Abstract
Bioinstructive scaffolds encode information in the physical shape and size of materials to direct cell responses. Electrospinning nanofibers is a process that offers control over scaffold architecture and fiber diameter, while providing extended linear length of fibers. This review summarizes tissue engineering literature that has utilized nanofiber scaffolds to direct stem cell differentiation for various tissues including musculoskeletal, vascular, immunological and nervous system tissues. Nanofibers are also considered for their extracellular matrix mimetic characteristics that can preserve stem cell differentiation capacity. These topics are considered in the context of focal adhesion and integrin signaling. Regenerative engineering will be enhanced by construction of scaffolds encoded with shape information to cause an attached cell to create the intended tissue at that region. Nanofibers are likely to be a bioinstructive scaffold in future regenerative engineering development as we pursue the Grand Challenges of engineering tissues.
Introduction
Tissue engineering and regenerative medicine seek to use materials and the body’s own regenerative capacity in synchrony to heal, replace and regenerate missing, defective or dysfunctional tissues. Tissue engineering is classically defined as the application of materials, stem cells, and soluble factors alone or in combination whereas regenerative medicine seeks to incorporate knowledge of the natural regenerative processes to affect the desired restoration of function. While materials have long been used in the body to aid in restoring function, such as wooden replacement prosthesis, only recently have we begun to appreciate the complexity of the approaches that are required to bring the efforts of regenerative tissue engineers to the next level.
A single tissue is made up of several regions even if only the vasculature and the tissue specific cellular groups are considered. Therefore creating a complete tissue requires many specialized cell types to be combined correctly. Creating tissue cell types from progenitors through differentiation into a specialized cell type is a complex process. Typically a cocktail of growth factors are applied to a stem cell in a specific temporal pattern to realize the characteristics of the desired cell type. The number and nature of the factors creates a formidable challenge for process control and therefore Food and Drug Administration approval. If this list of required factors can be reduced, or even eliminated, by encoding information into the engineered scaffold it may reduce the burden of bringing the treatments to market.
Cell differentiation is a process that occurs in the early stages of development through the end of life to varying degrees. Understanding differentiation is a key goal for biologists and tissue engineers, the latter of which seek to direct differentiation. The importance of the environment as an input into a cell’s decision process is an area of growing interest. A majority of cell biology has been conducted in well controlled in vitro environments, i.e. the petri dish[1]. However, discrepancies in the function of a cell in vitro versus in vivo are well recognized. The flat culture dish is a good substrate that provides reproducible data, however accuracy of the data could be improved by increasing the topological complexity of cell culture substrates[2–4]. Therefore, nanofibers have the potential to change the way differentiation is studied and therefore harnessed as a bioinstructive substrate.
It is likely that mechanisms which a bioinstructive substrate activate in cells to direct responses are utilizing pathways that the extracellular matrix physiologically stimulate. The extracellular matrix provides physical structure that cells interact with via sensing of signals as well as integration and application of physical stress. Various techniques have been utilized to make many kinds of cell adhesive substrates allowing for expansion of our knowledge of cell biology. Nanofibers are of particular interest for specific applications such as musculoskeletal engineering. Nanofiber substrates provide an easy to construct and long shelf life in vitro substrate that may mimic the in vivo environment better than flat polystyrene dishes. In addition, from a tissue engineering perspective, nanofibers are encountered by cells on many scaffolds.
While biochemical differences in a substrate control biological outcomes, it is possible that some of the effects observed are partially attributable to physical dissimilarities in the substrate[5]. The shape of the extracellular environment is often reflected in the shape of attached cells and the morphology of the cytoskeletal components. The size, shape and mechanical properties of the adhesive regions a cell attaches to instruct the cell in a process termed outside-in signaling. An example of this is the formation of focal adhesions that are known to form on 2D substrates, but were found to be different on 3D fibronectin[6]. On the other hand, investigation of cell attachment in 3D collagen gels called the formation of focal adhesions in vivo into question altogether, including with attachment to collagen[7,8]. Understanding how the curvature of an ECM size feature is sensed may reveal mechanisms by which 3D substrates communicate information to cells.
There are many possible molecular sensors for the membrane curvature around fibers. Molecules involved in endocytosing large microbes, macropinocytosis and phagocytosis of cell debris define a broad range of candidates. Interestingly clathrin is known to preferentially associate with certain integrins during the endocytosis process, where αVβ5 is the only known integrin to have a direct association with clathrin[9,10]. An exciting paper also recently revealed the fact that clathrin can bend during the process of clathrin mediated endocytosis[11]. Because this bending occurs during the maturation of an endocytic site, this raises the possibility that signaling occurs at certain bending radii and that clathrin could be a variable sensor for membrane curvature. Signaling molecules that bind clathrin include AP-180/CALM, β-arrestins, epsins, amphiphysins, synaptojanin 1, and auxilins[12]; rounding out a list of possible curvature sensors.
Flotillins, like caveolins, associate with the cell membrane. Because flotillins have a hydrophobic domain, it is possible that spacing in the inner leaflet of the curved cell membrane allows space for binding, although this has not been shown[13]. BAR domain proteins have been studied for their involvement in the formation of membrane curvature, however it is not completely clear what role they play[14]. F-BAR domain proteins bind to shallow membrane curvatures (r>100 nm). The possibility that they could sense, in concert with forming these curvatures, is plausible based on the current knowledge[14,15]. Thus, more investigation is needed into proteins that may have curvature binding and signaling capacities.
