Basal green tissue ABA widely affects plant morphology, water relations, and growth, inhibiting early growth and flowering, and does not directly suppress photosynthesis in the short term.
Abstract
Abscisic acid (ABA) levels increase significantly in plants under stress conditions, and ABA is thought to serve as a key stress-response regulator. However, the direct effect of ABA on photosynthesis and the effect of mesophyll ABA on yield under both well-watered and drought conditions are still the subject of debate. Here, we examined this issue using transgenic Arabidopsis (Arabidopsis thaliana) plants carrying a dominant ABA-signaling inhibitor under the control of a mesophyll-specific promoter (FBPase::abi1-1, abbreviated to fa). Under normal conditions, fa plants displayed slightly higher stomatal conductance and carbon assimilation than wild-type plants; however, these parameters were comparable following ABA treatment. These observations suggest that ABA does not directly inhibit photosynthesis in the short term. The fa plants also exhibited a variety of altered phenotypes under optimal conditions, including more vigorous initial growth, earlier flowering, smaller flowers, and delayed chlorophyll degradation. Furthermore, under optimal conditions, fa plant seed production was less than a third of that observed for the wild type. However, under drought conditions, wild-type and fa seed yields were similar due to a significant reduction in wild-type seed and no reduction in fa seed. These findings suggest that endogenous basal ABA inhibits a stress-escape response under nonstressed conditions, allowing plants to accumulate biomass and maximize yield. The lack of a correlation between flowering time and plant biomass combined with delayed chlorophyll degradation suggests that this stress-escape behavior is regulated independently and upstream of other ABA-induced effects such as rapid growth and flowering.
Plants are autotrophic organisms that rely on carbon assimilation for organic matter production, and thus CO2 accessibility is extremely important. However, this accessibility comes at a cost. Opening stomata to allow CO2 to enter allows water to leave through those same pores. In order to regulate the balance between water loss and carbon uptake, stomata can respond to environmental stimuli and hormonal signals. The phytohormone abscisic acid (ABA) is the main hormone known to regulate various stress responses in a variety of species from green algae to angiosperms (Takezawa et al., 2011). In angiosperms, the effects of ABA on stomatal guard cells have been well studied, particularly with regard to productivity parameters (e.g. plant water balance, stress response, and carbon assimilation). Nevertheless, the role of ABA in regulating stress responses in other nonstomatal productive tissues (e.g. mesophyll tissue) and its impact on plant water balance have received less attention, although new evidence suggests that mesophyll cells produce significant amounts of ABA under water-stress conditions (McAdam and Brodribb, 2018). These effects can be seen in transcriptomic analyses (Hoth et al., 2002; Seki et al., 2002; Matsui et al., 2008; Mizoguchi et al., 2010; Wang et al., 2011; González-Guzmán et al., 2012), in which ABA has been shown to affect thousands of genes (Nemhauser et al., 2006) involved in a wide range of processes. These effects, which are sometimes masked by the effect of ABA on gas exchange, can also be seen in organisms in which ABA does not affect stomata (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012), but does act as a stress hormone.
In the past, it was believed that ABA is synthesized in plant roots and carried through the xylem to the shoot, where it causes stomatal closure. However, in grafting experiments performed in tomato (Solanum lycopersicum) and Arabidopsis (Arabidopsis thaliana) using ABA-synthesis mutant root stocks and wild-type scions, stomata were able to close regardless of impaired ABA synthesis in the root (Holbrook et al., 2002; Christmann et al., 2007). These findings raised the question of how plant stomata respond to drying soil. It has been suggested that the bundle sheath may act as a control center, regulating leaf hydraulic conductance in response to environmental stimuli (Sade et al., 2014b). The existence of root-derived, hydraulic long-distance signaling has also been suggested (Christmann et al., 2013). In Arabidopsis, bundle sheath cells constitute a barrier to apoplastic water transport from the xylem to the mesophyll. In these cells, ABA reduces membrane permeability to water through the regulation of aquaporins (Shatil-Cohen et al., 2011). Following Shatil-Cohen et al. (2011), Pantin et al. (2013) examined responses of different ABA-sensing mutants, as well as the ost2-2 mutant to ABA, and found that although the mutants’ stomata were insensitive to ABA, entire leaves still responded to the hormone (Pantin et al., 2013). These findings suggest that manipulation of ABA signaling in green tissues could affect gas exchange without directly affecting stomatal sensitivity to ABA.
In addition to its well-documented effects on angiosperm stomata and gas exchange, the direct effect of ABA on short-term photosynthetic activity is still not clear. It is difficult to distinguish whether the reduction in photosynthesis seen under stress conditions is exclusively related to the decrease in substomatal CO2 (Ci) concentration caused by the impact of ABA on stomatal closure, or if there is some other direct biochemical inhibition of photosynthesis. This issue has long been debated (Mawson et al., 1981; Raschke and Hedrich, 1985; Downton et al., 1988; Popova et al., 1996; Liang et al., 1997) and, to the best of our knowledge, no conclusive physiological evidence has been reported. Several reviews have concluded that ABA might have differential modes of action, according to which, during mild drought stress, the decrease in photosynthesis correlates mainly with Ci (i.e. is affected by stomatal regulation) and, under more severe stress, there is additional, direct biochemical inhibition of photosynthesis in the mesophyll (Flexas and Medrano, 2002; Flexas et al., 2004). Morover, under severe or prolonged stress, ABA is involved in the regulation of leaf senescence. Examples of this can be found in wheat (Triticum aestivum), in which rising ABA concentrations lead to the movement of carbohydrates from stems to seeds (Yang et al., 2003); in barley (Hordeum vulgare), in which lines exhibiting delayed stress-induced senescence had lower ABA levels (Seiler et al., 2014); and in tomato, in which ABA concentration-dependent promotion of senescense was also seen (Tung et al., 2008). In Arabidopsis, some of the mechanisms underlying ABA-regulated leaf senescence have recently been revealed. Gao et al. (2016) found that ABA-activated transcription factors bind to the promoter of chlorophyll-catabolizing proteins and promote their expression. In addition, different ABA-sensing mutants displayed a “stay green” phenotype in response to ABA treatment (Gao et al., 2016).
One of the most pronounced effects of ABA involves drought tolerance. ABA mediates many drought-related processes from reduction of transpiration (Iuchi et al., 2001; Schroeder et al., 2001) through the inhibition of shoot growth and thus reducing the plants’ consumption of resources, especially water, to osmotic adjustment (Mason et al., 1990). The final outcome of these effects is enhanced tolerance to drought (Iuchi et al., 2001; Qin and Zeevaart, 2002; Fujita et al., 2005). However, this drought tolerance comes at a price. Even though ABA's induction of drought tolerance is a multitiered process, as has been mentioned, one of ABA's fastest effects on the plant is the reduction of stomatal conductance (Taiz and Zeiger, 2006), which is the trait most strongly correlated with yield (de Wit, 1958; Tanner and Sinclair, 1983; Sinclair et al., 1984; Lu et al., 1994; Fischer et al., 1998; Richards, 2000; Kemanian et al., 2005; Blum, 2009). Consequently, drought-tolerant crops usually have lower yields under well-watered conditions (for review, see Negin and Moshelion, 2017). Therefore, manipulation of ABA signaling might be a promising approach for increasing crop yield under drought conditions (Joshi-Saha et al., 2011).
Yet, the present understanding of ABA’s effects on drought responses that emerges from the literature is not clear cut, partially due to the different definitions of drought and drought tolerance and the different ABA levels and responses caused by such conditions. This leads us to the following question: Does ABA affect yield under well-watered and drought conditions and, if so, how? For example, a line of ABA-hypersensitive transgenic canola (Brassica napus) showed no difference in yield relative to that of wild-type plants under well-watered conditions. However, under mild drought conditions, the transgenic plants had significantly higher yields than control plants (Wang et al., 2005). In wheat exposed to moderate drought, both shoot dry biomass and seed yield were greater in plants treated with exogenous ABA (Travaglia et al., 2010). Tomato plants with impaired ABA synthesis (flacca and sitiens mutants) had lower fresh biomass levels than did wild-type plants under both well-watered (Sagi et al., 2002) and drought (Aroca et al., 2008) conditions. Furthermore, ABA-deficient flacca scions grafted onto wild-type roots exhibited reduced biomass, as compared to wild-type self-grafts. However, biomass was not reduced when wild-type scions were grafted onto flacca roots (Chen et al., 2002). Taken together, it seems that ABA has a positive effect on plant biomass and yield under mild drought conditions. However, under more severe stress, the advantages ABA provides through stomatal closure and plant adaptation to water loss are transformed into an adverse effect due to the inhibition of growth and the promotion of leaf senescence (Sreenivasulu et al., 2012).
Following the elucidation of the ABA signal transduction pathway in 2009 (Ma et al., 2009; Park et al., 2009), it was found that the previously described dominant ABA-insensitive mutant abi1-1 (Koornneef et al., 1984), in which a SNP led to a Gly to Asp substitution (Leung et al., 1994; Meyer et al., 1994), is insensitive to ABA due to steric hindrance that reduces the affinity of abi1-1 for the ABA receptor, and thus abi1-1 continues to inhibit SnRK activity even though ABA is present and bound to the receptor (Joshi-Saha et al., 2011).
ABA’s complex and intertwined regulation of gas exchange and stress responses leads to the question of whether and to what extent the ABA in green tissues inhibits photosynthesis and how it affects plant growth under both unstressed and drought conditions. To the best of our knowledge, these questions have not been fully addressed using a whole-plant model in which ABA insensitivity is limited to the green tissues. Therefore, we examined these questions using transgenic plants that express the abi1-1 protein under a green tissue-specific promoter, which leads to dominant ABA insensitivity in mesophyll and bundle sheath cells. We initially hypothesized that ABA directly inhibits carbon assimilation in green tissue (i.e. in addition to the inhibition caused by the reduced availability of CO2) and that insensitivity to ABA in green tissues would lead to enhanced growth and yield under well-watered conditions, but a somewhat impaired stress response under drought conditions.
