Abstract
Background and Objectives
While tyrosine kinase inhibitors (TKIs) have transformed CP-CML management, limited data exist on their use in clinical practice.
Methods
SIMPLICITY (NCT01244750) is an observational study in CP-CML patients, exploring first-line (1L) TKI use and management patterns in the US and Europe. Over half of the patients recruited in Europe are from Italy (n=266). This is an analysis of the Italian cohort and a comparison with the rest of the European SIMPLICITY population. Baseline demographic, factors influencing the choice of first-line TKI, response monitoring patterns and predictors of monitoring, and treatment interruptions, discontinuations and switching by index TKIs are presented for the Italian cohort in the first year of treatment and compared with that for the overall European SIMPLICITY cohort.
Results
Italian patients received 1L imatinib (IM; retrospective [(n=31]; prospective [n=106]), dasatinib (DAS; n=56) or nilotinib (NIL; n=73). Documented cytogenetic response monitoring by 12 months was lower than expected, but almost all patients had documented molecular response monitoring. Fewer patients discontinued first-line TKI by 12 months in Italy compared with the rest of the European SIMPLICITY population (p=0.003). Of those with ≥12 months follow-up since the start of 1L TKI, only 7.1% (n=19) of Italian patients switched to a second-line TKI, a third less than in the rest of the European SIMPLICITY population. Of interest, intolerance as opposed to resistance, was the main reason for switching.
Conclusions
This analysis provides valuable insights into management and treatment patterns in Italian patients with CML within routine clinical practice.
Keywords: SIMPLICITY, Chronic-Phase Chronic Myeloid Leukaemia, Response Monitoring, TKI switching patterns, Italy
Introduction
Over the last two decades, tyrosine kinase inhibitors (TKIs) have transformed the management of chronic-phase chronic myeloid leukaemia (C-P CML) from a terminal disease to a chronic illness. 1, 2 Survival rates in patients with newly-diagnosed C-P CML are thus approximating to rates in age-adjusted general populations. 3–5 Imatinib (Gleevec®/Glivec®, Novartis), dasatinib (Sprycel®, Bristol-Myers Squibb) and nilotinib (Tasigna®, Novartis) are recommended as first-line TKI therapy for C-P CML. 6–8 Once initiated, careful monitoring of cytogenetic response (CyR) and molecular response (MR), as well as adjustments in therapy, using time-based ‘milestone’ testing, is necessary to ensure optimal outcomes. 9 While the efficacy of TKIs in the management of CML has been demonstrated, some patients will either experience intolerance, achieve a suboptimal response, or fail treatment. In such patients, TKI treatment may be adjusted by dose modification, treatment interruption, or discontinuation, followed by switching to the next most appropriate TKI. 7, 10
European LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN) have published evidence-based recommendations for the management of CML. Of particular importance for the haematological community is to determine how closely these recommendations are followed, to identify any influential factors that may be involved, and to understand the impact that compliance with practice recommendations has on patient outcomes. Insights on the rationale for TKI treatment patterns in routine clinical practice may also better inform how treatment decisions are made.
SIMPLICITY (NCT01244750) is an ongoing observational study of patients with C-P CML seen in routine clinical practice receiving first-line treatment with imatinib, dasatinib or nilotinib. The primary objective of SIMPLICITY is to understand TKI use and management in clinical practice. Information derived from the whole SIMPLICITY population has shown that monitoring practices are not entirely in accordance with the published recommendations of ELN and NCCN. Patients may not be monitored by CyR or MR as frequently as recommended. 11 Almost a quarter of all patients who were followed for at least 12 months had discontinued or switched first-line TKI therapy during the first 12 months, and intolerance or resistance was the most common primary reason for discontinuation and switching of first-line TKI. 12
In addition to data reported for SIMPLICITY, there are several other studies of patients with CML treated within routine clinical practice; 13–25 however, most are of patients treated with imatinib only, and of patients who are elderly with severe comorbidities. While these studies support the use of imatinib in an older population, the results align with those from the whole SIMPLICITY population, where treatment and monitoring practices are not entirely in accordance with guidelines. Importantly, studies observing response monitoring and TKI treatment patterns in patients with CML treated in Europe are limited. The need to follow these patterns is crucial to identify any discord between guidelines and clinical practice and to understand the reasons behind these discordances fully so that the management of CML in the routine clinical practice setting can be improved.
Here we report SIMPLICITY data for the first year of treatment of the Italian population (data cut: September 06, 2016). SIMPLICITY includes 241 sites (Europe, n=91; US; n=150). Of the 91 European sites included in SIMPLICITY, Italian sites make up almost a third of these (29/91). For patients with C-P CML, the first year of treatment – and how they respond to treatment during it – is of particular relevance. Treatment response and tolerance are likely to influence adherence, which ultimately has an impact on long-term clinical outcomes. 6, 7 Here, we report baseline demographic and clinical characteristics, factors influencing the choice of first-line TKI, response monitoring patterns (CyR and MR), and predictors of monitoring, within the Italian population. We also report on patterns of treatment interruptions, discontinuations and switching, stratified by index TKI, including the reasons for discontinuation and switching observed in these patients. These findings are compared with those for the rest of the SIMPLICITY European population excluding Italian patients. To our knowledge, this article is the first to report on management patterns, and TKI use in patients with C-P CML treated in an observational setting in Italy.
Material and Methods
Study design and patient enrolment
The design of SIMPLICITY has been described previously. 11 It includes three prospective cohorts of patients newly diagnosed with CP-CML, ≥18 years of age at the time of diagnosis, receiving first-line therapy with imatinib, dasatinib or nilotinib on or after October 01, 2010, and a retrospective imatinib cohort (January 02, 2008–September 30, 2010). Study sites include academic and community practices in Italy. Community practices are defined as small-size practices run by an independent physician, or group of physicians, who offer patient care on a local or countywide basis. Academic centres are defined as large-size, hospital-based clinics (includes both public and private practice), cancer centre or universities), including centres of excellence, offering care on a regional or national basis. Patients involved in ongoing interventional CML clinical trials were excluded. The study protocol was reviewed and approved by the relevant institutional review boards, and patient consent was obtained. Data were collected using an electronic case report form (eCRF).
Demographic data collection
Baseline demographics include data on patient comorbidities derived from a defined checklist of 15 system organ classes, including cardiovascular (CV), respiratory, gastrointestinal, and endocrine/metabolic disorders. The total number of baseline comorbidities is defined by a total count of body systems/organ classes affected by comorbid conditions.
Physicians’ selection of first-line TKI
Physicians were asked to record the primary reason for their choice of first-line TKI (namely: familiarity with TKI, cost efficiency, comorbid conditions, effectiveness, tolerability, dosing schedule or other).