This review will focus on summarizing examples of tissues where nanofibers have been shown to play a productive role in efforts to tissue engineer functional tissues through differentiation of progenitor cells. This will include musculoskeletal, vascular, adipose, internal organs and the nervous system. Before beginning that discussion, we consider the role of integrins in natural extracellular matrix adhesions and a brief discussion of methods to fabricate nanofibers. We also point out the possibility that rather than promote differentiation, carefully designed nanofibers can preserve ‘stemness’.
Interaction with the Extra Cellular Matrix through Integrins
Nanofibers mimic extracellular matrix providing attachment sites for cells and a similar architecture. Type 1 collagen organizes into fibers that are approximately 100nm in diameter[16], while elastin based fibers are approximately 800nm in diameter[17–19]. Since the protein ligands presented in collagen and elastin are distinct and correspond to different integrin dimers [20], the effect of fiber diameter in natural ECM is intimately linked to protein composition. In addition, collagen binding integrins are thought to rely heavily on the helix structure of collagen. This raises the possibility that some integrins are more likely to bind based on shape rather than molecular sequence. Specific known sequences have been utilized by themselves to enhance integrin based cellular binding including RGD, QAGDV and IKVAV for fibronectin / vitronectin, fibrinogen, and laminin respectively. Synthetic electrospun nanofibers offer the ability to change the dimensions and architecture without changing the composition of the fiber. Binding sites are then provided to cells by a mixture of proteins that bind in an uncontrolled adsorption process from serum in media or with a specific adhesive protein such as fibronectin prior to the addition of cells. If properly designed, these experiments can still test the dimensions and architecture changes of scaffolds since the protein coatings would be largely consistent between groups.
Integrins involved in cell attachment in vitro
Vitronectin is a known component of fetal bovine serum that mediates cell attachment[21]. The principal source of serum vitronectin is the liver[22]. The integrin dimer pairs involved in binding to vitronectin include αVβ5 and αVβ6 [23]. The αV subunits have been associated with binding to the RGD sequence found in matrix proteins including fibronectin, vitronectin, osteopontin, collagens, thrombospondin, fibrinogen and von Willebrand factor[24]. The integrin β-subunit that pairs with αV may be related to the conformation of the RGD sequence in the specific matrix protein. β5 integrin mRNA expression was increased 4-fold compared to a fibronectin coated substrate when cells were cultured on octadecanethiolate monolayers (without a specifically added adhesion protein)[25]. P21-activated kinase 4 has been shown to interact with integrin αVβ5, but not αVβ1, during human breast carcinoma cell migration on fibronectin coated surfaces[26]. Recent evidence differentiates α5β1 from αVβ3 in that α5β1 supports centripetally oriented fibers while αVβ3 allows a more random orientation of cytoskeletal stress fibers[27]. In epithelial cells Rho activity is modulated by the balance between α5β1 and αVβ3, where β1 favors random and β3 persistent migration[28]. Interestingly, the ability of cells to migrate on nanofibers made of Poly-N-acetyl glucosamine (pGlcNAc) is greatly reduced when function blocking antibodies to αVβ3 and α5β1 are added [29].
Mouse mesenchymal stem cell attachment and development of actin stress fibers has been shown to be significantly reduced by integrin β1 blockade on PLGA nanofiber scaffolds[30]. β1 integrin has also been implicated in attachment to collagen/PCL blend nanofibers, which may be primarily related to the collagen content, as the authors of this study did not compare against smooth surfaces[31]. The proliferation of cells on nanofiber scaffolds is a common method to determine the suitability of a new scaffold. β1 and α6 integrins are associated with increased or decreased proliferation in many cell types depending on the splice variant involved[32]. When expressed in CHO cells, the αVβ1 receptor was able to mediate cell attachment to fibronectin, while α5β1 mediated fibronectin matrix assembly and migration in addition to attachment[33]. Thus the involvement of specific integrins during attachment to nanofiber substrates is an area of open investigation.
Methods to fabricate nanofiber scaffolds
Nanofiber mats find application in a variety of settings including as filter media, reaction substrates, biomedical applications, and material reinforcement when embedded in another material. While electrospinning is a widely used technique for scientific studies, several other techniques exist. Airbrushing creates nanofiber mats with greater alignment and higher porosity than conventional electrospinning [34], that can have varied effects on cultured hBMSCs [35]. Fiber drawing can be manipulated to create long fibers in the micrometer and in theory the nanometer range [36]. Individual fibers can be drawn out of melted polymer, but this is limited by throughput [37]. Short nanofibers can also be made using a molding process [38]. Phase separation is a useful technique where the polymer is dissolved and a second solution is added that the polymer is insoluble in, causing the polymer to fall out of solution and form fibers. This has the advantage of being able to create nanofiber structures through the pores of another structure [39,40]. Self-assembling nanofibers offer the advantage of being able to form in a biocompatible way allowing full encapsulation of cells[41,42]. However, none of these techniques offer the ability to control fiber diameter with the same length of individual fibers that electrospinning can produce.
