Abstract
Hydroxylated polychlorinated biphenyls (OH-PCBs) are toxic contaminants produced via biotic or abiotic transformation of PCBs. In this study, we have tested the toxicity of 2,5- dichlorobiphenyl (2,5-DCB) and three of its OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5- DCB toward the model plant, Arabidopsis thaliana. Toxicity tests showed that the parent 2,5- DCB (5 mg L−1) had little effect on the plants, while all three OH-metabolites (5 mg L−1) exhibited a significant toxicity, with 4’-OH-2,5-DCB being the most potent (inhibition concentration 50%-IC50 in germination tests = 9.8 mg L−1 for 2’-OH-2,5-DCB, 9.5 mg L−1 for 3’- OH-2,5-DCB, and 4.8 mg L−1 for 4’-OH-2,5-DCB). Whole-genome expression microarrays (Affymetrix) showed that exposure to the three OH-PCBs resulted in rather similar expression patterns, which were distinct from the one developing in response to 2,5-DCB. Searching an Arabidopsis microarray database (Genevestigator) revealed that, unlike the parent compound, the three OH-derivatives induced expression profiles similar to inhibitors of brassinosteroid synthesis (i.e., brassinazole, propiconazole, and uniconazole), resulting in severe iron deficiency in exposed plants. Our results suggest that the higher phytotoxicity of OH-derivatives as compared to 2,5-DCB is at least partly explained by the inhibition of the brassinosteroid pathway.
Keywords: polychlorinated biphenyls–PCBs, hydroxylated PCBs, Arabidopsis thaliana, brassinosteroids, iron deficiency
Introduction
Hydroxylated polychlorinated biphenyls (OH-PCBs) have been widely detected in the environment, including air, water, sediments, and animal tissues (Kawano et al., 2005; Tehrani and Van Aken, 2014; Awad et al., 2016). OH-PCBs have raised environmental concerns because they are in some cases more toxic than the parent PCBs (Grimm et al., 2015; Montano et al., 2013). OH-PCBs are formed by the biological or abiotic transformation of PCBs. The metabolism of PCBs in higher organisms is often initiated by hydroxylation reactions mediated by oxidative enzymes, such as the cytochrome P-450 monooxygenase (Grimm et al., 2015). OH-PCBs have also been suggested to be co-contaminants in commercial formulations and/or be formed in the atmosphere through reaction with the hydroxyl radical (Anderson and Hites, 1996; Marek et al., 2013).
As many organic contaminants, PCBs are susceptible to be taken up and, to some extent, metabolized by plants (Sanderman, 1994; Schnoor et al., 1995; Van Aken et al., 2010). Within plant tissues, PCBs are thought to be metabolized through a three-step process known as ‘green liver model’ (Sanderman, 1994), which is typically initiated by oxidation to OH-derivatives (Chroma et al., 2003; Van Aken et al., 2010). Different studies have shown that PCBs exert moderate to severe effects on plants, depending on the PCB congener and the plant species (Weber and Mozek, 1979; Mahanty, 1986).
Even though OH-PCBs are widespread in the environment, little information is available about their toxicity for plants. Understanding the effects of PCBs and OH-PCBs on plants is significant because plants are the basis of the terrestrial food web and can be used for soil remediation (i.e., phytoremediation). In a prior publication, we reported that a suite of lower-chlorinated OH-PCBs were highly toxic for the model plant, Arabidopsis thaliana, while most parent PCBs did not show significant effects (Subramanian et al., 2017). Gene expression analysis using 2,5-dichlorobiphenyl (2,5-DCB) and its OH-derivative, 4’-OH-2,5-DCB, showed that the parent compound – unlike the OH-derivative – induced multiple xenobiotic response genes, such as cytochrome P-450 and glutathione S-transferases, potentially involved in the PCB metabolism, which may explain the lower phytotoxicity of PCBs as compared with their OH-derivatives.
The objective of the present study is to understand further the toxicity of PCB and OH-PCBs by comparing the effects of three OH-isomers of 2,5-DCB, 2’-OH-2,5-DCB, 3’-OH-2,5-DCB, and 4’-OH-2,5-DCB, on Arabidopsis plants. We used whole-genome microarrays (Affymetrix) to explain why different OH-PCB isomers have different toxic effects on plants.
Materials & Methods
Toxicity tests
A. thaliana, ecotype Columbia (Col-0/Redei-L211497, Ohio State University, Columbus, OH) was grown as we described in Subramanian et al. (2017). In short, surface-sterilized seeds were germinated in Petri dishes containing semi-solid, half-strength Murashige & Skoog (MS) medium (0.3% sucrose) supplemented with increasing concentrations of 2,5-DCB, 2’-OH-, 3’- OH-, and 4’-OH-2,5-DCBs. The toxicants were added to the medium as an acetone solution (5,000-mg L−1) to final concentrations from 0.0 to 20 mg L−1 for OH-2,5-DCBs and 0.0 to 100 mg L−1 for 2,5-DCB. Control seeds were grown in the nutrient medium supplemented with the solvent carrier only (acetone, < 1% v/v). Seeds were incubated at 25°C under white (cool) fluorescent light (0.4 ± 0.05 W ft−2) with a 16-h light/8-h dark photoperiod. After 7 days, the germination rate was determined by visual observation (plantlets with two visible green leafs were counted as germinated). After 21 days, surviving plantlets were removed from the medium, washed and weighed to determine the fresh biomass. Three replicate dishes containing at least 20 seeds per dish were used for each treatment. The phytotoxic effect of PCBs and OH-PCBs was expressed by the inhibition concentration 50% (IC50) obtained by plotting the experimental dose – response curve (percent inhibition vs. contaminant concentration) and computing a 3-parameter sigmoidal regression (Prism 6.0, GraphPad, La Jolla, CA).
