Abstract
Objective
Supra therapeutic doses of methylphenidate (MPH) activate mu opioid receptors, which are linked to euphoria. This study assessed whether naltrexone, a mixed mu opioid antagonist, may attenuate the euphoric effects of stimulants, thereby minimizing their abuse potential in subjects with Attention Deficit Hyperactivity Disorder (ADHD).
Methods
We conducted a six-week, double-blind, placebo-controlled, randomized clinical trial of naltrexone in adults with DSM-IV ADHD receiving open treatment with a long acting formulation of MPH (January 2013 to June 2015). Spheroidal Oral Drug Absorption System (SODAS)–MPH was administered BID and titrated to ~1 mg/kg/day over three weeks and continued for three additional weeks depending on response and adverse effects. Subjects were adults with ADHD preselected for having experienced euphoria with an oral test dose of 60 mg of immediate release (IR) MPH. The primary outcome measure was Question 2 (liking) on the Drug Rating Questionnaire (DRQ-S) after additional oral test doses of 60 mg of IR-MPH at the 3rd and 6th week of treatment with SODAS-MPH. Thirty-seven subjects who experienced stimulant-induced (mild) euphoria on a baseline visit were started in the open trial of SODAS-MPH and randomized to 50 mg naltrexone or placebo.
Results
Thirty-one subjects completed through Week 3, twenty-five through Week 6. Naltrexone significantly diminished the euphoric effect of IR-MPH during the heightened risk titration phase (primary outcome; first three weeks) (χ2=5.07, p=0.02) but not the maintenance phase (Weeks 4 to 6) (χ2=0.22, p=0.64) of SODAS-MPH treatment.
Conclusion
Preclinical findings are extended to humans showing that naltrexone may mitigate stimulant-associated euphoria. These findings extend to humans preclinical findings showing that naltrexone may mitigate stimulant-associated euphoria. Our findings provide support for further studies combining opioid receptor antagonists with stimulants to reduce abuse potential.
Clinical Trials Registration
ClinicalTrials.gov identifier: NCT01673594
Keywords: ADHD, Stimulants, Substance Abuse
Introduction
While stimulants remain the mainstay of the treatment of Attention Deficit Hyperactivity Disorder (ADHD), their use is marred by persistent concerns about abuse potential. 1 Wilens et al. reported rates of past-year nonprescribed stimulant use ranging from 5%–35% in college-aged individuals. 1 Individuals at greatest risk are those with either pre-existing conduct or substance use disorders. 1
Recent investigations indicate that stimulants activate brain opioid mu receptors. 2 Areas of the brain involved in the reward and addiction circuitry, such as the caudate-putamen, nucleus accumbens, frontal cortex and ventral midbrain, are enriched in opioid receptors. 3 Interactions of opioids and neurotransmitters, including dopamine and noradrenaline, facilitate different aspects of reward circuits. Activation of the mu opioid receptor (MOPR) is associated with euphoria. 3
In a mouse model we found that supra therapeutic but not therapeutic doses of methylphenidate (MPH) produced conditioned place preference, a well-known animal behavioral model of addiction 2, as well as enhanced striatal MOPR activity. 2 We showed that naltrexone, an opioid receptor antagonist, blocked MPH-induced place preference. Thus, an opioid antagonist can block rewarding effects of MPH in a mouse model. Naltrexone is a mixed opiate antagonist that is currently FDA approved for the treatment of opioid use disorder as well as the treatment of alcoholism.
In a previous publication, we showed that the co-administration of naltrexone with Spheroidal Oral Drug Absorption System (SODAS)-MPH was well tolerated and did not interfere with the clinical benefits of MPH. 4 Yet, whether the co-administration of naltrexone to MPH attenuates stimulant induced euphoric effects and drug abuse liability in humans remained to be established.
The main aim of this study was to assess whether the co-administration of naltrexone to treatment with a stimulant would attenuate stimulant-induced euphoria. To this end we conducted a six-week, double-blind, placebo controlled, randomized clinical trial of naltrexone in adults with ADHD receiving open-label treatment with therapeutic oral doses of SODAS-MPH. We used an enriched sample approach in which we only included participants who experienced euphoria with a test dose of immediate release (IR)-MPH. Because there may be a period of heightened risk for euphoric effects during a titration phase when the dose of SODAS-MPH is being increased, we tested subjective response to IR-MPH in two periods: after 3 weeks of titration of SODAS-MPH and again at week 6 after an additional 3 weeks of stable treatment with SODAS-MPH. Based on its pharmacological properties, we hypothesized that treatment with naltrexone would attenuate MPH-induced euphoria in general, and during the titration phase in particular.
