The acid-growth theory was proposed over 40 years ago based on early observations that low apoplastic pH promotes growth in a pathway involving the plant hormone auxin (Rayle and Cleland, 1992; Hager, 2003; Arsuffi and Braybrook, 2018). Since this discovery, a clearer understanding of the mechanism behind acid growth and its regulation is beginning to surface. Apoplast acidification is mediated by proton-pumping ATPases (H+-ATPases) in the plasma membrane (PM). Auxin induces acid growth via a signaling cascade that (among many other effects) induces expression of PM H+-ATPases and stimulates H+-ATPase activation via phosphorylation (Hager et al., 1971; Hager, 2003; Arsuffi and Braybrook, 2018). Low apoplastic pH causes loosening of the cell wall, and the activation of ion channels drives osmotic water uptake and cell expansion (Rayle and Cleland, 1992; Hager, 2003; Majda and Robert, 2018). Secretory vesicle traffic provides a membrane source and delivers cell wall materials to the expanding cell and also controls the density of PM transporters through regulation of exocytosis and endocytosis (Geldner et al., 2003; Grefen and Blatt, 2008). While the connection between auxin and H+-ATPase activity/PM density is well known, there are many questions remaining about how these critical transporters are trafficked to the PM.
SNARE (Soluble NSF Attachment protein Receptor) proteins are essential for intracellular vesicle trafficking and mediate fusion of vesicles to target membranes (Grefen and Blatt, 2008). SNAREs are classified based on the presence of either a conserved Glu residue (Q-SNARE) or Arg residue (R-SNARE) within the four-helix bundle SNARE motif. Q-SNAREs are typically localized to target membranes, while R-SNAREs are found in vesicle membranes. During membrane fusion, Q- and R-SNAREs form a ternary complex containing three Q-SNAREs, one from each of the Qa, Qb, and Qc classes (defined by the location of the Q residue within the four-helix bundle), as well as their cognate R-SNARE (Grefen and Blatt, 2008; Baker and Hughson, 2016). Three PM-localized Qa-SNAREs in Arabidopsis (Arabidopsis thaliana), SYNTAXIN OF PLANTS121 (SYP121), SYP122, and SYP132, are expressed throughout plant development, although SYP132 is present at relatively lower levels. SYP121 and SYP122 mediate the majority of PM trafficking and have some overlapping and distinct functions (Karnik et al., 2017). In this issue of Plant Physiology, Xia et al. (2019) identify a role for the Qa-SNARE SYP132 in trafficking of H+-ATPases to the PM to facilitate acid growth.
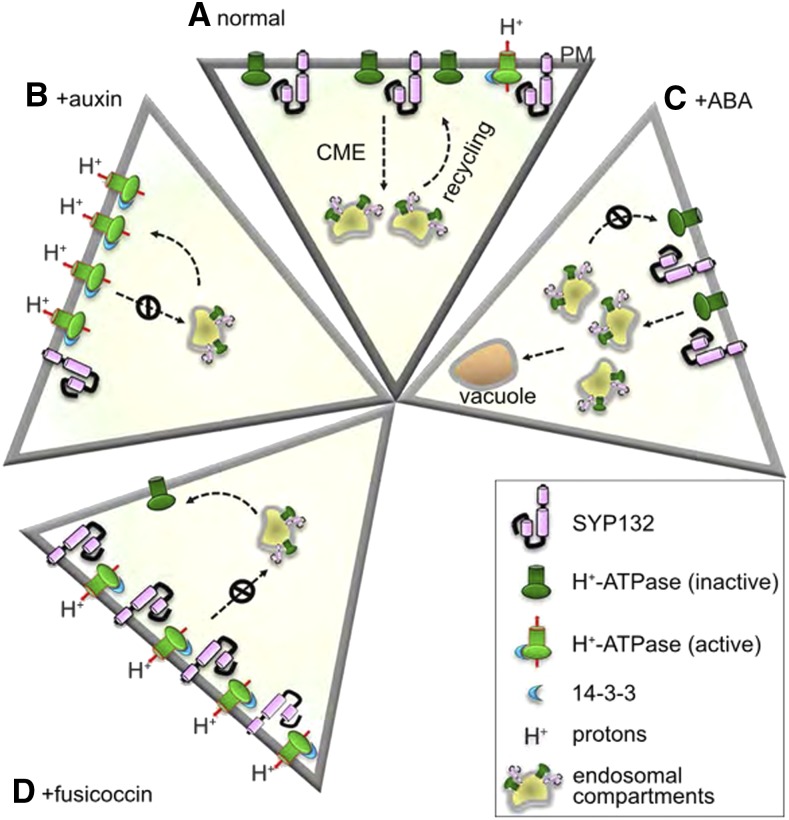
To investigate the effects of SYP132 on trafficking of one of the major H+-ATPases, Arabidopsis H+-ATPase 1 (AHA1), fluorescent protein-fused versions of both proteins were transiently coexpressed in tobacco (Nicotiana tabacum) epidermal cells. When expressed with SYP132, AHA1 exhibited a punctate fluorescence pattern characteristic of internalization, in contrast to the PM localization of AHA1 when expressed on its own. Internalization was not observed when either SYP121 or a dominant negative version of SYP132 (SYP132HabcΔ) was coexpressed with AHA1, confirming that this effect is due to SYP132 activity. SYP132 (and SYP132 HabcΔ) coexpression had similar effects on AHA1 in Arabidopsis root hairs, where internalization of AHA1 was coincident with the endocytic dye FM4-64, confirming that SYP132-dependent internalization of AHA1 occurs via endocytosis. AHA1 trafficking was further explored in two Arabidopsis mutants defective in clathrin-mediated endocytosis: chc1, a clathrin H chain mutant; and HUB1, an