Maintenance of Stem Cell Phenotype on Nanofibers
A properly selected cell culture surface can provide stem cells with signals that discourage differentiation. Cell culture materials can be combined with natural polymers to affect cell responses. Hashemi et al. cultured mouse embryonic stem cells on a nanofiber scaffold made of collagen-grafted polyethersulfone and showed improved maintenance of SSEA-1 and Oct-4 positive colonies when compared to unconjugated polyethersulfone fibers and flat gelatin surfaces[43]. Lee et al. examined the presentation of integrin specific ligands, selected to stimulate the integrin heterodimers that are highly expressed on pluripotent cells, within a PEG hydrogel and showed a differential percentage of Nanog expression based on which integrins were stimulated[44]. Clearly the protein composition of the substrate a stem cell attaches to can affect the cell response, however the material properties are also integrated by the cell.
Many experiments have investigated material properties in the stem cell response. Wei et al. utilized microarray analysis to show differences in gene expression for stemness related genes between 3D and 2D systems[45]. Using patterned substrates, cell area has been linked to the maintenance of stemness and the ability to differentiate after re-seeding to another substrate [46]. Low stiffness hydrogels promote the maintenance of stemness as measured on polyacrylamide gels with measurements of nanog[47]. Stiffness, although not always measured by investigators, is one of the most well known regulators of stem cell fate[48]. While it is possible to estimate relative stiffness based on material type used to make nanofibers, such as hydrogels [44,45,47] verses solid polymers [43,45,49,50], it should be given more attention as a co-factor in studies of other material properties directing stem cell propagation or differentiation. In support of the idea that soluble signals may not be necessary for maintenance of stem cell phenotypes, Zhou et al. found that a low fixation of a MEF feeder cell layer (resulting in a high membrane fluidity) supported stemness better than the more rigidly fixed MEFs [51].
Electrospinning is not the only method to create large culture surfaces of solid polymer nanofibers. Hofmeister et al. showed that a molding technique could create fibers of sufficient length to lay over and create a surface with randomly oriented fiber morphology that increased expression of Nanog and Oct4A genes in hMSCs (indicators of self renewal and stemness) [49]. In another study, carbon nanofibers were produced in an aligned and random pattern with the aligned pattern showing signs of satellite cell (a muscle specific stem cell) properties arising from adipose derived stromal cells [50]. A discussion on nanofiber orientation as a promoter of differentiation occurs later in this review. Overall, material properties are able to reduce stem cell differentiation and the opposite is also true.
Musculoskeletal Tissue Cell Differentiation
Nanofibers have been used to support bone tissue engineering. Shin et al. utilized PCL to make a MSC seeded nanofiber scaffold about 1mm thick that became mineralized[52]. Bone marrow derived MSCs are likely candidates for musculoskeletal tissue differentiation, but it also turns out that nanofibers support the differentiation of umbilical cord and adipose derived MSCs [53]. Peripheral blood mesenchymal stem cells have been encapsulated in a self-assembling peptide scaffold to fill a bone defect with better results than the gel alone [54]. In another study, alkaline phosphatase activity was significantly upregulated by 2 and 3 weeks in culture on nanofibers compared to a flat control [55]. PLGA nanofibers have also been shown to induce osteogenic differentiation without the use of differentiation media. Sonomoto et al. showed that this effect may be due to the release of lactic acid from the fibers as addition of lactic acid to culture of stem cells in regular media on a flat surface also increased the alkaline phosphatase activity at Day 7 [56].
Nanofibers intrinsically drive osteogenic differentiation, but the mechanism is not fully understood. Possibilities include limited adhesive regions, the arrangement of those adhesive regions and/or the shape of nanofibers. The shape of cells can be manipulated by micropatterns of adhesive molecules, which Kilian et al. used to show that osteogenic differentiation is stimulated when cells are encouraged into a shape such as a star with points that accumulate focal adhesions[57]. Using cell seeding density to affect cell shape, Eychmans et al. have shown that osteogenic, adipogenic and chondrogenic differentiation are regulated by cell shape [58].
A collection of mechanotransduced signals may be involved in the ability of nanofibers to support osteogenic differentiation. Indeed, POR1 has been shown to increase Rac1 activity on 100nm nanofibers, reducing the ability of cells to undergo bone differentiation[59]. Some signals may be more sensitive at a particular diameter than others. While in other cases, there may be varied modulation of already known mechanotransduction pathways, or new signals may arise that had only been known in different contexts, such as POR1, a membrane stabilizing factor.
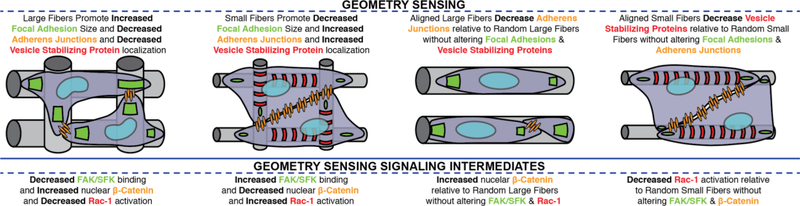
Our future understanding of environmental factors in cell differentiation are also likely to come from cell-cell interactions [60]. Electrospun nanofibers do not prevent cell-cell interaction at any scale, but rather may promote or suppress interaction depending on many factors. For instance, cell-cell interactions might be encouraged if a limited adhesion area is presented, or if the adhesive area is large enough that two cells can spread out adjacent to one another (Figure 1). In the case of fibers, if the cell density exceeds the binding capacity of the fibers, some cells will be forced to adhere to cells rather than the fiber surface. Wide fibers would present adjacent cells with ample surface area to form cell-cell junctions while still being fully engaged with the nanofiber surface. The formation of cell-cell junctions on nanofibers would therefore be affected by many factors including, fiber density, fiber diameter, fiber orientation and cell seeding density.
