles. To establish agreement between culture and molecular method in monitoring therapy, correlation coefficient and bacterial load decline rate were also extracted.
Data analysis and synthesis
Characteristics of articles and molecular methods identified are summarized in Tables 1 and 2. Meta-narratives for different molecular methods from different articles were catalogued to illuminate the clinical applications and research opportunities in TB-endemic settings. They were featured to describe the principle of the test, technical performance (accuracy), advantages and limitations based on simplicity, turnaround time, laboratory infrastructure, and logistics required.
Table 1:
Characteristics of articles and molecular methods evaluated in adults with multidrug resistance tuberculosis
| Author and year | Country | Purpose | Target biomarker | Specimen tested | Sample size | Performance |
|---|---|---|---|---|---|---|
| Rice et al., 2017[26] | USA | Detects MTBC and | A 560 region of MTBC DNA and 81-bp of RRDR in the codons 507–533 of the rpoB gene | Sputum | 751 | 100% sensitivity and 98% specificity |
| Guenaoui et al., 2016[27] | Algeria | DST of RIF | Sputum | 50 | Sensitivity and specificity arc 100% | |
| Chikaonda et al., 2017[28] | Malawi | Sputum | 348 | Sensitivity and specificity of 100% | ||
| Huang et al., 2018[29] | China | 1062 | 97% sensitivity and 98% specificity | |||
| Chakravorty et al., 2017[32] | USA | rpoB gene, IS6110 and IS 1081 | Sputum | 277 | 93% sensitivity and 98% specificity | |
| Dorman et al., 2018[33] | South Africa, Uganda, Kenya, India, China, Georgia, Belarus, and Brazil | Sputum | 314 | 95% sensitivity and 98% specificity | ||
| Chakravorty et al., 2017[34] | USA | Detects MTBC and DST of FQs and SLIDs (AMK and KAN), and INH | gyrA, gyrB, katG. and rrs | Sputum | 24 | 100% sensitivity for all targets and >94% specificity compared to sanger sequencing. Using phenotypic DST, Sensitivity for FQ is 75% and 100% for INH and AMK, KAN. Specificity 100% for INH and FQ and 94% for SLIDS |
| Xie et al., 2017[35] | China and South Korea | MTBC isolates | 308 | Using culture/genotypic DST, sensitivity for INH, FQs and SLIDs is 83/96%, 88/98% and 71/93% respectively. Specificity was 94/99% | ||
| Chen et al., 2014[37] | China | Detects MTBC and DST of RIF and INH, the MDR-TB defining anti-TB agents | rpoB. katG, inhA genes | Sputum | 427 | 86% sensitivity and 96% specificity for RIF; and 77 and 95% for INH. 70% sensitivity and 97% specificity for detecting MDR-TB and >70% accuracy against culture |
| Karimi et al., 2018[38] | Morocco | Sputum | 70 | 92 and 97% sensitivity for RIF and INH respectively with 100% Specificity | ||
| Lin et al., 2017[39] | Taiwan | Sputum | 5838 | Sensitivity and specificity for RIF were 92 and 97% and 78% and 100% for INH. 83% sensitivity and 100% specificity for detecting MDR-TB, and >95% test accuracy | ||
| Maharjan et al., 2017[40] | South Africa | Sputum | 69 | Sensitivity and specificity for RIF, INH and MDR-TB were 89% and 100% | ||
| Maningi et al., 2017[41] | Nepal | MTBC Isolates | 100 | Sensitivity for detecting RIF and INH and MDR were 100%. Specificity was 88%, 94% and 100% respectively, and >70% accuracy with culture | ||
| Seifert et al., 2016[42] | USA, India, Moldova, and South Africa | Sputum | 1128 | Sensitivity and specificity for RIF were 97% and 98% and 94% and 100% for INH. 95% sensitivity and 99% specificity for detecting MDR-TB | ||
| Abanda et al., 2017[43] | Cameroon | Sputum | 225 | Sensitivity for detecting RR, INH, and MDR-TB 98%, 92$, 94% respectively, and specificity over 99% with 96% accuracy with culture | ||
| Maeza et al., 2017[44] | Ethiopia | Sputum | 274 | sensitivity for detecting RR, INH and MDR-TB 88, 92, 96% respectively, and specificity over 99% | ||
| Singh et al., 2017[45] | India | Sputum | 572 | Sensitivity for RIF and INH were 100 and 99%, with 99% specificity | ||
| Tagliani et al., 2015[46] | Europe (Germany, Italy and Sweden) | Detect MTBC and DST to SLIDs and FQs (either XDR or pre-XDR-TB) | rrs, eis, gyrA and gyrB on the DNA of MTBC | MTBC Isolates | 228 | 86% and 90% Sensitivity and specificity for SLIDs, 83%−94$ and 100% for FQs, and 80$ and 82% for detecting MDR-TB, respectively |
| Sputum | 231 | Sensitivity and specificity for SLIDs were 90% and 92%, and 93$ and 98% for FQs and for detecting MDR-TB was 82% and 98% respectively | ||||
| Gardee et al, 2017[47] | South Africa | MTBC Isolates | 268 | 89% and 99% sensitivity and specificity for SLIDs and 100% and 99% for FQs, and 87% sensitivity for detecting XDR-TB, with 96% test accuracy for both target drugs | ||
| Yadav et al., 2018[48] | India | Sputum and isolates | 431 | 93% sensitivity and 100% specificity for both SLIDs and XDR-TB, and 97% and 99% for FQs respectively | ||
| Hillemann et al., 2018[50] | Germany | Detects MTBC and DST of RIF and INH | rpoB, katG, inhA genes | Isolates | 180 | 99% and 92% sensitivity for RIF and INH, respectively with 100% specificity compared to phenotypic DST |
| de Vos et al., 2018[51] | South Africa | Sputum | 448 | 100% sensitivity for both RIF and INH is 100% with 97% and 98% specificity for RIF and INH respectively, compared to genotypic DST | ||