RESULTS
FBPASE::abi1-1 Transgenes
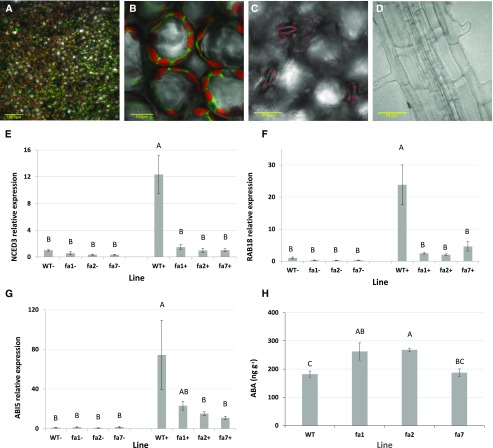
In order to assess the effect of ABA on photosynthesis in angiosperms, we used the mutant PP2C abi1-1 protein. This protein dominantly inhibits ABA sensing, driven by the wheat FBPase promoter, which, as a nucleus-expressed chloroplastic protein, is expressed in green tissues (see “Materials and Methods”). The FBPase::abi1-1 transgene (fa) was inserted into plants using Agrobacterium-mediated transformation, and three independent lines were selected (fa1, fa2, and fa7). The FBPase promoter expression pattern was confirmed to be mesophyll specific, as shown previously by Sade et al. (2014a), using FBPase::GFP expressing plants. In these, we indeed saw green fluorescence in the mesophyll tissue (Fig. 1, A and B), but not in stomata (Fig. 1C) or roots (Fig. 1D). The dominant nature of the abi1-1 mutation is dependent on the ratio between the nonmutated ABI1 and mutated abi1-1 proteins. We, therefore, quantified this ratio through restriction enzyme digestion of the PCR product of ABI1 complementary DNA (cDNA), which is cut in the endogenous form but not in the mutated form. We found that the original abi1-1 gene was 100% uncut and that 6.8% of the gene in the wild type was uncut, indicating the efficiency of the restriction reaction. Among fa1, fa2, and fa7, 72.7%, 78.5%, and 66.7% of the gene was uncut, respectively (Supplemental Fig. S1).
Figure 1.
FBPase::abi1-1 transgene characterization. A to D, Composite images of different tissues of FBPase::GFP-expressing plants. Mesophyll (A and B; Bar = 100 µm and 10 µm, respectively), stomata (C; Bar = 20 µm), and root (D; Bar = 50 µm) are shown. Composite mesophyll and stomata images are composed of overlays of bright-field, GFP (520 nm emission), and auto-fluorescence (650 nm emission) images. Composite root images are composed of bright-field and GFP images. E to G, RT-qPCR expression analysis of the ABA reporter genes NINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3 (NCED3; E), RESPONSIVE TO ABA 18 (RAB18; F), and ABA INSENSITIVE 5 (ABI5; G) before and following ABA induction in wild-type (WT) plants and the three independent fa lines: fa1, fa2, and fa7 (n = 5). H, Foliar ABA quantification of well-irrigated plants of wild type and three independent lines of fa (n = 3). Different letters represent a significant difference as determined using Tukey’s honestly significant difference (HSD) test (P < 0.05).
We next used reverse transcription-quantitative PCR (RT-qPCR) expression analysis of the ABA reporter genes NCED3 (Kelly et al., 2017), RAB18 (Lång and Palva, 1992), and ABI5 (Lopez-Molina et al., 2001) to evaluate the homogeneity of the selected lines. These genes were expressed similarly in the three fa lines. Furthermore, whereas ABA treatment significantly induced the expression of these genes in wild-type plants, there was far less induction in the fa lines and the induction that did occur was insignificant. These differences also led to a statistically significant interaction between line and ABA treatment (Fig. 1, E-G). In order to assess whether the difference in the ABA response was related to different ABA levels or dominant inhibition of ABA signaling by abi1-1, we quantified the concentration of ABA in wild-type and fa leaves (Fig. 1H). Two of the fa lines had higher levels of ABA than wild type, whereas the third had a concentration similar to that observed for the wild type.
Morphological and Phenological Characterization of Transgenic Plants
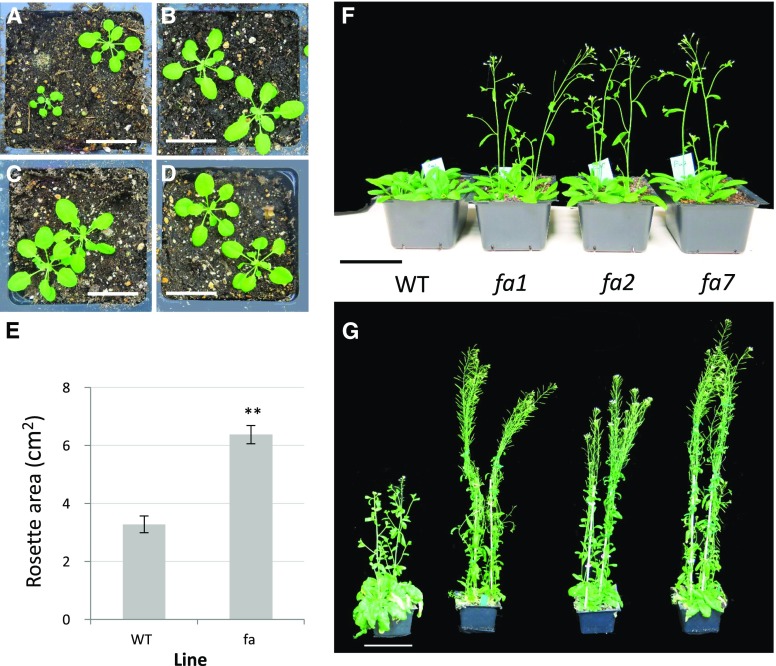
The first irregular phenotype that was observed in fa plants was their faster growth. This could be seen from early stages of vegetative growth (Fig. 2, A–E), through earlier flowering that led to taller plants during the first flowering stages (Fig. 2, F and G) and earlier seed production, drying, and death.
Figure 2.
Morphological and phenological fa plant characterization. A to D, Rosettes of 6-week-old wild-type (WT; A) and fa plants (B, fa1; C, fa2; D, fa7). Bars = 3 cm. E, Mean + SE rosette area of wild-type and fa plants. Statistical analysis was performed using Student's t test; **significant difference at P < 0.01. Wild type: n = 36; fa: n = 72. F, Four-week-old wild-type and fa plants grown under long-day conditions. Bar = 6 cm. G, Approximately 10-week-old wild-type and fa plants grown under short-day conditions, digitally extracted for comparison. Bar = 10 cm.
In order to assess early growth vigor, we photographed 6-week-old plants (grown under short-day conditions) and leaf area was measured. At that age, fa plants had a significantly larger rosette area than wild-type plants (6.38 ± 0.31 cm2 as compared with 3.28 ± 0.29 cm2; Fig. 1).
In addition, fa plants flowered earlier, which led to earlier bolting and a shorter life cycle, that is, they also began senescence earlier and had a shorter life span (Fig. 2). We repeated the short-day experiment in a temperature-regulated greenhouse in 4-L pots. Here again, fa plants flowered significantly earlier (at 36.7 ± 0.2 d after sowing as compared with 47 d after sowing in wild type; Fig. 3A).
Figure 3.
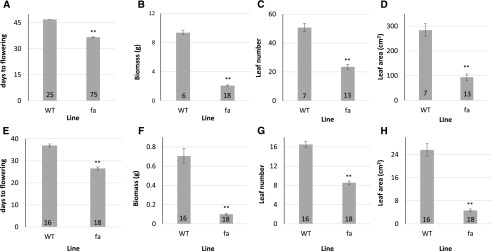
Flowering time, biomass, leaf number, and leaf area at flowering of wild-type (WT) and fa plants grown under short- or long-day conditions. A and E, Days from sowing to flower meristem emergence of plants grown under short-day conditions in a temperature-controlled greenhouse (A) and plants grown under long-day conditions in a growth room (E). B and F, Average fresh rosette biomass at the time of flowering of plants grown under short-day (B) or long-day conditions (F). C and G, Number of leaves at flowering under short- (C) or long-day (G) conditions. D and H, Leaf area at flowering under short- (D) or long-day (H) conditions. Numbers at the bottom of the columns indicate the number of plants tested. **Significant difference relative to that in the wild type (P < 0.01) as determined using Student's t test.
Our first hypothesis was that the fa plants' rapid initial growth led to early accumulation of the critical mass needed for flowering under short-day conditions. This critical mass could be caused by elevated sugar concentrations that would trigger a flowering signal. To test that hypothesis, we compared the transgenic plants’ rosette biomass at flowering time to that of wild-type plants. The average biomass of the transgenic plants at the time of flowering was 4.5 times less than that of wild-type plants (2.08 ± 0.09 g as compared with 9.35 ± 0.25 g in wild type). This difference was reflected in both dry and fresh weights (Fig. 3B). The ratio of dry to fresh biomass was not significantly different between the two lines (10 ± 0.16% in wild type as compared with 9.6 ± 0.16% in fa plants).
We next examined whether the early flowering phenotype of fa plants was related to the plants reaching a leaf-number threshold. If there was such a threshold, even though the wild-type biomass was far greater than the transgenic plants at flowering, the number of leaves at flowering would be similar across the two lines. After only leaves longer than 1 cm of plants grown in a short-day growth room were counted, the fa plants' leaf number figures were found to be significantly lower than those of wild-type plants (23.5 ± 1.7 in fa plants as compared with 50.7 ± 2.8 in wild-type plants; Fig. 3C). Leaf area at the time of flowering was also significantly smaller among fa plants (94 ± 13 cm2 among fa plants as compared with 284 ± 26 cm2 among wild-type plants; Fig. 3D).
These findings led us to believe there was a more direct connection between the insensitivity to ABA in the mesophyll and flowering time. In order to further test this connection, we examined the time of flowering under long-day conditions. As they did in the greenhouse during short days, fa plants also flowered significantly earlier when exposed to long days in a growth room (fa plants began to flower at 26.6 ± 0.2 d and wild-type plants began to flower at 37 ± 0.7 d; Fig. 3E). They did so despite their far smaller biomass (0.1 ± 0.01 g, as compared with 0.7 ± 0.08 g; Fig. 3F), leaf number (8.6 ± 0.035, as compared with 16.5 ± 0.6; Fig. 3G), and leaf area (4.7 ± 0.5 cm2, as compared with 25.6 ± 2.2 cm2; Fig. 3H).