Gene expression analysis using microarrays
A. thaliana was grown in 10 × 10-cm Magenta boxes as described above. Plantlets were exposed to sub-inhibitory concentration of 2,5-DCB, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB (5 mg L−1). Control plantlets were grown in the presence of the solvent carrier (acetone) only. Six boxes containing five plantlets each were used for each treatment. After 21 days of growth, plantlets were removed from the medium, treated with RNAlater™ (Ambion, Foster City, CA) and stored at −80°C until RNA extraction. RNA was extracted from the whole plants using TRIzol® Plus RNA Purification kit with on-column PureLink® DNase treatment (Thermofisher, Waltham, MA) as described in Subramanian et al. (2017). RNA was analyzed by the absorbance ratios OD(optical density) 260/OD280 and OD260/OD230 (NanoDrop™ ND-2000, Vernon Hills, IL). RNA integrity numbers (RIN) (Agilent 2100 Bioanalyzer, Santa Clara, CA) were between 6.6 and 7.4. RNA was labeled and hybridized to the Arabidopsis Gene 1.0 ST Arrays according to the manufacturer’s instructions (Affymetrix, Santa Clara, CA). Three biological replicates per treatment were utilized. Scanned microarray images were analyzed using the Expression Console™ and Transcriptome Analysis Console™ version 3.0 (Affymetrix). Analysis of gene expression level was conducted using One-Way Between-Subject ANOVA (unpaired) with p-value < 0.05.
Reverse-transcription real-time PCR
Quantitative analysis of gene expression was performed for a subset of genes using reverse-transcription real-time PCR (RT-qPCR) as described in Subramanian et al. (2017). Six genes – three upregulated and three downregulated across all treatments – were selected for the analysis. The internal standard was the 18S ribosomal DNA (rDNA) gene. RT-qPCRs were conducted using the same RNA as used for the microarray experiments. RNA was reverse-transcribed into cDNA using SuperScript® III First-Strand Synthesis system and oligo-dT primers (Invitrogen, Foster City, CA). Negative controls were generated by running the reactions without reverse-transcriptase. Gene sequences were retrieved from The Arabidopsis Information Resource (TAIR) database (www.arabidopsis.org/) and used to design gene-specific real-time primers using PrimerQuest (IDT, Coralville, IA) (Table S1). Real-time PCR quantification of cDNA was performed on a StepOnePlus™ Real-Time PCR System using SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA). CT (cycle threshold) values were computed by the StepOnePlus™ Software (version 2.1; Applied Biosystems).
MIAME compliance
This article is written in compliance with the Minimum Information About a Microarray Experiment (MIAME) guidelines (http://www.mged.org/miame). Microarray data have been submitted to the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo).
Results and discussion
Toxicity testing
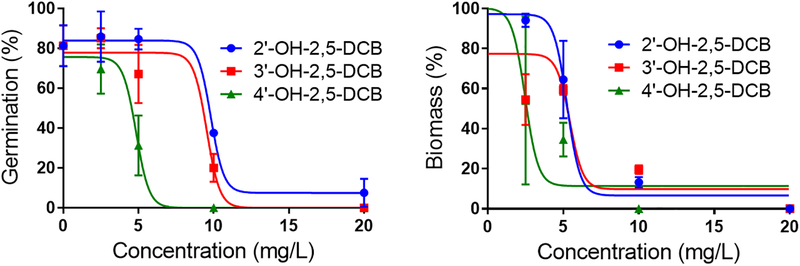
The toxicity of 2,5-DCB and its three OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, for A. thaliana was determined based on germination and growth tests conducted over 7 and 21 days, respectively. Based on both germination and growth tests, the three OH-derivatives exhibited a significant toxicity at the concentration of 5 mg L−1 and higher (Figure 1). On the other hand, exposure to the parent 2,5-DCB did not exhibit significant effects at concentration up to 100 mg L−1 (data not presented). In both germination and growth tests, 4’-OH-2,5-DCB exhibited a significantly higher toxicity than 2’-OH- and 3’-OH-2,5-DCB: inhibition concentration 50% (IC50) in germination tests = 4.8 mg L−1 (confidence interval-CI 95% = 4.2 – mg L−1) for 4’-OH-2,5-DCB vs. 9.8 and 9.5 mg L−1 (CI 95% = 9.5 – 10.1 mg L−1 and 8.0 – mg L−1) for 2’-OH- and 3’-OH-2,5-DCB, respectively. The data fitted generally well the toxicity model, with coefficients of determination (R2) ranging from 0.80 to 0.99.
Figure 1.
Dose-response curves of A. thaliana exposed to 2'-OH-2,5-DCB, 3'-OH-2,5-DCB, and 4'-OH-2,5-DCB as determined through germination tests (left panel) and growth tests (right panel). The solid lines were obtained by fitting to a 3-parameter sigmoidal inhibition model (Prism 6.0, Graphpad). The error bars represent standard deviations between biological replicates.
