Abstract
Objective:
To study a broad range of FMR1 CGG repeat lengths and assisted reproduction technology (ART) outcomes.
Design:
Retrospective cohort study.
Setting:
Private ART practice.
Patient(s):
Fresh autologous ART stimulation cycles.
Intervention(s):
None.
Main Outcome Measure(s):
Oocyte yield, live birth.
Result(s):
We screened 14,088 fresh autologous ART cycles from 2012 to 2015, of which 4,690 cycles in 3,290 patients met the inclusion criteria. The FMR1 repeat length was statistically significantly but weakly associated with oocyte yield and other markers of ovarian response. The receiver operating characteristic curve analysis suggested extremely limited predictive ability. Moreover, the FMR1 repeat length was not statistically significantly associated with outcomes in multivariable models, including other markers of ovarian reserve. The FMR1 repeat length was not associated with embryo quality or live birth. Only patient age had a strong ability to predict live birth.
Conclusion(s):
The FMR1 repeat length is associated with ART response, but only weakly. It provides no incremental predictive ability beyond the conventionally used predictors, including patient age, antimuüllerian hormone concentration, antral follicle count, and follicle-stimulating hormone level. These data suggest a possible role of the FMR1 repeat length within the normal range in ovarian response but demonstrate no clinically relevant indication for testing FMR1 as a predictor of ART outcomes.
Keywords: ART, CGG repeats, FMR1, IVF, ovarian reserve
The fragile X mental retardation 1 (FMR1) gene located at Xq27.3 codes for fragile X mental retardation protein, an RNA-binding protein important for regulation of transport and translation of messenger RNA (mRNA) involved in neurologic processes (1–4). Its role in neuronal function is multifaceted, complex, and has been well studied (5). However, the role of FMR1 in ovarian function and fertility is poorly understood. The CGG repeat length in the 5′ untranslated region is associated with three known disease phenotypes: fragile X syndrome, fragile X tremor ataxia syndrome, and fragile X associated premature ovarian insufficiency (POI) (6).
Fragile X syndrome, the most common heritable cause of intellectual disability, occurs in children hemizygous or heterozygous for >200 CGG repeats, with 100% of males affected and up to 70% of female carriers showing some degree of intellectual impairment (4, 7). Hypermethylation of the gene promoter site results in silencing of the gene and a deficiency of fragile X mental retardation protein. In contrast, fragile X tremor ataxia syndrome and POI occur in individuals with FMR1 premutations (55–200 CGG repeats), which may result in increased translation of FMR1 (8–12). Additionally, women with FMR1 premutations are at risk of having a child with fragile X syndrome due to repeat expansion through the maternal germ line (13, 14). Women with FMR1 CGG repeats in the 45–54 range are considered to carry a borderline or ‘‘grey-zone’’ repeat number, with a risk of repeat expansion in one generation to the premutation range (14, 15).
Previous works have suggested that women with the FMR1 premutation (CGG repeats of 55–200) have a 20% risk of POI (11). Premutation carriers also have higher follicle-stimulating hormone (FSH) and lower antimuüllerian hormone (AMH) levels compared with the general population (10,16–20). The reproductive consequences of intermediate CGG repeat size as well as a “high-normal” range of 35–44 are a contentious subject of debate in the literature. A number of small, case-control studies have shown increased rates of premutation or high-normal repeats in POI and diminished ovarian reserve groups (21–23). In contrast with this work, a number of larger studies did not find a relationship between high-normal or intermediate CGG repeat length and POI or diminished ovarian reserve (24–27). Most recently, Schufreider et al. (28) retrospectively examined 1,287 women with infertility and found no relationship between CGG repeats 35–54 and the ovarian reserve markers FSH, AMH, and antral follicle count (AFC).
Gleicher et al. (29) have suggested a new normal range of 26–34 repeats based upon their extensive work on FMR1 and ovarian function. In a series of studies of women undergoing assisted reproduction technology (ART) treatment for infertility, egg donors, or patients undergoing ART for preimplantation genetic diagnosis, Gleicher and colleagues analyzed the FMR1 repeat number genotypes, ovarian reserve markers, embryo quality, and in vitro fertilization (IVF) outcomes. Some of their studies agreed with previous work that a high repeat number was associated with an increased risk of diminished ovarian reserve (30, 31). Surprisingly, the group also found an association between low CGG repeat alleles and poorer ovarian reserve testing, decreased day-3 embryo quality, and lower clinical pregnancy rates (29, 32, 33).
Beyond the Gleicher group, few studies have examined ART outcomes and the number of FMR1 CGG repeats. We analyzed a broad range of CGG repeat lengths below the pre-mutation range and the ART outcomes in an infertile population undergoing ART.
MATERIALS AND METHODS
Study Design
This was a retrospective cohort analysis of fresh autologous IVF cycles from 2012 to 2015. Cycles were included if FMR1 gene testing was performed on the patient. The FMR1 testing was included in a comprehensive genetic testing package offered to all couples undergoing ART. The FMR1 testing performed by triplet repeat capillary electrophoresis polymerase chain reaction (Counsyl). The study was performed at Shady Grove Fertility Reproductive Science Center in Rockville, MD, and was approved by the institutional review board.