Fig 1. Cell-Cell Interactions in Nanofiber Attached Cells.
Fiber alignment and diameter present differential attachment patterns to cells that may cause differential focal adhesion, cytoskeletal, and adherens junction arrangements. The fiber alignment may dictate the shape and availability of area for adherens junctions to form, where larger fibers (A,C) encourage fewer than smaller fibers (B,D). Vesicle stabilizing proteins form on smaller fibers (B,D), while larger fibers favor larger focal adhesions.
Fiber alignment is a useful parameter that can be designed into nanofiber scaffolds. When looking at tissues in the body the ECM is organized differently based on the tissue function in many cases. One example is the need for neurons to reach long distances, which then logically results in aligned fibers being more useful to guide directed cell extensions. Similarly, due to the aligned nature of the ECM in tendon and ligaments, nanofibers constructed with electrospinning methods designed to create alignment have proven useful in many studies. Chen et al. blended silk fibroin with PCL to create a scaffold, which they were able to validate in an in vivo model [61]. The reader is directed to reviews on the subject of fiber alignment in tissue engineering scaffolds for further consideration of this subject [62–64]. Linear but tubular tissues exist in several places in the body and benefit from an additional level of complexity in the scaffold design.
Vascular Tissue
Small diameter vascular grafts have been studied extensively in part because of the great need for treatment of coronary artery disease in the United States and other developed countries. Early tissue engineered substitutes for autografts, have focused on seeding cells to materials including ePTFE, collagen gels and Dacron [65]. One way to utilize scaffold design for vessel replacements is to create scaffolds that have two domains. A domain designed to instruct and perform as the endothelial lining and the other designed to function as the smooth muscle layer. Ju et al. demonstrate the in vivo function of one such vessel graft. They show that with a low porosity small fiber tube surrounded by a high porosity large fiber layer an endothelial monolayer will form on the inside and SMCs will populate the thickness of the outer layer. This construct remained patent in vivo for 6 months [66].
A major challenge in vascular grafts is getting a graft to grow with a pediatric patient instead of requiring multiple surgeries to replace the graft with a larger version. Work by Syedain et al. from Robert Tranquillo’s group demonstrates a graft made of natural materials that was able to grow in diameter and volume by 56% and 216% respectively over the course of 42 weeks in an ovine model [67], promising to be an important basis for further research. While blood vessels are present in hard tissues such as bone, they are also present in other tissues including internal organs, a tissue need that outpaces the suitable donor tissues every year.
Internal Organs
Generation of tissues that could replace internal organs is a strategy to address the rising need for organ transplants in end stage organ failure patients. Ghaedi et al. have shown enhanced differentiation of hMSCs into hepatocyte like cells when the cells were cultured on PLLA/collagen scaffolds during the differentiation process as compared to a flat surface [68]. A nanofibrillar cellulose hydrogel also supported hepatocyte culture [69]. In an extension of this work, a comparison of the cellulose hydrogel and a hyaluronic acid hydrogel showed that the presence of a 3D culture condition was comparably more effective at encouraging organotypic arrangements of hepatocytes than a 2D culture [70]. To stimulate hepatocyte culture on chitosan nanofibers galactose was conjugated to the fibers that were fabricated with an average diameter of 160nm, and supported better aggregate formation than a flat galactose chitosan control[71].
Using electrospun nanofibers as a basement membrane for epithelial cells can be challenging. Dankers et al. demonstrated that incorporation of ECM peptides enabled the maintenance of polarized monolayers that still contained tight junctions even after 19 days of culture, whereas the nanofibers without ECM peptides lost their polarized monolayer characteristics [72]. In trying to encourage kidney cell specification, nanotopographies have been used in combination with surface chemistry modification, where it was shown that higher spatial frequency of nanotopography along with an amine rich surface chemistry, lead to a higher differentiation into podocytes[73]. This experiment is a good example of how both chemistry and topography can affect cell phenotypes.
Bladder smooth muscle cells have also been tissue engineered using random and aligned PLLA nanofibers, where over time the effect of alignment was overcome by the cultured cells, suggesting that fiber alignment may most affect cells at earlier time points [74]. Despite positive in vitro results in another complementary study using Poly (L- Lactide- Co— Caprolactone) nanofibers for bladder regeneration, in vivo results proved more difficult to achieve [75]. Transitions from in vitro to in vivo to human trials are an area of great need in the biomedical research enterprise that better in vitro substrates may greatly improve.
Immune Organs / Cells
There are many internal organs that have not received much attention from a tissue engineering perspective, highlighting areas that may prove to be useful avenues of new research. Immune system organs, such as the thymus and lymph nodes, have not been the focus of broad tissue engineering efforts, however there are important medical needs that could be addressed by tissue engineered versions of these organs, either to directly treat a disease or as an in vitro model to study new therapies. Tuckett et al. used electrospun PCL nanofibers alternating with salt leached macrofibers to create a multi-zone model of the thymus. Using the construct that also contained specific factors in the individual layers, the authors were able to show that donor cells were at the thymus site and also populated the recipient animal with a full set of CD4+, CD8+, CD4+CD8+ Tcells and NKT cells [76]. Due to the large number of diseases that have an underlying incorrectly activated or insufficient immune response, it seems clear that this should be an area of intensified research focus [77,78]. Lymphoid organs, like most organs, are not fully functional without central nervous system intervention.