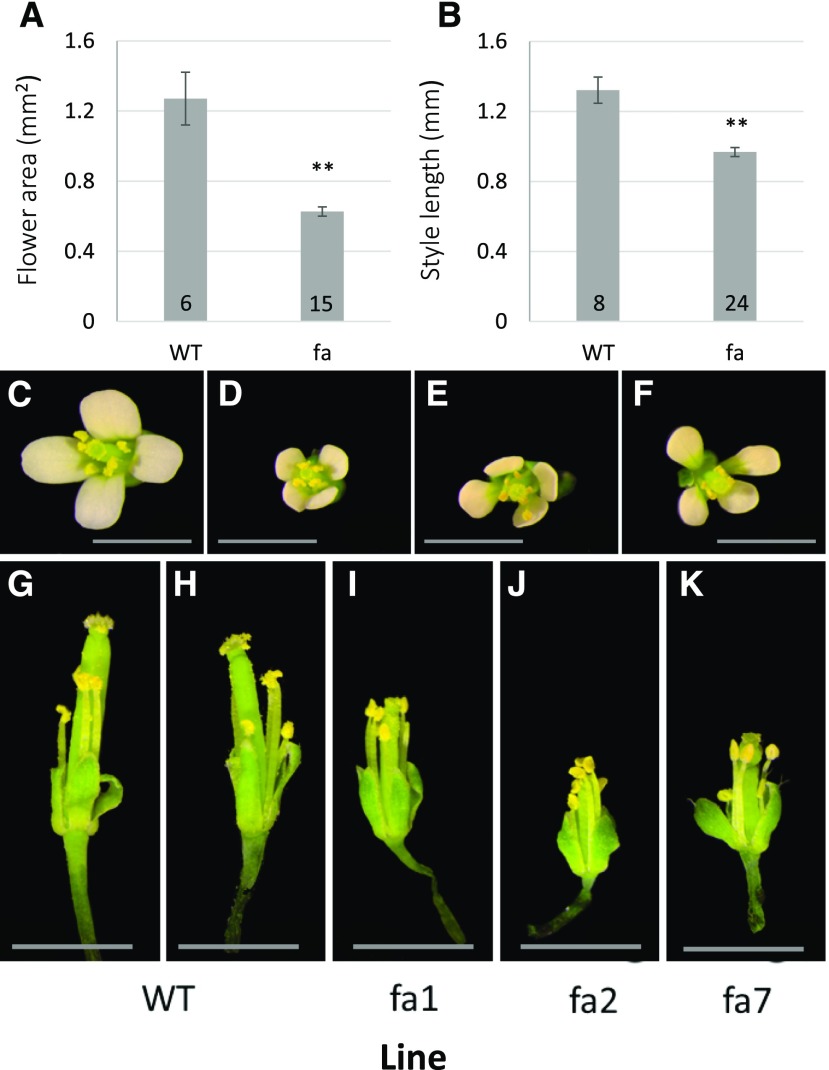
Another morphological feature of fa plants was that although their leaves were larger than those of wild-type plants (Supplemental Fig. S2), their flowers were smaller. We photographed flowers from above and from the side with petals removed, and analyzed flower area and style length. The fa flower area and styles length were significantly smaller than those of the wild type (0.63 ± 0.03 mm2 for fa flowers as compared with 1.27 ± 0.15 mm2 for wild-type flowers, and 0.97 ± 0.03 mm as compared with 1.32 ± 0.075 mm for styles length; Fig. 4). In contrast with leaves and flowers, fa seedling roots did not differ in length from those of wild-type seedlings (Supplemental Fig. S3).
Figure 4.
Flower size and style length. A, Flower area of wild-type (WT) and fa plants. B, Style length of wild-type and fa plants. Numbers at the bottom of the columns indicate the sample size. **Significant difference at P < 0.01, as determined using Student's t test. B to F, Pictures of representative wild-type (C) and fa (D to F) flowers. G to K, Pictures of representative wild-type (G and H) and fa (I to K) styles. Bars = 1 mm.
Gas Exchange of Well-Watered Plants
In order to test whether ABA inhibits photosynthesis directly in green tissues and to try to better understand the greater growth vigor and earlier flowering of fa plants, we used gas-exchange measurements to examine their photosynthesis and water relations.
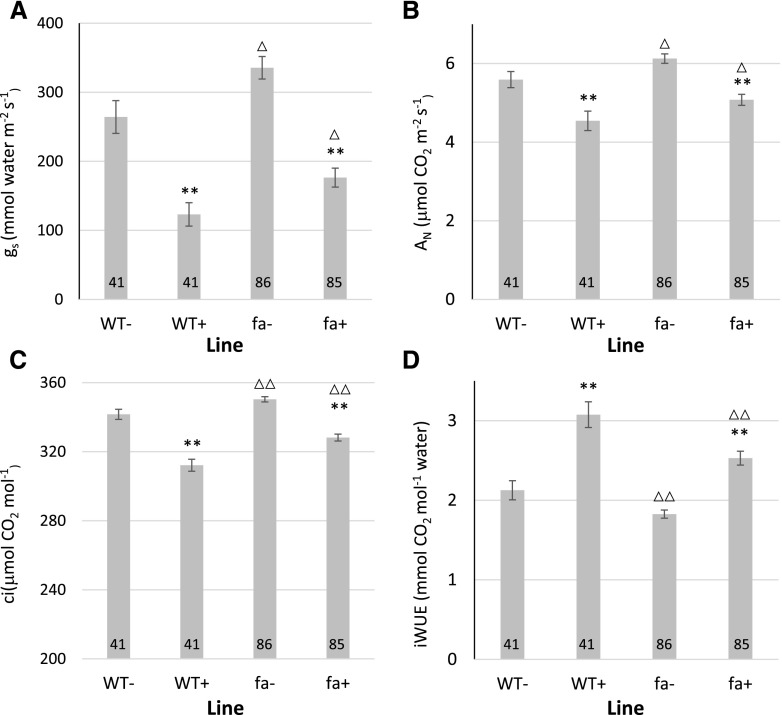
Under control conditions (i.e. no added ABA), fa stomatal conductance (gs) was higher than that of the wild type (335 ± 16 mmol m−2 s−1 as compared with 264 ± 23 mmol m−2 s−1; Fig. 5A). ABA perfusion to the xylem reduced the gs of both lines. Here again, fa gs was significantly higher than that of the wild type (176 ± 14 mmol m−2 s−1 as compared with 123 ± 17 mmol m−2 s−1). Carbon assimilation (AN) was slightly higher in fa leaves both when treated with ABA and when left untreated (6.13 ± 0.12 µmol m−2 s−1 as compared with 5.59 ± 0.2 µmol m−2 s−1, respectively, in leaves that were not treated with ABA, and 5.08 ± 0.14 µmol m−2 s−1 compared with 4.54 ± 0.25 µmol m−2 s−1, respectively, in ABA-treated leaves). In leaves that were not treated with ABA, the ratio of transpiration to carbon assimilation (instantaneous water use efficiency) was higher in wild-type leaves (2.13 ± 0.12 µmol CO2 mol−1 H2O in wild type and 1.83 ± 0.05 mmol CO2 mol−1 H2O in fa leaves). This difference was maintained when leaves were treated with ABA (3.08 ± 0.16 in the wild type as compared with 2.53 ± 0.09 in fa leaves; Fig. 5D). Although fa plants had higher gs, AN, and Ci before ABA treatment, their response to ABA was similar to that of the wild type and there was no statistically significant interaction between plant type and response to the ABA treatment (Supplemental Table S1). We next examined the possibility that although the responses to ABA were similar, fa plants that were not treated with ABA may have had altered photosynthetic efficiency. We constructed A/Ci curves and calculated the maximum capacity of Rubisco carboxylation and maximum electron transport rate. No significant differences were found in these parameters (Supplemental Fig. S4).
Figure 5.
Wild-type (WT) and fa gas exchange in response to ABA treatment. A, gs. B, AN. C, Ci. D, instantanious water use ifficiency (iWUE; AN/E). Numbers at the bottom of the columns indicate the sample size. “+” indicates plants that were treated with ABA; “-” indicates plants that were not. **Significant difference between ABA-treated and untreated leaves of the same genotype; △ and △△, significant difference among similarly treated leaves of different genotypes. △Significant difference at P < 0.05; ** and △△, significant difference at P < 0.01. Comparisons were performed using Student’s t test.