inducible dominant negative mutant (Kitakura et al., 2011; Larson et al., 2017). In both of these mutant backgrounds, coexpression with SYP132 no longer promoted AHA1 internalization and AHA1 remained mostly at the PM, suggesting that SYP132-dependent internalization of AHA1 is due to an increase in endocytosis. Using Arabidopsis plants stably expressing varying levels of SYP132 either by overexpression of SYP132 with a constitutive promoter (SYP132-OX) or use of the syp132 heterozygous mutant (syp132T), levels of AHA1 internalization were shown to positively correlate with SYP132 levels. This result was confirmed more generally for PM H+-ATPases using cell fractionation. Furthermore, SYP132 overexpression (which induces AHA1 internalization) resulted in an increase in apoplastic pH, while apoplastic pH in syp132T was decreased relative to the wild type. In line with these observations, SYP132-OX plants were smaller and had shorter hypocotyls in dark-grown seedlings. Altogether, these results support the hypothesis that SYP132 is a negative regulator of H+-ATPase trafficking to the PM by stimulating their internalization via endocytosis (Fig. 1A).
Figure 1.
Model of SYP132 function in H+-ATPase trafficking. A, During normal growth, SYP132 promotes internalization and recycling of inactive H+-ATPases to regulate apoplastic pH. B, In response to auxin, SYP132 levels are decreased, which results in increased density of active H+-ATPases at the PM. Proton pumping lowers the pH of the apoplast, facilitating cell wall loosening and cell expansion. C, In the presence of ABA, inactive H+-ATPases are internalized and targeted to the vacuole for degradation, likely along with SYP132. D, FC treatment constitutively activates H+-ATPases at the PM, which prevents SYP132-stimulated internalization. CME, clathrin-mediated endocytosis. Figure from Xia et al. (2019).
Given that auxin regulates acid growth, the authors examined the relationship between auxin and SYP132. Taking advantage of the known differential effects of high auxin treatment (∼10 μm) on shoot and root growth (Evans et al., 1994; Velasquez et al., 2016), SYP132 transcript levels were measured following high auxin treatment. SYP132 expression decreased in the shoot where growth is promoted and increased in the root where growth is inhibited, consistent with the observed correlation between SYP132 levels and H+-ATPase trafficking and acid growth (Fig. 1B). Similarly, SYP132 levels correlated with stomatal aperture size, which is heavily dependent upon auxin and active H+-ATPase density at the PM (for review, see Jezek and Blatt, 2017): SYP132-OX plants had smaller stomatal apertures on average relative to syp132T. Fusicoccin (FC) is a fungal phytotoxin that activates H+-ATPases (and other transporters) by stabilizing their interaction with 14-3-3 binding proteins (Olsson et al., 1998). FC increased stomatal opening in all plants including SYP132-OX, demonstrating that the negative effect of SYP132 overexpression can be rescued through constitutive activation of H+-ATPase channels. FC treatment increased total H+-ATPase levels; however, in SYP132-OX plants, this translated into an increase in the PM:internal membrane density of H+-ATPases, indicating that FC prevents SYP132-dependent internalization of active H+-ATPases (Fig. 1D). Together, these data suggest that internalization of H+-ATPases is also tied to their activation state. In the activated state, H+-ATPases undergo less SYP132-dependent internalization/recycling than in their deactivated state, possibly providing a mechanism for fine-tuning active H+-ATPases at the PM.