Response monitoring
Testing for CyR is based either on chromosome banding analysis (CBA) or fluorescence in situ hybridisation (FISH). CyR monitoring is categorised according to whether analysis was done with a date present or not done. CyR monitoring that was done with a date present was further classified into results available (excludes data with the reported number of evaluated nuclei or number of examined metaphases but missing FISH/bone marrow %Ph+ cells) or not (may include data with known number of evaluated nuclei or number of examined metaphases). CyR monitoring may concern FISH or bone marrow data with missing testing dates and may include patients who were not tested due to progression. Quantitative polymerase chain reaction (qPCR) was used for MR and was recorded. The vast majority (94%) of patients were monitored based on the International Scale (IS). MR monitoring is categorised according to whether analysis was done with a date present (includes tests with recorded dates that are available on the IS, available not on IS or unavailable) or not done (no time reported). Patients with at least 3, 6, and 12 months of follow-up, since initiation of first-line TKI, respectively, underwent to testing for CyR or MR with a frequency, respectively, of 3, 6, and 12 months and to assessments performed between ≥30 days from baseline and each respective time-point; the reporting of ‘any test done’ includes MR or CyR assessments during the specified timeframe. CyR and MR monitoring were analysed for the selected population, for which there was a follow-up of ≥12 months since initiation of index TKI, by year of TKI initiation.
Treatment patterns
TKI treatment changes of the first year since initiating first-line TKI, are summarised and include treatment interruptions or first-line discontinuations, duration of treatment interruptions and the primary reason for discontinuation of first-line TKI within the first year. ‘Treatment interruption’ was defined as a gap in treatment of >1 day before restarting the same TKI. ‘Treatment discontinuation’ was defined as cessation of TKI treatment that did not qualify as a treatment interruption. Discontinuations just before data download (within 60 days) are considered treatment interruptions. TKI switch is defined as a discontinuation of a first-line TKI within one year, followed by initiation of a second-line TKI. Patients for whom a date of first-line TKI discontinuation is missing but who switched to a second-line TKI within one year of initiating first-line TKI are counted as discontinuations. TKI treatment changes for patients who switched to a second-line TKI within one year are summarised, including information on the second-line TKI, days from first- to second-line TKI, and the primary reason for switching. Intolerances leading to discontinuation are presented for those patients who switched during the first year since initiating first-line TKI.
Events concurrent with TKI treatment interruptions were defined as events that occurred between the TKI start date (and two weeks before the date of treatment interruptions) and the end of the treatment interruption window, signified by the start of the same TKI. The same event was summarised once per patient and per unique event, date if concurrent with multiple TKI treatment interruptions.
Statistical analyses
Descriptive statistics were presented and P values calculated using a chi-square test for categorical comparisons and Fisher’s exact test, in the case of low cell counts; no corrections were made for multiple comparisons. Saturated multivariable logistic regression models were performed separately for Italy and for all other European countries included in SIMPLICITY, to assess predictors of whether or not CyR or MR monitoring was done among patients with at least 12 months of follow-up since initiating a first-line TKI. The saturated model included the following predictors: age at diagnosis, sex, practice type, first-line TKI, Eastern Cooperative Oncology Group (ECOG) performance status, and an indicator of whether patients were still on their first-line TKI at the end of 12 months’ follow-up.
Saturated multivariable logistic regression models were also performed to assess predictors of discontinuation and switching. The following predictors were included in the models: age at diagnosis, sex, practice type, first-line TKI, total comorbidity counts, ECOG performance status, and Sokal category.
Results
Study population
1,242 patients were enrolled prospectively into the study between October 01, 2010 and September 06, 2016 (data download) and 252 patients retrospectively. Of the 482 patients enrolled at the European sites, 266 (55%) were recruited, at 29 sites across Italy (Supplemental Figure S1). Most of these patients (n=249; 94%) were enrolled in the study through academic centres. Patients received first-line imatinib (retrospective [n=31]; median follow-up [interquartile range; IQR] 60.2 [59.4–61.1] months), prospective imatinib (n=106; median follow-up [IQR] 54.0 [48.0–59.5] months), dasatinib (n=56; median follow-up [IQR] 39.4 [31.1–46.4] months) or nilotinib (n=73; median follow-up [IQR] 38.5 [27.0–50.9] months).
Patient demographics are shown in Table 1. The overall median age (IQR; min., max.) of Italian patients at the time of initiation of first-line treatment was 57.1 (44.9–69.6; 17.7, 90.0) years. Italian patients in the dasatinib cohort were older than those in the imatinib (retrospective and prospective) and nilotinib cohorts (P=0.01). Demographics and clinical characteristics of the Italian population were similar to those reported for the rest of the European SIMPLICITY population, except the fact that Italian patients had fewer missing Sokal (20% vs. 43%, respectively) and Hasford (28% vs. 43%, respectively) data.
Table 1.
Patient demographics according to first-line TKI therapy and all patients.
| Italy | Europe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First-line TKI | First-line TKI | |||||||||
| Imatinib (Retro) | Imatinib (Pro) | Dasatinib | Nilotinib | All patients | Imatinib (Retro) | Imatinib (Pro) | Dasatinib | Nilotinib | All patients | |
| Cohort, N | 31 | 106 | 56 | 73 | 266 | 35 | 65 | 51 | 65 | 216 |
| Sex, n (%) | ||||||||||
| Male | 18 (58.1) | 64 (60.4) | 25 (44.6) | 37 (50.7) | 144 (54.1) | 19 (54.3) | 39 (60.0) | 32 (62.7) | 34 (52.3) | 124 (57.4) |
| Median (IQR) age at diagnosis (years) | ||||||||||
| 49.9 (40.6–69.2) | 61.4 (46.1–70.6) | 62.3 (47.3–73.1) | 53.1 (40.6–63.5) | 57.1 (44.8–69.5) | 46.7 (39.2–65.5) | 61.8 (49.2–74.7) | 57.9 (44.7–73.8) | 53.9 (47.2–65.6) | 57.8 (44.8–69.4) | |