| Pholwat et al., 2015[52] | Bangladeshi, Thailand and Tanzania | Detects MTBC and DST for both first- and second-line drugs including PZA | rpoB. katG. inhA, pncA. emb, rrs, eis. tlyA, rpIC, gyrA, gyrB etc.) | MTBC Isolates | 230 | 87% and 96% accuracy against culture and Sanger sequencing respectively. Accuracy for detecting PZA 81% |
| Foongladda et al., 2016[53] | Bangladeshi, Thailand and Tanzania | MTBC Isolates | 212 | 75%−87% sensitivity and 91 %−98% specificity for SLD and≥91% for detecting MDR-TB with 87% accuracy for all drugs tested | ||
| Banu et al., 2017[54] | Bangladeshi and Thailand | sputum and MTBC Isolates | 71 | 98% sensitivity and 92% specificity. Sensitivity in smear positive and negative was 89% and 33%, with 96%accuracy against Sanger sequencing | ||
| Walker et al., 2015[57] | UK | Detection of MTBC up to species level and associated phenotypic drug resistance | SNP on entire MTBC genome or target region of a gene | MTBC isolate | 2099 | 92% sensitivity, 98% specificity, and 89% accuracy or detecting resistance |
| Quan et al., 2018[58] | UK | MTBC isolates | 2039 | 94% sensitivity, 99% specificity, and 99% accuracy for detecting resistance | ||
| Chatterjee et al., 2017[59] | India | MTBC isolates | 74 | 100% sensitivity, 94% specificity, and 97% accuracy for detecting MDR-TB | ||
| Shea et al., 2017[60] | New York State, USA | MTBC isolates | 608 | Sensitivity for speciation, detecting and predicting resistance for all drugs was 99, 96% and 83% respectively. Specificity and accuracy were 99% | ||
| Nikolayevskyy et al., 2015[62] | UK, Italy, Russia, Lithuania, Latvia | Detect viable MTBC DNA during treatment | PMA free DNA (viable DNA) | Serial sputa | 1937 | Sensitivity and specificity for detecting viable MTBC DNA was 98% and 71%−80% |
| Kayigire et al., 2016[63] | South Africa | 151 | Sensitivity and specificity for detecting viable MTBC DNA was 95% and 63% compared to 95% and 42% in samples without PMA respectively | |||
| Honeyborne et al., 2011[64] | Tanzania and Germany | Monitoring treatment response | MTBC 16S rRNA | Serial sputa | 148 | Biphasic decline of bacterial load in response to anti-TB treatment comparable o culture |
| Honeybome et al., 2014[65] | Tanzania | 111 | Biphasic decline as observed longitudinally during anti-TB therapy at a mean of 0.99–0.81 log |
MTBC: Mycobacterium tuberculosis complex, MDR-TB: Multidrug-resistant tuberculosis, PZA: Pyrazinamide, PMA: Propidium monoazide, DST: Drug susceptibility testing, SLIDs: Second-line injectable drug, rRNA: Ribosomal RNA, SNP: Single nucleotide polymorphism, RIF: Rifampicin, INH: Isoniazid, XDR-TB: Extensively drug-resistant tuberculosis, AMK: Amikacin, KAN: Kanamycin, RRDR: Rifampicin resistant determining region
Table 2:
Summary of molecular methods for either diagnosis or monitoring of drug resistant tuberculosis patients
| Variable & methods | Xpert® MTB/RIF | Xpert®- Ultra | Xpert®- XDR | Genotype MTBDRplus | Genotype MTBDRsl | FlouroType MTBDR | TAC-HRM | DNA Sequencers | Xpert-PMA | MBLA |
|---|---|---|---|---|---|---|---|---|---|---|
| Maker | Cepheid, USA | Hain Life Science, Germany | Thermal fisher, USA | Several | Cepheid, USA | UK | ||||
| WHO status | Approved | Not approved | Approved | Not approved | ||||||
| Purpose | DR-TB diagnosis | monitoring therapy | ||||||||
| Target genes or biomarker | rpoB | rpoB, IS6110& IS 1081 | kalG, inhA, gyrA, gyrB, rrs | rpoB, katG & inhA | gyrA, gyrB, rrs & eis | rpoB,katG & inhA | rpoB, katG, inhA, gyrA, gyrB, rrs, pncA etc. | All or several target genes | DNA for viable MTBC | I6S rRNA for viable MTBC |
| Anli-TB drugs | RIF | RIF | INH, FQs, KAN, AMK | RIF & INH | FQs, KAN & AMK | RIF & INH | Several e.g., PZA | All or target | Not applicable | Not applicable |
| TAT (days) | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 5–10 | 1 | 2 |
| Specimen | Sputum & isolates | Sputum & isolates | Sputum & isolates | Sputum & isolates | Sputum & isolates | Sputum & isolates | Sputum & isolates | Isolates | sputum | sputum |
| Minimum Location | Peripheral laboratory | reginal and reference laboratory | reference laboratory | Peripheral laboratory | reginal laboratory | |||||
| Infra structurer | BSL2 with BSC | BSL2 with BSC | BSL2 with BSC | BSL2 with BSC | BSL2 with BSC | BSL2 with BSC | BSL2 with BSC | BSL3 | BSL2 with BSC | BSL3 |
| Personnel required | less skilled laboratorian | Skilled laboratorian with molecular biology knowledge | highly Skilled | less skilled | skilled | |||||
| Reagents storage | rT | 2–8 °C and freezer (either −20 or-80 °C) | rT | 2–8 °C and freezer | ||||||
| Reference | [26–29] | [32,33] | [34,35] | [37–45] | [46–48] | [50,51] | [52–54] | [57–60] | [62,63] | [64,65] |
AMK: amikacin; BSC: biosafety cabinet; BSL: biosafety laboratory level; FQs: fluoroquinolones; INH: Isoniazid; KAN: Kanamycin; MBLA: Molecular bacterial load assay; PZA: Pyrazinamide; rT: room Temperature; TAT: turnaround time and TAC-HRM: TaqMan® array card-high resolution melts
Results
Selection of studies included
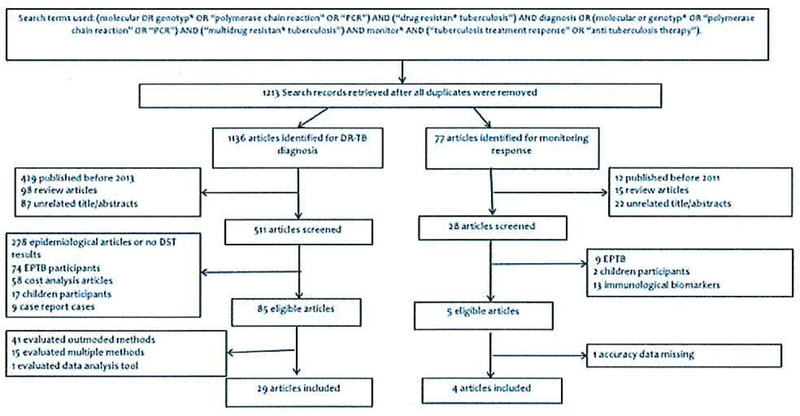
A total of 1213 articles were retrieved from all electronic databases. Of these, 92 articles (87 for diagnosis and 5 for monitoring therapy) were read in full. A total of 29 and four articles were included in the review of methods for diagnosis and monitoring anti-TB therapy, respectively [Figure 1], Reasons listed in Figure 1 are used to exclude irrelevant articles. Common molecular methods are summarized in Tables 1 and 2.