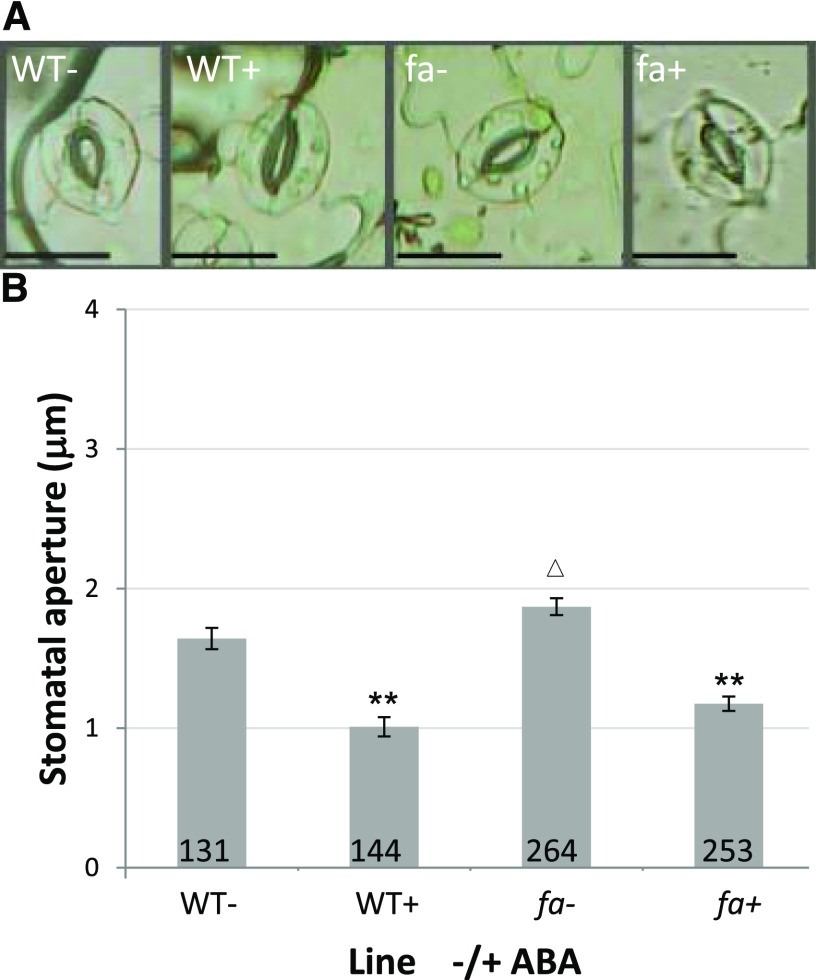
Our results raised the possibility that elevated gs in fa plants was due to reduced stomatal sensitivity to ABA, even though FBPase::GFP fluorescence could not be detected in guard cells. In order to test this hypothesis, we examined the response of stomata to ABA in epidermis peels from fa and the wild type. Untreated fa stomata had slightly although significantly larger apertures than that of wild-type stomata (1.87 ± 0.06 µm as compared with 1.64 ± 0.08 µm; Fig. 6B). In response to treatment with 10 µM ABA, the apertures of fa stomata were reduced significantly by 37% (from 1.87 ± 0.06 µm to 1.175 ± 0.03 µm; Fig. 6B). This was similar to the 38% reduction in wild-type stomatal aperture observed following ABA treatment (from 1.64 ± 0.08 µm to 1 ± 0.07 µm). When cross-interactions were tested statistically, no significant difference was found in the response patterns to ABA among fa and wild-type stomata. These results showed that fa stomata respond to ABA much like the stomata of wild-type plants, even though fa stomata had slightly larger initial apertures.
Figure 6.
Stomatal aperture. A, Stomata of the different lines with (+) and without (-) ABA treatment as photographed during the experiments. Stomata chosen for this figure were those whose apertures were most similar to the average for their treatment. Bars = 20 µm. B, Stomatal apertures observed in wild-type (WT) and fa epidermal peels with (+) and without (-) 10 µM ABA treatment. **Significant difference (P < 0.01) between treated and untreated stomata of the same genotype; △ significant difference (P < 0.05) between the untreated fa stomata and the untreated wild-type stomata. Numbers at the bottom of the columns indicate the sample size.
Following these results, we examined how mesophyll insensitivity to ABA affects leaf water potential (ψw) in wild-type and fa plants under ABA treatment and under drought. Under ABA treatment, a significant cross-interaction existed, meaning that fa leaves responded differently than wild-type leaves to ABA treatment and exhibited an increase rather than a decrease in their ψw (Supplemental Fig. S5B). Under drought conditions, whereas wild-type plants exhibited significantly reduced ψw, among fa plants, the reduction in ψw was insignificant (Supplemental Fig. S5D).
Seed Yield of Plants Grown in a Growth Room
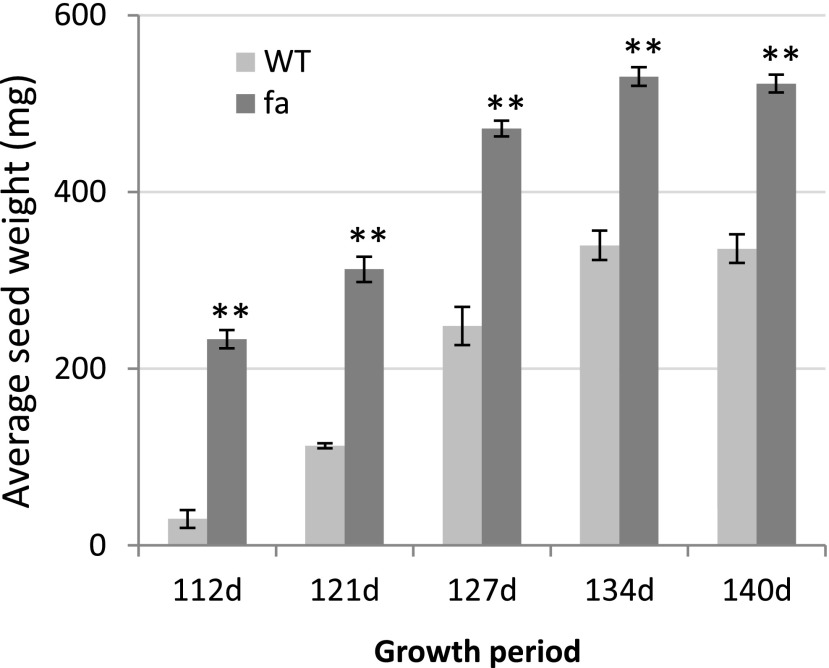
While first characterizing fa plants, we wanted to evaluate how their tissue-specific insensitivity to ABA would affect yield. Seeds were collected approximately once a week for 4 weeks, and irrigation was stopped 2 weeks into seed collection, creating conditions of terminal drought stress. Throughout the seed-collection period, fa yield was significantly higher than wild-type yield (Fig. 7). However, that difference can be attributed to irrigation stoppage that favored the faster-growing fa plants. When plants were allowed to complete their life cycle during the drought experiments, the yield of well-watered wild-type plants was far greater than that of fa plants.
Figure 7.
Average seed weight of fa and wild-type (WT) plants grown under short-day conditions in a growth room and exposed to terminal drought. Mean seed weight (± se) wild type n = 4, fa n = 9 at the different collection dates (days after sowing). **Significant difference (P < 0.01) relative to that in the wild type on the same date, as determined using Student's t test.
Greenhouse Drought Treatment
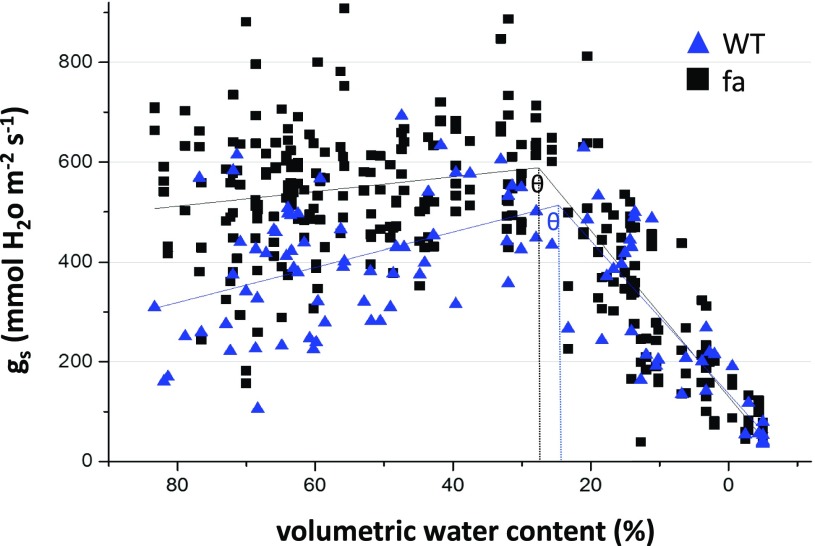
The fa plants' vigorous initial growth and slightly raised gs together with their stomata’s normal response to ABA led us to test their behavior under “field” conditions, that is growing plants in much bigger pots (4 L) in a climate-controlled greenhouse (natural sunlight) and allowing them to complete their life cycle under well-watered as well as water-limited conditions. Our initial results suggested that under well-watered conditions, fa plants would transpire more and along with their enhanced initial growth vigor they would also have higher yields. To examine the gs response to pot relative water content over the course of the drought treatment, we plotted gs against soil water content. Stress conditions were considered to have been initiated after plants had reached the soil water limitation point (θcrit; Halperin et al., 2017). Here again, fa gs was higher than that of the control. The θcrit of fa plants was not significantly different than that of wild-type plants (27.58 ± 1.65% as compared with 24.68 ± 2.65%; Fig. 8).
Figure 8.
Stomatal conductance of wild-type (WT) and fa plants in response to volumetric soil water content. Comparison of the gs patterns of wild-type and fa plants exposed to terminal drought or exposed to 25% irrigation or well-watered, in relation to their soil water content. Trend lines represent best fits in piecewise-linear model as calculated using an Origin program (see “Materials and Methods”). θ, Critical point at which plants began to respond to drought by reducing gs. Wild type n = 38; fa n = 114; wild type R2 = 0.52; fa R2 = 0.685.