| Median (IQR) age at first-line TKI (years) | ||||||||||
| 50.0 (40.7–69.3) | 61.4 (46.2–70.6) | 62.4 (47.4–73.1) | 53.1 (40.6–63.5) | 57.1 (44.9–69.6) | 49.6 (39.2–65.6) | 61.9 (49.2–74.8) | 57.9 (44.8–73.8) | 53.9 (47.3–65.6) | 57.8 (44.8–69.8) | |
| Age at first-line TKI (years), n (%) | ||||||||||
| <50 | 16 (51.6) | 32 (30.2) | 17 (30.4) | 31 (42.5) | 96 (36.1) | 20 (57.1) | 18 (27.7) | 18 (35.3) | 22 (33.8) | 78 (36.1) |
| 50–64 | 7 (22.6) | 30 (28.3) | 13 (23.2) | 26 (35.6) | 76 (28.6) | 6 (17.1) | 18 (27.7) | 15 (29.4) | 26 (40.0) | 65 (30.1) |
| ≥65 | 8 (25.8) | 44 (41.5) | 26 (46.4) | 16 (21.9) | 94 (35.3) | 9 (25.7) | 29 (44.6) | 18 (35.3) | 17 (26.2) | 73 (33.8) |
| Race/ethnicity, n (%) | ||||||||||
| White non-Hispanic | 23 (74.2) | 89 (84.0) | 35 (62.5) | 50 (68.5) | 197 (74.1) | 21 (60.0) | 48 (73.8) | 33 (64.7) | 41 (63.1) | 143 (66.2) |
| Other/unknown | 8 (25.8) | 17 (16.0) | 21 (37.5) | 23 (31.5) | 69 (25.9) | 14 (40.0) | 17 (26.2) | 18 (35.3) | 24 (36.9) | 73 (33.8) |
| Baseline comorbidities | 21 (67.7) | 75 (70.8) | 43 (76.8) | 38 (52.1) | 177 (66.5)* | 21 (60.0) | 53 (81.5) | 34 (66.7) | 51 (78.4) | 159 (73.6) |
| Median no. comorbidities (IQR) | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 1.0 (1.0–3.0) | 1.0 (0.0–2.0) | 1.0 (0.0–3.0) | 1.0 (0.0–2.0) | 2.0 (1.0–4.0) | 1.0 (0.0–3.0) | 1.0 (1.0–3.0) | 1.0 (0.0–3.0) |
| ECOG performance status,† n (%) | ||||||||||
| 0 – fully active | 14 (77.8) | 33 (54.1) | 19 (59.4) | 38 (69.1) | 104 (62.7) | 14 (53.8) | 20 (45.5) | 21 (58.3) | 31 (66.0) | 86 (56.2) |
| 1 – restricted strenuous activity | 2 (11.1) | 16 (26.2) | 8 (25.0) | 10 (18.2) | 36 (21.7) | 10 (38.5) | 14 (31.8) | 9 (25.0) | 12 (25.5) | 45 (29.4) |
| 2 – ambulatory and capable of all self-care, no work | 0 (0.0) | 0 (0.0) | 2 (6.3) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 3 (6.8) | 2 (5.6) | 0 (0.0) | 5 (3.3) |
| 4 – completely disabled | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (1.8) | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not assessed | 2 | 11 | 3 | 6 | 22 | 2 | 7 | 4 | 4 | 17 |
| Cohort, N | 31 (11.1) | 106 (18.0) | 56 (9.4) | 73 (10.9) | 266 (13.3) | 35 (7.7) | 65 (15.9) | 51 (11.1) | 65 (8.5) | 216 (11.1) |
| Sokal category at diagnosis‡ | ||||||||||
| Low (<0.8) | 13 (41.9) | 24 (22.6) | 8 (14.3) | 20 (27.4) | 65 (24.4) | 5 (14.3) | 14 (21.5) | 9 (17.6) | 16 (24.6) | 44 (20.4) |
| Intermediate (0.8–1.2) | 11 (35.5) | 36 (34.0) | 20 (35.7) | 29 (39.7) | 96 (36.1) | 6 (17.1) | 20 (30.8) | 8 (15.7) | 8 (12.3) | 42 (19.4) |
| High (>1.2) | 3 (9.7) | 14 (13.2) | 16 (28.6) | 19 (26.0) | 52 (19.5) | 6 (17.1) | 7 (10.8) | 13 (25.5) | 11 (16.9) | 37 (17.1) |
| Missing | 4 (12.9) | 32 (30.2) | 12 (21.4) | 5 (6.8) | 53 (19.9) | 18 (51.4) | 24 (36.9) | 21 (41.2) | 30 (46.2) | 93 (43.1) |
| Hasford score at diagnosis¶ | ||||||||||
| Low-risk, n (%) | 10 (32.3) | 30 (28.3) | 14 (25.0) | 30 (41.1) | 84 (31.6) | 8 (22.9) | 25 (38.5) | 21 (41.2) | 21 (32.3) | 75 (34.7) |
| Intermediate-risk, n (%) | 4 (12.9) | 33 (31.1) | 23 (41.1) | 30 (41.1) | 90 (33.8) | 5 (14.3) | 15 (23.1) | 9 (17.6) | 9 (13.8) | 38 (17.6) |
| High-risk, n (%) | 1 (3.2) | 4 (3.8) | 6 (10.7) | 6 (8.2) | 17 (6.4) | 3 (8.6) | 2 (3.1) | 3 (5.9) | 2 (3.1) | 10 (4.6) |
| Missing, n (%) | 16 (51.6) | 39 (36.8) | 13 (23.2) | 7 (9.6) | 75 (28.2) | 19 (54.3) | 23 (35.4) | 18 (35.3) | 33 (50.8) | 93 (43.1) |
| Practice type, n (%) | ||||||||||
| Academic centre | 19 (61.3) | 101 (95.3) | 56 (100.0) | 73 (100.0) | 249 (93.6) | 14 (40.0) | 23 (35.4) | 25 (49.0) | 19 (29.2) | 81 (37.5) |
| Private / community practices | 12 (38.7) | 5 (4.7) | 0 (0.0) | 0 (0.0) | 17 (6.4) | 21 (60.0) | 42 (64.6) | 26 (51.0) | 46 (70.8) | 135 (62.5) |
Percentages are calculated using the total number of patients for whom data on ECOG performance status are available as the denominator. ECOG performance status is defined as: 0, fully active; 1, restricted strenuous activity; 2, ambulatory and capable of all self-care, no work; 3, capable of only limited self-care; 4, completely disabled.
Sokal score categories; low-risk: Sokal score <0.8; intermediate-risk: Sokal score 0.8–1.2; high-risk: >1.2.
Hasford score categories; low-risk: Hasford score ≤780); intermediate-risk: Hasford score >780–≤1480; high-risk: Hasford score >1480.
ECOG: Eastern Cooperative Oncology Group; IQR: interquartile range; Pro: prospective; Retro: retrospective; TKI: tyrosine kinase inhibitor.
Of the total patients, 41% (n=110) had CV comorbidities (imatinib retrospective: 36% [n=11]; imatinib prospective: 46% [n=49]; dasatinib: 48% [n=27]; nilotinib: 32% [n=23]). These results are similar to those observed in Europe with some between-TKI variations (overall: 44% [n=94]; imatinib retrospective: 34% [n=12]; imatinib prospective: 55% [n=36]; dasatinib: 35% [n=18]; nilotinib: 43% [n=28]).
Physicians’ selection of first-line TKI
The primary reason cited by the treating physician for selecting the first-line TKI was perceived ‘effectiveness’ in both Italy and the rest of the European SIMPLICITY population (35% and 46%, respectively). Other reasons that were primary drivers for treatment choice in Italy and the rest of the European SIMPLICITY population included familiarity with TKI (15% and 13%, respectively), cost efficiency (19% and 14%, respectively) and the presence of comorbidities (18% and 10%, respectively).
Response monitoring patterns
Among patients followed for at least 12 months, the median (IQR) time from initiation of first-line TKI to the end of follow-up was 50.5 (36.1–59.1) months and was comparable with the other European countries (47.2 [34.7–58.2] months). All Italian patients (100%), and 97% of the rest of the European SIMPLICITY population had documentation of monitoring for either CyR or MR by 12 months.
CyR monitoring patterns
The proportion of patients with documentation of CyR monitoring increased, as expected, with longer patient follow-up (Table 2). By 3 months, the percentage of patients who had documentation of CyR was low in both Italy and the rest of the European SIMPLICITY population (25% and 16%, respectively By 12 months, a greater proportion of Italian patients had documentation of CyR compared with the European populations (80% vs. 53%; P<0.001) and the proportion of patients with ‘not done/recorded’ status decreased for both populations. Of those patients with documentation of CyR, similar proportions were classified with ‘results available’ in the Italian and European populations (95% and 89%).
Table 2.