Nervous System
The nervous system, which reaches and controls almost all parts of the body, can benefit from tissue engineering techniques including nanofibers. Presentation of peptides that mimic the laminin rich environment of the nervous system is one approach likely to be useful. IKVAV presented by peptide amphiphile nanofibers was much more effective at inducing the differentiation of cells into neurons when compared to soluble IKVAV or laminin itself [79]. Mahairaki et al. differentiated neurons from human embryonic stem cells on nanofibers to show that the smaller aligned fibers, followed by larger aligned fibers and then the small and large random fibers, induced the most efficient production of neurons as measured by the early neuronal marker TUJ1 [80]. In a very interesting study, Lim et al. examined the differentiation of adult neural stem cells on nanofibrous substrates of 3 diameters in aligned and random orientations. The results showed that aligned fibers of about 480 nm in diameter promoted differentiation by decreasing stemness and increasing Wnt signaling [81]. Connections of substrate properties to phenotypic outcomes are of greater interest when biochemical signals connect the input to the observed output.
Conclusion
Regenerative tissue engineers continue to parse out effects of material properties across the relevant parameter ranges, driving more effective implant designs, as the field matures. Physical as well as biochemical aspects of nanofiber scaffolds play roles in providing information to attached cells. This is true for the nervous, vascular, musculoskeletal, and soft tissues described in this review, but is also true for many other cell types. Basic science studies are essential to uncover “rules” for how cells interpret and respond to changes in scaffold environments. As a field, mechanobiologists have established stiffness as a major material property that drives cellular responses including migration and differentiation. However, there are many more mechanical properties that have not received such widespread attention, and may be just as crucial to understanding how to design a biomaterial. Professor David Tirrell issued a grand challenge in 2010 to “harness the principles of developmental biology to control collective cell movement and differentiation in vivo and in vitro.” [75] While this goal has not yet been achieved, it is clear through the studies reviewed here and those that are forthcoming that regenerative engineering is moving ever closer to a day when scaffolds can be effectively designed to instruct cell behavior.
Acknowledgements
Funding for this work is from the U.S. National Institutes of Health (NIBIB: R21EB019230, NIAMS: R03AR065192).
Works Cited
- [1].Schwartz MA, Chen CS. Deconstructing Dimensionality. Science (80- ) 2013;339:402–4. doi: 10.1126/science.1233814. [DOI] [PubMed] [Google Scholar]
- [2].Levinger I, Ventura Y, Vago R. Life is three dimensional-as in vitro cancer cultures should be. Adv Cancer Res 2014;121:383–414. doi: 10.1016/B978-0-12-800249-0.00009-3. [DOI] [PubMed] [Google Scholar]
- [3].Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Viswanathan P, Ondeck MG, Chirasatitsin S, Ngamkham K, Reilly GC, Engler AJ, et al. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015;52:140–7. doi: 10.1016/j.biomaterials.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barradas AMC, Monticone V, Hulsman M, Danoux C, Fernandes H, Tahmasebi Birgani Z, et al. Molecular mechanisms of biomaterial-driven osteogenic differentiation in human mesenchymal stromal cells. Integr Biol 2013;5:920. doi: 10.1039/c3ib40027a. [DOI] [PubMed] [Google Scholar]
- [6].Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 2001;294:1708–12. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- [7].Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nat Cell Biol 2011;13:3–5; author reply 5–7. doi: 10.1038/ncb0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fraley SI, Feng Y, Krishnamurthy R, Kim D-H, Celedon A, Longmore GD, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 2010;12:598–604. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol 2009;10:843–53. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- [10].De Deyne PG, O’Neill A, Resneck WG, Dmytrenko GM, Pumplin DW, Bloch RJ. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J Cell Sci 1998;111 ( Pt 18):2729–40. [DOI] [PubMed] [Google Scholar]
- [11].Avinoam O, Schorb M, Beese CJ, Briggs JAG, Kaksonen M. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science (80- ) 2015;348:1369–72. doi: 10.1126/science.aaa9555. [DOI] [PubMed] [Google Scholar]
- [12].Dell’Angelica EC. Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol 2001;11:315–8. [DOI] [PubMed] [Google Scholar]
- [13].Bodin S, Planchon D, Rios Morris E, Comunale F, Gauthier-Rouvière C. Flotillins in intercellular adhesion - from cellular physiology to human diseases. J Cell Sci 2014;127:5139–47. doi: 10.1242/jcs.159764. [DOI] [PubMed] [Google Scholar]
- [14].Antonny B Mechanisms of Membrane Curvature Sensing. Annu Rev Biochem 2011;80:101–23. doi: 10.1146/annurev-biochem-052809-155121. [DOI] [PubMed] [Google Scholar]