Methods
Subjects
Subjects were medication-naïve 18–30 year old adults with ADHD preselected by the experience of euphoria with a test dose of IR-MPH who were willing to reliably participate and understood all study procedures. Main exclusion criteria included any current non-ADHD clinically significant psychiatric condition, any chronic or clinically significant medical illness, current or recent substance abuse/dependence or psychotropic use, current or prior adequate treatment with MPH, and/or a known hypersensitivity to MPH. Informed consent was obtained from subjects after the study procedures and possible side effects were fully explained. This study was approved by the institutional review board at Massachusetts General Hospital and was conducted from 01/2013 to 07/2015. Clinical Trials Registration: ClinicalTrials.gov identifier: NCT01673594
Assessments Measures
Sociodemographic assessment
This was a brief interview to collect information on education and occupation to estimate socioeconomic status, as well as information about educational accommodations.
Assessment of ADHD and comorbid psychopathology
An expert clinician assessed the diagnosis of ADHD and exclusionary comorbid Axis I DSM-IV disorders. Subjects were also assessed with the Structured Clinical Interview for DSM-IV (SCID) supplemented with modules from the Kiddie-Schedule for Affective Disorders and Schizophrenia (K-SADS-E) to assess childhood DSM-IV disorders.5
AISRS & CGI
The Adult ADHD Investigator System Report Scale (AISRS) 6 is a validated DSM-IV investigator-rated assessment widely used in clinical trials of adults with ADHD. 7, 8 The Clinical Global Impression (CGI) Scale for ADHD 9 is a rating scale used to measure the overall severity of ADHD, and has been shown to be drug sensitive in psychopharmacology research. 9
Drug Rating Questionnaire (DRQ-S)
The Drug Rating Questionnaire (DRQ-S) is a simple questionnaire used to measure factors in abuse liability. Each subscale (feeling, liking, disliking) is a Likert scale (1 to 29). 10 This measure and related scales have been used in over 27 published studies assessing the abuse liability of MPH. 11, 12
Study Procedures
Eligible subjects were randomized to 50 mg naltrexone daily (active or placebo) and entered a six week, open-label treatment protocol with daily, therapeutic doses of long-acting (SODAS-MPH). At baseline, and at the end of weeks 3 and 6, subjects underwent one day likeability assessments with single supratherapeutic doses (60 mg) of IR-MPH and then (IR-MPH) placebo (order randomized). On the likeability assessment days, the subjects took their blinded doses of naltrexone (active or placebo) but did not take SODAS-MPH on those days.
Likeability Assessment Procedures
Subjects were tested for a euphoric effect in response to an oral 60 mg bolus dose of IR-MPH under double-blind conditions. On Likeability Assessment days the MGH Research Pharmacy assigned the randomization of the order of IR-MPH administration (active- placebo; placebo-active). Likeability was assessed three times: 1) at pre-baseline assessment for eligibility, 2) Titration Phase: at the end of Week 3 when subjects are expected to have reached the optimal and tolerated dose of SODAS-MPH and 3) Maintenance Phase: at the end of the clinical trial (Week 6). On the Weeks 3 and 6 visits, subjects took their naltrexone (or placebo) in the morning but did not take SODAS-MPH (for that day only).
At the pre-baseline visit, subjects received one oral blinded test dose (60 mg) of IR-MPH (active/placebo) in the morning and the other treatment in the afternoon (order randomized) and completed the DRQ-S hourly for four hours after each dose. On subsequent likeability assessment days (end of Week 3 and the end of Week 6), subjects did not take the usual SODAS MPH but did receive their blinded, randomized dose of naltrexone or placebo in the morning and then a blinded test dose (60 mg IR-MPH or placebo) in the morning and afternoon (order randomized). They completed the DRQ-S hourly for four hours after each dose.