Patients
All patients who underwent a fresh autologous ART cycle with FMR1 testing during the study period were included in the analysis. Because the study was designed to evaluate the impact of FMR1 repeats within the normal range, patients with >55 CGG repeats on either allele were also excluded from the analysis.
Stimulation Protocol
The ovarian stimulation treatment used mixed FSH/luteinizing hormone (LH) protocols under gonadotropin-releasing hormone (GnRH) antagonist or GnRH agonist pituitary suppression. In general, oral contraceptive treatment was initiated 21 days before stimulation. For GnRH antagonist cycles, the antagonist (Ganirelix Acetate; Merck) was initiated when the lead follicle was 14 mm in size. For GnRH agonist cycles, 20 units of leuprolide acetate (Lupron; AbbVie) was initiated during the last 3 days of oral contraceptive use. The leuprolide acetate dose was decreased to 5 units when ovarian suppression had been confirmed with ultrasound and the serum estradiol level was <5 pg/mL. Ovarian stimulation was achieved by employing recombinant FSH and human menopausal gonadotropin. When the lead follicle was ≥18 mm, we triggered final oocyte maturation with 10,000 IU of human chorionic gonadotropin or with 4 mg of GnRH agonist. Oocyte retrieval occurred 36 hours later, and fertilization was achieved with conventional IVF or intracytoplasmic sperm injection as clinically indicated.
Ultrasound-guided embryo transfer was performed on day 3 or on day 5. In general, embryos were cultured to the blastocyst stage if more than two high-grade cleavage embryos were available. Embryos could also be transferred on day 6 if no high-quality blastocysts were available by day 5. Patients who had an embryo transfer on day 3 or 5 could have supernumerary embryos cultured to day 6 for assessment for vitrification. Embryos were graded as good, fair, or poor according to the simplified Society for Assisted Reproductive Technology (SART) scoring system (34). In all pregnant patients, the serum human chorionic gonadotropin levels were assessed at 4 weeks’ gestational age followed by ultrasonography confirmation of a gestational sac. Live birth was defined as a live-born infant after the 23rd week of gestation.
FMR1 Groups
Testing for FMR1 gives results for CGG repeats on both FMR1 alleles. Previous publications have suggested that X chromosome inactivation may not impact the clinical manifestation of POI (28, 35). However, it is unclear whether X inactivation may impact ovarian response to exogenous gonadotropin stimulation. To ensure that both alleles were accounted for, we performed all analyses in duplicate, evaluating both the shorter and longer allele for each patient.
Other publications have accounted for both alleles by evaluating the heterozygosity of FMR1 CGG repeats based on groupings within the normal repeat length (29, 32). The repeat length was classified as low (≤25), normal (26–34), or high (≥35). Patients were grouped into results based on allele combinations of low-low, low-normal, low-high, normal-normal, normal-high, and high-high. Prior studies have suggested that FMR1 allele groupings are associated with ovarian reserve and embryo quality (29, 32).
It also has been suggested that certain FMR1 CGG repeat groups may have different effects across the age spectrum that are not linear in nature (33). To account for a nonlinear effect of repeat length on oocyte yield, FMR1 groups were further analyzed in patients <30 years old, 30 to 38 years old, and >38 years old.
Outcomes
To test the hypothesis that FMR1 is associated with ovarian response, the primary outcome was oocyte yield. Ovarian reserve was assessed with FSH, AMH, and AFC, and serum assays did not change over the study period. Secondary outcomes included days of gonadotropin stimulation, total dose of gonadotropin stimulation (IU), peak estradiol (pg/mL), total follicles measured on the last day of stimulation, metaphase 2 oocytes, the number of good-quality blastocysts on day 5, the total number of good-quality blastocysts that developed by day 6, and the number of embryos vitrified. To test the hypothesis that FMR1 is associated with embryo quality, the primary outcome was live birth. This primary outcome was based on studies associating embryo quality with live birth (36, 37). Secondary embryo quality outcomes included embryo grade in cleavage transfers, embryo grade in blastocyst transfers, and the likelihood of achieving blastocyst transfer. Cleavage and blastocyst embryos were graded as poor, fair, or good based on the simplified SART scoring system (34).
Statistics
Histograms were used to demonstrate the prevalence of FMR1 repeats and groups in the sample population. The normality of the data distribution was examined with the Shapiro-Wilk test, and non-normally distributed data were reported as median with interquartile range. The relationships of FMR1 repeat length with AMH, and AFC with oocyte yield were illustrated with scatter plots, and regression was used to assess linear relations and estimate R2. Generalized estimating equations (GEE) were used to evaluate the association of FMR1 with primary and secondary outcomes while accounting for covariates to address the correlations in the data arising from some patients contributing multiple cycles.