Consistently with our results, several studies have shown that PCBs exert low to moderate toxicity toward terrestrial plants, except when exposed to high concentrations (Weber and Mozek, 1979; Mahanty, 1986). For instance, exposure of soybean plants to Aroclor 1254 for 26 days resulted in approx. 11%, 27%, and 22% growth inhibition at concentration of 10, 100, and 1,000 mg L−1, respectively. In fescue plants, exposure to Aroclor 1254 for 42 days resulted in growth stimulation at low concentration (10 – 100 mg L−1) and 16% inhibition at high concentration (1,000 mg L−1) (Weber and Mozek, 1979). On the other hand, several studies have reported more severe effects of PCBs on plants at concentrations well below the maximum concentration (100 mg L−1) used in our study (Jin et al., 2011; Ahammed et al. 2013; Wang et al., 2017). For instance, exposure of Arabidopsis plants to increasing concentration of 2,2’,3,3’-tetrachlorobiphenyl resulted in growth stimulation low concentrations (< 10 mg L−1) and growth inhibition at higher levels (40 to 100 mg L−1) (Jin et al., 2011).
Although higher organisms are known to metabolize PCBs into OH-derivatives (Van Aken et al., 2010; Chroma et al., 2002), little information is available about the effects of OH-PCBs on plants. In a prior study, we tested the toxicity of 17 mono-hydroxylated PCBs and 11 parent compounds carrying one to four chlorine atoms toward A. thaliana (Subramanian et al., 2017). In this prior study, we observed none of the parent PCBs tested (except one congener) were significantly toxic at concentration up to 100 mg L−1, which is consistent with the results presented here. On the other hand, all OH-PCBs with one to three chlorine atoms exhibited a recordable toxicity at the concentration of 20 mg L−1 or lower (IC50 values ranging from 2.2 mg L−1 to 10.7 mg L−1).
Studies conducted in other organisms, including animals and bacteria, also reported that OH-PCBs can be more toxic than the parent PCBs. The higher toxicity of OH-PCBs could be explained partially by their higher solubility and bioavailability (Bhalla et al., 2016). On the other hand, OH-PCBs are known to be further oxidized in vivo into toxic products, such as quinones, and generate reactive oxygen species (ROS), which can damage DNA and form adducts with proteins, lipids, and nucleic acids (Grimm et al., 2015).
Gene expression microarrays
The transcriptional response of Arabidopsis plantlets exposed to 2,5-DCB and its three OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, was investigated using Affymetrix whole-genome expression microarrays. After filtering out low-quality and non-significant expression results – based on One-Way ANOVA (p < 0.05), 387 genes were shown to be differentially expressed by exposure to 2,5-DCB (as compared with non-exposed controls), including 252 (65.1%) upregulated genes (defined as genes showing an expression level at least two times higher in exposed plants than in untreated controls: i.e., fold change > 2.0) and 135 (34.9%) downregulated genes (similarly as defined genes showing an expression level at least two times lower in exposed plants than in untreated controls: i.e., fold change < 0.5). Exposure to the three OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, resulted in differential expression of 214, 162, and 172 genes, including 133 (62.1%), 46 (28.4%), and 72 (41.9%) upregulated genes, and 81 (37.9%), 116 (71.6%), and 100 (58.1%) downregulated genes, respectively. Thirty seven (37) genes were differentially expressed by exposure to all four compounds (representing 12.1 – 29.0% of the total differentially-expressed genes, depending on the congener). Fifty one (51) genes were differentially expressed by exposure to all three OH-derivatives (representing 23.8 – 31.5% of the total differentially-expressed genes). Hierarchical clustering showed good consistency between replicate microarray results associated with each treatment (Figure S1). It also revealed that exposure to the three OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, induced rather similar gene expression profiles as compared with the plants exposed to the parent 2,5-DCB and the control, non-exposed plants. This suggests that hydroxylation of 2,5-DCB resulted in distinct transcriptomic responses, as indicated by the toxicity testing.
In order to validate the microarray results, quantitative analysis of gene expression was performed using RT-qPCR on six selected genes, including three downregulated and three upregulated genes upon exposure to each compound. Because the purpose of the RT-qPCR was solely to confirm the microarrays results, these genes were not selected based on their biological significance. Expression levels obtained by microarrays were plotted against expression levels obtained by RT-qPCR (Figure 2), showing good correlations (Pearson’s correlation coefficients = 0.95, 1.00, 0.92, and 0.73 for exposure to 2,5-DCB, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, respectively).
Figure 2.
Comparison of relative gene expression levels in A. thaliana exposed to 2'-OH-2,5- DCB (upper left), 3'-OH-2,5-DCB (upper right), 4'-OH-2,5-DCB (lower left), and 2,5-DCB (lower right) using reverse-transcription real-time PCR (RT-qPCR) and microarrays (Arabidopsis Gene 1.0 ST Arrays, Affymetrix). Error bars represent the standard deviations of three biological replicates.