- [15].Shimada A, Niwa H, Tsujita K, Suetsugu S, Nitta K, Hanawa-Suetsugu K, et al. Curved EFC/F BAR-domain dimers are joined end to end into a filament for membrane invagination in endocytosis. Cell 2007;129:761–72. doi: 10.1016/j.cell.2007.03.040. [DOI] [PubMed] [Google Scholar]
- [16].Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF. Collagen fibrillogenesis in vitro: interaction of types I and V collagen regulates fibril diameter. J Cell Sci 1990;95 ( Pt 4):649–57. [DOI] [PubMed] [Google Scholar]
- [17].Frances C, Branchet MC, Boisnic S, Lesty CL, Robert L. Elastic fibers in normal human skin. Variations with age: a morphometric analysis. Arch Gerontol Geriatr n.d.;10:57–67. [DOI] [PubMed] [Google Scholar]
- [18].Ushiki T Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol 2002;65:109–26. [DOI] [PubMed] [Google Scholar]
- [19].Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: The roles of short fibulins. Int J Biochem Cell Biol 2010;42:1084–93. doi: 10.1016/j.biocel.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tuckwell D, Humphries M. Integrin–collagen binding. Semin Cell Dev Biol 1996;7:649–57. doi: 10.1006/scdb.1996.0079. [DOI] [Google Scholar]
- [21].Hayman EG, Pierschbacher MD, Suzuki S, Ruoslahti E. Vitronectin--a major cell attachment-promoting protein in fetal bovine serum. Exp Cell Res 1985;160:245–58. [DOI] [PubMed] [Google Scholar]
- [22].Seiffert D, Keeton M, Eguchi Y, Sawdey M, Loskutoff DJ. Detection of vitronectin mRNA in tissues and cells of the mouse. Proc Natl Acad Sci U S A 1991;88:9402–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lehmann M, Rabenandrasana C, Tamura R, Lissitzky JC, Quaranta V, Pichon J, et al. A monoclonal antibody inhibits adhesion to fibronectin and vitronectin of a colon carcinoma cell line and recognizes the integrins alpha v beta 3, alpha v beta 5, and alpha v beta 6. Cancer Res 1994;54:2102–7. [PubMed] [Google Scholar]
- [24].Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science 1987;238:491–7. [DOI] [PubMed] [Google Scholar]
- [25].Sobers CJ, Wood SE, Mrksich M. A gene expression-based comparison of cell adhesion to extracellular matrix and RGD-terminated monolayers. Biomaterials 2015;52:385–94. doi: 10.1016/j.biomaterials.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang H, Li Z, Viklund E-K, Strömblad S. P21-activated kinase 4 interacts with integrin alpha v beta 5 and regulates alpha v beta 5-mediated cell migration. J Cell Biol 2002;158:1287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Balcioglu HE, van Hoorn H, Donato DM, Schmidt T, Danen EHJ. The integrin expression profile modulates orientation and dynamics of force transmission at cell-matrix adhesions. J Cell Sci 2015;128:1316–26. doi: 10.1242/jcs.156950. [DOI] [PubMed] [Google Scholar]
- [28].Danen EHJ, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, et al. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol 2005;169:515–26. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vournakis JN, Eldridge J, Demcheva M, Muise-Helmericks RC. Poly-N-acetyl glucosamine nanofibers regulate endothelial cell movement and angiogenesis: dependency on integrin activation of Ets1. J Vasc Res 2008;45:222–32. doi: 10.1159/000112544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zanatta G, Rudisile M, Camassola M, Wendorff J, Nardi N, Gottfried C, et al. Mesenchymal stem cell adherence on poly(D, L-lactide-co-glycolide) nanofibers scaffold is integrin-beta 1 receptor dependent. J Biomed Nanotechnol 2012;8:211–8. [DOI] [PubMed] [Google Scholar]
- [31].Huang C, Fu X, Liu J, Qi Y, Li S, Wang H. The involvement of integrin β1 signaling in the migration and myofibroblastic differentiation of skin fibroblasts on anisotropic collagen-containing nanofibers. Biomaterials 2012;33:1791–800. doi: 10.1016/j.biomaterials.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Moreno-Layseca P, Streuli CH. Signalling pathways linking integrins with cell cycle progression. Matrix Biol 2014;34:144–53. doi: 10.1016/j.matbio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 1993;122:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Srinivasan S, Chhatre SS, Mabry JM, Cohen RE, McKinley GH. Solution spraying of poly(methyl methacrylate) blends to fabricate microtextured, superoleophobic surfaces. Polymer (Guildf) 2011;52:3209–18. doi: 10.1016/j.polymer.2011.05.008. [DOI] [Google Scholar]
- [35].Tutak W, Sarkar S, Lin-Gibson S, Farooque TM, Jyotsnendu G, Wang D, et al. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013;34:2389–98. doi: 10.1016/j.biomaterials.2012.12.020. [DOI] [PubMed] [Google Scholar]
- [36].Shih-Min Chuo, Meng-Hsun Wan, Wang LA, Jau-Sheng Wang. Multistage Modified Fiber Drawing Process and Related Diameter Measuring System. J Light Technol 2009;27:2983–8. doi: 10.1109/JLT.2009.2015059. [DOI] [Google Scholar]
- [37].Xing X, Wang Y, Li B. Nanofibers drawing and nanodevices assembly in poly(trimethylene terephthalate). Opt Express 2008;16:10815–22. doi: 10.1364/OE.16.010815. [DOI] [PubMed] [Google Scholar]