Unadjusted models evaluated FMR1 repeat length as a continuous variable or in allele groupings directly with outcomes. Multivariable models were also evaluated to assess predictive ability of FMR1 incremental to other established predictors including patient age, FSH, AMH, and AFC. The number of embryos transferred was also included as a covariate in all analyses of live birth.
Receiver operator characteristic (ROC) curves were used to assess the predictive ability of FMR1 repeats and other ovarian reserve markers as reflected by the area under the curve (AUC). Separate ROCs were evaluated to assess the predictors of poor ovarian response, hyperovarian response, and live birth. Poor ovarian response was defined as ≤4 oocytes based on the Bologna criteria (38). Hyperovarian response was defined as >15 based on studies demonstrating higher rates of ovarian hyperstimulation syndrome without improvement in birth outcomes (39–41).
The results are expressed as the mean difference or odds ratios with 95% confidence intervals. P<.05 was considered statistically significant. Statistical analysis was performed using SPSS (IBM). Histograms were generated using Statcrunch.com.
RESULTS
Population Summary
There were 14,088 fresh autologous ART cycles screened during the study period, of which 4,690 cycles in 3,290 patients met inclusion criteria. There were 34 excluded cycles in patients with testing results in the premutation range on at least one allele. The patients for the remainder of the 9,364 cycles (66%) elected not to have genetic testing as part of their ART screening. Other cycles were excluded due to frozen embryo transfer, donor oocyte, and no FMR1 testing.
The median age of the patients with FMR1 testing was 35 years. The furthest developed embryo attained the cleavage stage in 944 cycles, the morula stage in 395 cycles, and the blastocyst stage in 3,027 cycles. In 324 cycles, no embryos were available for evaluation. The population was 62% Caucasian, 17% Asian, 9% African American, 4% Hispanic, and 9% other or not identified. The baseline ovarian reserve results are shown in Supplemental Table 1 (available online).
The FMR1 repeat length on the long allele was inversely associated with basal FSH values, and this persisted when adjusting for patient age (Supplemental Table 2, available online). The FMR1 repeat length was not statistically significantly associated with AMH or AFC on either the short or long allele.
Ovarian Response
The distribution of FMR1 short and long repeats is shown in Supplemental Figure 1 (available online). Both alleles had median repeat lengths of 30. The short allele averaged 27 repeats (range: 8–49), and the long allele averaged 32 repeats (range: 19–51). The average difference between the alleles was 5 (range: 0–24).
In unadjusted GEE analyses, FMR1 repeats were associated with the primary outcome of oocyte yield, with a mean weighted difference of 0.07 (95% CI, 0.01–0.14) on the short allele and 0.05 (95% CI, 0.01–0.10) on the long allele (Table 1). This association corresponds to an increase of one additional oocyte retrieved for every increase of 14 to 20 FMR1 repeats. In models that adjusted only for age, the estimated association of FMR1 repeats with oocyte yield remained similar.
TABLE 1.
Unadjusted and adjusted generalized estimating equation (GEE) models demonstrating associations of the short and long FMR1 alleles with ovarian response and assisted reproduction outcomes.
| FMR1 short allele | FMR1 long allele | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Unadjusted association (95% CI) |
P value | Adjusted association (95% CI) |
P value | Unadjusted association (95% CI) |
P value | Adjusted association (95% CI) |
P value |
| Continuous | ||||||||
| Gonadotropins (d) | −0.05 (−0.10 to −0.01) | .04 | −0.02 (−0.05 to 0.01) | .10 | −0.01 (−0.06 to 0.04) | .15 | 0.01 (−0.01 to 0.04) | .30 |
| Gonadotropins (IU) | −25 (−43 to −9) | .003 | −24 (−45 to −3) | .02 | −11 (−25 to 4) | .14 | −4 (−23 to 16) | .71 |
| Estradiol (pg/mL) | 17 (8–25) | < .001 | 10 (3–22) | .13 | 11 (4–18) | .001 | 10 (−2 to 22) | .11 |
| Follicles | 0.07 (0.03–0.11) | .002 | 0.07 (0.01–0.12) | .02 | 0.04 (0.01–0.07) | .02 | 0.05 (0.00–0.10) | .05 |
| Oocytes retrieved | 0.07 (0.01–0.14) | .02 | 0.08 (−0.01 to 0.16) | .06 | 0.05 (0.01–0.10) | .04 | 0.04 (−0.03 to 0.11) | .29 |
| MII oocytes | 0.06 (0.01–0.11) | .02 | 0.06 (−0.01 to 0.13) | .07 | 0.05 (0.01–0.09) | .02 | 0.06 (−0.01 to 0.11) | .08 |
| Blastocysts (d5) | 0.02 (0.01–0.04) | .01 | −0.02 (−0.01 to 0.04) | .18 | 0.02 (0.01–0.03) | .01 | 0.01 (−0.01 to 0.03) | .45 |
| Blastocysts (d6) | 0.03 (0.01 −0.05) | .004 | 0.01 (−0.02 to 0.04) | .54 | 0.03 (0.01–0.05) | .02 | 0.01 (−0.01 to 0.04) | .29 |
| Vitrified embryos | 0.03 (0.01 −0.05) | .007 | 0.01 (−0.02 to 0.05) | .40 | 0.04 (0.01–0.06) | .002 | 0.01 (−0.01 to 0.04) | .29 |
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | Unadjusted OR (95% CI) | Adjusted OR (95% CI | |||||
| Outcome | ||||||||
| Good embryo grade | 0.99 (0.97–1.01) | .43 | 1.00(0.98–1.02) | .96 | 0.99(0.97–1.01) | .24 | 1.00 (0.98–1.02) | .62 |
| Blastocyst transfer | 0.98 (0.97–1.01) | .13 | 1.00(0.97–1.03) | .91 | 0.99(0.97–1.01) | .10 | 1.01 (0.98–1.03) | .40 |
| Live birth | 0.98 (0.96–1.01) | .33 | 1.00(0.98–1.02) | .97 | 0.99(0.98–1.01) | .33 | 1.00 (0.99–1.01) | .85 |
Note: Continuous and dichotomous variables were adjusted for age, antimullerian hormone (AMH), antral follicle count (AFC), and follicle-stimulating hormone (FSH). Live birth was adjusted for age, AMH, AFC,FSH, and the number of embryos transferred.