Open-Label Treatment with SODAS-MPH
All study subjects underwent six weeks of open treatment with SODAS-MPH administered BID. Titration Phase. Subjects were started on 20 mg SODAS-MPH BID, increased to 30 mg BID by Week 2, and to 40 mg BID by Week 3, based on response and adverse effects, up to a maximum daily dose of 80 mg/day (~ 1 mg/kg/day). Maintenance Phase. In weeks 4–6 they were continued at the highest tolerated dose (≤ 80 mg/day).
Placebo controlled, randomized clinical trial of naltrexone
The MGH Research Pharmacy assigned randomization for (daily) naltrexone (active vs placebo; 50:50) to eligible and consenting subjects for a six-week period combined with the open treatment with SODAS-MPH. Naltrexone-masked placebo was matched to an identically appearing naltrexone formulation in lactose filled capsules
Statistical Analysis
We compared demographics and clinical features among the placebo and naltrexone groups using Student’s t-tests and Pearson’s χ2 tests for parametric data and Wilcoxon rank-sum tests for non-parametric data. Analyses pertaining to likability testing days and the six-week clinical trial were performed using mixed-effects Poisson regression, linear regression, Wilcoxon signed-rank tests, and Spearman correlations. Regression models used robust standard errors to account for the repeated measures on each subject. We performed backwards selection to arrive at the final mixed-effects Poisson regression models used to examine feeling a drug effect, euphoria, and dysphoria at Weeks 3 and 6. All models started with the following variables: naltrexone, IR-MPH, session (morning or afternoon), hours (1–4), the IR-MPH x naltrexone interaction, the IR-MPH x hours interaction, the IR-MPH x session interaction, the naltrexone x hours interaction, and the IR-MPH x naltrexone x hours interaction. Insignificant higher order variables were removed from the model successively until only those that were significant at the 0.05 alpha level remained. We kept the IR-MPH x naltrexone interaction term in all models regardless of significance because it was our effect of interest. All tests were two-tailed and performed at the 0.05 alpha level. We did not control for any demographic or clinical characteristics since none reached statistical significance. Analyses were performed using Stata® (version 14) StataCorp LLC.
A priori analyses included the effects of naltrexone on MPH-induced Detection, Euphoria and Dysphoria in the titration phase (week 3) and maintenance phase (week 6). The primary outcome analysis was the effects of naltrexone on MPH-induced Euphoria in the titration phase (week 3).
Results
Subjects
As depicted in Figure 1, 64 subjects were consented and enrolled. 56 subjects completed all screening procedures. 44 subjects participated in the baseline Drug Feeling Visit, of which 86% (n=38/44) experienced stimulant-induced euphoria. Of those 38, 37 were started in the open trial of SODAS-MPH and randomized to naltrexone or placebo. 31 subjects completed to Week 3. 25 subjects completed through Week 6.
Figure 1.
CONSORT diagram.
39 subjects did not complete the study for various reasons. Six subjects were ineligible after they consented due to cardiovascular concerns about using stimulant treatment, a positive urine drug screen, or comorbidity. An additional six subjects failed to experience stimulant-induced euphoria (n=6/44) on the baseline Drug Feeling Visit. A total of 23 subjects withdrew or were later dropped due to the demanding time commitment of participating in the study or due to relocation. Finally, four subjects were terminated from the study during the treatment phase due to adverse events. Of these subjects, one developed negative mood side effects, one was discovered to have previously asymptomatic lymphoma, one experienced a reoccurrence of her peptic stress ulcers, and one subject experienced nausea and vomiting. In no case were the adverse events judged to be due to naltrexone.
Demographic and Clinical Characteristics of Randomized Sample
As described in Table 1, there were no significant differences in age, weight, or sex between the naltrexone and (naltrexone) placebo groups. There also were no significant differences in baseline ADHD severity on the AISRS or in ratings of anxiety symptoms (HAM-A) and depression symptoms (HAM-D and BDI). Furthermore, pre-baseline ratings for feelings of any effect, euphoria, and dysphoria on the DRQ-S did not significantly differ between the naltrexone and placebo groups (Table 1).
Table 1.