Functional categories of differentially expressed genes
The enrichment of transcripts in different Gene Ontology (GO) categories was interrogated using the Remote Term Enrichment tool with Bonferroni’s correction (http://amigo.geneontology.org/rte). To avoid redundancy, only the most specific categories (i.e., at the lowest hierarchical level) were considered for the discussion (Table 1). Genes upregulated in response to 2,5-DCB and its OH-derivatives showed strong enrichment in several Biological Processes related to response to abiotic stresses (e.g., high light intensity, salt, osmotic stress, water deprivation, cold, oxidative stress) and biotic stresses (e.g., nematodes, chitin, fungus), indicating the deleterious effects of the compounds for plants. Exposure to 2,5-DCB and its OH-derivatives induced stress-response genes not directly related to toxic chemicals. Different types of biotic and abiotic stresses may cause similar cellular damages and therefore induce similar plant responses. This may explain why PCBs activated genes involved in different types of stresses. Xenobiotic sensing by plant cells may occur directly by recognition of toxic molecules or indirectly by detection its deleterious effects (Singh et al., 2002; Ramel et al., 2012). For instance, toxic compounds can activate genes similar to the ones involved in response to oxidative stress and pathogens, which frequently cause accumulation of toxins inside the host cells (Ramel et al., 2012). Unlike the three OH-derivatives, exposure to 2,5-DCB resulted in enrichment of genes involved in regulation and hormone signaling, including jasmonic acid, abscisic acid, and ethylene (Table 1). Jasmonic acid, abscisic acid, and ethylene are plant hormones involved in many plant processes, including response to biotic and abiotic stresses (Singh et al., 2002; Cao et al., 2007; Ramel et al., 2012; Ramegowda and Senthil-Kumar, 2015). The induction of genes involved in jasmonic acid, abscisic acid, and ethylene signaling pathway by 2,5-DCB may in turn stimulate toxic response genes, which are capable to counteract the deleterious effects of the compound. On the contrary, the failure of the OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, to induce such signaling cascade may explain their much higher toxicity to the plants. Although limited information is available about the toxic mechanisms of PCBs on plants, several studies have shown that exposure to PCBs resulted in decrease of plant biomass associated with photosynthesis inhibition (e.g., reduction of chlorophyll content and CO2 assimilation), lipid peroxidation, and oxidative stress (e.g., generation of reactive oxygen species-ROS) (Ahammed et al., 2013; Wang et al., 2017).
Table 1:
Enrichment analysis of genes up- and downregulated upon exposure to 2,5-DCB and 4’-OH-2,5-DCB based on Biological Processes and Molecular Functions. Only gene ontology (GO) terms of special interest are shown. The full list of significantly enriched categories in presented in Supplemental Information (Table S2).
| GO biological process | Enrich. | P-value |
|---|---|---|
| 2'-OH-2,5-DCB | ||
| Cellular response to iron ion starvation | 67.41 | 3.20E-05 |
| Hyperosmotic salinity response | 11.83 | 9.48E-05 |
| Transition metal ion homeostasis | 10.97 | 5.87E-06 |
| Response to nematode | 9.23 | 2.81E-04 |
| Response to chitin | 8.17 | 9.80E-06 |
| Response to cold | 5.75 | 3.85E-08 |
| Response to water deprivation | 5.33 | 1.71E-06 |
| Response to wounding | 5.09 | 2.38E-04 |
| Response to oxidative stress | 3.93 | 3.93E-05 |
| Response to osmotic stress | 3.05 | 2.66E-04 |
| Defense response to other organism | 2.84 | 8.83E-05 |
| 3'-OH-2,5-DCB | ||
| Photosynthesis, light harvesting in photosystem I | 30.08 | 1.66E-05 |
| Cellular response to salt stress | 20.35 | 6.66E-05 |
| Response to red light | 15.72 | 3.75E-06 |
| Response to blue light | 13.48 | 8.60E-06 |
| Cellular response to light stimulus | 10.64 | 1.41E-06 |
| Response to cold | 4.61 | 8.25E-05 |
| 2,5-DCB | ||
| Protein autoubiquitination | 28.98 | 2.79E-06 |
| Response to chitin | 17.12 | 8.63E-26 |
| Photosynthesis, light harvesting in photosystem I | 16.38 | 2.88E-05 |
| Jasmonic acid biosynthetic process | 16.38 | 2.88E-05 |
| Response to high light intensity | 9.54 | 2.67E-07 |
| Response to blue light | 8.81 | 1.95E-06 |
| Cold acclimation | 8.19 | 5.37E-04 |
| Negative regulation of defense response | 7.24 | 8.98E-04 |
| Response to wounding | 7.11 | 3.81E-11 |
| Response to red light | 6.85 | 3.66E-04 |
| Response to water deprivation | 6.64 | 6.86E-15 |
| Ethylene-activated signaling pathway | 5.2 | 6.90E-06 |
| Response to reactive oxygen species | 5.08 | 2.01E-05 |
| Response to heat | 4.29 | 8.57E-05 |
| Immune system process | 3.53 | 4.10E-05 |
| Response to bacterium | 3.01 | 8.51E-05 |
| Response to auxin | 2.8 | 4.57E-04 |
| Response to fungus | 2.79 | 5.77E-05 |
| Response to salt stress | 2.63 | 1.87E-04 |
| Response to abscisic acid | 2.61 | 2.04E-04 |
| Oxidation-reduction process | 3.12 | 9.11E-07 |
| 4'-OH-2,5-DCB | ||
| Cellular response to iron ion starvation | > 100 | 4.41E-11 |