Long allele FMR1 was also associated with other markers of ovarian reserve, including the amount of gonadotropins used, peak estradiol, metaphase 2 oocytes, the number of blastocysts developed by days 5 and 6, and the number of embryos vitrified (Table 1). The unadjusted associations for the secondary outcomes remained weak, with an additional 33 repeats needed to have one additional vitrified embryo.
In unadjusted and multivariable GEE models, age, AMH, AFC, and FSH were each independently associated with oocyte yield (Supplemental Table 3, available online). In multivariable GEE analyses including other markers of ovarian reserve (age, AMH, AFC, FSH), the association of FMR1 with markers of ovarian reserve were weak and generally statistically not significant (Table 1, Supplemental Table 2). Linear regression demonstrated the weak linear relation of FMR1 repeat length on the short allele (R2= 0.002) and the long allele (R2= 0.001) with oocyte yield (Fig. 1). Conversely, strong associations were observed for both AFC (R2 = 0.27) and AMH (R2 = 0.28) with oocyte yield.
FIGURE 1.

Scatter plots demonstrating the weak association of (A) FMR1 short alleles and (B) FMR1 long alleles and oocyte yield, and the strong association of (C) antral follicle count and (D) antimüllerian hormone with oocyte yield.
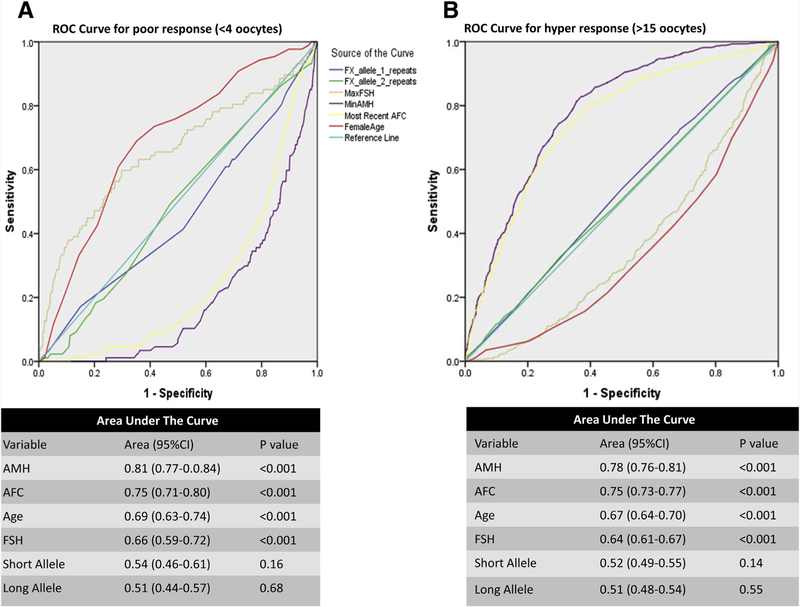
The utility of FMR1 and other considered covariates for prediction of ovarian response was also evaluated in ROC curve analyses with poor ovarian response and hyperovarian response considered as the outcomes. In analyses that considered each factor individually, age, AMH, AFC, and FSH were each observed to have good predictive ability for ovarian response based on AUC from ROC curves. We found that AMH and AFC were most predictive of poor ovarian response and hyperovarian response with AUCs of 0.75–0.81 (Fig. 2). Age and FSH also performed as a good test in predicting ovarian response with AUCs of 0.64–0.69. Conversely, FMR1 repeat length had limited predictive ability for ovarian response with AUCs of 0.51–0.54 (Fig. 2).
FIGURE 2.