The number and percentage of patients followed for a minimum of 12 months tested for CyR (FISH, BM, or both) or MR (including IS and non-IS). Includes assessments performed after index TKI start date, between 30 days and 3, 6 and 12 months, respectively.
| Monitoring patterns | ||||||
|---|---|---|---|---|---|---|
| During first 3 months of first-line TKI therapy | During first 6 months of first-line TKI therapy | During first 12 months of first-line TKI therapy | ||||
| Italy (n=266) | Europe (n=216) | Italy (n=266) | Europe (n=216) | Italy (n=266) | Europe (n=216) | |
| CyR monitoring Patterns | ||||||
| Done, date present, n (%) | 67 (25.2) | 35 (16.2) | 172 (64.7) | 81 (37.5) | 213 (80.1) | 114 (52.8) |
| Done/recorded with results available* | 64 (95.5) | 29 (82.9) | 160 (93.0) | 72 (88.9) | 202 (94.8) | 101 (88.6) |
| Done/recorded with no results available | 3 (4.5) | 6 (17.1) | 12 (7.0) | 9 (11.1) | 11 (5.2) | 13 (11.4) |
| Not done/recorded, n (%) | 199 (74.8) | 181 (83.8) | 94 (35.3) | 135 (62.5) | 53 (19.9) | 102 (47.2) |
| MR monitoring patterns | ||||||
| Done, date present, n (%) | 89 (33.5) | 73 (33.8) | 216 (81.2) | 185 (85.6) | 263 (98.9) | 206 (95.4) |
| Done/recorded with results on IS, n (%) | 81 (91.0) | 53 (72.6) | 197 (91.2) | 134 (72.4) | 247 (93.9) | 156 (75.7) |
| Done/recorded with results not on IS†, n (%) | 4 (4.5) | 20 (27.4) | 8 (3.7) | 49 (26.5) | 10 (3.8) | 48 (23.3) |
| Done/not recorded, n (%) | 4 (4.5) | 0 (0.0) | 11 (5.1) | 2 (1.1) | 6 (2.3) | 2 (1.0) |
| Not done/recorded, n (%) | 177 (66.5) | 143 (66.2) | 50 (18.8) | 31 (14.4) | 3 (1.1) | 10 (4.6) |
The denominator is the total number of patients with a CyR test done with date present.
The proportion of MR tests not on the IS includes ‘no’ and ‘unknown’.
BM: bone marrow; CyR: cytogenetic response; FISH: fluorescence in situ hybridization; IS: international scale; MR: molecular response; TKI: tyrosine kinase inhibitor.
MR monitoring patterns
The proportion of patients with documentation of MR monitoring increased, as expected, with longer patient follow-up (Table 2). By three months, the proportion of patients who had documentation of MR was low in both Italy and Europe (34% for both). By 12 months, most patients in the Italian and European populations had MR monitoring (99% and 95%, respectively) and the proportion of patients with ‘not done/recorded’ status decreased for both populations. Among patients tested for MR by 12 months, a greater proportion of Italian patients had MR assessments on the IS, compared with those in the rest of Europe (94% vs 76%; P<0.001). This was most likely due to haematological centres in Italy having better access to referral labs through the LabNet network than other centres in Europe.
CyR and MR monitoring stratified by year of first-line TKI initiation in SIMPLICITY
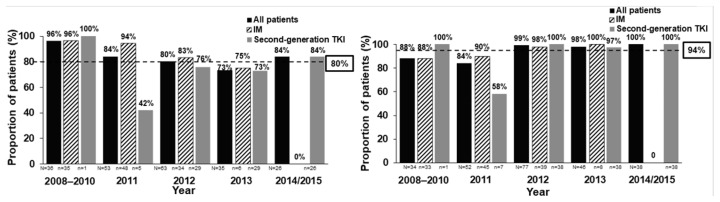
Figure 1 shows the proportion of Italian patients from SIMPLICITY with documented response monitoring throughout the study. Documentation of CyR monitoring decreased somewhat, while that for MR monitoring on the IS remained steady overall (90–100%) between 2008 and 2015, except in 2011, when the rate was lower.
Figure 1.
The proportion (%) of Italian patients from SIMPLICITY with documented response monitoring. (A) CyR monitoring for the overall population, and for those patients receiving IM and second-generation TKIs, over the years of first-line TKI initiation. Both FISH and BM cytogenetic tests were included as long as a date was documented. Patients had to be followed for ≥12 months. Includes assessments performed after index TKI start date, between 30 days and 12 months later. (B) MR monitoring patterns during the first 12 months of treatment according to the year of first-line TKI initiation – result on IS. Dashed line corresponds to the proportion of patients with CyR or MR monitoring during the first 12 months across the entire study period. N indicates the number of patients per cohort. BM: bone marrow; CyR: cytogenetic response; FISH: fluorescence in situ hybridisation; IM: imatinib; IS: international scale; MR: molecular response TKI: tyrosine kinase inhibitor.
Predictors of monitoring
Logistic regression analysis could not be performed for the Italian population because all patients in the cohort had documentation of monitoring for either CyR or MR by 12 months. In the rest of the European SIMPLICITY population, the model showed that there were no statistically significant predictors of monitoring.
Treatment Interruptions
Differences in treatment interruptions were observed between the Italian and the rest of the SIMPLICITY European population, as well as between first-line TKIs. Of the patients in Italy who had ≥12 months of follow-up since initiating first-line TKI, 16.2% (n=43) had a treatment interruption within 1 year of initiating first-line TKI, compared with a slightly lower proportion (11.1%; n=24) in the rest of the SIMPLICITY European population. For both Italian and the rest of the SIMPLICITY European population, the proportion of patients interrupting first-line TKI treatment was greatest in the imatinib prospective cohort (22.6% [n=24] and 13.8% [n=9], respectively) vs. other cohorts (imatinib retrospective: 16.1% [n=5] and 8.6% [n=3], respectively; dasatinib: 12.5% [n=7] and 9.8% [n=5]; nilotinib: 9.6% [n=7] and 10.8% [n=7]).
For patients in Italy, the median duration of treatment interruption (IQR) was 24.0 (14.0–118.0) days and was longer in comparison with the rest of the SIMPLICITY European population (14.0 [10.0–36.5] days). Patients in Italy receiving first-line imatinib (prospective) had the shortest median duration of treatment interruption (16.5 [12.0–52.5] days), whilst those receiving first-line dasatinib had the longest median duration of treatment interruption (124.0 [28.0–209.0] days); the results were different in comparison with the results for the rest of the SIMPLICITY European population (imatinib prospective: 12.0 [10.0–31.0] days; dasatinib: 12.0 [9.0–14.0] days). Similarly, there were disparities between Italy and the rest of the SIMPLICITY European population in regard to the median duration of treatment interruption for imatinib (retrospective; 18.0 [7.0–32.0] vs. 50.0 [21.0–270.0] days, respectively) and nilotinib (65.0 [20.0–152.0] vs. 20.0 [13.0–30.0] days, respectively) cohorts.
A total of 91 events were recorded as concurrent with TKI treatment interruption in the first year of treatment in Italy, the most common of which were thrombocytopenia (16 events) and neutropenia (11 events). In the rest of the SIMPLICITY European population, more than half the number of events reported in Italian patients (41 events) were recorded as concurrent with TKI treatment interruption, the most common being thrombocytopenia (6 events).