| Iron ion homeostasis | 27.54 | 1.64E-09 |
| Response to cold | 6.04 | 1.43E-07 |
| Response to water deprivation | 4.43 | 2.57E-04 |
| Response to light stimulus | 3.24 | 1.39E-04 |
| Response to abiotic stimulus | 2.98 | 3.70E-09 |
Comparison between 2’-OH-, 3’-OH-, 4’-OH-2,5-DCB, and 2,5-DCB
Exposure of Arabidopsis plants to the parent compound 2,5-DCB and the three OH-derivatives, 2’-OH-, 3’-OH-, and 4’-OH-2,5-DCB, resulted in overexpression of many genes involved in response to stress, including toxin catabolic processes (e.g., copper/zinc superoxide dismutase 2–AT2G28190), oxidative stress (e.g., superoxide dismutase-AT1G08830, peroxidase-AT1G49570), osmotic stress (e.g., low-temperature-responsive protein 78/desiccation-responsive protein 29A–AT5G52310, stress-induced protein KIN2–AT5G15970), defense response to organisms (e.g., defensin-like protein 4–AT2G02120), and transcription factors involved in abiotic stress response (e.g., dehydration-responsive element-binding protein 2A–AT5G05410). Overall, genes involved in response to stress accounted for 13.9 – 21.7% of the total upregulated genes and 7.4 – 13.6% of the total downregulated genes by exposure to 2,5-DCB and OH-DCBs. In addition, the parent 2,5-DCB – and not its OH-derivatives – resulted in upregulation of multiple genes involved in response to hormones, including ethylene-responsive elements (e.g., ethylene-responsive transcription factor ERF109–AT4G34410, ethylene responsive element binding factor 6–AT4G17490), genes responsive to jasmonic acid stimulus (e.g., ethylene-responsive transcription factor 13–AT2G44840, calcium-binding protein CML38–AT1G76650), and genes responsive to abscisic acid stimulus (e.g., E3 ubiquitin-protein ligase ATL31–AT5G27420, putative WRKY transcription factor 40–AT1G80840). Overall, genes involved in response to ethylene accounted for 21.8% of the upregulated genes by 2,5-DCB (vs. 1.5 – 4.3% of the upregulated genes by OH-DCBs) and 1.5% of the downregulated genes by 2,5-DCB (vs. 2.5 – 5.0% of the downregulated genes by OH-DCBs). Jasmonic acid, abscisic acid, and ethylene are plant hormones involved in response to many types of biotic and abiotic stresses (Singh et al., 2002; Ramel et al., 2012; Ramegowda and Senthil-Kumar, 2015).
Looking at the OH-derivatives of 2,5-DCB, we observed that exposure to the most toxic congeners, 4’-OH-2,5-DCB, resulted in expression of specific genes not significantly up- or down-regulated by the two other isomers, 2’-OH-2,5-DCB and 3’-OH-2,5-DCB: among the 40 genes the most overexpressed by exposure to 4’-OH-2,5-DCB, only five were only induced by this compound and not by the two other isomers; four of these genes are involved in response to iron deficiency: transcription factor bHLH100 (AT5G04150, fold change 7.36), transcription factor ORG2 (AT3G51560, fold change 6.29), transcription factor ORG3 (AT3G56980, fold change 5.55), and zinc ion binding protein (AT1G74770, fold change 2.54) (Sivitz et al., 2012; Kobayashi and Nishizawab, 2014). This observation is consistent with the enrichment analysis, showing that the two Biological Process categories with the highest enrichment values are cellular response to iron ion starvation (enrichment > 100) and iron ion homeostasis (enrichment 27.54). The second gene the most downregulated by exposure to 4’-OH-2,5-DCB (and not by the two other isomers) is a rubredoxin family protein (AT5G17170, fold change −4.28) involved in iron ion binding (Kobayashi and Nishizawab, 2014). The reduction of rubredoxin synthesis may constitute an attempt by the plant to limit iron uptake in biological molecules, which would further lower iron availability. Genes involved in iron deficiency and homeostasis were also differentially regulated in response to the 2’-OH- and 3’-OH-2,5-DCB, but to a lesser extent, possibly explaining their lower toxicity as comparted with 4’-OH-2,5-DCB.
Comparison of expression profiles of 2,5-DCB and OH-2,5-DCBs with other chemicals
The transcriptomic profiles of Arabidopsis exposed to 2,5-DCB, 2’-OH-, 3’-OH-, and 4’-OH-2,5- DCB were then searched against the Arabidopsis expression microarray database, Genevestigator (genevestigator.com/gv/). The analysis was restricted to expression profiles recorded with wild-type A. thaliana and the Perturbation Category, Chemicals. Searching expression profiles similar to the ones induced by the three OH-derivatives (based on the 25 most upregulated genes for each treatment) returned a suite of 13 chemicals, which were all among the 20 highest hits for each compounds (Table S3). (Only two of these 13 chemicals were among the 20 highest hits for 2,5-DCB.) For each OH-derivatives, the two highest hits – based on the similarity of transcription profiles – were two compounds (referred to as ‘7606596’ and ‘5929745’) described as ‘albino chemicals’ because they induce an albino phenotype in exposed plants. This is consistent with the observation that exposure to OH-derivatives resulted in enrichment of genes involved with response to high light intensity. Unfortunately, no more information can be found about compounds ‘7606596’ and ‘5929745’.