Receiver operating characteristic (ROC) curves associating predictor variables with (A) poor ovarian response (<4 oocytes) and (B) hyper ovarian response (>15 oocytes). The area under the curve (AUC) is shown under each ROC curve to demonstrate the association of the predictor variable with the outcome. For demonstrative purposes, an unconventional graphic display is demonstrated, as negative predictive variables are shown as dropping below the 0.50 null line. Note the further the ROC line drifts from the 0.50 null line (either up or down), the stronger it was for predicting the outcome.
Serial ROC curves were analyzed first without and then with FMR1 as an incremental predictor of oocyte yield. Adding FMR1 to the ROC analyses resulted in no incremental improvement in the ability of conventional ovarian reserve markers to predict either poor response or hyperresponse.
To relax assumptions of linearity, we also analyzed FMR1 repeat length in groups, as previously described. The distribution of FMR1 allele groupings is shown in Supplemental Figure 1. The majority of cycles were in the normal-normal (54%), low-normal (25%), and normal-high (14%) FMR1 allele groups. There were only four women with high-high FMR1 alleles. Due to the low number of subjects, this group was excluded from analyses, leaving low-low, low-normal, normal-normal, low-high, and normal-high as the five groups compared. These analyses were consistent with analyses using FMR1 repeats as a continuous variable, and the groups were not different with regard to markers of ovarian response.
When <30 years old, the women in the FMR1 low-low group had four fewer oocytes retrieved compared with the normal-normal group (Supplemental Table 4, available online). Similarly the low-low group had five fewer oocytes when >38 years old. Patients in the low-high group aged 30 to 38 years had four fewer oocytes compared with the normal-normal group. In adjusted GEE models, only the low-low group >38 years old had a statistically significantly different oocyte yield compared with the normal-normal group.
Embryo Quality
In unadjusted GEE models, FMR1 repeats were not associated with the likelihood of achieving a blastocyst transfer (Table 1) or with the likelihood of having a good quality embryo transfer for either the short allele (odds ratio [OR] 1.00; 95% confidence interval [CI], 0.98–1.02) or the long allele (OR 1.00; 95% CI, 0.98–1.02). Conversely, patient age, AMH, AFC, and FSH were all independently associated with the likelihood of having a good-quality blastocyst to transfer (Table 1). The FMR1 repeats were not associated with live birth for both the short allele (OR 1.00; 95% CI, 0.97–1.03) and the long allele (OR 1.00; 95% CI, 0.98–1.02).
In adjusted GEE models including age, AMH, AFC, FSH, and FMR1 repeats, only female age was associated with the likelihood of live birth (OR 0.92; 95% CI, 0.89–0.94). Similarly, in ROC curve analysis, age had a moderate ability to predict live birth with an AUC of 0.62 (Supplemental Fig. 2, available online); by comparison, AUCs for other markers were weaker: 0.57 for AMH, 0.57 for AFC, 0.53 for FSH, and 0.5 for FMR1 repeat lengths for both the short and the long allele (Supplemental Fig. 2).
The FMR1 groupings were considered for analysis of embryo quality end points, including cleavage embryo quality, good-quality embryo transfer, blastocyst formation, good-quality blastocyst formation, and live birth (Fig. 3; Supplemental Table 4). The incidence of having a good-quality embryo transferred in cleavage transfers was 85% for low-low, 87% for low-normal, 87% for low-high, 87% for normal-normal, and 91% for normal-high (Fig. 3). The GEE models demonstrated no difference between the groups with cleavage embryo quality.
FIGURE 3.

Graph demonstrating the incidence of the best embryo being good (blue), fair (red), or poor (green) for (A) cleavage embryo transfers and (B) blastocyst embryo transfers/blastocyst freezing cycles.
The incidence of having a good-quality embryo transferred in blastocyst transfers was 84% for low-low, 87% for low-normal, 87% for low-high, 87% for normal-normal, and 85% for normal-high (Fig. 3). The distribution of blastocyst embryo quality across the four FMR1 groups was not statistically significantly different. The GEE models demonstrated no difference between the groups for blastocyst embryo quality (Supplemental Table 5, available online).
The incidence of forming a blastocyst and forming a good-quality blastocyst for all cycle starts is shown in Supplemental Figure 3 (available online). The incidence of forming a blastocyst per cycle start was 71% for low-low, 74% for low-normal, 67% for low-high, 72% for normal-normal, and 74% for normal-high. The incidence of forming a good-quality blastocyst per cycle start was 58% for low-low, 58% for low-normal, 57% for low-high, 61% for normal-normal, and 64% for normal-high. The adjusted GEE models demonstrated no difference between the groups for blastocyst embryo quality (Supplemental Table 5).
Live birth was also evaluated as a marker of embryo quality and the most important end point of an ART cycle. Live birth occurred in 46.8% of blastocyst transfers, 24.4% of cleavage transfers, and 20.2% of morula transfers. Unadjusted and adjusted GEE models demonstrated no difference between the FMR1 groups for live-birth outcomes (Supplemental Table 5).