Demographic and clinical characteristics of subjects who completed through at least Week 3.
| Characteristic Placebo | Placebo N = 16 | Naltrexone N = 15 | Test Statistic | P-Value |
|---|---|---|---|---|
| Age | 24.4 ± 3.2 | 25.1 ± 2.9 | t = −0.63 | 0.53 |
| Gender (Male) | 8 (50) | 6 (40) | χ2 = 0.31 | 0.58 |
| Weight | 154.7 ± 23.2 | 162.5 ± 41.1 | z = −0.20 | 0.84 |
| HAM-A | 3.9 ± 3.5 | 7.0 ± 8.3 | z = −0.86 | 0.39 |
| HAM-D | 2.8 ± 3.6 | 4.7 ± 6.0 | z = −0.75 | 0.45 |
| BDI | 2.4 ± 2.1 | 4.5 ± 4.4 | z = −0.92 | 0.34 |
| AISRS | 36.4 ± 9.0 | 38.5 ± 9.8 | z = −0.61 | 0.54 |
| DRQ-S | ||||
| Feel Effect | 5.4 ± 5.5 | 5.4 ± 5.9 | z = 0.14 | 0.89 |
| Euphoria | 5.9 ± 6.1 | 6.1 ± 7.4 | z = 0.57 | 0.57 |
| Dysphoria | 1.9 ± 2.9 | 1.7 ± 1.9 | z = −0.86 | 0.39 |
Data are presented as mean ± SD or N (%).
Week 3 DRQ-S Findings
“Feeling a drug” effect (Detection)
The final model for Feeling a Drug Effect at Week 3 included naltrexone, IR-MPH, session, hours, the IR-MPH x hours interaction, the naltrexone x hours interaction, and the IR-MPH x naltrexone interaction. Although the effect of naltrexone on IR-MPH-associated Feeling a Drug Effect did not reach our a priori threshold for statistical significance (χ2=3.65, p=0.06), the trend favored naltrexone (lower feeling). There was less of a difference in average Feeling a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone (difference=1.92) compared to those on (naltrexone) placebo (difference=2.65). Additionally, average Feeling a Drug Effect scores significantly differed by session (AM and PM) with higher ratings in the PM compared to those in the AM (5.15 vs. 3.88; χ2=4.61, p=0.03).
“Liking a drug” effect (Euphoria)
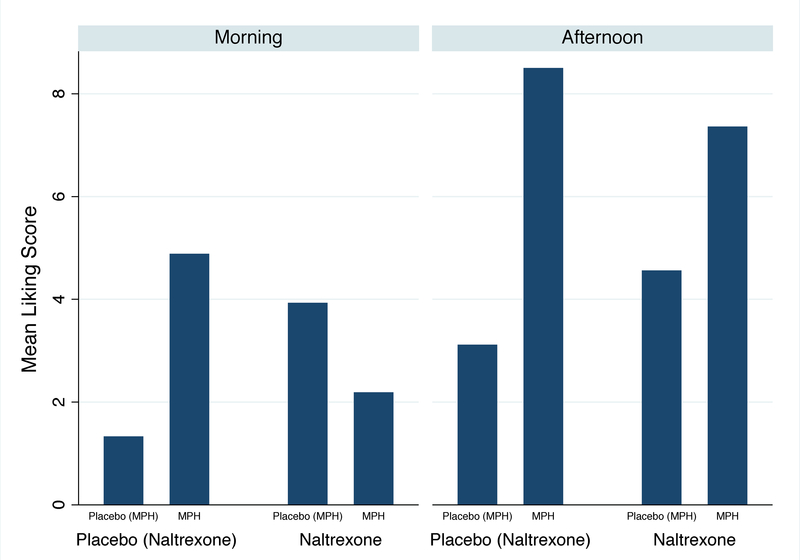
For Liking a Drug, the final model included naltrexone, IR-MPH, baseline euphoria, hours, session, and the IR-MPH x naltrexone interaction. Baseline Liking a Drug Effect was significantly associated with Week 3 liking (z=4.99, p<0.001). Controlling for baseline findings, the effect of IR-MPH on Liking a Drug Effect significantly differed between (naltrexone) placebo and naltrexone groups (χ2=5.07, p=0.02). There was less of a difference in average Liking a Drug Effect scores between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone compared to those on (naltrexone) placebo (Figure 2a). In addition, average Liking a Drug Effect scores were significantly higher during the afternoon session compared to the morning session (5.33 vs. 3.80; χ2=6.31, p=0.01). A three-way interaction between IR-MPH, naltrexone, and session revealed that naltrexone suppressed the Liking a Drug Effect score significantly more when IR-MPH was given in the morning compared to when IR-MPH was administered in the afternoon (χ2=5.20, p=0.02) (Figure 2b). In addition, we found that the difference in average Liking a Drug Effect scores between the IR-MPH and (IR-MPH) placebo groups was decreased at Week 3 (difference=2.35) compared with baseline (difference=6.45) regardless of naltrexone (χ2=4.20, p=0.04).