Three other compounds inducing expression profiles similar to the OH-derivatives are brassinazole, propiconazole, and uniconazole, which all are characterized by a triazole group and a chlorinated benzylic ring. The expression profile developing in response to 2’-OH-2,5-DCB is in addition similar to paclobutrazole, which is characterized by a similar structure (Figure 3). These four compounds, brassinazole, propiconazole, uniconazole, and paclobutrazole, are inhibitors of the brassinosteroid synthesis. Brassinosteroids are signaling molecules involved in many plant functions, such as extreme dwarfism, delayed senescence, male sterility, and constitutive photomorphogenesis in the dark (Mussig et al., 2002). Interestingly, it has been demonstrated that the structural elements responsible for the inhibitory activity include not only the triazole ring and chlorinated benzylic ring, but also an hydroxyl group – or, in the case of propiconazole, a dioxlane group (Hartwig et al., 2012) (Figure 3). None of these compounds were shown to induce an expression profile similar to the parent PCB, which also lacks the hydroxyl (or dioxlane) group (Table S3). Consistently with these observations, exposure to all three OH-derivatives resulted in strong downregulation of several genes responsive to brassinosteroid stimulus or involved in brassinosteroid synthesis: e.g., phosphate-responsive 1 family protein (AT4G08950), ethylene-responsive transcription factor ERF012 (AT1G21910) and ERF013 (AT1G77640), xyloglucan endotransglucosylase/hydrolase protein 22 (AT5G57560), 24-methylenesterol C-methyltransferase 3 (AT1G76090), probable pectinesterase/pectinesterase inhibitor 41 (AT4G02330) (Table 2). All these six genes were most strongly repressed in plants exposed to 4’-OH-2,5-DCB, which is also the most toxic of the OH-derivatives under study. On the contrary, none of these genes were downregulated in plants exposed to the parent compound. Most interestingly, brassinosteroids have been found to be involved in iron uptake and homeostasis (Brumbarov et al., 2014). Indeed, many genes involved in response to iron starvation were upregulated in plants exposed to the three OH-derivatives (e.g., copper/zinc superoxide dismutase 2–AT2G28190, transcription factor bHLH100–AT5G04150, bHLH transcription factor POPEYE–AT3G47640) (Table 2). Conversely, many genes involved in iron binding and homeostasis were downregulated in plants exposed to the three OH-derivatives (e.g., NEET group protein–AT5G51720, metal-nicotianamine transporter YSL3–AT5G53550, glutamyl-tRNA reductase 1–AT1G58290). On the other hand, only very few genes involved in iron metabolism were differentially expressed by exposure to the parent 2,5-DCB (Table 2). Besides iron deficiency, the inhibition of brassinosteroid synthesis is likely to have additional effects of plants. For instance, brassinosteroid deficiency is also known to repress genes involved in cell elongation and resistance to stress factors, such as response to cold (Mussig et al., 2002). Indeed, exposure to OH-2,5-DCBs downregulated expansins genes (e.g., expansin-like A1–AT3G45970, expansin-like A2–AT4G38400). Also, enrichment analysis has shown that exposure to the three OH-derivatives resulted in enrichment of genes involved in response to cold (Table 1). Besides, brassinosteroids have been shown to play a role in plant responses to a variety of biotic and abiotic stresses, including pathogens, drought, heavy metals, organic pollutants, and nanomaterials (Ahammed et al., 2013). Intriguingly, brassinosteroids have been shown to alleviate the specific toxicity of PCBs on plants (Ahammed et al., 2013; Wang et al., 2017). The inhibition of brassinosteroids by OH-derivatives of PCBs is therefore expected to increase to toxicity for the plants.
Figure 3.
Chemical structure of the brassinosteroid inhibitors, brassinazole, propiconazole, uniconazole, and paclobutrazole
Table 2:
Genes involved in brassinosteroid and iron response differentially expressed in response to 2'-OH-, 3'-OH-, 4'-OH-2,5-DCB, and 2,5-DCB.
| Tair # | Description | Biological function | Fold change | ||
|---|---|---|---|---|---|
| 2'-OH- | 3'-OH- | 4'-OH- | |||
| At2g28190 | Copper/zinc superoxide dismutase | Response to iron deficiency | 4.38 | 2.34 | |
| At5g04150 | bhlh100 | Response to iron deficiency | 4.17 | 5.44 | |
| At3g51560 | ORG2 | Response to iron deficiency | |||
| At3g56980 | ORG3 | Response to iron deficiency | 5.55 | ||
| At3g18290 | Putative E3 ligase BRUTUS | Response to iron deficiency | 2.30 | 2.70 | |
| At3g47640 | bhlh Transcription factor POPEYE | Response to iron deficiency | 2.09 | 2.15 | |
| At4g11280 | 1-Aminocyclopropane-1-carboxylate synthase | Response to iron | 2.02 | ||
| At1g08830 | Superoxide dismutase [Cu-Zn] | Response to iron | 2.34 | ||