- [38].Jeong Hoon Eui, Lee Sung Hoon, Kim Pilnam and, Suh* KY. Stretched Polymer Nanohairs by Nanodrawing 2006. doi: 10.1021/NL061045M. [DOI] [PubMed] [Google Scholar]
- [39].Brown JL, Nair LS, Laurencin CT. Solvent/non-solvent sintering: A novel route to create porous microsphere scaffolds for tissue regeneration. J Biomed Mater Res Part B Appl Biomater 2008;86B:396–406. doi: 10.1002/jbm.b.31033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown JL, Peach MS, Nair LS, Kumbar SG, Laurencin CT. Composite scaffolds: Bridging nanofiber and microsphere architectures to improve bioactivity of mechanically competent constructs. J Biomed Mater Res Part A 2010;95A:1150–8. doi: 10.1002/jbm.a.32934. [DOI] [PubMed] [Google Scholar]
- [41].Shah RN, Shah NA, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A 2010;107:3293–8. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, et al. A self-assembly pathway to aligned monodomain gels. Nat Mater 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hashemi SM, Soudi S, Shabani I, Naderi M, Soleimani M. The promotion of stemness and pluripotency following feeder-free culture of embryonic stem cells on collagen-grafted 3-dimensional nanofibrous scaffold. Biomaterials 2011;32:7363–74. doi: 10.1016/j.biomaterials.2011.06.048. [DOI] [PubMed] [Google Scholar]
- [44].Lee ST, Yun JI, Jo YS, Mochizuki M, van der Vlies AJ, Kontos S, et al. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials 2010;31:1219–26. doi: 10.1016/j.biomaterials.2009.10.054. [DOI] [PubMed] [Google Scholar]
- [45].Wei J, Han J, Zhao Y, Cui Y, Wang B, Xiao Z, et al. The importance of three-dimensional scaffold structure on stemness maintenance of mouse embryonic stem cells. Biomaterials 2014;35:7724–33. doi: 10.1016/j.biomaterials.2014.05.060. [DOI] [PubMed] [Google Scholar]
- [46].Zhang D, Kilian KA. The effect of mesenchymal stem cell shape on the maintenance of multipotency. Biomaterials 2013;34:3962–9. doi: 10.1016/j.biomaterials.2013.02.029. [DOI] [PubMed] [Google Scholar]
- [47].Lü D, Luo C, Zhang C, Li Z, Long M. Differential regulation of morphology and stemness of mouse embryonic stem cells by substrate stiffness and topography. Biomaterials 2014;35:3945–55. doi: 10.1016/j.biomaterials.2014.01.066. [DOI] [PubMed] [Google Scholar]
- [48].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [49].Hofmeister LH, Costa L, Balikov DA, Crowder SW, Terekhov A, Sung H-J, et al. Patterned polymer matrix promotes stemness and cell-cell interaction of adult stem cells. J Biol Eng 2015;9:18. doi: 10.1186/s13036-015-0016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Newman P, Galenano-Niño JL, Graney P, Razal JM, Minett AI, Ribas J, et al. Relationship between nanotopographical alignment and stem cell fate with live imaging and shape analysis. Sci Rep 2016;6:37909. doi: 10.1038/srep37909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhou Y, Mao H, Joddar B, Umeki N, Sako Y, Wada K-I, et al. The significance of membrane fluidity of feeder cell-derived substrates for maintenance of iPS cell stemness. Sci Rep 2015;5:11386. doi: 10.1038/srep11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shin M, Yoshimoto H, Vacanti JP. In vivo bone tissue engineering using mesenchymal stem cells on a novel electrospun nanofibrous scaffold. Tissue Eng 2004;10:33–41. doi: 10.1089/107632704322791673. [DOI] [PubMed] [Google Scholar]
- [53].Xue R, Qian Y, Li L, Yao G, Yang L, Sun Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res Ther 2017;8:148. doi: 10.1186/s13287-017-0588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wu G, Pan M, Wang X, Wen J, Cao S, Li Z, et al. Osteogenesis of peripheral blood mesenchymal stem cells in self assembling peptide nanofiber for healing critical size calvarial bony defect. Sci Rep 2015;5:16681. doi: 10.1038/srep16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ruckh TT, Kumar K, Kipper MJ, Popat KC. Osteogenic differentiation of bone marrow stromal cells on poly(ε-caprolactone) nanofiber scaffolds. Acta Biomater 2010;6:2949–59. doi: 10.1016/j.actbio.2010.02.006. [DOI] [PubMed] [Google Scholar]
- [56].Sonomoto K, Yamaoka K, Kaneko H, Yamagata K, Sakata K, Zhang X, et al. Spontaneous Differentiation of Human Mesenchymal Stem Cells on Poly-Lactic-Co-Glycolic Acid Nano-Fiber Scaffold. PLoS One 2016;11:e0153231. doi: 10.1371/journal.pone.0153231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 2010;107:4872–7. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Eyckmans J, Lin GL, Chen CS. Adhesive and mechanical regulation of mesenchymal stem cell differentiation in human bone marrow and periosteum-derived progenitor cells. Biol Open 2012;1:1058–68. doi: 10.1242/bio.20122162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Higgins AM, Banik BL, Brown JL. Geometry sensing through POR1 regulates Rac1 activity controlling early osteoblast differentiation in response to nanofiber diameter. Integr Biol 2015;7:229–36. doi: 10.1039/C4IB00225C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials 2010;31:2470–6. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [61].Chen C-H, Chen S-H, Kuo C-Y, Li M-L, Chen J-P. Response of Dermal Fibroblasts to Biochemical and Physical Cues in Aligned Polycaprolactone/Silk Fibroin Nanofiber Scaffolds for Application in Tendon Tissue Engineering. Nanomater (Basel, Switzerland) 2017;7:219. doi: 10.3390/nano7080219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ingavle GC, Leach JK. Advancements in Electrospinning of Polymeric Nanofibrous Scaffolds for Tissue Engineering. Tissue Eng Part B Rev 2014;20:277–93. doi: 10.1089/ten.teb.2013.0276. [DOI] [PubMed] [Google Scholar]