Figure 1.
The processes and procedures used to retried relevant articles in electronic data. It also depicts the numbers of articles identified, screened for eligibility, and inclusion into the review
Characteristics of articles included
This review included 33 articles published from 2011 to 2018 [Table 1 and Figure 1]. Of 33 articles, 16 (48%) were conducted in high TB-endemic settings, 12 (36%) in lower TB-endemic settings, and 5 (16%) had collaborators from both settings [Table 1]. About 64% (21/33) of articles reported methods that analyzed sputa. The target biomarkers, clinical application, and strengths and limitations of molecular methods are summarized in Table 1.
Molecular methods for detecting Mycobacterium tuberculosis complex and multi/extensively drug-resistant tuberculosis
Xpert® MTB/RIF assay (Cepheid, Sunnyvale, California, USA)
The Xpert® MTB/RIF is a cartridge-based real-time polymerase chain reaction (RT-PCR) assay approved by the WHO for dual detection of MTBC and RIF susceptibility.[24] It amplifies the target 560 region of MTBC and 81-bp RIF-resistant determining region (RRDR) in the codons 507–533 of the rpoB gene, a proxy biomarker for RIF-resistant TB (RR-TB).[25] Xpert® MTB/RIF is robust and rapid, providing results within 24 h, and has sensitivity and specificity of over 95% and 99% in detecting RR-TB as compared to culture-based DST [Table 1].[26,29] In addition, the test is simple to use and semi-automated with minimal risk of contamination and infection to laboratorians.[30] However, susceptibility testing is limited to RIF only.[24] It also requires laboratory infrastructure such as a stable electrical supply and a consistent temperature-humidity range necessary to prevent module malfunctions.[31]
Xpert® MTB/RIF Ultra assay (Cepheid, Sunnyvale, California, USA)
Xpert® MTB/RIF Ultra assay (Ultra) is a new generation assay that is more sensitive than Xpert® MTB/RIF (Cepheid, Sunnyvale, California, USA). The Ultra detects two additional multi-copy amplification targets (IS6110 and IS 1081) and has a larger PCR chamber to accommodate 50 µL of a sample compared with 25 µL in Xpert® MTB/RIF. This design lowers the limit of detection from 131 CFU/mL for Xpert® MTB/RIF to 16–20 CFU/mL, accounting for sensitivity of 93% for Ultra compared to culture [Table 1].[32,31] It detects MTBC even in patients with paucibacillary load. Unlike Xpert® MTB/RIF, Ultra uses melting temperatures instead of the RT-PCR curve analysis, which allows detection of silent mutations within RRDR that may or may not be associated with resistance. Therefore, Ultra is robust and has improved ability to detect mutations predictive of phenotypic RIF resistance (i.e., rpoB 533 C-to-G mutations), while avoiding false positives in samples with low bacterial load [Table 1].
Xpert XDR assay (Cepheid, Sunnyvale, California, USA)
The XDR assay, also called Xtend XDR, is a new CEPHEID platform for detecting pre-XDR and XDR-TB. Principally, the assay is designed into three phases: 8-plex nested PCR, melt curve analysis, and 10 sloppy beacon detection probes. The assay can differentiate 32 mutations in genes predictive of phenotypic resistance to isoniazid (katG and inhA promoter genes), FQs (gyrA and gyrB), and aminoglycosides (rrs and eis promoter). Compared to sequencing, XDR assay has sensitivity of 98%, 96%, 93%, and 97% in detecting isoniazid, FQ, and aminoglycoside (kanamycin and amikacin) resistance, respectively, with specificity of 100%. The sensitivity is lower when compared with Conventional MGIT 960 System at 71%, 83%, and 88% in detecting aminoglycoside, isoniazid, and FQ resistance, respectively [Table 1].[34,35] Unfortunately, this assay has not yet been evaluated in clinical settings or in implementation studies. The XDR assay also cannot reliably predict susceptibility for the cyclic polypeptide capreomycin, an alternative drug to the aminoglycosides.
GenoType® MTBDR assays (Hain Lifescience GmbH, Nehren, Germany)
The GenoType® MTBDR is DNA-strip line probe assay (LPA) that amplifies MTBC DNA and drug RDRs and detects mutation(s) on target genes predictive of MDR-TB or XDR-TB. For example, the genotype MTBDRplus version 2.0 dually detects MTBC and mutations predictive of phenotypic resistant to RIF on rpoB and isoniazid on both katG and inhA genes.[36] Compared to culture-based DST, it has sensitivity and specificity of 84% and 98%, with accuracy of 83% in detecting MDR-TB [Table 1].[37–45] This performance is also similar for genotype MTBDRsl version 2.0 in detecting mutations on rrs and eis promoter regions, predictive of phenotypic resistance to amikacin and kanamycin, and gyrA and gyrB genes for FQs [Table 1].[46–48] Compared to the Xpert® MTB/RIF platforms, the GenoType MTBDR assays are more labor intensive. They require a skilled laboratorian, adequate laboratory infrastructure compatible with at least BSL2, biosafety cabinets, three separate rooms to accommodate all steps and minimize cross-contamination risk, constant power supply, refrigerator or freezer to store reagents and centrifuges.[36,49]
FluoroType® MTBDR assays (Hain Lifescience GmbH, Nehren, Germany)
The FluoroType® MTBDR assay is a semi-automated LPA that detects MTBC DNA and mutations on rpoB for RIF and both katG and inhA genes for isoniazid from both isolates and sputum samples.[50,51] This detection is made in a closed system using melting-curve analysis and the results are read and provided by FluoroSoftware in 3–4 h.[50,51] Compared to phenotypic DST, sensitivity in detecting RIF and isoniazid resistance is 99% and 92%, respectively, with specificity of 100%.[50] In sputum samples, the assay has excellent sensitivity of 100% and specificity of 97%, compared to GenoType® MTBDRplus or targeted Sanger sequencing.[51] Its main advantage over other LPAs is its closed system and automation that reduces the risk of contamination and erroneous results interpretation. Like other LPAs, it requires different workstations for DNA extraction and preparation of PCR mix and hybridization.