| At1g74770 | Zinc ion binding protein | Response to iron deficiency | 2.54 | ||
| At3g14940 | Phosphoenolpyruvate carboxylase | Response to iron deficiency | 2.24 | ||
| At5g51060 | Respiratory burst oxidase homolog protein c | Iron binding/homeostasis | 2.23 | ||
| At4g13310 | Cytochrome p450 71A20 | Iron binding | 2.06 | ||
| At5g51720 | NEET group protein | Iron binding/homeostasis | −2.06 | −2.04 | −2.58 |
| At5g53550 | Metal-nicotianamine transporter YSL3 | Iron binding/homeostasis | −2.09 | ||
| At1g58290 | Glutamyl-trna reductase | Response to iron | −2.24 | −2.51 | −3.91 |
| At5g52570 | Beta-carotene hydroxylase | Iron binding/homeostasis | −2.57 | −3.17 | |
| At1g33720 | Cytochrome P450, family 76 | Iron binding/homeostasis | −2.82 | ||
| At1g44446 | Chlorophyllide a oxygenase | Iron binding/homeostasis | −2.03 | ||
| At1g02205 | Protein eceriferum | Iron binding/homeostasis | −2.07 | ||
| At2g34770 | Fatty acid hydroxylase | Iron binding/homeostasis | −2.27 | ||
| At5g23980 | Ferric reduction oxidase | Iron binding/homeostasis | −2.61 | ||
| At5g25130 | Cytochrome P450 71B12 | Iron binding/homeostasis | −2.03 | ||
| At3g48310 | Cytochrome P450 71A22 | Iron binding/homeostasis | −2.31 | ||
| At4g04770 | ATP binding cassette protein | Response to iron | −2.43 | ||
| At5g17170 | Rubredoxin family protein | Iron binding/homeostasis | −4.28 | ||
| At4g08950 | Phosphate-responsive 1 family protein | Response to brassinosteroid | −2.55 | −2.35 | −2.80 |
| At1g21910 | Ethylene-responsive transcription factor ERF012 | Response to brassinosteroid | −2.73 | 3.29 | −4.10 |
| At1g77640 | Ethylene-responsive transcription factor ERF013 | Response to brassinosteroid | −2.75 | −2.17 | −3.18 |
| At5g57560 | Xyloglucan endotransglucosylase/hydrolase | Response to brassinosteroid | −2.92 | −2.78 | |
| At1g76090 | 24-methylenesterol C-Methyltransferase | Brassinosteroid biosynthesis | −2.22 | ||
| At4g02330 | Probable pectinesterase/pectinesterase inhibitor | Response to brassinosteroid | −2.43 | ||
Other chemicals inducing expression profiles similar to the OH-derivatives include broad-spectrum herbicides and pesticides (e.g., glyphosate, imazapyr, rotenone, sulfometuron methyl), plant growth regulators (aminoethoxyvinylglycine–AVG, prohexadione, benzothiadiazole), inducers of systemic acquired resistance (dichloroisoicotinic acid–INA, MgCl2+glycerol-3-phosphate–G3P), and various phytotoxic compounds (e.g., syringolin, N-octyl-3-nitro-2,4,6-trihydroxybenzamide–PNO8, ibuprofen, dexamethasone, phenanthrene) (Table S3). This suggests that OH-derivatives of 2,5-DCB act on plants through various mechanisms in addition to inhibition of brassinosteroid synthesis.
As it was reported in a prior publication (Subramanian et al., 2017), we observed that exposure to 2,5-DCB resulted in an expression profile similar to plant safeners (e.g., 4-chloro-6-methyl-2-phenylpyrimidine–CMP, fenclorim, isoxadifen, menadione sodium bisulphite-MSB). Safeners are non-toxic chemicals used to protect crops against deleterious effects of herbicides and pesticides (Skipsey et al., 2011; Behringer et al., 2011) (Table S3). Safeners are thought to act through the induction of detoxification enzymes, known as xenobiotic response genes (XRGs), such as cytochrome P-450 monooxygenases, glucosyltransferases, glutathione ^-transferases, and ABC transporter proteins (DeRidder et al., 2002; Behringer et al., 2011), therefore further explaining the low toxicity of 2,5-DCB for plants (Subramanian et al., 2017). Other compounds inducing expression profiles similar to 2,5-DCB include a radio-protective agent (4-thiazolidinone/acetic acid), an inhibitor of the response to indole acetic acid (triiodobenzoic acid-TIBA), a flowering agent (furyl acrylate ester), and an antifungal antibiotic (trichostatin A) (Table S3).
The results presented here increase our understanding of the effects of PCBs and OH-PCBs on plants, which may have important implications for human health and the environment. It is noteworthy that the major findings of this study were obtained using resource of a microarray database, emphasizing the importance of standardizing transcriptomic data and developing searchable databases. However, the evidence presented in this article is entirely based on the analysis of transcriptomic results. Biochemical analyses are warranted to confirm the toxic mechanism of OH-PCBs through the inhibition of brassinosteroid synthesis. Further investigation is also needed to identify the xenobiotic receptors and transcription factors that are involved in the plant response to PCBs and OH-PCBs.
Supplementary Material
Acknowledgements
This work was funded by the Iowa Superfund Basic Research Program, National Institute of Environmental Health Sciences (NIEHS), Grant P42ES013661.