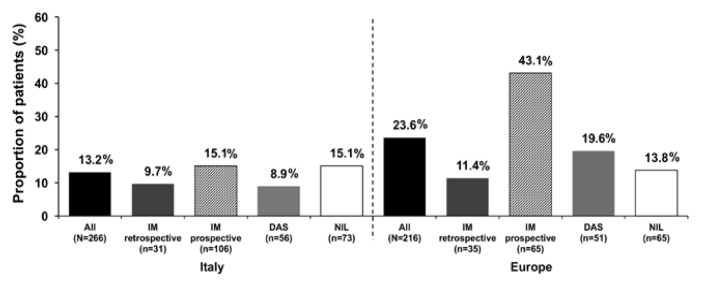
Treatment discontinuations
Treatment discontinuations are presented in Figure 2. A smaller proportion of patients in Italy discontinued their first-line TKI during the first year since initiating first-line TKI compared with those in the rest of the European SIMPLICITY population (13.2% vs. 23.6%; P=0.003). In Italy, most patients remained on first-line TKI for ≥12 months (86.8%; n=231); results were similar for the rest of the European SIMPLICITY population (76.4%; n=165). In the first year of treatment in Italy, the median time to discontinuation of first-line TKI (IQR) was 3.9 (1.6–7.0) months. For imatinib (retrospective), imatinib (prospective), dasatinib and nilotinib, median times (IQR) were, respectively, 2.3 (1.7–3.6), 4.6 (1.8–7.8), 4.6 (1.6–5.7) and 5.5 (1.3–7.0) months. In the rest of the European SIMPLICITY population, in the first year of treatment, the median time to discontinuation of first-line TKI (IQR) was 3.7 (1.6–8.4) months. For imatinib (retrospective), imatinib (prospective), dasatinib and nilotinib, median times to discontinuation (IQR) were, respectively, 7.6 (4.6–10.5), 3.6 (2.1–8.5), 4.4 (1.7–7.2) and 1.4 (0.9–4.1) months.
Figure 2.
Proportion (%) of patients in Italy (left hand panel) and the rest of the European SIMPLICITY population (excluding Italy; right hand panel) who discontinued TKI treatment within the first 12 months of first-line TKI. DAS: dasatinib; IM: imatinib; NIL: nilotinib; TKI: tyrosine kinase inhibitor.
Intolerance was the most common primary reason for discontinuation of first-line TKI, reported in 70.4% (n=19; imatinib retrospective: 100% [n=1]; imatinib prospective: 53.3% [n=8]; dasatinib: 66.7% [n=2]; nilotinib: 100.0% [n=8]) of all patients who discontinued; this was slightly lower than in the rest of the European SIMPLICITY population (75.6% [n=31]; imatinib retrospective: 100.0% [n=2]; imatinib prospective: 66.7% [n=16]; dasatinib: 100.0% [n=8]; nilotinib: 71.4% [n=5]). Primary resistance was the second most common primary reason for discontinuation of first-line TKI, reported in 14.8% (n=4) of all patients who discontinued: all four patients were from the imatinib prospective cohort. Results were similar observations for the rest of the European SIMPLICITY population, in which 7.3% (n=3) of all patients discontinued first-line TKI because of primary resistance: all three patients were from the imatinib prospective cohort. Other reasons for discontinuation in the Italian population included acquired resistance (7.4% [n=2]), insurance/financial reasons (3.7% [n=1]) and unrelated medical conditions (3.7% [n=1]).
Predictors of discontinuation
Logistic regression analysis showed that there were no statistically significant predictors of first-line TKI discontinuation in Italian patients. However, in the rest of the European SIMPLICITY population, In Europe, however, female vs. male patients were more likely to discontinue first-line TKI treatment (odds ratio [OR; 95% CI] 2.60 [1.26, 5.36]; P=0.01), as were patients on prospective imatinib vs. dasatinib (OR [95% CI] 3.04 [1.21, 7.62]; P=0.018).
TKI switching patterns
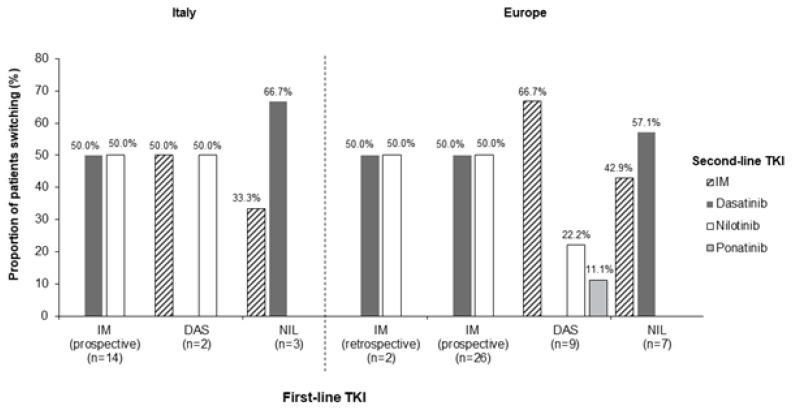
Of the Italian patients with ≥12 months of follow-up since initiating first-line TKI, 7.1% (n=19) switched to a second-line TKI – a smaller proportion than in the rest of the European SIMPLICITY population, where almost three times as many patients switched to a second-line TKI (20.4% [n=44]). In Italy, a greater proportion of patients initiating prospective imatinib as a first-line TKI switched to a second-line TKI within 12 months, compared with the imatinib retrospective, dasatinib and nilotinib cohorts (13.2% [n=14] vs. 0% [n=0] vs. 3.6% [n=2] vs. 4.1% [n=3], respectively). Of those who switched from imatinib prospective, six (42.9%) patients switched within the first 3 months and six (42.8%) switched between 6 and 12 months of first-line TKI initiation. Patients on first-line dasatinib switched either between 3 and 6 months (50.0% [n=1]) or 6 and 9 months (50.0% [n=1]). Of those who switched from nilotinib, one patient (33.3%) switched within the first 3 months and two patients (66.7%) switched between 6 and 9 months of initiating a first-line TKI.
The median time (IQR) to switch from first-line TKI in Italy was 172.0 (73.0–239.0) days. Between-TKI differences were noted for the median time (IQR) to switch from first-line TKI: this was longest in the nilotinib cohort (239.0 [61.0–255.0] days), followed by the dasatinib cohort (163.5 [140.0–187.0] days), and finally the imatinib prospective cohort (149.0 [73.0–214.0] days). In the rest of the European SIMPLICITY population, the median time (IQR) to switch from first-line TKI was 135.5 (65.5–265.0) days. Between-TKI differences were also noted: the longest time was for the imatinib retrospective cohort (292.5 [235.0–350.0] days), followed by the dasatinib (181.0 [134.0–281.0] days), imatinib prospective (131.5 [73.0–262.0] days) and nilotinib (44.0 [19.0–137.0] days) cohorts.