TaqMan® array card for tuberculosis
The TaqMan® array card for TB (TB-TAC) is a customizable 384-well microfluidic RT-PCR system that compartmentalizes each sample into 48 different PCRs simultaneously for detecting mutations on multiple genes associated with phenotypic resistance of MTBC to anti-TB drugs.[52] These genes include inhA and katG (isoniazid), rpoB (RIF), embB (ethambutol), rrs (kanamycin, amikacin, and capreomycin), eis (low-level kanamycin), gyrA and gyrB (FQs), 23S and rplC (linezolid), and pncA (pyrazinamide). TB-TAC has two layers of detection: the probe-based layer, containing over 40 sequence-specific probes, and the second layer, high-resolution melt (FIRM) analysis interrogated into at least 20 primer pairs and 27 amplicons for detecting MTBC and the presence of wild-type and mutant genes encoding these drugs. It also characterizes pncA mutations which are not possible with probe-based assays. The assay performs more accurately in smear-positive sputum than smear-negative samples at 89% and 33%, respectively, as compared to culture and Sanger sequencing. The overall accuracy for MTBC susceptibility to all anti-TB drugs is 87% [Table 1].[52–54] However, it requires an expensive RT-PCR platform and skilled personnel to interpret FIRM software results and has only been used in the research settings.
DNA sequencing technologies
DNA sequencing technologies have gained popularity, not only in research settings but also in clinical applications and public health and epidemiological investigations.[55] Principally, all sequencing technologies involve DNA extraction, library preparation by breaking down genomic DNA into small base paired fragments, sequencing to 100–300 bp reads, and ultimately curating of sequence reads. Adequate quality reads are then mapped to published M tuberculosis reference genome sequences to identify single nucleotide polymorphisms and insertions-deletions.[55] Finally, bioinformatic analyses are carried out to interpret results and predict strain lineage and drug resistance using different software tools.[56] Sequencing is accurate, robust, and average turnaround time is 7 days [Table 1]. For example, whole genome sequencing (WGS) by Illumina MiSeq platform can differentiate MTBC species, detect, and predict ding resistance phenotypes at a sensitivity of 99%, 96%, and 83%, respectively. Compared to either culture or genotypic-based DST, the concordance, sensitivity, and specificity of WGS in detecting phenotypic resistance to anti-TB drugs range from 83% to 99%, 83% to 100%, and 78% to 99%, respectively [Table 1].[57–60] Sequencing allows tracing of genetic relatedness and transmission dynamics of MTBC strains during an outbreak.[55] It is expensive, mostly done in reference research or clinical laboratories by skilled bioinformaticians and requires several software for analysis, stable internet access, and a regularly maintained hardware server for online storage of biological data. In addition, sequencing has no standardized protocol or testing algorithms across the globe. Nevertheless, as the technology moves closer to point of care, there will be numerous opportunities for implementation studies.
Molecular methods for monitoring anti-tuberculosis treatment
Xpert® MTB/RIF assay and propidium monoazide
DNA-based molecular methods such as Xpert and LPA are not recommended for monitoring treatment response in patients with tuberculosis because they cannot differentiate viable and dead MTBC DNA.[ 25] However, pretreatment of sputum samples with propidium monoazide (PMA) (Biotium Inc., Hayward, California, USA) increases the specificity of Xpert® MTB/RIF in the detection of viable DNA. PMA selectively intercalates the dead MTBC DNA and inhibits its amplification and detection.[61] Monitoring anti-TB treatment by Xpert-PMA has been evaluated in two studies. The first study measured bacterial load from 1937 sputum samples that were collected before treatment, 2 weeks after treatment, and monthly thereafter, during the intensive and continuation phases of non-MDR-TB and MDR-TB treatment.[62] In the second study, participants produced 151 sputa at eight time points before treatment and then at days 3, 7, 14, 28, 35, 56, and 84 of treatment.[63] Compared to culture, both studies achieved 53%−80% specificity for detecting viable MTBC DNA [Table 1].
Molecular bacterial load assay
Molecular bacterial load assay (MBLA) is a RT-PCR that detects and quantifies 16S ribosomal RNA (16S rRNA) of viable MTBC from sputa.[64] When MTBC cells are killed by anti-TB drugs, the amount of rRNA also decreases, making it possible to estimate the number of viable cells in sputum sample. rRNA decline has been interpreted as a surrogate biomarker of bactericidal activity for anti-TB therapy. For instance, two studies documented mean MTBC load decline rate and correlation of MBLA with culture from sequential sputum samples for at least 14 days of intensive phase of treatment in patients treated for drug-susceptible TB of 90% and 84%, respectively [Table 1].[64,65] MBLA is rapid, robust, and accurate with minimal or no risk of contamination [Table 1]. Nevertheless, logistics for handling sputum samples have not yet been optimized for use in clinical settings. It is also expensive and requires skilled personnel for several manual steps and good laboratory infrastructure compatible with reference-level laboratories.
Discussion
In this meta-narrative review, we report rapid and accurate molecular methods for MDR-TB diagnosis and monitoring anti-TB therapy. Their rapidity shorten the time to diagnosis and treatment from 2 to 3 months by phenotypic culture to 1–7 days.[66] They accurately guides early treatment options that both minimize transmission and further development of drug resistant strains in the community.[67] Although implementation of molecular methods for diagnosis reduced time to treatment of MDR-TB in South Africa and Georgia,[68,69] high cost and unavailability of comprehensive implementation and impact assessment plans in most TB-endemic settings have been the main constraints to incorporate in clinical practices.[69,70] Furthermore, a cluster-randomized clinical trial in South Africa found that usage of Xpert® MTB/RIF in initial TB diagnosis did not provide a mortality benefit as compared to smear microscopy.[71] Further evaluation of these diagnostics especially those with expanded DST in clinical settings is required to improve patient care and reduce global TB burden.