References
- Ahammed G, Ruan Y, Zhou J, Xia X, Shi K, Zhou Y, Yu J. 2013. Brassinosteroid alleviates polychlorinated biphenyls-induced oxidative stress by enhancing antioxidant enzymes activity in tomato. Chemosphere 90(11):2645–2653. [DOI] [PubMed] [Google Scholar]
- Anderson P, Hites R. 1996. OH radical reactions: The major removal pathway for polychlorinated biphenyls from the atmosphere. Environ Sci Technol 30(5):1756–1763. [Google Scholar]
- Awad A, Martinez A, Marek R, Hornbuckle K. 2016. Occurrence and distribution of two hydroxylated polychlorinated biphenyl congeners in Chicago air. Environ Sci Technol Lett 3(2):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer C, Bartsch K, Schaller A. 2011. Safeners recruit multiple signalling pathways for the orchestrated induction of the cellular xenobiotic detoxification machinery in Arabidopsis. Plant Cell Environ 34(11):1970–85. [DOI] [PubMed] [Google Scholar]
- Bhalla R, Tehrani R, Van Aken B. 2016. Toxicity of hydroxylated polychlorinated biphenyls (HO-PCBs) using the bioluminescent assay Microtox®. Ecotoxicology 25(7):1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbarova T, Bauer P, Ivanov R. 2015. Molecular mechanisms governing Arabidopsis iron uptake. Trends Plant Sci 20(2):124–33. [DOI] [PubMed] [Google Scholar]
- Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, Zhang JS. 2007. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143(2):707–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chroma L, Moeder M, Kucerova P, Macek T, Mackova M 2003. Plant enzymes in metabolism of polychlorinated biphenyls. Fresen Environ Bull 12:291–295. [Google Scholar]
- DeRidder B, Dixon D, Beussman D, Edwards R, Goldsbrough P. 2002. Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol 130(3): 1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm F, Hu D, Kania-Korwel I, Lehmler H, Ludewig G, Hornbuckle K, Duffel M, Bergman A, Robertson L. 2015. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45(3):245–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig T, Corvalan C, Best NB, Budka JS, Zhu JY, Choe S, Schulz B. 2012. Propiconazole is a specific and accessible brassinosteroid (BR) biosynthesis inhibitor for Arabidopsis and maize. PLoS One 7(5):e36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X-F, Shuai J-J, Peng R-H, Zhu B, Fu X-Y, Tian Y-S, Zhao W, Han H-J, Chen C, Xu J et al. 2011. Identification of candidate genes involved in responses of Arabidopsis to polychlorinated biphenyls based on microarray analysis. Plant Growth Regul 65(1): 127–135. [Google Scholar]
- Kawano M, Hasegawa J, Enomoto T, Onishi H, Nishio Y, Matsuda M, Wakimoto T. 2005. Hydroxylated polychlorinated biphenyls (OH-PCBs): Recent advances in wildlife contamination study. Environ Sci 12:315–324. [PubMed] [Google Scholar]
- Kobayashi T, Nishizawa NK. 2014. Iron sensors and signals in response to iron deficiency. Plant Sci 224:36–43. [DOI] [PubMed] [Google Scholar]
- Mahanty HK. 1986. Polychlorinated biphenyls: Accumulation and effects upon plants In: Waid JS, editor. PCBs and the Environment, Vol. 2 Florida (USA) CRC Press; p. 1–8. [Google Scholar]
- Marek R, Martinez A, Hornbuckle K. 2013. Discovery of Hydroxylated Polychlorinated Biphenyls (OH-PCBs) in Sediment from a Lake Michigan Waterway and Original Commercial Aroclors. Environ Sci Technol 47(15):8204–8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano M, Gutleb A, Murk A. 2013. Persistent Toxic Burdens of Halogenated Phenolic Compounds in Humans and Wildlife. Environ Sci Technol 47(12):6071–6081. [DOI] [PubMed] [Google Scholar]
- Mussig C, Fischer S, Altmann T. 2002. Brassinosteroid-regulated gene expression. Plant Physiol 129:1241–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramegowda V, Senthil-Kumar M. 2015. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J Plant Physiol 176:47–54. [DOI] [PubMed] [Google Scholar]
- Ramel F, Sulmon C, Serra A, Gouesbet G, Couee I. 2012. Xenobiotic sensing and signalling in higher plants. J Exp Bot 63(11):3999–4014. [DOI] [PubMed] [Google Scholar]
- Sanderman H 1994. Higher plant metabolism of xenobiotics: The “green liver” concept. Pharmacogenetics 4:225–241. [DOI] [PubMed] [Google Scholar]
- Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH. 1995. Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29(7):318A–23A. [DOI] [PubMed] [Google Scholar]
- Singh K, Foley R, Onate-Sanchez L. 2002. Transcription factors in plant defense and stress responses. Cur Op Plant Biol 5(5):430–436. [DOI] [PubMed] [Google Scholar]
- Sivitz AB, Hermand V, Curie C, Vert G. 2012. Arabidopsis bHLH100 and bHLH101 control iron homeostasis via a FIT-independent pathway. PLoS One 7(9):e44843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipsey M, Knight K, Brazier-Hicks M, Dixon D, Steel P, Edwards R. 2011. Xenobiotic responsiveness of Arabidopsis thaliana to a chemical series derived from a herbicide safener. J Biol Chem 286(37):32268–32276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Schnoor JL, Van Aken B. 2017. Effects of polychlorinated biphenyls (PCBs) and their hydroxylated metabolites (OH-PCBs) on Arabidopsis thaliana. Environ Sci Technol 51(12):7263–7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehrani R, Van Aken B. 2014. Hydroxylated polychlorinated biphenyls in the environment: sources, fate, and toxicities. Environ Sci Pollu Res 21(10):6334–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Aken B, Correa P, Schnoor J. 2010. Phytoremediation of Polychlorinated Biphenyls: New Trends and Promises. Environ Sci Technol 44(8):2767–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Teng Y, Zhang N, Christie P, Li Z, Luo Y, Wang J. 2017. Rhizobial symbiosis alleviates polychlorinated biphenyls-induced systematic oxidative stress via brassinosteroids signaling in alfalfa. Sci Total Environ 592:68–77. [DOI] [PubMed] [Google Scholar]
- Weber JB, Mrozek E. 1979. Polychlorinated biphenyls: Phytotoxicity, absorption and translocation by plants, and inactivation by activated carbon. Bull Environ Contam Toxicol 23(3):412–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.