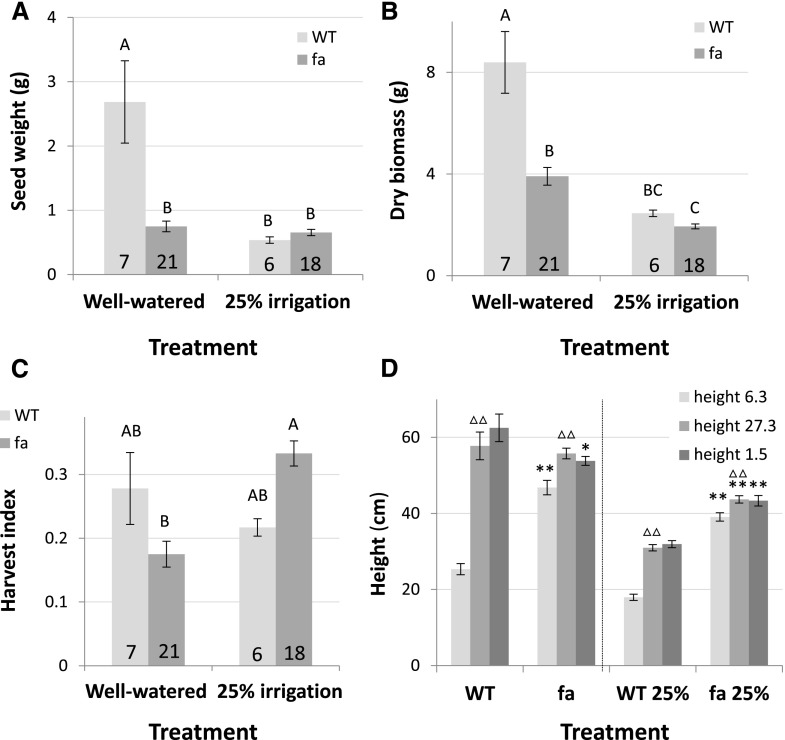
These first results from the drought experiment seemed to support our initial findings obtained in the growth chamber conditions. However, to our surprise, under well-watered conditions, wild-type average seed yield was more than triple that of the fa lines (2.69 ± 0.64 g as compared with 0.75 ± 0.08 g; Fig. 9A) and the dry biomass of wild-type plants was more than double that of fa plants (8.39 ± 1.21 g as compared with 3.9 ± 0.35 g; Fig. 9B). This also led to a greater harvest index in wild-type plants (0.28 ± 0.06 as compared with 0.175 ± 0.02; Fig. 9C). Nevertheless, under water-limited conditions (25% irrigation volume relative to the well-watered control), wild-type seed yield was significantly reduced by almost 80% to 0.54 ± 0.05 g. The seed yield of the transgenic plants was slightly and insignificantly reduced by 13.3% to 0.65 ± 0.05 g. This difference in yield reduction was also expressed in a significant interaction between the line and the irrigation regime (Supplemental Table S1), illustrating that the different genotypes responded differently to drought. Under water-limited conditions, wild-type dry biomass was reduced by 70.8% (to 2.45 ± 0.13 g), as compared with a 49.7% reduction (to 1.94 ± 0.1 g) among fa plants. Here too, there was a significant interaction between the line and the irrigation regime. These different response patterns led to opposite responses in terms of the harvest index, with the harvest index of the wild type reduced under water-limited conditions to 0.22 ± 0.01 and the fa harvest index raised to 0.33 ± 0.02 under those same conditions. This also led to the fa harvest index being significantly higher than that of the wild type under the 25% irrigation regime, and a significant Line × Irrigation Treatment interaction was also seen for that parameter.
Figure 9.
Wild-type (WT) and fa plants' yield, biomass, and plant height in response to drought treatment. A, Seed yield. B, Dry biomass as measured at harvest time. C, Harvest index, as calculated by dividing seed yield by dry shoot biomass. Numbers at the bottom of the columns indicate the sample size. Data are means ± se. Different letters represent a significant difference as determined using Tukey HSD test (P < 0.05). Interactions were tested using a two-way ANOVA. D, wild-type and fa plant height, as measured at three dates over the course of their life cycle (6 March, 27 March, and 1 May) wild type n = 7, wild type25% n = 6, fa n = 18, fa25% n = 18. **Significant difference (P < 0.01) between fa plants and the wild type at the same date and under the same irrigation regime, as determined using Student's t test; triangles represent a significant difference (△△: P < 0.01) between two consecutive measuring dates for the same genotype and irrigation regime, as determined using a Student's t test. Triangles were placed above the later of the two dates.
An additional parameter measured in this experiment was plant height, which was a good way to monitor the growth rates of the different genotypes in a nondestructive manner. Plant height was measured twice during the experiment and again at its conclusion, when plants were cut for biomass measurements. As shown in growth-chamber measurements (Fig. 7), under well-watered as well as water-limited conditions, the vigorous initial growth of fa plants led to their being significantly taller than wild-type plants at the first measurement point. However, under well-watered conditions, the second set of measurements revealed no statistically significant difference between the heights of wild-type and fa plants. Furthermore, at the third measurement point, wild-type plants were significantly taller than fa plants. In contrast, under water-limited conditions, fa plants were taller than wild-type plants at all three measurement points (Fig. 9D).
Chlorophyll Degradation
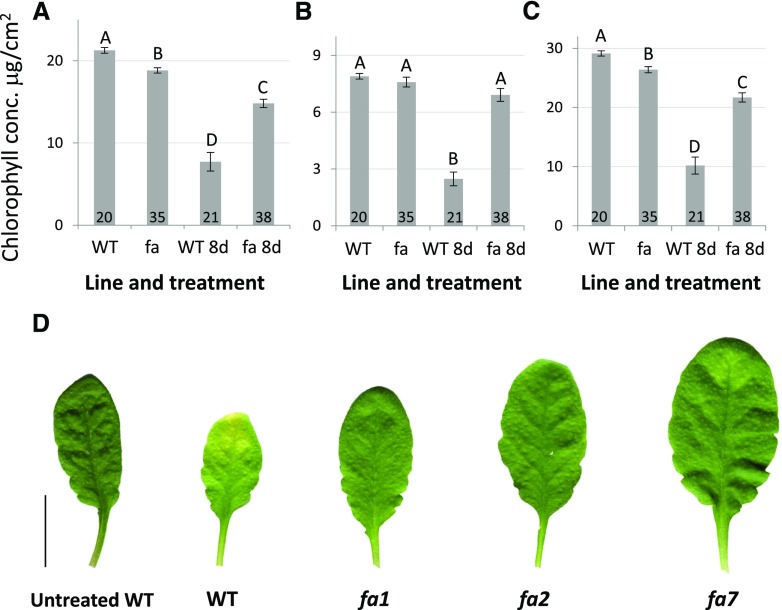
The better performance of fa plants under stress conditions led us to examine their senescence response by measuring chlorophyll degradation. We measured chlorophyll content in light-grown leaves and in leaves that were dark-treated for 8 d. Interestingly, light-grown fa leaves had a significantly lower total chlorophyll content than did light-grown wild-type leaves (29.13 ± 0.45 µg cm−2 as compared with 26.4 ± 0.5 µg cm−2; Fig. 10C). However, following dark treatment, the reduction in the chlorophyll content of fa leaves was much smaller (reduced to 10.2 ± 1.4 µg cm−2 in wild type as compared with 21.7 ± 0.8 µg cm−2 in fa leaves). This led fa total chlorophyll content following dark treatment to be significantly higher than that observed for the wild type, and this differential response was found to be statistically significant (Fig. 10C). This differential response to senescence treatment could also be seen in leaf coloring, with wild-type leaves turning yellow following the senescence treatment, whereas fa leaves remained green (Fig. 10D).
Figure 10.
Chlorophyll concentration (conc.) of wild-type (WT) and fa light-grown leaves or those kept in darkness for 8 d. A, Chlorophyll a. B, Chlorophyll b. C, Total chlorophyll. 8d = 8-d dark treatment. Three separate repetitions were performed for each treatment. Different letters represent a significant difference as determined using the Tukey HSD test (P < 0.05). Numbers at the bottom of the columns indicate the sample size. D, Representative scanning of leaves following senescence treatment, digitally extracted for comparison. Bar = 2 cm.
The response pattern of chlorophyll a was similar to that observed for total chlorophyll and was significantly higher in the wild type before dark treatment (21.3 ± 0.35 µg cm−2 as compared with 18.8 ± 0.3 μg cm−2). Following dark treatment, chlorophyll a content was significantly reduced in both wild-type and fa leaves, although this reduction was far less significant in fa leaves (to 7.7 ± 1.1 µg cm−2 in wild-type leaves and to 14.8 ± 0.5 µg cm−2 in fa leaves; Fig. 10A). This led to fa leaves having significantly higher chlorophyll a levels following dark treatment. Chlorophyll b content behaved differently than chlorophyll a content; there was no significant difference between the chlorophyll b contents of the two lines before dark treatment (7.9 ± 0.15 µg cm−2 as compared with 7.59 ± 0.26 µg cm−2) and chlorophyll b levels of fa leaves were not significantly reduced following dark treatment (wild type: 2.48 ± 0.36 µg cm−2; fa: 6.91 ± 0.34 µg cm−2; Fig. 10B). In wild-type leaves following dark treatment, chlorophyll a and b and total chlorophyll contents were reduced by 63.7%, 68.6%, and 65%, respectively, whereas the chlorophyll a and b and total chlorophyll contents of fa leaves were reduced by 21.3%, 8.9%, and 17.8%, respectively.
DISCUSSION
ABA has been reported to affect the transcription of thousands of genes (Nemhauser et al., 2006) and is reported to have significant redundancy in its signal transduction pathway (Umezawa et al., 2010). Moreover, the ABA response varies in different tissues and under different environmental conditions (Saab et al., 1990; Sade and Moshelion, 2017). This complexity makes it hard to isolate the role of ABA in specific processes. In this study, we used fa plants carrying the ABA-insensitive abi1-1 transgene under the control of a green tissue-specific promoter to isolate ABA responses in those tissues without affecting other key tissues such as stomata and roots (Fig. 1, A–D). The expression of ABA reporter genes did not increase in fa plants following ABA treatment, thus validating fa plants experimental suitability. As could be expected, due to the several negative feedback loops triggered by the ABA response, two out of the three fa lines had higher concentrations of foliar ABA compared with that of the wild type (Fig. 1H). These plants did not exhibit an elevated ABA response due to the abi1-1 mutant’s dominant nature. This enabled us to explore how mesophyll ABA affects shoot growth, photosynthesis, and stress response.