DISCUSSION
The origin of the classic CGG repeat categories arose in the context of defining fragile X syndrome, or the risk of anticipation and expansion of the CGG repeat through the maternal line. It was later discovered that the premutation also conferred risk of POI and fragile X tremor ataxia syndrome. Investigations into markers of ovarian function in populations, largely ascertained based upon family history of fragile X syndrome, found a relationship between premutation carrier status and both FSH and AMH (16–20). Similar investigations examining intermediate repeat lengths were mixed, with some studies showing evidence of ovarian dysfunction and others showing no relationship (21, 22, 24–27). This body of work remained strongly tied to the classic categorizations of CGG repeats.
Initial attempts to stratify CGG repeat lengths in the context of ovarian function suggested a new framework with 26–34 repeats proposed as normal, >34 repeats as high and <26 repeats as low. The apparent significance of these values rested on prior studies, in which individuals with at least one low repeat allele demonstrated a lower likelihood of pregnancy with IVF, poorer embryo morphology, and an accelerated decline in functional ovarian reserve (32, 33). These reports were extended to suggest that FMR1 CGG repeat length could be used clinically to predict IVF outcome and identify women at risk for diminished ovarian reserve (32, 42–44). These findings were based upon a relatively small number of patients and have not been confirmed by other independent researchers.
Our study of FMR1 CGG repeat lengths across the spectrum, obtained as a part of routinely offered preconception genetic screening, is the largest data set available to date. In multivariable models that included age, FSH, and AMH, the FMR1 CGG repeat length was not an independent predictor of most clinically relevant findings in an infertile population undergoing ART. In particular, the FMR1 repeat length was not a predictor of clinically relevant ART outcomes: oocyte yield, achieving blastocyst transfer, vitrification of supernumerary embryos, or live birth. These findings were robust across multiple disparate analyses, including continuous modeling and modeling using possible allele combinations.
The observation of a statistically significant relationship between repeat length and some aspects of ovarian function, suggests the possibility that FMR1 plays an important biological role in ovarian physiology. Our data are limited for assessment of such a hypothesis. However, our results provide a strong indication that, regardless of underlying biological roles, the clinical utility of FMR1 for predicting IVF outcomes is questionable at best. This finding was consistent across a wide array of statistical analyses that evaluated FMR1 as predictor of outcomes both with and without consideration of more established predictors. The ROC curve analyses of single predictors demonstrated that FMR1 gene testing for the purpose of ovarian reserve measurement is inferior to the current commonly measured markers of AMH, AFC, and FSH. We found that FMR1 testing did not add any additional clinically significant benefit to other forms of ovarian reserve screening.
A recent study from Schufreider et al. (28) evaluated FMR1 CGG repeats and their relationship to markers of ovarian reserve in ART patients. They found no significant association with FMR1 CGG repeat length and FSH, AFC, or AMH. Our findings were overall similar, with the exception that we did find an inverse relationship with FMR1 CGG repeat length on the long allele with FSH. This discrepancy may be due to the greater power we had to detect such a relationship, especially given the relatively weak association with every 1 unit increase in FMR repeat length being associated with a reduction in FSH of 0.04 IU/L. Our study also analyzed end points beyond ovarian reserve by evaluating ovarian response, embryo quality, and live birth.
The FMR1 repeat length is commonly tested as one aspect of preconception genetic testing due to the risk of premutation expansion and birth of a child with fragile X syndrome. Patients and clinicians are able to readily access FMR1 repeat numbers with the increasing availability of expanded preconception screening, particularly as large IVF centers more routinely offer such testing. Clinicians need more information to best counsel their patients and decide how, if at all, the FMR1 CGG repeat number should impact clinical care aside from genetic counseling.
Our study adds to the present literature of FMR1 testing and ART outcomes in several areas. This is the largest study to date, allowing for a greater statistical power to detect relatively small outcomes. Given that statistically significant associations with FMR1 and ART outcomes were not demonstrated in a sample size of over 4,600 cycles, it is doubtful that larger studies would demonstrate a clinically meaningful utility for FMR1 testing predicting ART response or outcomes. Post hoc power analysis demonstrated that we were powered to detect a 2.6% absolute difference in high-quality blastocyst formation, a 1.9% absolute difference in having any high-quality embryo for transfer, and a 3.7% absolute difference in live birth. The data are limited to an ART population, which may limit their applicability to the general infertile population.
Additional screening through ovarian reserve testing before ART may also have affected patient selection and the observed outcomes. Analyses were performed on both the short and long allele of FMR1 and by grouping previously reported FMR1 allele combinations. The results of the study were persistent, regardless of how FMR1 was grouped. Similar results were observed across different statistical methods used for evaluation including GEE models and ROC curves. The GEE models allowed our study to account for other commonly performed assessments of ovarian reserve. When age, AMH, AFC, and FSH were accounted for, any minimal association of FMR1 with ovarian reserve became statistically nonsignificant. The unadjusted models demonstrate a biologic role of FMR1 in ovarian function and response. The adjusted models were performed to assess if FMR1 testing offered incremental value to clinical ovarian reserve testing already commonly used. The adjusted models did not demonstrate a clinical advantage to adding FMR1 CGG repeat length testing for the purpose of predicting ART outcomes.