- [63].James R, Laurencin CT. Nanofiber technology: its transformative role in nanomedicine. Nanomedicine (Lond) 2016;11:1499–501. doi: 10.2217/nnm.16.44. [DOI] [PubMed] [Google Scholar]
- [64].Santoro M, Shah SR, Walker JL, Mikos AG. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv Drug Deliv Rev 2016;107:206–12. doi: 10.1016/j.addr.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nerem RM, Seliktar D. Vascular Tissue Engineering. Annu Rev Biomed Eng 2001;3:225–43. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- [66].Ju YM, Ahn H, Arenas-Herrera J, Kim C, Abolbashari M, Atala A, et al. Electrospun vascular scaffold for cellularized small diameter blood vessels: A preclinical large animal study. Acta Biomater 2017;59:58–67. doi: 10.1016/j.actbio.2017.06.027. [DOI] [PubMed] [Google Scholar]
- [67].Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Tranquillo RT. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun 2016;7:12951. doi: 10.1038/ncomms12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ghaedi M, Soleimani M, Shabani I, Duan Y, Lotfi AS. Hepatic differentiation from human mesenchymal stem cells on a novel nanofiber scaffold. Cell Mol Biol Lett 2012;17:89–106. doi: 10.2478/s11658-011-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bhattacharya M, Malinen MM, Lauren P, Lou Y-R, Kuisma SW, Kanninen L, et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J Control Release 2012;164:291–8. doi: 10.1016/j.jconrel.2012.06.039. [DOI] [PubMed] [Google Scholar]
- [70].Malinen MM, Kanninen LK, Corlu A, Isoniemi HM, Lou Y-R, Yliperttula ML, et al. Differentiation of liver progenitor cell line to functional organotypic cultures in 3D nanofibrillar cellulose and hyaluronan-gelatin hydrogels. Biomaterials 2014;35:5110–21. doi: 10.1016/j.biomaterials.2014.03.020. [DOI] [PubMed] [Google Scholar]
- [71].Feng Z-Q, Chu X, Huang N-P, Wang T, Wang Y, Shi X, et al. The effect of nanofibrous galactosylated chitosan scaffolds on the formation of rat primary hepatocyte aggregates and the maintenance of liver function. Biomaterials 2009;30:2753–63. doi: 10.1016/j.biomaterials.2009.01.053. [DOI] [PubMed] [Google Scholar]
- [72].Dankers PYW, Boomker JM, Huizinga-van der Vlag A, Wisse E, Appel WPJ, Smedts FMM, et al. Bioengineering of living renal membranes consisting of hierarchical, bioactive supramolecular meshes and human tubular cells. Biomaterials 2011;32:723–33. doi: 10.1016/j.biomaterials.2010.09.020. [DOI] [PubMed] [Google Scholar]
- [73].MacGregor-Ramiasa M, Hopp I, Bachhuka A, Murray P, Vasilev K. Surface nanotopography guides kidney-derived stem cell differentiation into podocytes. Acta Biomater 2017;56:171–80. doi: 10.1016/j.actbio.2017.02.036. [DOI] [PubMed] [Google Scholar]
- [74].Derakhshan MA, Pourmand G, Ai J, Ghanbari H, Dinarvand R, Naji M, et al. Electrospun PLLA nanofiber scaffolds for bladder smooth muscle reconstruction. Int Urol Nephrol 2016;48:1097–104. doi: 10.1007/s11255-016-1259-2. [DOI] [PubMed] [Google Scholar]
- [75].Pokrywczynska M, Jundzill A, Adamowicz J, Kowalczyk T, Warda K, Rasmus M, et al. Is the poly (L- lactide- co- caprolactone) nanofibrous membrane suitable for urinary bladder regeneration? PLoS One 2014;9:e105295. doi: 10.1371/journal.pone.0105295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].In Vivo Generation of Thymus-Independent T Cells in a Tissue-Engineered T Cell Development Supporting Microenvironment. Biol Blood Marrow Transplant 2013;19:S208–9. doi: 10.1016/J.BBMT.2012.11.235. [DOI] [Google Scholar]
- [77].Singh A Biomaterials innovation for next generation ex vivo immune tissue engineering. Biomaterials 2017;130:104–10. doi: 10.1016/j.biomaterials.2017.03.015. [DOI] [PubMed] [Google Scholar]
- [78].Fan Y, Tajima A, Goh SK, Geng X, Gualtierotti G, Grupillo M, et al. Bioengineering Thymus Organoids to Restore Thymic Function and Induce Donor-Specific Immune Tolerance to Allografts. Mol Ther 2015;23:1262–77. doi: 10.1038/mt.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Silva GA, Czeisler C, Niece KL, Beniash E, Harrington DA, Kessler JA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004;303:1352–5. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- [80].Mahairaki V, Lim SH, Christopherson GT, Xu L, Nasonkin I, Yu C, et al. Nanofiber Matrices Promote the Neuronal Differentiation of Human Embryonic Stem Cell-Derived Neural Precursors In Vitro. Tissue Eng Part A 2011;17:855–63. doi: 10.1089/ten.tea.2010.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lim SH, Liu XY, Song H, Yarema KJ, Mao H-Q. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials 2010;31:9031–9. doi: 10.1016/j.biomaterials.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]