Plant Morphology
An interesting phenotype observed in fa plants was their particularly vigorous initial growth and faster life cycle. In the first weeks after germination, fa plants had a greater leaf area than wild-type plants, and they flowered, produced seed, and died earlier than wild-type plants (Figs. 2 and 3), a phenotype we identified as stress-escape behavior. This phenomenon supports the suggestion that under nonstressed conditions, basal endogenous ABA might play a role in the inhibition of shoot growth (Tung et al., 2008). Thus, the stress-escape behavior of fa plants may be the result of insensitivity to basal ABA levels. Early flowering under short-day conditions is one of the most striking phenotypes of fa plants. Because fa plants also grew faster than wild-type plants, we initially hypothesized that their earlier flowering was induced by the plants reaching a critical mass at which point assimilate accumulation triggers flowering (Sachs and Hackett, 1969; Roldán et al., 1999; Wahl et al., 2013) or their reaching a critical leaf number, similar to what is seen in tomato (Lifschitz and Eshed, 2006). However, we ruled out these hypotheses as fa plants flowered at a much smaller biomass and when they had far fewer leaves (Fig. 3). Few studies have revealed a link between ABA and flowering. Most recently, a link was found between the drought-induced flowering inhibitor SHORT VEGETATIVE PHASE, reduced ABA catabolism, and flowering (Wang et al., 2018). Increased ABA levels have also been reported to delay flowering, although this could be attributed to a general reduction in growth (Lefebvre et al., 2006), and ABA has been found to inhibit floral initiation in Polianthes tuberosa (Su et al., 2002). In Arabidopsis, a possible mechanism was suggested by which ABA inhibits flowering via phosphorylation of ABA-responsive kinase substrate 1 inhibiting ABA-responsive kinase substrate 1 from binding to DNA, which leads to down-regulation of the CONSTANS gene (Takahashi et al., 2016). Phloem-localized FLOWERING LOCAS T expression (Corbesier et al., 2007) coincides with leaf insensitivity to ABA-inducing flowering even though floral meristems are unaffected in fa plants.
It is interesting to note that although CONSTANS does not affect flower morphology (Pidkowich et al., 1999), fa flowers were significantly smaller than wild-type flowers (Fig. 4). Barrero et al. (2005) noted smaller flowers among ABA-deficient mutants and attributed this to the mutants’ generally inhibited growth (Barrero et al., 2005). However, because the growth of fa plants was not inhibited at stages before flowering, this does not seem to be the reason for the smaller flowers observed in our study.
Gas Exchange
ABA is known to reduce transpiration and photosynthesis via stomatal closure. Nevertheless, there has been some debate as to whether ABA has a direct effect on photosynthesis in mesophyll tissue in a pathway that is not related to limited availability of CO2 (due to stomatal closure). Gas-exchange experiments were designed to answer this question. The insensitivity of the fa mesophyll tissue to ABA together with normal ABA-induced stomatal closure meant that if ABA does indeed affect the biochemistry of angiosperm photosynthesis, the transgenic plants would show elevated carbon assimilation due to insensitivity to the hormone. Initially, we verified that fa plants exhibited reduced stomatal conductance in response to ABA treatment in a manner similar to that observed for the wild type (Figs. 5A and 6). Although the gs of fa plants was greater than that of the wild type when ABA was not applied, the two sets of plants responded similarly to ABA treatment (no statistical interaction between plant type and ABA treatment). The results showed that among fa and wild-type plants, there was no difference in the reduction of AN in response to ABA (Fig. 5B). We therefore concluded that ABA does not directly inhibit photosynthesis under the examined conditions. These results received further validation from the A/Ci curve results showing that photosynthetic efficiency was unaltered in fa plants (Supplemental Fig. S4).
One question that arose from these experiments was as follows: If fa stomata respond normally to ABA, what is the reason for their wider stomatal apertures and higher gs? The wide-ranging developmental and hydraulic effects of ABA could affect this phenotype, for example, by altering leaf hydraulic conductance (Shatil-Cohen et al., 2011) and cell wall development (Wakabayashi et al., 1989). However, further investigation of this question is beyond the scope of this study.
fa “Field” Experiments
The fa plants had higher yields (i.e. dry seed biomass) than did wild-type plants under short-day conditions in the growth room, under conditions of terminal drought stress (Fig. 7). We wanted to test whether fa plants would maintain those yield levels under well-watered and water-limited conditions that more closely resembled field conditions (i.e. larger soil volume, daylight radiation intensity, and a day/night temperature gradient). We hypothesized that fa insensitivity to ABA would lead to higher yields under nonterminal drought conditions via a stay green-like phenotype (Rivero et al., 2007). In the yield experiments performed in the greenhouse, we allowed plants to complete their life cycle under well-irrigated conditions. In our preliminary experiment in the growth room, irrigation was stopped before that point, which resulted in fa plant yields exceeding those of wild-type plants due to their faster growth and earlier flowering stress-escape behavior (Fig. 7). However, in the greenhouse, well-watered wild-type plants yielded far more than fa plants and had a better harvest index as well (Fig. 9). In fact, fa plants seemed to have a more rapid life cycle not only in terms of early growth vigor and flowering, but also in terms of earlier senescence and drying. However, under water-limited conditions, the yield of wild-type plants was limited and similar to that of fa plants, probably due to limitation of the wild-type reproductive phase by shortening of the plant life cycle. Furthermore, whereas drought treatment reduced the wild-type harvest index, it improved the harvest index of fa plants.
Taken together, these results somewhat surprised us. Because ABA is a hormone that regulates the response to stress, we assumed that high levels of ABA would induce an escape mechanism and would be in line with the understanding that ABA promotes senescence and the allocation of resources to seeds. ABA's role in regulating plant stress-escape behavior is still the subject of debate (Riboni et al., 2013). In fa plants, insensitivity to ABA caused what can be seen as a stress-escape mechanism: an accelerated and shorter life cycle that is beneficial under terminal drought conditions (as seen in the growth-room yield experiment; Fig. 7; Negin and Moshelion, 2017). This stress-escape mechanism may be beneficial under water-limited conditions, but it limits yield potential under nonstressed conditions. This stress-escape behavior is exemplified by the plant height measurements; fa plants initially grew faster than wild-type plants, but stopped growing earlier under well-watered conditions and, at the end of the experiment, were significantly shorter than wild-type plants. However, under water-limited conditions, fa plants were taller at all three measurement points, apparently due to their initial rapid growth before the onset of drought (Fig. 9D).
These results are congruent with those of Franks (2011), who examined drought escape mechanisms in Brassica rapa and found that stress-escape mechanisms were coupled with increased transpiration and reduced water-use efficiency. Those findings hint at a reduction in ABA synthesis or sensitivity. In addition, Franks (2011) mentioned the trade-off between drought tolerance and escape, meaning that plants that use drought escape mechanisms cannot be drought-tolerant and vice versa (Franks, 2011).
The embodiment of the different stress responses can be seen in the response of the plants’ harvest index to drought treatment. Wild-type plants, which do not use stress-escape mechanisms, reach maximal yields under well-watered conditions and, therefore, develop a massive shoot as compared with fa plants. Pending completion of their life cycle, biomass is directly related to seed yield. However, under drought conditions, wild-type plants’ initial growth of a large shoot cannot be translated into seed yield and their harvest index is reduced. In fa plants, on the other hand, because a stress-escape mechanism is used, the seed yield is hardly affected by drought and the reduction in plant biomass under water-limited conditions leads to an improved harvest index (Fig. 9).
In addition, although fa plants had significantly higher gs compared with that of the wild type, their response to drought was unimpaired due to normal stomatal closure. Such behavior, that is, the uncoupling of gs from the stress response under well-watered conditions, could be better suited for dealing with mild stress conditions, since it allows for high levels of transpiration and photosynthesis under well-watered conditions, together with a normal stress response.
Yield Stability
Another aspect of the stress-escape mechanism is its effect on yield stability. Whereas wild-type plants exhibited a severe 80% reduction in their yield following drought treatment (as compared with the well-irrigated control), the yield of fa plants was reduced by only 13% (Fig. 9). Furthermore, these results were obtained under relatively mild stress and it can be expected that under more severe stress wild-type plants would show an even greater yield penalty, whereas fa plants would suffer less. Stress-escape mechanisms are especially beneficial in drought-prone environments where, following initial rainfall events, there is no certainty that there will be any additional rain. In such a situation, plants that use early rain to complete their life cycle, although not achieving high yield in rainy years, will produce a small, but stable yield, which is beneficial in uncertain environments. That being said, the examination of ABA’s involvement in stress-escape mechanisms in terms of yield measurements should be approached cautiously until the relevant relationships can be examined in additional plant (preferably crop) species.
Leaf Senescence
Under a senescence-inducing dark treatment, fa leaves exhibited delayed chlorophyll degradation as compared with wild-type leaves (Fig. 10), supporting ABA's role in promoting leaf senescence. It seems as if fa plants exhibit a contradicting stay-green phenotype and an escape phenotype simultaneously. These results and those of the yield experiments may together be explained by the escape mechanism, which leads to a programmed shorter life cycle regardless of the stay-green phenotype. Similar to early flowering, the apparent contradiction between the stay-green phenotype and the stress-escape response suggests that this stress-escape mechanism is not a side effect of ABA’s regulation of flowering and senescence, but rather a separate and independent stress response.
CONCLUSIONS
The role of basal endogenous ABA in the green tissues (mainly the mesophyll) of plants growing under nonstressed conditions may be involved in restraining mechanisms of early growth vigor and flowering. This enables the inhibition of the stress-escape response that is exhibited by plants insensitive to green-tissue ABA.
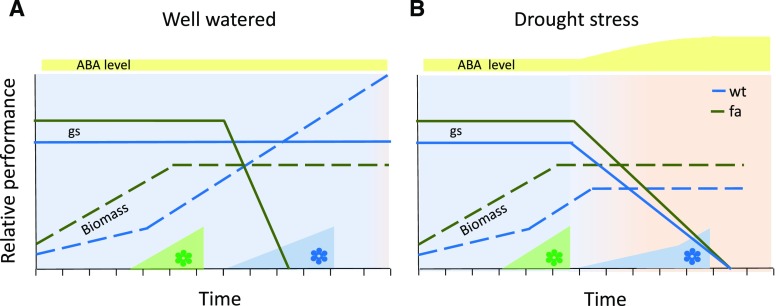
This inhibition of a stress-escape mechanism under optimal conditions allows plants to reach maximum potential yields, as exhibited by the wild-type plants in this work. In contrast, under stress, the plants that were insensitive to ABA and escaped the stress reached a lower than optimal, but stable yield, comparable with the lower yield of the stressed wild-type plants. The fact that both sets of plants reached similar yields under stress explains the evolutionary advantage of the wild type’s opportunistic behavior (Fig. 11).