Figure 2a.
Poisson regression model predicting liking a drug effect at Week 3 controlling for baseline euphoria: the MPH x naltrexone interaction (N=31).
*There is a significant interaction between MPH and naltrexone (p=0.02).
Figure 2b.
Poisson regression model predicting liking a drug effect at Week 3: the MPH x naltrexone x session interaction (N=31).
*There is a significant interaction between MPH, naltrexone, and session (p=0.02).
“Disliking a drug” effect (Dysphoria)
The final model for Disliking a Drug Effect included naltrexone, IR-MPH, hours, the IR-MPH x hours interaction, the naltrexone x hours interaction, and the IR-MPH x naltrexone interaction. Not controlling for naltrexone, we found that Disliking a Drug Effect ratings did not significantly differ between the IR-MPH and (IR-MPH) placebo groups (2.03 vs. 1.82; χ2=0.57, p=0.45). The effect of IR-MPH on dysphoria did not significantly differ between (naltrexone) placebo and naltrexone groups (χ2=0.11, p=0.75). There was no difference in average Disliking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone (difference=0.28) compared to those on placebo (difference=0.15). Additionally, three weeks of open SODAS-MPH treatment did not significantly change the IR-MPH associated Disliking a Drug Effect score (χ2=1.02, p=0.31). There was no significant difference in the average Disliking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups at Week 3 (difference=0.21) versus baseline (difference=0.72).
Euphoria/dysphoria relationship
At Week 3, IR-MPH associated Euphoria and Dysphoria were positively correlated at each hour (Figure 3) and most strongly so at hour three (rs=0.57; p<0.001). In contrast, we found no significant correlation between Euphoria in the morning and Dysphoria in the afternoon (rs=−0.02, p=0.92).
Figure 3.
Correlation between MPH associated euphoria and MPH associated dysphoria by hour.
Week 6 DRQ-S Findings
“Feeling a drug” effect (Detection)
The final model for feeling a drug effect at Week 6 included naltrexone, IR-MPH, hours, the IR-MPH x hours interaction, and the IR-MPH x naltrexone interaction. The effect of IR-MPH on feeling a drug effect did not significantly differ between (naltrexone) placebo and naltrexone groups at Week 6 (χ2=1.14, p=0.29). There was no difference in the average Disliking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone (difference=5.52) compared to those on placebo (difference=3.15).
“Liking a drug” effect (Euphoria)
The final model for euphoria at Week 6 included naltrexone, IR-MPH, hours, the IR-MPH x hours interaction, the naltrexone x hours interaction, the IR-MPH x naltrexone interaction, and the IR-MPH x naltrexone x hours interaction. There was no main effect of the IR-MPH x naltrexone interaction on euphoria at Week 6 (χ2=0.22, p=0.64). There was no difference in the average Liking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone (difference=5.72) compared to those on placebo (difference=4.35). The three-way interaction including IR-MPH x naltrexone x hours shows that the effect of naltrexone and IR-MPH on euphoria varied by hour (χ23=12.24, p = 0.007). There was a greater difference in the average Liking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone versus placebo at hour three compared to hours one (naltrexone: difference=4.67, placebo: difference=3.92) and two (naltrexone: difference=4.83, placebo: difference=6.92). Examining the effect of IR-MPH on Liking a Drug Effect across time (baseline, Week 3 and Week 6), we found a significant interaction between IR-MPH and time regardless of naltrexone (χ23=6.71, p=0.03). The difference in Liking a Drug Effect scores between the IR-MPH and (IR-MPH) placebo groups significantly differed between Week 3 and baseline and between Week 3 and Week 6 (Figure 4).
Figure 4.
Poisson regression model predicting liking from the MPH x time interaction (Week 6 vs. Week 3 vs. Baseline) (N=31).
*There is a significant interaction between MPH and time (p=0.03). Week 3 significantly differs from baseline (p=0.04) and Week 6 significantly differs from Week 3 (p=0.02).