Multiple molecular methods such as TAC-HRM and DNA sequencing technologies, which extend DST to most anti-TB drugs including pyrazinamide, have been appraised in this review.[57,60] Unlike TAC-HRM and other probe-based assays, sequencing technologies can categorize mutations into high, moderate, or low confidence resistance patterns that may or may not be associated with phenotypic drug resistance. Technological advances from Sanger sequencing to next-generation sequencing (NGS) enhance detection of heteroresistance that can occur at very low levels within a specimen. In a multi-country study, amplicon-based NGS detected over 5% and 21% heteroresistant strains that were deemed wild-type and mutant by Sanger sequencing.[72] While expanding the scope of anti-TB DST adds clinical value, these strains may have relevance in informing drug-susceptibility for antibiotics such as pyrazinamide and FQs and can contribute to the decision to initiate patients on a bedaquiline-based regimen and/or one supplemented by other drug classes.[4] Generally, probe-based assays (with or without HRM) and sequencing methods have diagnostic value but have not been widely used in designing DR-TB treatment regimens. Unlike sequencing, which has a wider reach, probe-based methods target specific RDRs of a gene such as vpoB for RIF. This explains why some methods have limited DST capability. Noting that not all mutations lead to phenotypic resistance, their accuracy, and clinical impact requires parallel testing with conventional phenotypic DST methods.[73]
Even if the treatment regimen is well design for individual patient, regular microbiological monitoring, a key clinical practice to foresee health outcomes, is required throughout the duration of anti-TB therapy. Despite challenges related to smear microscopy for acid-fast bacilli and isolation of MTBC on culture, these methods remain the worldwide gold standard for monitoring anti-TB therapy. In this review, two applications of molecular methods used for monitoring anti-TB therapy have been highlighted. In the first method, serially measured MTBC 16S rRNA, a proxy biomarker for viability that assesses mycobactericidal decline during anti-TB treatment by MBLA, was compared to phenotypic culture-based methods.[74] MBLA has not been recommended by the WHO, but its potential clinical value was reported in one case report that documented favorable outcomes after MBLA results were used to modify the anti-TB regimen. In this case, a 12-year-old child with TB/HIV coinfection had prolonged smear positivity beyond 2 months of standard treatment for drug-susceptible TB. MBLA showed high bacterial load in the 1st month. After substitution of moxifloxacin for rifabutin, mycobacterial decline by MBLA was demonstrated, and culture was negative for the duration of treatment.[75] In the second method, PCR inhibitors such as PMA (which bind the DNA of dead bacilli and allow for serial measurement of viable DNA) were used in conjunction with Xpert® MTB/RIF. However, specificity in detecting viable mycobacteria was low favoring serial measurement of 16S rRNA [Table 1]. Thus, there remains no molecular method recommended by the WHO to replace or complement phenotypic methods for monitoring anti-TB therapy.[12] Yet, this meta-narrative review favors further clinical and implementation studies of both Xpert/PMA assay and MBLA to evaluate applicability in different settings and relevant patient outcomes.
This review also found unanswered questions on molecular methods necessary to guide choices for MDR-TB regimen, which merit further attention.[76,77] Possibilities include further studies of TAC on direct sputum along with additional biomarkers that would improve detection in smear-negative individuals. Similarly, it is worth evaluating the Xpert-XDR assay and optimizing protocols for DNA extraction, sequencing, bioinformatics tools, and analysis to augment or replace conventional DST methods in patients who are currently being treated for DR-TB or are failing their regimens. Unlike drug-susceptible TB, treatment failure and relapse are more common in MDR-TB, and patients with pre-XDR and XDR-TB remain culture positive for long periods. These are resource-intensive conditions that require tests such MBLA for monitoring mycobactericidal activity and altering regimens for patients who are poorly responding to anti-TB therapy. In this review, 36% of the molecular methods were performed in lower TB-endemic settings. Because mortality is higher in high TB-endemic settings in comparison to low TB-endemic settings, this meta-narrative review supports recommendations that these settings require more investment and should lead the TB research agenda, a key step toward achieving the 2035 World End-TB strategy.[78,79]
Strength and limitations
The main strength of this review is that it provides timely and relevant information on various molecular methods for diagnosis of MDR-TB and monitoring anti-TB therapy, and it explains potential clinical impact and research opportunities. Focusing on articles published in English language only may have limited the scope of information presented. However, previous systematic reviews documented that language restriction has no significant effect.[80,81]
Conclusion
We found potential molecular diagnostic methods for MDR-TB diagnosis, expanded DST to tailor individualized regimens, and monitoring of treatment response. We urge funders to support efforts to evaluate these technologies for their various clinical applications. Meticulous introduction of these technologies in differing clinical settings will likely be a major step toward fulfilling the End TB Strategy.
Acknowledgments
We acknowledge that this review was conducted within the framework of the DELTAS Africa Initiative (Afrique One-ASPfRE /DEL-15-008) as part of Dr. Peter Mbelele’s PhD thesis at the Nelson Mandela-African Institution of Science and Technology in Tanzania, who is currently working in this field. Afrique One-ASPIRE is funded by a consortium of donors including the African Academy of Sciences, Alliance for Accelerating Excellence in Science in Africa, the New Partnership for Africa’s Development Planning and Coordinating Agency, the Wellcome Trust (107753/A/15/Z), and the UK Government. Furthermore, PMM and SH partly benefited from the EDCTP2 programme supported by the European Union project of Stellah Mpagama (TMA2016-1463), and U01 AI115594 that is evaluating the role of a molecular diagnostic in drug-resistant tuberculosis respectively.