The switching patterns (Figure 3) were largely comparable between Italy and the rest of the European SIMPLICITY population. In Italy, intolerance was the most common primary reason for discontinuation of a first-line TKI and switching to a second-line TKI, reported in 56.3% (n=9; imatinib retrospective: 0%; imatinib prospective: 46.2% [n=6]; dasatinib: 100.0% [n=1]; nilotinib: 100.0% [n=2]) of all patients who discontinued first-line TKI; this was lower in comparison with the rest of the European SIMPLICITY population (76.3% [n=29]; imatinib retrospective: 100.0% [n=2]; imatinib prospective: 63.6% [n=14]; dasatinib: 100.0% [n=8]; nilotinib: 83.3% [n=5]). Table 3 shows the events concurrent with treatment discontinuation and switching in Italy. Primary resistance was the second most common primary reason for discontinuation of first-line TKI and switching to a second-line TKI in Italy, reported in 25.0% (n=4) of all patients who discontinued: all four patients were from the imatinib prospective cohort.
Figure 3.
TKI switching patterns in SIMPLICITY in Italy (left hand panel) and the rest of the European SIMPLICITY population (excluding Italy; right hand panel) within the first 12 months of first-line TKI. No patients switched from the imatinib retrospective cohort in Italy. IM: imatinib; TKI: tyrosine kinase inhibitor.
Table 3.
Intolerances leading to discontinuation of first-line TKI within 1 year of initiation among patients who switched to a second-line TKI within 1 year from initiating first-line TKI. The denominator is the number of patients in the column whose primary reason for discontinuation of first-line TKI is intolerance.
| Italy | Europe | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Event, N (%) | Imatinib (Retro) (N=0) | Imatinib (Pro) (N=6) | Dasatinib (N=1) | Nilotinib (N=2) | All patients (N=9) | Imatinib (Retro) (N=2) | Imatinib (Pro) (N=14) | Dasatinib (N=8) | Nilotinib (N=5) | All patients (N=29) |
| Arthralgia | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 2 (14.3) | 0 (0.0) | 0 (0.0) | 2 (6.9) |
| Erythema | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Generalised oedema | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Neutropenia | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (50.0) | 2 (22.2) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.4) |
| Oedema | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 1 (3.4) |
| Platelet count decreased | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Thrombocytopenia | 0 (0.0) | 1 (16.7) | 0 (0.0) | 2 (100.0) | 3 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Pro: prospective; Retro: retrospective; TKI: tyrosine kinase inhibitor.
Predictors of Switching
Logistic regression analysis showed that patients who were on imatinib prospective were more likely to switch from a first-line TKI than those on dasatinib (OR=5.62, P=0.036). Patients who had three or more comorbidities were less likely to switch from first-line TKI than those who had no comorbidities (OR=0.17, P=0.048). In the rest of the European SIMPLICITY population, female vs. male patients (OR=2.11, P=0.060) and those prospectively treated with imatinib vs dasatinib (OR=2.97, P=0.025) were more likely to switch from a first-line TKI.
Discussion
Here, we report response monitoring and TKI treatment patterns in patients with C-P CML treated in sites in Italy from the SIMPLICITY study and make detailed comparisons with the rest of the European SIMPLICITY population.
This manuscript furthers our knowledge by focusing on an analysis of the Italian cohort of 266 patients, which is the largest European cohort within the SIMPLICITY population and, importantly, is the first to report on management practices of the first year of TKI therapy. These data, from a country with high first-line usage of imatinib, dasatinib and nilotinib, reveals valuable insights into treatment and monitoring patterns in CP-CML patients by exploring TKI choice, switching pattern and reason for the switching in the first 12 months of TKI therapy.
As with the SIMPLICITY population as a whole, 11 SIMPLICITY patients in Italy are older (median 57.1 years) than the CML patients studied in the three pivotal clinical trials of the TKIs under investigation in newly diagnosed C-P CML (median 46.0–50.0 years), and in the three investigator-initiated randomised controlled trials evaluating use of imatinib (51.0–53.0 years). 6, 26–31 Unsurprisingly, given the interdependent nature of age and comorbidity, two-thirds of the Italian population had baseline comorbidities, indicating that Sokal and Hasford prognostic measurements are frequently carried out in Italy. Prognostic scores are of crucial importance, and guidelines recommend determining a patient’s score before making any first-line treatment decisions. 7 While risk score was not an individual category on the eCRF when capturing the rationale of treating physicians for TKI selection; it may be considered under the wider category of perceived effectiveness, which was the primary reason for treatment selection.
Management of C-P CML requires early and routine monitoring of CyR and MR and it is essential to identify whether or not patients are responding to treatment. 6, 7 In this analysis, the proportion of patients who were monitored by CyR and MR by 3 months was low. It is possible that, for a proportion of patients, testing had been carried out but with no date recorded and thus falling into the ‘not done’ category. Similarly, for some patients, testing may not have been possible for reasons of disease progression, or initial temporary drug interruption/reduced dosage, or other patient-related factors so that testing may have occurred outside of the strict 3-month timeframe.
Standardised MR assessments are gaining increased recognition for their importance; 32, 33 in SIMPLICITY this was particularly evident for the Italian population, demonstrating accordance with the ELN recommendations, regarding MR testing on the IS. Most patients were enrolled in the study through academic centres – a factor that may have influenced adherence to treatment recommendations on MR testing on the IS. A network of more than 50 standardised laboratories (LabNet) performs BCR-ABL analysis for hematologic clinics, and the availability of this resource may have also contributed to greater adherence to MR testing on the IS.
For patients not responding to their first-line TKI, and those who experience intolerance to treatment, guidelines recommend several options: dose modification, treatment interruption, or discontinuation followed by switching to the next most appropriate treatment. 6,7 In Italy and Europe, the proportion of patients who interrupted their first-line TKI was generally low, although a lower proportion of patients discontinued first-line TKI in Italy, compared with the rest of the European SIMPLICITY population. This finding may reflect differences in management between sites in Italy and the rest of Europe. In Italy, CML care is centralised: almost all haematologists operate within hospitals, and TKIs can be dispensed only by hospital pharmacies. 34 The significant variations observed in duration of treatment interruptions between TKIs may result from patients’ variability in speed of recovery, following any concurrent adverse events.
Intolerance was the most common primary reason for discontinuation of first-line TKI. The ELN and NCCN recommendations suggest a clinical interpretation of BCR-ABL levels >10% at 3 months before changing TKIs as result of resistance, 6,7 since there is currently no evidence to demonstrate any advantage for patients switching their TKI by 3 months because of BCR-ABL levels >10%. The adherence to this “careful” approach, paired with a low rate of early monitoring, could explain the higher percentage of discontinuations due to intolerance compared with resistance. The primary resistance observed in the imatinib (prospective) cohort is not surprising, given that primary resistance to imatinib is generally seen in 15–25% of patients. 7 In one instance, it was reported that the discontinuation was due to the patient’s insurance/financial reasons, and it is important to highlight that the Italian healthcare system is a regionally based National Health Service that provides universal coverage, mainly free of charge. 35
Plausible explanations for imatinib (prospective) being a predictor of switching, in addition to primary resistance, include that patients who achieve a suboptimal response, therefore, switch to a second-line TKI. Additionally, as second-generation TKIs became more widely available, and clinicians gained experience with them, further treatment options were then available for patients who were imatinib-intolerant, or who had suboptimal outcomes, increasing the likelihood of patients switching from first-line imatinib. Interestingly, primary resistance was not observed in the imatinib (retrospective) cohort, and this could be a result of selection bias associated with the retrospective nature of this cohort. Interpretation of switching patterns is not possible from this study, due to low numbers of patients.