“Disliking a drug” effect (Dysphoria)
The final model for dysphoria at Week 6 included naltrexone, IR-MPH, hours, the IR-MPH x hours interaction, the naltrexone x hours interaction, the IR-MPH x naltrexone interaction, and the IR-MPH x naltrexone x hours interaction. There was no main effect of the IR-MPH x naltrexone interaction on dysphoria at Week 6 (χ2=0.36, p = 0.55). There was no difference in average Disliking a Drug Effect score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone (difference=0.40) compared to those on placebo (difference=0.67). The three-way interaction including IR-MPH x naltrexone x hours shows that the effect of naltrexone and IR-MPH on disliking varied by hour (χ23=13.47, p=0.004). There was less of a difference in the disliking score between the IR-MPH and (IR-MPH) placebo groups for those on naltrexone versus placebo at hour three compared to hour two.
Discussion
The clinical trial aimed to assess whether the mixed opiate antagonist naltrexone, combined with treatment with SODAS-MPH, mitigates stimulant-associated euphoria in adults with ADHD. Our study hypothesis was partially confirmed; naltrexone significantly diminished the euphoric effect of IR-MPH during the titration phase (first three weeks) but not the stabilization phase (Weeks 4 to 6) of SODAS-MPH treatment.
The titration phase of open label treatment with SODAS-MPH appears to be a period of heightened vulnerability. These findings are consistent with prior work by us 13 and Volkow et al. 14, 15 that emphasized the importance of the rate of delivery of stimulants to the brain for abuse liability. Also consistent with this notion is the finding that naltrexone had little effect on euphoria during the last 3 weeks of the trial in which subjects remained on a stable optimized therapeutic dose of SODAS-MPH.
The measures of abuse liability (DRQ-S) consisted of subscales that ranged from 1 to 29. Despite administration of 60 mg of IR-MPH, the subjective responses on these rating scales were in the mild range. It is possible that the experience of euphoria attenuated because the subjects had only mild euphoria at study outset. Our non-significant effect for euphoria at week 6 should be viewed with caution due to the possibility of a floor effect which would have reduced statistical power. Our results are consistent with a previous study that reported decreased subjective effects of single doses of amphetamine with naltrexone pretreatment in 12 healthy volunteers. 16
The finding that naltrexone diminished IR-MPH-associated euphoria was significantly greater in the morning than in the afternoon is noteworthy. Considering that naltrexone was administered in the morning, this finding suggests that the euphoria blocking effect of naltrexone may be maximally beneficial if administered proximally to the stimulant dosing. The mean elimination half-life (T1/2) values for naltrexone and 6-ß-naltrexol are 4 hours and 13 hours, respectively. However, clinical studies indicate that 50 mg of naltrexone will block the pharmacologic effects of 25 mg of intravenously administered heroin for periods as long as 24 hours. 17, 18 More work is needed to confirm this intriguing finding.
The euphoric response to the acute bolus, supra therapeutic dose of IR-MPH was significantly decreased at Week 3 regardless of naltrexone. This finding is consistent with the hypothesis that chronic treatment with MPH is associated with some desensitization to IR-MPH associated euphoria. Despite the lower euphoric response to IR-MPH at Week 3, naltrexone was associated with a further decrease in euphoric response.
While subjects reported both euphoria and some degree of dysphoria simultaneously, there was no effect of either IR-MPH or naltrexone on dysphoria. This finding is surprising considering that dysphoria is associated with activation of opiate kappa receptors and that naltrexone blocks kappa receptors. Additionally, our results do not support the time-lagged association of euphoria and dysphoria, suggesting that dysphoria in the afternoon does not seem to be related to “crashing” after euphoria in the morning. More work is needed to further examine the relationship of euphoria to dysphoria and the effects of naltrexone on dysphoria.
The observed positive effects of naltrexone on IR-MPH induced euphoria are particularly important when coupled with the previously reported observation that treatment with naltrexone does not interfere with the clinical benefits of SODAS-MPH on ADHD. 4
Our study has important strengths. Notably, this study translates programmatic work on the relationship of euphoria to stimulant induced opioid activation from animals to humans. Additionally, this is a double-blind study of both naltrexone and test doses of IR-MPH in an enriched sample of ADHD subjects who register a euphoric response to IR-MPH. However, our findings need also to be seen in light of limitations. Future studies should examine whether naltrexone reduces “liking” and “estimated monetary street value” within populations of non-treatment seeking poly - substance use disorder volunteers. Because naltrexone was added to open label treatment with SODAS-MPH, further studies are needed to examine the effect of naltrexone on euphoric response without chronic stimulant treatment. While our results suggest some desensitization to euphoria after open treatment with stimulants, naltrexone was associated with a further decrease in euphoric response, particularly at Week 3. Because our study was restricted to referred Caucasian adults, our findings cannot be extrapolated to a younger populations or community samples. Because the sample was largely Caucasian and referred, our findings do not generalize to community samples or other ethnic groups.