Financial support and sponsorship
Nil.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis 2016 Geneva: World Health Organization; 2016. [Google Scholar]
- 3.Sotgiu G, Tiberi S, Centis R, D’Ambrosio L, Fuentes Z, Zumla A, et al. Applicability of the shorter ‘Bangladesh regimen’ in high multidrug-resistant tuberculosis settings. Int J Infect Dis 2017;56:190–3. [DOI] [PubMed] [Google Scholar]
- 4.Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, et al. Effect of bedaquilinc on mortality in South African patients with drug-resistant tuberculosis: A retrospective cohort study. Lancet Respir Med 2018;2600:1–8. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Rapid Communication : Key Changes to Treatment of Multidrug- and Rifampicin-Resistant Tuberculosis Geneva: World Health Organization; 2018. [Google Scholar]
- 6.Horsburgh CR Jr., Barry CE 3rd, Lange C Treatment of tuberculosis. N Engl J Med 2015;373:2149–60. [DOI] [PubMed] [Google Scholar]
- 7.Walzl G, Mcnerney R, Plessis N, Bates M, Mchugh TD, Chegou NN, et al. Tuberculosis : Advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis 2018;3099:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Kuo CY, Wang WH, Huang CH, Chen YH, Lu PL. Resistance to first- and second-line antituberculosis drugs in Southern Taiwan: Implications for empirical treatment. J Microbiol Immunol Infect 2018;51:88–93. [DOI] [PubMed] [Google Scholar]
- 9.Dookie N, Rambaran S, Padayatchi N, Mahomed S, Naidoo K. Evolution of drug resistance in Mycobacterium tuberculosis: A review on the molecular determinants of resistance and implications for personalized care. J Antimicrob Chemother 2018;73:1138–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. The END TB Strategy 2015–2035 Geneva: World Health Organization; 2015. Available from: http://www.who.int/tb/End_TB_brochure.pdf?ua=l. [Last access on 2018 Oct 14]. [Google Scholar]
- 11.Lienhardt C, Lönnroth K, Menzies D, Balasegaram M, Chakaya J, Cobelens F, et al. Translational research for tuberculosis elimination: Priorities, challenges, and actions. PLoS Med 2016; 13:e 1001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expert opinion of the European Tuberculosis Laboratory Initiative core group members for the WHO European Region. Algorithm for laboratory diagnosis and treatment-monitoring of pulmonary tuberculosis and drug-resistant tuberculosis using state-of-the-art rapid molecular diagnostic technologies Geneva, Switzerland: World Health Organization; 2017. Available from: http://www.euro.who.int/—data/asscts/pdf_file/0006/333960/ELIAlgorithm.pdf?ua=l. [Last accessed on 2018 Oct 14]. [Google Scholar]
- 13.Goletti D, Petruccioli E, Joosten SA, Ottenhoff TH. Tuberculosis biomarkers: From diagnosis to protection. Infect Dis Rep 2016;8:6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Treatment of Tuberculosis Guideline 4th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 15.Rageade F, Picot N, Blanc-Michaud A, Chatellier S, Mirandc C, Fortin E, et al. Performance of solid and liquid culture media for the detection of Mycobacterium tuberculosis in clinical materials: Meta-analysis of recent studies. EurJ Clin Microbiol Infect Dis 2014;33:867–70. [DOI] [PubMed] [Google Scholar]
- 16.Reddy M, Gounder S, Reid SA. Tuberculosis diagnostics in Fiji: How reliable is culture? Public Health Action 2014;4:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heilig CM, Feng PJ, Joloba ML, Johnson JL, Morgan K, Gitta P, et al. How we determined the most reliable solid medium for studying treatment of tuberculosis. Tuberculosis (Edinb) 2014;94:317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhillon J, Fourie PB, Mitchison DA. Persister populations of Mycobacterium tuberculosis in sputum that grow in liquid but not on solid culture media. J Antimicrob Chemother 2014;69:437–40. [DOI] [PubMed] [Google Scholar]
- 19.Torrey HL, Keren I, Via LE, Lee JS, Lewis K. High persister mutants in Mycobacterium tuberculosis. PLoS One 2016; 11 :e0155127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pai M, Nicol MP, Boehme CC. Tuberculosis diagnostics: State of the art and future directions. Microbiol Spectr 2016;4:TBTB2-0019-2016. [DOI] [PubMed] [Google Scholar]
- 21.Noor KM, Shephard L, Bastian I. Molecular diagnostics for tuberculosis. Pathology 2015;47:250–6. [DOI] [PubMed] [Google Scholar]
- 22.Pae CU. Why systematic review rather than narrative review? Psychiatry Investig 2015;12:417–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. Rameses publication standards: Meta-narrative reviews. BMC Med 2013; 11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Xpert MTB/R1F Implementation Manual: Technical and Operational ‘How-to’: Practical Considerations Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 25.Steingart K, Schiller I, Dj H, Pai M, Boehme CC, Dendukuri N, et al. Xpcrt® MTB/R1F assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Libr 2014; 1:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice JP, Seifert M, Moser KS, Rodwell TC. Performance of the Xpert MTB/R1F assay for the diagnosis of pulmonary tuberculosis and rifampin resistance in a low-incidence, high-resource setting. PLoS One 2017; I2:e0186139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guenaoui K, Harir N, Ouardi A, Zeggai S, Sellam F, Bekri F, et al. Use of geneXpert Mycobacterium tuberculosis/infampicin for rapid detection of rifampicin resistant Mycobacterium tuberculosis strains of clinically suspected multi-drug resistance tuberculosis cases. Ann Transl Med 2016;4:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chikaonda T, Nguluwe N, Barnett B, Gokhale RH, Krysiak R, Thengolose I, et al. Performance of Xpert® MTB/RIF among tuberculosis outpatients in Lilongwe, Malawi. Afr J Lab Med 2017;6:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang II, Zhang Y, Li S, Wang J, Chen J, Pan Z, et al. Rifampicin resistance and multidrug-resistant tuberculosis detection using Xpert MTB/RIF in Wuhan, China: A retrospective study. Microb Drug Resist 2018;24:675–9. [DOI] [PubMed] [Google Scholar]