Figure 11.
Schematic model of the relative development and physiological performance of wild-type (wt) and fa plants under drought-stress and well-watered conditions. A, Under well-watered conditions (i.e. no drought stress induction), ABA levels are relatively constant. The fa plants exhibit stress-escape behavior that includes greater initial growth vigor and gs, and earlier flowering time, as compared with that in the wild type. However, the growth rate and gs of fa plants decline more quickly as the plant senesces, whereas the wild-type gs remains constant and wild-type plant biomass surpasses that of fa plants. These differences are mirrored in yield levels. B, Under drought conditions, plants reduce their gs at a similar point and with a similar pattern. The fa stress-escape behavior results in a less severe yield penalty, as compared with the wild type. The fa flowering initiated under well-watered conditions is not affected by drought. Wild-type plants exhibit delayed flowering at the beginning of drought, but later reach a high flowering percentage (wild-type flowering under drought stress based on Su et al., 2013). Taken together, this model exemplifies the payoff of the stress-escape mechanism. Under optimal conditions, this mechanism severely limits yield potential. However, under drought conditions that begin in the middle of the growing season, this mechanism reduces the yield penalty and maintains yield stability.
We suggest that ABA’s role in leaf senescence, which is in opposition to its role in the inhibition of the stress-escape response, suggests that this inhibition involves a mechanism independent of a stay-green phenotype. Similarly, the lack of correlation between flowering and productivity measurements such as photosynthetic rate and biomass indicates that the observed inhibition of flowering is part of a signal transduction pathway that inhibits the stress-escape response and, in this case, is not caused by the inhibition of vegetative growth. Finally, we conclude that although ABA promotes chlorophyll degradation, in the short term this phytohormone does not directly inhibit the photosynthetic machinery.
MATERIALS AND METHODS
Plant Material and Growing Conditions
All Arabidopsis (Arabidopsis thaliana) plants used in this study were of the Colombia (col) ecotype, and this ecotype is referred to as the wild type. In addition to wild-type plants, in all of the Arabidopsis experiments, we used transgenic FBPase::abi1-1 (fa) plants. For physiological experiments, Arabidopsis plants were grown in a growth room with short-day conditions (10 h light/14 h dark) at temperatures of 18°C to 22°C. Plants grown for seed, and long-day flowering experiments were grown in a growth room kept at a temperature of 22°C with 16 h of light. For drought and yield experiments, plants were grown in a temperature-controlled greenhouse with temperatures ranging between 18°C and 22°C from December through April (2016). When grown in Murashige and Skoog plates, plants were grown in a cell-culture room at a temperature of 22°C with 16 h of light each day.
FBPase::abi1-1 Constructs and Plant Transformation
For construct assembly, the MultiSite Gateway Three-Fragment Vector Construction Kit (Invitrogen) was used according to the manufacturer's instructions. For green tissue-specific expression, the FBPase promoter (Lloyd et al., 1991; Sade et al., 2014a) was used. FBPase is a chloroplastic protein encoded in the nucleus and expressed specifically in green tissues, but not in stomata (Fig. 1). The gene used was the abi1-1 gene, which was PCR amplified from abi1-1 plants (Koornneef et al., 1984). The DONR plasmids were inserted into the binary pB7M24GW (Invitrogen) plasmid containing the 35S terminator and the BAR glufosinate-resistance gene. Plasmids were inserted into Arabidopsis plants from the col ecotype using the floral-dip method (Clough and Bent, 1998). Four lines that exhibited segregation indicative of the presence of one insert (fa1, fa2, fa6, and fa7) were chosen, although in the end, experiments were performed without the fa6 line. Homozygote t3 lines were selected and used throughout the study. The presence of the transgene containing the G to A substitution was confirmed by sequencing t3 plant DNA.
Physiological and Morphological Characterization of Transgenic Plants
For quantitative analysis of RAB18, NCED3, and ABI5 expression by RT-qPCR, leaves were harvested from each plant and immediately immersed into liquid nitrogen. Total RNA was extracted using total RNA mini kit (Geneaid Biotech). cDNA was prepared using the qPCRBIO cDNA synthesis kit (PCR Biosystems) according to the manufacturer’s instructions. qPCR was performed in the presence of SYBR Green ROX Mix (Thermo Fisher Scientific) in a Corbett Research Rotor-Gene 6000 cycler. The reaction was 30 s at 94°C followed by 40 cycles consisting of 10 s at 94°C, 30 s at 60°C and 20 s at 72°C. Analysis was performed using Rotor-Gene 6000 series software 1.7. The PCR primers used for amplification of RAB18 were forward: 5′ TTACCAGAACCGTCCAGGAG 3′ and reverse: 5′ ACCACCACCAGTTCCGTATC 3′, for NCED3 were forward: 5′ AAAGCCATCGGTGAGCTTCA 3′ and reverse: 5′ GCAGCTCTGGCGTAGAATAG 3′, and for ABI5 were forward: 5′ TGGAGAGGAAGAGGAAGCAA 3′ and reverse 5′ CTCGGGTTCCTCATCAATGT 3′. The specificity of the primers was determined by a melting curve analysis; a single, sharp peak in the melting curve indicates that a single, specific DNA sequence was amplified. Arabidopsis TUB2 (b-tubulin; Kelly et al., 2017) was used as a reference for the standardization of quantities of cDNA.
Foliar ABA concentrations were measured using a liquid chromatography-mass spectrometry system, which consisted of Dionex Ultimate 3000 RS HPLC coupled to Q Exactive Plus hybrid Fourier Transform mass spectrometer equipped with heated electrospray ionization source (Thermo Fisher Scientific). The HPLC separations were carried out using an Acclaim C18 column (2.1 × 150 mm, particle size 2.2 µm; Dionex). The mass spectrometer was operated in negative ionization mode, with a spray voltage of 3 kV, capillary temperature of 300°C, electrospray ionization capillary temperature of 300°C, sheath gas rate (arb) of 40, and auxiliary gas rate (arb) of 10. Mass spectra were acquired in the m/z 130-600 D range at a resolving power of 70,000. Data analysis was carried out using Xcalibur software (Thermo Fisher Scientific). For calibration and analysis, abscisic acid and abscisic acid-D6 (internal standard) were purchased from Toronto Research Chemicals.
Short-day flowering was evaluated in a temperature-controlled greenhouse during January and February (2016). Plants were considered to be “flowering” when flower buds could be seen, before bolting. Among these plants, six mini-blocks were used for biomass evaluation at flowering. In these blocks, once budding was noticed, the plant rosette was cut at midday and put into a zipper-locked bag. These rosettes were weighed, and then dried in a 60°C oven and weighed again to determine dry biomass. For the evaluation of flowering under long-day conditions, both budding and bolting were evaluated, due to the plants’ small size at the time of flowering, which made recognition of flowering buds before bolting difficult. For the evaluation of leaf number at flowering, plants were grown in a short-day growth room. Once the plants reached the bolting stage, rosettes were harvested and leaves were separated and scanned using an Epson Perfection v37 scanner (www.epson.com). Leaves longer than 1 cm were counted, and leaf area was analyzed using ImageJ software (https://imagej.nih.gov/ij/).
Flower size was evaluated in well-watered plants from the temperature-controlled greenhouse. Flowers were cut and photographed through an Olympus SZX7 binocular scope (http://www.olympus-global.com) with an Olympus LC20 camera. Flower area and style height were then calculated using ImageJ software. For leaf area and root length, see Supplemental Methods.
Leaf Gas-Exchange Measurements
Gas-exchange measurements, including stomatal conductance (gs), carbon assimilation (AN), Ci, and transpiration (E) were performed on leaves of 6- to 8-week-old plants grown in a short-day growth room. Leaves were excised before the light in the growth room was turned on. Two leaves were cut from each plant and were put into control tubes containing artificial xylem sap (1 mm K2HPO4, 1 mm KH2PO4, 1 mm CaCl, 0.1 mm MgSO4, 3 mm KNO3, 0.1 mm MnSO4, and 0.01% [v/v] dimethyl sulfoxide, titrated to pH 5.8 using KOH; Shatil-Cohen et al., 2011) or artificial xylem sap with ABA at a final concentration of 10 µM. The leaves were then put into hermetically sealed transparent boxes and left under lighting for 1 h. Following that period, the lids were opened for 5 min, after which gas exchange was measured using the LI-6400xt portable gas-exchange system (LI-COR). Cuvette conditions were set to 400 µL L−1 CO2, photosynthetically active radiation 200 µE m−2 s−1, vapor pressure deficit ∼1.5, and a flow of 200 µmol s−1. For A/Ci curves and ψw, see Supplemental Methods.
Stomatal Aperture
Leaves of 6- to 8-week-old plants grown in a short-day growth room were cut during the morning hours. Two epidermal strips were peeled from each leaf. One strip was put into stomatal opening buffer (containing 20 mm KCl, 1 mm CaCl2, and 5 mm MES hydrate, and titrated to pH 6.15 using KOH; Acharya et al., 2013), and the other was put into the same buffer that would later be supplemented with ABA. Following 2 h in the light, ABA was added to a final concentration of 10 µM and dimethyl sulfoxide was added to the control treatment to a final concentration of 0.01% (v/v). After another 1.5 h in the light, stomata were photographed at a magnification of 40× and stomatal aperture was analyzed using ImageJ software.
Arabidopsis Greenhouse Drought Experiment: Setup
Plants were grown in a temperature-controlled greenhouse in 4-L pots, with an upside-down 1-L pot inserted in the middle of each pot in order to reduce pot volume while maintaining surface area. Each pot contained a block of all of the experimental plants: wild type, fa1, fa2, and fa7. Plant location in the pot was randomized. Until flowering, all pots received the same irrigation regime, using 4 L/h dripper heads. For experimental setup, also see Supplemental Fig. S11. Once flowering began, pots were divided into two irrigation regimes: well-watered and 25% of well-watered (this was done by changing the dripper head from 4 L/h to 1 L/h). Plants were then grown until the end of their life cycle.