While observational studies can capture the management of patients within the routine clinical practice setting; they are associated with inherent limitations, which need to be considered when interpreting results. 36 Such limitations, regarding this study, include selection bias related to the method of patient enrolment, as well as bias related to the year of enrolment and choice of TKI. Results should be interpreted in the context of shifting practices that may ultimately be influenced by evolving treatment recommendations. An artefact of observational studies is the capture of management practices over time. The update to the ELN recommendations in 2013, which specifically concerned routine response monitoring by MR, 6 could only influence monitoring practices after that date, so this might explain the pattern of observations reported. It is also worth noting that the European SIMPLICITY population is not representative of Europe as a whole, with the majority of patients enrolled in either Italy or Germany. Finally, the numbers in the patients who switched from first-line TKI within the first 12 months of treatment was small, which might be considered a positive result, but caution needs to be taken when making such inferences from the results, for the reasons stated above.
Monitoring practices in Italy, and the rest of the European SIMPLICITY population, are not in full accordance with treatment recommendations. These results are consistent with those reported previously for the whole SIMPLICITY population. 11, 12 The detailed information regarding the switching patterns and the reason for switching during the first year of CML therapy are presented here for the first time. These data provide insight into the “dynamic” real-life picture of the CML population during the most important time-frame, where patients are characterised and stabilised on the most appropriate therapy according to their results and tolerability to current TKI therapy. Future analyses will assess the relationship between response monitoring patterns, TKI switching patterns and clinical response in the SIMPLICITY population.
Supplementary Materials
Acknowledgements
We thank all SIMPLICITY study investigators, the patients who consented to be part of the study and LATITUDE (AXON Communications) who provided medical writing services on behalf of the authors and Bristol-Myers Squibb Pharmaceuticals Ltd.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Hochhaus A. Educational session: managing chronic myeloid leukemia as a chronic disease. Hematology Am Soc Hematol Educ Program. 2011;2011:128–35. doi: 10.1182/asheducation-2011.1.128. [DOI] [PubMed] [Google Scholar]
- 2.Jabbour E, Saglio G, Radich J, Kantarjian H. Adherence to BCR-ABL inhibitors: issues for CML therapy. Clin Lymphoma Myeloma Leuk. 2012;12(4):223–9. doi: 10.1016/j.clml.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambacorti-Passerini C, Antolini L, Mahon F-X, Guilhot F, Deininger M, Fava C, Nagler A, Della Casa C, Morra E, Abruzzese E, D’Emilio A, Stagno F, le Coutre P, Hurtado-Monroy R, Santini V, Martino B, Pane F, Piccin A, Giraldo P, Assouline S, Durosinmi M, Leeksma O, Pogliani E, Puttini M, Jang E, Reiffers J, Valsecchi M, Kim D-W. Multicenter Independent Assessment of Outcomes in Chronic Myeloid Leukemia Patients Treated With Imatinib. Journal of the National Cancer Institute. 2011;103(7):553–561. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–3127. doi: 10.1002/cncr.26679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki K, Strom SS, O’Brien S, Jabbour E, Ravandi F, Konopleva M, Borthakur G, Pemmaraju N, Daver N, Jain P, Pierce S, Kantarjian H, Cortes JE. Relative survival in patients with chronic-phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patient data from six prospective clinical trials. The Lancet Haematology. 2015;2(5):e186–e193. doi: 10.1016/S2352-3026(15)00048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Muller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saussele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–84. doi: 10.1182/blood-2013-05-501569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network (NCCN) NCCN Guidelines Chronic Myelogenous Leukemia Version 1. 2016. 2016. [cited 2017 May]; Available from: http://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. [DOI] [PubMed]
- 8.Abruzzese E, Breccia M, Latagliata R. Second-generation tyrosine kinase inhibitors in first-line treatment of chronic myeloid leukaemia (CML) BioDrugs. 2014;28(1):17–26. doi: 10.1007/s40259-013-0056-z. [DOI] [PubMed] [Google Scholar]
- 9.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, Apperley J, Cervantes F, Cortes J, Deininger M, Gratwohl A, Guilhot F, Horowitz M, Hughes T, Kantarjian H, Larson R, Niederwieser D, Silver R, Hehlmann R. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 10.Sundar HJ Radich. Optimizing Patient Care in Chronic Phase Chronic Myelogenous Leukemia: A Multidsciplinary Approach. Journal of the National Comprehensive Cancer Network: JNCCN. 2016;14(Supplement 1):S1–S6. doi: 10.6004/jnccn.2016.0197. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg SL, Cortes J, Gambacorti-Passerini C, Hehlmann R, Khoury HJ, Michallet M, Paquette R, Simonsson B, Zyczynski T, Foreman A, Abruzzese E, Andorsky D, Beeker A, Cony-Makhoul P, Hansen R, Lomaia E, Olavarria E, Mauro M. First-line treatment selection and early monitoring patterns in chronic phase-chronic myeloid leukemia in routine clinical practice: SIMPLICITY. Am J Hematol. 2017;92(11):1214–1223. doi: 10.1002/ajh.24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hehlmann R, Cortes J, Gambacorti-Passerini C, Goldberg SL, Khoury HJ, Mauro M, Michallet M, Mohamed H, Paquette R, Simonsson B, Subar M, Turner M, Zyczynski T. Tyrosine kinase inhibitor (tki) switching: Experience from simplicity, a prospective observational study of chronic-phase chronic myeloid leukemia (cp-cml) patients in clinical practice. 19th Congress of the European Hematology Association (EHA); 2014; Milan, Italy. [Google Scholar]
- 13.Bollu V, Quintas-Cardama A, Flamm M, Lill M, Thirman M, Ravandi-Kashani F, Akard LP, Talpaz M. PCN12 Resource utilization and perceptions of major molecular response in chronic myeloid leukemia (CML): results of a Delphi panel study. Value in Health. 2011;14:A156–A157. doi: 10.1016/j.jval.2011.02.870. [DOI] [Google Scholar]
- 14.Chen L, Guérin A, Xie J, Wu EQ, Yu AP, Ericson SG, Jabbour E. Monitoring and switching patterns of patients with chronic myeloid leukemia treated with imatinib in community settings: a chart review analysis. Curr Med Res Opin. 2012;28(11):1831–9. doi: 10.1185/03007995.2012.741577. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg SL, Chen L, Guerin A, Macalalad AR, Liu N, Kaminsky M, Ericson SG, Wu EQ. Association between molecular monitoring and long-term outcomes in chronic myelogenous leukemia patients treated with first line imatinib. Curr Med Res Opin. 2013;29(9):1075–82. doi: 10.1185/03007995.2013.812034. [DOI] [PubMed] [Google Scholar]