Despite these limitations, this double-blind, randomized controlled study showed that treatment with naltrexone diminished the euphoric effect of IR-MPH during the initial MPH titration period of heightened vulnerability. If confirmed, these findings could lead to the development of a non-addictive form of stimulant treatment for ADHD, which could facilitate access to an effective ADHD treatment.
Clinical Points.
Animal studies have shown Mu opioid antagonists, such as naltrexone, may minimize the abuse potential of stimulants
In our study of adults with ADHD, the addition of naltrexone to daily methylphenidate decreased abuse potential (subjective “liking”) during the heightened risk titration phase but not the maintenance phase.
Our findings provide support for the concept of combining opioid receptor antagonists with stimulants to provide an effective stimulant formulation with less abuse potential.
Acknowledgments
Funding Source: This work was supported by grant W81XWH-12–1-0510 from the Department of Defense in Fort Detrick, Maryland. The funding supporter had no role in the design, analysis, interpretation, or publication of this study.
Financial Disclosures:
Dr. Thomas J. Spencer: Dr. Thomas Spencer receives research support or is a consultant from the following sources: Alcobra, Avekshan, Heptares, Impax, Ironshore, Lundbeck, Shire Laboratories Inc, Sunovion, VayaPharma/ Enzymotec, the FDA and the Department of Defense. Consultant fees are paid to the MGH Clinical Trials Network and not directly to Dr. Spencer. Dr. Thomas Spencer is on an advisory board for the following pharmaceutical companies: Alcobra, Dr. Spencer receives research support from Royalties and Licensing fees on copyrighted ADHD scales through MGH Corporate Sponsored Research and Licensing. Dr. Spencer has a US Patent Application pending (Provisional Number 61/233,686), through MGH corporate licensing, on a method to prevent stimulant abuse.
Dr. Pradeep Bhide: Dr. Bhide is a consultant to Avekshan LLC, Tallahassee, FL
Dr. Stephen V. Faraone: In the past year, Dr. Faraone received income, potential income, travel expenses and/or research support from Lundbeck, Rhodes, Arbor, KenPharm, Ironshore, Shire, Akili Interactive Labs, CogCubed, Alcobra, VAYA Pharma and NACE. With his institution, he has US patent US20130217707 A1 for the use of sodium-hydrogen exchange inhibitors in the treatment of ADHD. In previous years, he received income or research support from: Shire, Neurovance, Alcobra, Otsuka, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. Dr. Faraone receives royalties from books published by Guilford Press: Straight Talk about Your Child’s Mental Health, Oxford University Press: Schizophrenia: The Facts and Elsevier: ADHD: Non-Pharmacologic Interventions. He is principal investigator of www.adhdinadults.com.
Dr. Amy M. Yule: Dr. Yule received grant support from the Massachusetts General Hospital Louis V. Gerstner III Research Scholar Award from 2014 to 2016. She currently has funding through the American Academy of Child and Adolescent Psychiatry Physician Scientist in Substance Abuse Award, 5K12DA000357–17. She has served as a consultant for Phoenix House (Clinical Services).
Dr. Andrea E. Spencer: I have received funding for research in the past 3 years from the Louis Gerstner Research Scholar Award, the Dupont-Warren Fellowship, the Livingston Award, and the Fuss Family Foundation.