- 30.Clinical Laboratory Standard Institute (CLSI). Laboratory detection and identification of mycobacteria; approved standard. In: CLSI document M48-A Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 31.Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011;377:1495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravorly S, Simmons AM, Rowneki M, Parmar II, Cao Y, Ryan J, et al. The new Xpert MTB/RIF ultra: Improving detection of Mycobacterium tuberculosis and resistance to rifampin in an suitable for point-of-carc testing. MBio 2017;8 pii: e00812–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorman SE, Schumacher SG, Alland D, Nabeta F, Armstrong DT, King B, et al. Xpert MTB/RIF ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect Dis 2018;18:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakravorty S, Roll SS, Glass J, Smith LE, Simmons AM, Lund K, et al. Detection of isoniazid-, fluoroquinolone-, amikacin-, and kanamycin-resistant tuberculosis in an automated, multiplexed 10-color assay suitable for point-of-care use. J Clin Microbiol 2017;55:183–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie YL, Chakravorty S, Armstrong DT, Hall SL, Via LE, Song T, et al. Evaluation of a rapid molecular drug-susceptibility test for tuberculosis. N Engl J Med 2017;377:1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Molecular Line Probe Assays for Rapid Screening of Patients at Risk of Multidrug-Resistant Tuberculosis (MDR-TB): Policy Statement Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 37.Chen C, Kong W, Zhu L, Zhou Y, Peng H, Shao Y, et al. Evaluation of the genoType(®) MTBDRplus line probe assay on sputum-positive samples in routine settings in China. Int J Tuberc Lung Dis 2014; 18:1034–9. [DOI] [PubMed] [Google Scholar]
- 38.Karimi H, En-Nanai L, Oudghiri A, Chaoui I, Laglaoui A, Bourkadi JE, et al. Performance of genoType® MTBDRplus assay in the diagnosis of drug-resistant tuberculosis in Tangier, Morocco. . T Glob Antimicrob Resist 2018;12:63–7. [DOI] [PubMed] [Google Scholar]
- 39.Lin HC, Perng CL, Lai YW, Lin FG, Chiang CJ,Lin HA, et al. Molecular screening of multidrug-resistance tuberculosis by a designated public health laboratory in Taiwan. Eur J Clin Microbiol Infect Dis 2017;36:2431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maningi NE, Malinga LA, Antiabong JF, Lekalakala RM, Mbelle NM. Comparison of line probe assay to BACTEC MGIT 960 system for susceptibility testing of first and second-line anti-tuberculosis drugs in a referral laboratory in South Africa. BMC Infect Dis 2017; 17:795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maharjan E, Pant ND, Neupane S, Amatya J, Shrestha B. Use of genotype MTBDRplus assay for diagnosis of multidrug-resistant tuberculosis in Nepal. Int Sch Res Notices 2017;2017:1635780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seifert M, Ajbani K, Georghiou SB, Catanzaro D, Rodrigues C, Crudu V, et al. A performance evaluation of MTBDRplus version 2 for the diagnosis of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2016;20:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abanda NN, Djieugoue JY, Lim E, Pefura-Yone EW, Mbachanr WF, Vemet G, et al. Diagnostic accuracy and usefulness of the genotype MTBDRplus assay in diagnosing multidrug-resistant tuberculosis in Cameroon? A cross-sectional study. BMC Infect Dis 2017; 17:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meaza A, Kebede A, Yaregal Z, Dagne Z, Moga S, Yenew B, et al. Evaluation of genotype MTBDRplus VER 2.0 line probe assay for the detection of MDR-TB in smear positive and negative sputum samples. BMC Infect Dis 2017; 17:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh BK, Sharma SK, Sharma R, Sreenivas V, Myneedu VP, Kohli M, et al. Diagnostic utility of a line probe assay for multidrug resistant-TB in smear-negative pulmonary tuberculosis. PLoS One 2017; 12:e0182988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tagliani E, Cabibbe AM, Miotto P, Borroni E, Toro JC, Mansjo M, et al. Diagnostic performance ofthe new version(v2.0) of genoType MTBDRsl assay for detection of resistance to fluoroquinolones and second-line injectable drugs: A multicenter study. J Clin Microbiol 2015;53:2961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardee Y, Dreyer AW, Koomhof HJ, Omar SV, da Silva P, Bhyat Z, et al. Evaluation of the GenoType MTBDR.s/version 2.0 assay for second-line drug resistance detection of Mycobacterium tuberculosis isolates in South Africa. J Clin Microbiol 2017;55:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yadav R, Saini A, Kaur P, Behera D, Sethi S. Diagnostic accuracy of genoType® MTBDRsl VER 2.0 in detecting second-line drug resistance to M. tuberculosis. Int J Tuberc Lung Dis 2018;22:419–24. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. The Use of Molecular Line Probe assays for Detection of Resistance to Second Line Anti-Tuberculosis Drugs: Policy guidance Geneva, Switzerland: World Health Organization; 2016. Available from: http://www.who.int/iris/handlc/l0665/246131. [Last accessed on 2018 Oct 30]. [Google Scholar]
- 50.Hillcmann D, Haasis C, Andres S, Behn T, Kranzer K. Validation of the fluoroType MTBDR assay for detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol 2018;56 pii: e00072–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Vos M, Derendinger B, Dolby T, Simpson J, van Helden PD, Rice JE, et al. Diagnostic accuracy and utility of fluoroType MTBDR, a new molecular assay for multidrug-resistant tuberculosis. J Clin Microbiol 2018;56 pii: e00531–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pholwat S, Liu J, Stroup S, Gratz J, Banu S, Rahman SM, et al. Integrated microfluidic card with TaqMan probes and high-resolution melt analysis to detect tuberculosis drug resistance mutations across 10 genes. MBio 2015;6:e02273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foongladda S, Banu S, Pholwat S, Gratz J, O-Thong S, Nakkcrd N, et al. Comparison of taqMan(®) array card and MYCOTB (TM) with conventional phenotypic susceptibility testing in MDR-TB. Int J Tuberc Lung Dis 2016;20:1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banu S, Pholwat S, Foongladda S, Chinli R, Boonlert D, Ferdous SS, et al. Performance of TaqMan array card to detect TB drug resistance on direct specimens. PLoS One 2017; 12:e0177167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witney AA, Cosgrove CA, Arnold A, Hinds J, Stoker NG, Butcher PD, et al. Clinical use of whole genome sequencing for Mycobacterium tuberculosis. BMC Med 2016; 14:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Beek J, Haanperä M, Smit PW, Mentula S, Soini H. Evaluation of whole genome sequencing and software tools for drug susceptibility testing of Mycobacterium tuberculosis. Clin Microbiol Infect 2018. pii: S1198. [DOI] [PubMed]