In addition to regulation by auxin, it has been previously documented that abscisic acid (ABA) negatively regulates H+-ATPase levels, which involves the tonoplast-localized R-SNARE VAMP711 (Xue et al., 2018). In both wild-type and SYP132-OX plants, total H+-ATPases were decreased upon ABA treatment, likely via vesicle transport to the vacuole. SYP132 levels were also decreased in ABA-treated SYP132-OX plants, suggesting that they are also degraded by targeting to the vacuole (Fig. 1C).
Together, the results presented by Xia et al. (2019) clearly show that the Qa-SNARE SYP132 plays a role in intracellular trafficking of H+-ATPases and contributes to the regulation of auxin-mediated acid growth. The effects of SYP132 on H+-ATPase trafficking are somewhat counterintuitive, since SYP132 is a target SNARE localized to the PM. SYP132 as a negative regulator of H+-ATPases raises many questions of how, mechanistically, SYP132 may perform this function. There is evidence that SYP132 interacts directly with H+-ATPases (Fujiwara et al., 2014), and it would be interesting to investigate if other accessory factors contribute to the observed negative regulation of H+-ATPase targeting. Future work aimed at investigating SYP132 interactions and the link between H+-ATPase activation and SYP132-dependent trafficking will be important for understanding how apoplast acidification is fine-tuned by hormones during growth and development.
References
- Arsuffi G, Braybrook SA (2018) Acid growth: An ongoing trip. J Exp Bot 69: 137–146 [DOI] [PubMed] [Google Scholar]
- Baker RW, Hughson FM (2016) Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol 17: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Ishiwaka H, Estelle MA (1994) Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta 194: 215–222 [Google Scholar]
- Fujiwara M, Uemura T, Ebine K, Nishimori Y, Ueda T, Nakano A, Sato MH, Fukao Y (2014) Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol 55: 781–789 [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G (2003) The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Grefen C, Blatt MR (2008) SNAREs: Molecular governors in signalling and development. Curr Opin Plant Biol 11: 600–609 [DOI] [PubMed] [Google Scholar]
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: Historical and new aspects. J Plant Res 116: 483–505 [DOI] [PubMed] [Google Scholar]
- Hager A, Menzel H, Krauss A (1971) Experiments and hypothesis concerning the primary action of auxin in elongation growth. Planta 100: 47–75 [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174: 487–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik R, Waghmare S, Zhang B, Larson E, Lefoulon C, Gonzalez W, Blatt MR (2017) Commandeering channel voltage sensors for secretion, cell turgor, and volume control. Trends Plant Sci 22: 81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, Tanaka H, Friml J (2011) Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23: 1920–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson ER, Van Zelm E, Roux C, Marion-Poll A, Blatt MR (2017) Clathrin heavy chain subunits coordinate endo- and exocytic traffic and affect stomatal movement. Plant Physiol 175: 708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majda M, Robert S (2018) The role of auxin in cell wall expansion. Int J Mol Sci 19: E951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Svennelid F, Ek B, Sommarin M, Larsson C (1998) A phosphothreonine residue at the C-terminal end of the plasma membrane H+-ATPase is protected by fusicoccin-induced 14-3-3 binding. Plant Physiol 118: 551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE (1992) The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol 99: 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez SM, Barbez E, Kleine-Vehn J, Estevez JM (2016) Auxin and cellular elongation. Plant Physiol 170: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Marquès-Bueno MM, Bruce CG, Karnik RA (2019) Unusual roles of secretory SNARE SYP132 in plasma membrane H+-ATPase traffic and vegetative plant growth. Plant Physiol 180: 837–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Yang Y, Yang Z, Wang X, Guo Y (2018) VAMP711 is required for abscisic acid-mediated inhibition of plasma membrane H+-ATPase activity. Plant Physiol 178: 1332–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]