Previous studies had evaluated FMR1 and cleavage embryo quality (32). Our study had the advantage of evaluating blastocyst quality, which may be a more clinically meaningful end point of embryo quality. Finally, our study followed the outcomes to live birth, which is the most important outcome for any test attempting to predict ART outcomes.
To have clinical predictive utility, a measure must provide a meaningful addition to current ovarian reserve testing for predicting ovarian response or ART outcomes. These data suggest FMR1 repeat length has a weak association with ART response, which provides no incremental predictive ability beyond patient age, AMH, AFC, and FSH. However, FMR1 testing is typically available as part of genetic screening packages. The negative effect of FMR1 repeats in the premutation and full mutation range is clear, although further research is needed to improve our understanding of the biological role of FMR1 repeats within the normal range in ovarian function.
As the biological role of FMR1 is clarified, results from genetic screening may provide an explanation to patients who have both FMR1 testing and poor ovarian response as a possible root cause of their problem. Our study found no clinically relevant indication for testing FMR1 as a predictor of ART outcomes in a population already screened for treatment with autologous IVF.
Supplementary Material
Acknowledgments
Supported in part by the Intramural Research Program of the Program in Reproductive and Adult Endocrinology, NICHD, National Institutes of Health. The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U. S. Government.
Footnotes
N.B. has nothing to disclose. G.P. has nothing to disclose. K.D. has nothing to disclose. A.H.D. has nothing to disclose. E.W. has nothing to disclose. E.D.L. has nothing to disclose. B.W.W. has nothing to disclose. M.J.H. has nothing to disclose.
Discuss: You can discuss this article with its authors and with other ASRM members at http://fertstertforum.com/banksn-fmr1-cgg-repeats-art-outcomes/
REFERENCES
- 1.Usdin K, Kumari D. Repeat-mediated epigenetic dysregulation of the FMR1 gene in the fragile X-related disorders. Front Genet 2015;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 2008;60:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loesch D, Hagerman R. Unstable mutations in the FMR1 gene and the phenotypes. Adv Exp Med Biol 2012;769:78–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identifi cation of a gene (FMR-1) containing a CGG repeat coincident with a break-point cluster region exhibiting length variation in fragile X syndrome. Cell 1991;65:905–14. [DOI] [PubMed] [Google Scholar]
- 5.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol 2012;7:219–45. [DOI] [PubMed] [Google Scholar]
- 6.Lozano R, Rosero CA, Hagerman RJ. Fragile X spectrum disorders. Intractable Rare Dis Res 2014;3:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, et al. The FMR1 premutation and reproduction. Fertil Steril 2007; 87:456–65. [DOI] [PubMed] [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Leehey MA, Hall DA, Levine RA, Brunberg JA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA 2004;291:460–9. [DOI] [PubMed] [Google Scholar]
- 9.Uzielli ML, Guarducci S, Lapi E, Cecconi A, Ricci U, Ricotti G, et al. Premature ovarian failure (POF) and fragile X premutation females: from POF to to fragile X carrier identification, from fragile X carrier diagnosis to POF association data. Am J Med Genet 1999;84:300–3. [PubMed] [Google Scholar]
- 10.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab 2004;89:4569–74. [DOI] [PubMed] [Google Scholar]
- 11.Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med 2005;7:584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet 2001;10:1449–54. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Carvajal I, Lopez Posadas B, Pan R, Raske C, Hagerman PJ, Tassone F. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn 2009;11:306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991;67:1047–58. [DOI] [PubMed] [Google Scholar]
- 15.Nolin SL, Glicksman A, Ding X, Ersalesi N, Brown WT, Sherman SL, et al. Fragile X analysis of 1112 prenatal samples from 1991 to 2010. Prenat Diagn 2011;31:925–31. [DOI] [PubMed] [Google Scholar]
- 16.Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE, Conway GS. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod 1999;14: 1217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hundscheid RD, Braat DD, Kiemeney LA, Smits AP, Thomas CM. Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod 2001;16:457–62. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2005; 20:402–12 [DOI] [PubMed] [Google Scholar]
- 19.Rohr J, Allen EG, Charen K, Giles J, He W, Dominguez C, et al. Anti-Mullerian hormone indicates early ovarian decline in fragile X mental retardation (FMR1) premutation carriers: a preliminary study. Hum Reprod 2008;23:1220–5. [DOI] [PubMed] [Google Scholar]