- 57.Walker TM, Kohl TA, Omar SV, Hedge J, Del Ojo Elias C, Bradley P, et al. Whole-genome sequencing for prediction of Mycobacterium tuberculosis drug susceptibility and resistance: A retrospective cohort study. Lancet Infect Dis 2015; 15:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quan TP, Bawa Z, Foster D, Walker T, Del Ojo Elias C, Rathod P, et al. Evaluation of whole-genome sequencing for mycobacterial species identification and drug susceptibility testing in a clinical setting: A large-scale prospective assessment of performance against line probe assays and phenotyping. J Clin Microbiol 2018;56 pii: e00531–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chattcrjee A, Nilgiriwala K, Saranath D, Rodrigues C, Mistry N. Whole genome sequencing of clinical strains of Mycobacterium tuberculosis from Mumbai, India: A potential tool for detennining drug-resistance and strain lineage. Tuberculosis (Edinb) 2017;107:63–72. [DOI] [PubMed] [Google Scholar]
- 60.Shea J, liaise TA, Lapierre P, Shudl M, Kohlerschmidt D, Van Roey P, et al. Comprehensive whole-genome sequencing and reporting of drug resistance profiles on clinical cases of Mycobacterium tuberculosis in New York state. J Clin Microbiol 2017;55:1871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YJ, Lee SM, Park BK, Kim SS, Yi J, Kim HH, et al. Evaluation of propidium monoazide real-time PCR for early detection of viable Mycobacterium tuberculosis in clinical respiratory specimens. Ann Lab Med 2014;34:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nikolayevskyy V, Miotto P, Pimkina E, Balabanova Y, Kontsevaya I, Ignatyeva O, et al. Utility of propidium monoazide viability assay as a biomarker for a tuberculosis disease. Tuberculosis (Edinb) 2015;95:179–85. [DOI] [PubMed] [Google Scholar]
- 63.Kayigire XA, Friedrich SO, Karinja MN, van der Merwe L, Martinson NA, Diacon AH, et al. Propidium monoazide and Xpert MTB/ RIF to quantify Mycobacterium tuberculosis cells. Tuberculosis (Edinb) 2016;101:79–84. [DOI] [PubMed] [Google Scholar]
- 64.Honeyborne I, McHugh TD, Phillips PP.Bannoo S, Bateson A, Carroll N, et al. Molecular bacterial load assay, a culture-free biomarker for rapid and accurate quantification of sputum Mycobacterium tuberculosis bacillary load during treatment. J Clin Microbiol 2011;49:3905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Honeyborne I, Mtafya B, Phillips PP, Hoelscher M, Ntinginya EN, Kohlenberg A, et al. The molecular bacterial load assay replaces solid culture for measuring early bactericidal response to antituberculosis treatment. J Clin Microbiol 2014;52:3064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu W, Lyu J, Cheng P, Cheng Y, Zhang Z, Li L, et al. Improvement in clinical outcome and infection control using molecular diagnostic techniques for early detection of MDR tuberculous spondylitis: A multicenter retrospective study. Emerg Microbes Infect 2017;6:e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auld SC, Kasmar AG, Dowdy DW, Mathema B, Gandhi NR, Churchyard GJ, et al. Research roadmap for tuberculosis transmission science: Where do we go from here and how will we know when we’re there? J Infect Dis 2017;216:S662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kipiani M, Mirtskhulava V, Tukvadze N, Magee M, Blumberg HM, Kempker RR, et al. Significant clinical impact of a rapid molecular diagnostic test (Genotype MTBDRplus assay) to detect multidrug-resistant tuberculosis. Clin Infect Dis 2014;59:1559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacobson KR, Theron D, Kendall EA, Frankc MF, Barnard M, van Hclden PD, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis 2013;56:503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Albert H, Bwanga F, Mukkada S, Nyesiga B, Ademun JP, Lukyamuzi G, et al. Rapid screening of MDR-TB using molecular line probe assay is feasible in Uganda. BMC Infect Dis 2010; 10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: A cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 2015;3:e450–7. [DOI] [PubMed] [Google Scholar]
- 72.Operario DJ, Koeppel AF, Turner SD, Bao Y, Pholwat S, Banu S, et al. Prevalence and extent of heteroresistance by next generation sequencing of multidrug-resistant tuberculosis. PLoS One 2017; 12:e0176522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rabie G, Moktar S, Omar A. Real-time polymerase chain reaction as an alternative method for diagnosis of multidrug-resistant tuberculosis: Can it stand alone in this concern ? Egypt J Broncol 2017; 11:342–5. [Google Scholar]
- 74.Rockwood N, du Bruyn E, Morris T, Wilkinson RJ. Assessment of treatment response in tuberculosis. Expert Rev Respir Med 2016;10:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Evangelopoulos D, Whittaker E, Honeyborne I, McHugh TD, Klein N, Shingadia D, et al. Pediatric tuberculosis-human immunodeficiency virus co-infection in the United Kingdom highlights the need for belter therapy monitoring tools: A case report. J Med Case Rep 2017; 11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schito M, Maeurer M, Kim P, Hanna D, Zumla A. Translating the tuberculosis research agenda: Much accomplished, but much more to be done. Clin Infect Dis 2015;61 Suppl 3:S95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitnick CD, Rodriguez CA, Hatton ML, Brigden G, Cobclens F, Grobusch MP, et al. Programmatic management of drug-resistant tuberculosis: An updated research agenda. PLoS One 2016; 11 :e0155968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pai M Time for high-burden countries to lead the tuberculosis research agenda. PLoS Med 2018; 15:e1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.World Health Organization. Global Investments in Tuberculosis Research and Development: Past, Present, and Future Geneva: World Health Organization; 2017. [Google Scholar]
- 80.Jüni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta-analyses of controlled trials: Empirical study. IntJ Epidemiol 2002;31:115–23. [DOI] [PubMed] [Google Scholar]
- 81.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, et al. The effect of english-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int J Technol Assess Health Care 2012;28:138–44. [DOI] [PubMed] [Google Scholar]