Dr. Joseph Biederman: Dr. Joseph Biederman is currently receiving research support from the following sources: AACAP, The Department of Defense, Food & Drug Administration, Headspace, Lundbeck, Neurocentria Inc., NIDA, PamLab, Pfizer, Shire Pharmaceuticals Inc., Sunovion, and NIH. Dr. Biederman has a financial interest in Avekshan LLC, a company that develops treatments for attention deficit hyperactivity disorder (ADHD). His interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. Dr. Biederman’s program has received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Ingenix, Prophase, Shire, Bracket Global, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. In 2017, Dr. Biederman is a consultant for Akili, Guidepoint, and Medgenics. He is on the scientific advisory board for Alcobra and Shire. He received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He has a US Patent Application pending (Provisional Number #61/233,686) through MGH corporate licensing, on a method to prevent stimulant abuse. In 2016, Dr. Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses, and from Alcobra and APSARD. He was on the scientific advisory board for Arbor Pharmaceuticals. He was a consultant for Akili and Medgenics. He received research support from Merck and SPRITES. In 2015, Dr. Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses, and from Avekshan. He received research support from Ironshore, Magceutics Inc., and Vaya Pharma/Enzymotec. In 2014, Dr. Biederman received honoraria from the MGH Psychiatry Academy for tuition-funded CME courses. He received research support from AACAP, Alcobra, Forest Research Institute, and Shire Pharmaceuticals Inc.
Dr. Jinmin Zhu, Dr. Mai Uchida, Maura Fitzgerald, Anna M. Hall, Ariana J. Koster, Leah Feinberg, Sarah Kassabian, and Barbara Storch report no financial or other relationship relevant to the subject of this article.
References
- 1.Wilens T, Adler LA, Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47(1):21–31. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Spencer TJ, Liu-Chen LY, Biederman J, Bhide PG. Methylphenidate and mu opioid receptor interactions: a pharmacological target for prevention of stimulant abuse. Neuropharmacology. 2011. Jul-Aug;61(1–2):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trigo JM, Martin-Garcia E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend. 2010. May 1;108(3):183–194. [DOI] [PubMed] [Google Scholar]
- 4.Spencer TJ, Bhide P, Zhu J, et al. Do Opiate Antagonists Interfere with the Clinical Benefits of Stimulants in ADHD? A Double-Blind, Placebo-Controlled Trial of the Mixed Opioid Receptor Antagonist Naltrexone. J Clin Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orvaschel H Schedule for Affective Disorders and Schizophrenia for School-Age Children Epidemiologic Version. 5th Edition ed. Ft. Lauderdale: Nova Southeastern University, Center for Psychological Studies; 1994. [Google Scholar]
- 6.Spencer TJ, Adler LA, Meihua Q, et al. Validation of the adult ADHD investigator symptom rating scale (AISRS). J Atten Disord. 2010. July;14(1):57–68. [DOI] [PubMed] [Google Scholar]
- 7.Spencer T, Biederman J, Wilens T, et al. A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(5):456–463. [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Mick E, Surman C, et al. A randomized, 3-phase, 34-week, double-blind, long-term efficacy study of osmotic-release oral system-methylphenidate in adults with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2010. October;30(5):549–553. [DOI] [PubMed] [Google Scholar]
- 9.National Institute of Mental Health. CGI (Clinical Global Impression) Scale - NIMH. Psychopharmacol Bull. 1985;21(8):839–844. [Google Scholar]
- 10.Jasinski DR, Henningfield JE. Human abuse liability assessment by measurement of subjective and physiological effects. NIDA Res Monogr. 1989;92:73–100. [PubMed] [Google Scholar]
- 11.Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001. March;68(3):611–627. [DOI] [PubMed] [Google Scholar]
- 12.Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000. March;14(1):53–60. [DOI] [PubMed] [Google Scholar]
- 13.Spencer TJ, Biederman J, Ciccone PE, et al. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163(3):387–395. [DOI] [PubMed] [Google Scholar]
- 14.Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160(11):1909–1918. [DOI] [PubMed] [Google Scholar]
- 15.Volkow N Stimulant medications: How to minimize their reinforcing effects? Am J Psychiatry. 2006;163(3):359–361. [DOI] [PubMed] [Google Scholar]
- 16.Jayaram-Lindstrom N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J. Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology. 2008. July;33(8):1856–1863. [DOI] [PubMed] [Google Scholar]
- 17.Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984. September;45(9 Pt 2):15–19. [PubMed] [Google Scholar]
- 18.Naltrexone Hydrochloride [package insert]. Durham, NC: Accord Healthcare, Inc.; 2014. Last Update 2017. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=49aa3d6d-2270-4615-aafa-b440859ab870&type=display. Accessed August 18th 2017. [Google Scholar]