- 16.Hanfstein B, Müller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, Schnittger S, Haferlach C, Gohring G, Proetel U, Kolb HJ, Krause SW, Hofmann WK, Schubert J, Einsele H, Dengler J, Hanel M, Falge C, Kanz L, Neubauer A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Branford S, Hughes TP, Spiekermann K, Baerlocher GM, Pfirrmann M, Hasford J, Saussele S, Hochhaus A. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26(9):2096–102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 17.Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, Clark RE, Apperley JF, Milojkovic D, Bua M, Pavlu J, Paliompeis C, Reid A, Rezvani K, Goldman JM, Foroni L. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30(3):232–8. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanek E, Aubert RE, Sanders C, Frueh FW, Yao J, Franklin Lakes NJ RS EpsteinI Medco Health Solutions. Inadequate BCR-ABL monitoring in imatinib-treated patients with chronic myelogenous leukemia. Journal of Clinical Oncology. 2009;27(Suppl 15s):7077. [Google Scholar]
- 19.Henk HJ, Woloj M, Shapiro M, Whiteley J. Real-world analysis of tyrosine kinase inhibitor treatment patterns among patients with chronic myeloid leukemia in the United States. Clin Ther. 2015;37(1):124–33. doi: 10.1016/j.clinthera.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 20.Rashid N, Koh HA, Lin K, Dimaano C, Felber E. Real World Treatment Patterns in Chronic Myeloid Leukemia Patients Treated with Tyrosine Kinase Inhibitors in First Line in an Integrated Healthcare System. Blood. 2015;126:5157. [Google Scholar]
- 21.Sail KR, Chen L, Jackson J, Ericson SG, Haislip S, Ibison T, Gilmore J, Saleh MN. Treatment Patterns Among Patients with Philadelphia Chromosome Positive Chronic Myeloid Leukemia (Ph+ CML) Treated with Imatinib in a Community Setting. Blood. 2012;120(21):3179. [Google Scholar]
- 22.Vander Velde N, Chen L, Guo A, Sharma H, Marynchenko M, Wu EQ, Liu J, Yang H, Shi L. Study of imatinib treatment patterns and outcomes among US veteran patients with Philadelphia chromosome-positive chronic myeloid leukemia. J Oncol Pract. 2013;9(5):e212–9. doi: 10.1200/JOP.2012.000822. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Latremouille-Viau D, Guerin A, Nitulescu R, Gagnon-Sanschagrin P. Treatment Patterns and Healthcare Costs in Newly Diagnosed Patients with Chronic Myeloid Leukemia Receiving Dasatinib or Nilotinib As First-Line Therapy in the United States: A Retrospective Claims Databse Analysis. ASH 57th Annual Meeting & Exposition; 2015; Orlando, Florida. [DOI] [PubMed] [Google Scholar]
- 24.Latagliata R, Ferrero D, Iurlo A, Cavazzini F, Castagnetti F, Abruzzese E, Fava C, Breccia M, Annunziata M, Stagno F, Tiribelli M, Binotto G, Mansueto G, Gozzini A, Russo S, Cavalli L, Montefusco E, Gugliotta G, Cedrone M, Russo Rossi A, Avanzini P, Pregno P, Mauro E, Spadea A, Celesti F, Giglio G, Isidori A, Crugnola M, Calistri E, Sora F, Storti S, D’Addosio A, Rege-Cambrin G, Luciano L, Alimena G. Imatinib in very elderly patients with chronic myeloid leukemia in chronic phase: a retrospective study. Drugs Aging. 2013;30(8):629–37. doi: 10.1007/s40266-013-0088-6. [DOI] [PubMed] [Google Scholar]
- 25.Rosti G, Iacobucci I, Bassi S, Castagnetti F, Amabile M, Cilloni D, Poerio A, Soverini S, Palandri F, Rege Cambrin G, Iuliano F, Alimena G, Latagliata R, Testoni N, Pane F, Saglio G, Baccarani M, Martinelli G. Impact of age on the outcome of patients with chronic myeloid leukemia in late chronic phase: results of a phase II study of the GIMEMA CML Working Party. Haematologica. 2007;92(1):101–5. doi: 10.3324/haematol.10239. [DOI] [PubMed] [Google Scholar]
- 26.Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A, Proetel U, Pletsch N, Pfirrmann M, Haferlach C, Schnittger S, Einsele H, Dengler J, Falge C, Kanz L, Neubauer A, Kneba M, Stegelmann F, Pfreundschuh M, Waller CF, Spiekermann K, Baerlocher GM, Ehninger G, Heim D, Heimpel H, Nerl C, Krause SW, Hossfeld DK, Kolb HJ, Hasford J, Saussele S, Hochhaus A. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-study IV. J Clin Oncol. 2014;32(5):415–23. doi: 10.1200/JCO.2013.49.9020. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, Nakamae H, Huguet F, Boque C, Chuah C, Bleickardt E, Bradley-Garelik MB, Zhu C, Szatrowski T, Shapiro D, Baccarani M. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen J, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen J, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman J, Kantarjian H, Taylor K, Verhoef G, Bolton A, Capdeville R, Druker B I Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. The New England Journal of Medicine. 2003;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 29.Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, Coiteux V, Gardembas M, Berthou C, Vekhoff A, Rea D, Jourdan E, Allard C, Delmer A, Rousselot P, Legros L, Berger M, Corm S, Etienne G, Roche-Lestienne C, Eclache V, Mahon FX, Guilhot F S Investigators C France Intergroupe des Leucemies Myeloides. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363(26):2511–21. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 30.Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 31.Simonsson B, Gedde-Dahl T, Markevarn B, Remes K, Stentoft J, Almqvist A, Bjoreman M, Flogegard M, Koskenvesa P, Lindblom A, Malm C, Mustjoki S, Myhr-Eriksson K, Ohm L, Rasanen A, Sinisalo M, Sjalander A, Stromberg U, Bjerrum OW, Ehrencrona H, Gruber F, Kairisto V, Olsson K, Sandin F, Nagler A, Nielsen JL, Hjorth-Hansen H, Porkka K. CMLSG Nordic, Combination of pegylated IFN-alpha2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood. 2011;118(12):3228–35. doi: 10.1182/blood-2011-02-336685. [DOI] [PubMed] [Google Scholar]
- 32.Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, Radich JP, Saglio G, Pane F, Kamel-Reid S, Wang YL, Press RD, Lynch K, Rudzki Z, Goldman JM, Hughes T. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–8. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 33.Cross NC, White HE, Müller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–5. doi: 10.1038/leu.2012.104. [DOI] [PubMed] [Google Scholar]
- 34.monitor, Thsap. Health systems in transition (HiT) profile of Italy. January 2018[; Available from: http://www.hspm.org/countries/italy25062012/livinghit.aspx?Section=5.6%20Pharmaceutical%20care&Type=Section.
- 35.Ferré F, Giulio de Belvis A, Valerio L, Longhi S, Lazzari A, Fattore G, Ricciardi W, Maresso A. Italy Health system review. 2014. Jan, 2018[; Available from: http://www.euro.who.int/data/assets/pdffile/0003/263253/HiT-italy.pdf. [PubMed]
- 36.Mauro MJ, Davis C, Zyczynski T, Khoury HJ. The role of observational studies in optimizing the clinical management of chronic myeloid leukemia. Therapeutic Advances in Hematology. 2015;6(1):3–14. doi: 10.1177/2040620714560305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.