- 20.Spath MA, Feuth TB, Allen EG, Smits AP, Yntema HG, van Kessel AG, et al. Intra-individual stability over time of standardized anti-muüllerian hormone in FMR1 premutation carriers. Hum Reprod 2011;26:2185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet 2005;117:376–82. [DOI] [PubMed] [Google Scholar]
- 22.Bodega B, Bione S, Dalpr’a L, Toniolo D, Ornaghi F, Vegetti W, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod 2006;21:952–7. [DOI] [PubMed] [Google Scholar]
- 23.Pastore LM, Young SL, Baker VL, Karns LB, Williams CD, Silverman LM. Elevated prevalence of 35–44 FMR1 trinucleotide repeats in women with diminished ovarian reserve. Reprod Sci 2012;19:1226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett CE, Conway GS, Macpherson JN, Jacobs PA, Murray A. Intermediate sized CGG repeats are not a common cause of idiopathic premature ovarian failure. Hum Reprod 2010;25:1335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murray A, Schoemaker MJ, Bennett CE, Ennis S, Macpherson JN, Jones M, et al. Population-based estimates of the prevalence of FMR1 expansion mutations in women with early menopause and primary ovarian insufficiency. Genet Med 2014;16:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline JK, Kinney AM, Levin B, Brown SA, Hadd AG, Warburton D. Intermediate CGG repeat length at the FMR1 locus is not associated with hormonal indicators of ovarian age. Menopause 2014;21:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voorhuis M, Onland-Moret NC, Janse F, Ploos van Amstel HK, Goverde AJ, Lambalk CB, et al. The significance of fragile X mental retardation gene 1 CGG repeat sizes in the normal and intermediate range in women with primary ovarian insufficiency. Hum Reprod 2014;29:1585–93. [DOI] [PubMed] [Google Scholar]
- 28.Schufreider A, McQueen DB, Lee SM, Allon R, Uhler ML, Davie J, et al. Diminished ovarian reserve is not observed in infertility patients with high normal CGG repeats on the fragile X mental retardation 1 (FMR1) gene. Hum Reprod 2015;30:2686–92. [DOI] [PubMed] [Google Scholar]
- 29.Gleicher N, Kushnir VA, Weghofer A, Barad DH. How the FMR1 gene became relevant to female fertility and reproductive medicine. Front Genet 2014;5:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence: I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Muüllerian hormone. Fertil Steril 2009;91:1700–6. [DOI] [PubMed] [Google Scholar]
- 31.Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online 2009;19:385–90. [DOI] [PubMed] [Google Scholar]
- 32.Kushnir VA, Yu Y, Barad DH, Weghofer A, Himaya E, Lee HJ, et al. Utilizing FMR1 gene mutations as predictors of treatment success in human in vitro fertilization. PLoS One 2014;9:e102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gleicher N, Yu Y, Himaya E, Barad DH, Weghofer A, Wu YG, et al. Early decline in functional ovarian reserve in young women with low (CGGn < 26) FMR1 gene alleles. Transl Res 2015;166:502–7.e2. [DOI] [PubMed] [Google Scholar]
- 34.Heitmann RJ, Hill MJ, Richter KS, DeCherney AH, Widra EA. The simplified SART embryo scoring system is highly correlated to implantation and live birth in single blastocyst transfers. J Assist Reprod Genet 2013;30:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Geyter C, M’Rabet N, De Geyter J, Zürcher S, Moffat R, Boüsch N, et al. Similar prevalence of expanded CGG repeat lengths in the fragile X mental retardation I gene among infertile women and among women with proven fertility: a prospective study. Genet Med 2014;16:374–8. [DOI] [PubMed] [Google Scholar]
- 36.Hill MJ, Richter KS, Heitmann RJ, Graham JR, Tucker MJ, DeCherney AH, et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers. Fertil Steril 2013;99:1283–9.e1. [DOI] [PubMed] [Google Scholar]
- 37.Ahlström A, Westin C, Reismer E, Wikland M, Hardarson T. Trophectoderm morphology: an important parameter for predicting live birth after single blastocyst transfer. Hum Reprod 2011;26:3289–96. [DOI] [PubMed] [Google Scholar]
- 38.Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L, et al. ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Hum Reprod 2011;26:1616–24. [DOI] [PubMed] [Google Scholar]
- 39.Hill MJ, Chason RJ, Payson MD, Segars JH, Csokmay JM. GnRH antagonist rescue in high responders at risk for OHSS results in excellent assisted reproduction outcomes. Reprod Biomed Online 2012;25:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A. Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 2011;26:1768–74. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Velasco JA, Bosch E. The necessity to define the sub-optimal re sponders. Hum Reprod 2015;30:2958. [DOI] [PubMed] [Google Scholar]
- 42.Gleicher N, Weghofer A, Oktay K, Barad DH. Can the FMR1 (fragile X) gene serve as predictor of response to ovarian stimulation? Reprod Sci 2009;16: 462–7. [DOI] [PubMed] [Google Scholar]
- 43.Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand 2009;88:1024–30. [DOI] [PubMed] [Google Scholar]
- 44.Gleicher N, Kushnir VA, Barad DH. Prospectively assessing risk for premature ovarian senescence in young females: a new paradigm. Reprod Biol Endocrinol 2015;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.