Arabidopsis Drought-Treatment Measurements
The gs levels of plants were measured after the initiation of the drought treatment with the first measurement, performed at t0, considered “well-watered.” Measurements were taken using a leaf porometer (SC-1 Porometer, Decagon Devices) during the morning hours (9 to 11 am). At the same time, soil water content was measured using a Prochek probe (Decagon Devices).
Seeds of plants from the different watering regimes were continually collected (twice a week) due to different flowering, fruit ripening, and plant death times. Dry biomass was measured at the experiment's end by harvesting plants' aerial parts and drying that tissue in an oven at 60°C for a week. Seed weight was measured after a similar drying procedure.
Chlorophyll Content
Leaf discs with a radius of 3 mm were cut from 6- to 8-week-old Arabidopsis plants grown in a short-day growth room. Discs were cut from untreated leaves, as well as leaves that had undergone a senescence treatment that included incubation in the dark for 8 d (Markovich et al., 2017). The discs were put into 80 v/v% acetone and stored in a freezer (−20°C) for at least 1 week. Following that treatment, absorption was read at two different wave lengths (663 nm and 645 nm) using a spectrophotometer (MRC V-1100D). Chlorophyll content was then calculated using the following equations: chlorophyll a (mg) = 663 nm absorption × 12.4-645 nm absorption × 2.7; chlorophyll b (mg) = 645 nm absorption × 23-663 nm absorption × 4.7; and total chlorophyll was calculated as the sum of chlorophyll a + b (Bruinsma, 1963).
Statistical Analyses
To plot soil water content against transpiration in the Arabidopsis drought experiments, a two-piece linear fit through the origin program (http://www.originlab.com) was used. In the other experiments, when two variables were examined (e.g. line and ABA treatment), the interaction between those factors was evaluated using a two-way ANOVA. When no significant interaction existed in the ANOVA, Student's t test was used. In cases in which there was a significant cross interaction, the Tukey–HSD test was used. JMP 12 pro (SAS Institute; http://www.jmp.com/en_us/home.html) was used for all analyses, except for the comparison of soil water content with gs. The different p-values attained from the two-way ANOVAs can be found in Supplemental Table S1.
Accession Numbers
Accession numbers of major genes mentioned in this paper are FBPase promoter – AR390745; abi1-1 - AT4G26080.1 polymorphism 4769512; NCED3 - AT3G14440.1; RAB18 – AT5G66400.1; and ABI5 - AT2G36270; TUB2 – AT5G62690.
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Semiquantification of the native ABI1 gene vs. the abi1-1 mutant gene in wild type, fa and col-abi1-1 leaves.
Supplemental Figure S2. fa plants had significantly larger leaves compared to wild type.
Supplemental Figure S3. Root length of 9-d-old fa and wild type seedlings grown on perpendicularly placed plates.
Supplemental Figure S4. fa and wild type leaves exhibited similar CO2 assimilation/leaf intercellular CO2 (A/Ci) curves.
Supplemental Figure S5. Effects of ABA treatment and drought on the leaf water potentials of fa and wild type plants.
Supplemental Figure S6. Flowering data (as shown in Fig. 3) broken down to illustrate results for individual fa lines.
Supplemental Figure S7. Flower size and style-length data (as shown in Fig. 4) broken down to illustrate results for individual fa lines.
Supplemental Figure S8. Wild type and fa gas exchange in response to ABA treatment (as shown in Fig. 5) for the individual lines.
Supplemental Figure S9. Stomatal-aperture data, separated out for individual fa lines.
Supplemental Figure S10. Wild type and fa plants' yield, biomass and harvest-index response to drought treatment; data presented for individual fa lines.
Supplemental Figure S11. Experimental setup for the greenhouse drought treatment.
Supplemental Table S1. p values of two way anovas performed.
Footnotes
This work was supported by the Israel Ministry of Agriculture and Rural Development (Eugene Kandel Knowledge centers) as part of the “Root of the Matter” — The root zone knowledge center for leveraging modern agriculture; the Israel Science Foundation (ISF) (grant no. 876/16); and the United States-Israel Binational Science Foundation (BSF) (grant no. 2015100).
Articles can be viewed without a subscription.
References
- Acharya BR, Jeon BW, Zhang W, Assmann SM (2013) Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol 200: 1049–1063 [DOI] [PubMed] [Google Scholar]
- Aroca R, Del Mar Alguacil M, Vernieri P, Ruiz-Lozano JM (2008) Plant responses to drought stress and exogenous ABA application are modulated differently by mycorrhization in tomato and an ABA-deficient mutant (sitiens). Microb Ecol 56: 704–719 [DOI] [PubMed] [Google Scholar]
- Barrero JM, Piqueras P, González-Guzmán M, Serrano R, Rodríguez PL, Ponce MR, Micol JL (2005) A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot 56: 2071–2083 [DOI] [PubMed] [Google Scholar]
- Blum A. (2009) Effective use of water (EUW) and not water-use efficiency (WUE) is the target of crop yield improvement under drought stress. F Crop Res 112: 119–123 [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Bruinsma J. (1963) The quantitative analysis of chlorophylls a and b in plant extracts. Photochem Photobiol 2: 241–249 [Google Scholar]
- Chen G, Lips SH, Sagi M (2002) Biomass production, transpiration rate and endogenous abscisic acid levels in grafts of flacca and wild-type tomato (Lycopersicon esculentum). Funct Plant Biol 29: 1329–1335 [DOI] [PubMed] [Google Scholar]
- Christmann A, Weiler EW, Steudle E, Grill E (2007) A hydraulic signal in root-to-shoot signalling of water shortage. Plant J 52: 167–174 [DOI] [PubMed] [Google Scholar]
- Christmann A, Grill E, Huang J (2013) Hydraulic signals in long-distance signaling. Curr Opin Plant Biol 16: 293–300 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- de Wit CT. (1958) Transpiration and Crop Yields. Institute of Biological and Chemical Research on Field Crops and Herbage, Wageningen, The Netherlands, http://edepot.wur.nl/186445
- Downton WJS, Loveys BR, Grant WJR (1988) Stomatal closure fully accounts for the inhibition of photosynthesis by abscisic acid. New Phytol 108: 263–266 [DOI] [PubMed] [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475 [Google Scholar]
- Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann Bot 89: 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol (Stuttg) 6: 269–279 [DOI] [PubMed] [Google Scholar]
- Franks SJ. (2011) Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol 190: 249–257 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M, Hiratsu K, Ohme-Takagi M, Shinozaki K, Yamaguchi-Shinozaki K (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17: 3470–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Gao J, Zhu X, Song Y, Li Z, Ren G, Zhou X, Kuai B (2016) ABF2, ABF3 and ABF4 promote ABA-mediated chlorophyll degradation and leaf senescence by transcriptional activation of chlorophyll catabolic genes and senescence-associated genes in Arabidopsis. Mol Plant 9: 1272–1285 [DOI] [PubMed] [Google Scholar]
- González-Guzmán M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Pérez-Amador MA, Kollist H, et al. (2012) PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin O, Gebremedhin A, Wallach R, Moshelion M (2017) High‐throughput physiological phenotyping and screening system for the characterization of plant–environment interactions. Plant J 89: 839–850 [DOI] [PubMed] [Google Scholar]
- Holbrook NM, Shashidhar VR, James RA, Munns R (2002) Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J Exp Bot 53: 1503–1514 [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez J-P, Hanafey MK, Tingey SV, Chua N-H (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115: 4891–4900 [DOI] [PubMed] [Google Scholar]
- Iuchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27: 325–333 [DOI] [PubMed] [Google Scholar]
- Joshi-Saha A, Valon C, Leung J (2011) Abscisic acid signal off the STARting block. Mol Plant 4: 562–580 [DOI] [PubMed] [Google Scholar]
- Kelly G, Lugassi N, Belausov E, Wolf D, Khamaisi B, Brandsma D, Kottapalli J, Fidel L, Ben-Zvi B, Egbaria A, Acheampong AK, Zheng C, et al. (2017) The Solanum tuberosum KST1 partial promoter as a tool for guard cell expression in multiple plant species. J Exp Bot 68: 2885–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemanian AR, Stöckle CO, Huggins DR (2005) Transpiration-use efficiency of barley. Agric Meteorol 130: 1–11 [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid‐insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 377–383 [Google Scholar]
- Lång V, Palva ET (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion-Poll A (2006) Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45: 309–319 [DOI] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J (1994) Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264: 1448–1452 [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang J, Wong MH (1997) Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynth Res 51: 149–159 [Google Scholar]
- Lifschitz E, Eshed Y (2006) Universal florigenic signals triggered by FT homologues regulate growth and flowering cycles in perennial day-neutral tomato. J Exp Bot 57: 3405–3414 [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Raines CA, John UP, Dyer TAA (1991) The chloroplast FBPase gene of wheat: Structure and expression of the promoter in photosynthetic and meristematic cells of transgenic tobacco plants. Mol Gen Genet 225: 209–216 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Radin JW, Turcotte EL, Percy R, Zeiger E (1994) High yields in advanced lines of Pima cotton are associated with higher stomatal conductance, reduced leaf area and lower leaf temperature. Physiol Plant 92: 266–272 [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Markovich O, Steiner E, Kouřil Š, Tarkowski P, Aharoni A, Elbaum R (2017) Silicon promotes cytokinin biosynthesis and delays senescence in Arabidopsis and Sorghum. Plant Cell Environ 40: 1189–1196 [DOI] [PubMed] [Google Scholar]
- Mason Robertson J, Hubick KT, Yeung EC, Reid DM (1990) Developmental responses to drought and abscisic acid in sunflower roots I. Root growth, apical anatomy, osmotic adjustment. J Exp Bot 41: 325–327 [Google Scholar]
- Matsui A, Ishida J, Morosawa T, Mochizuki Y, Kaminuma E, Endo TA, Okamoto M, Nambara E, Nakajima M, Kawashima M, et al. (2008) Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol 49: 1135–1149 [DOI] [PubMed] [Google Scholar]
- Mawson BT, Colman B, Cummins WR (1981) Abscisic acid and photosynthesis in isolated leaf mesophyll cell. Plant Physiol 67: 233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2012) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2018) Mesophyll cells are the main site of abscisic acid biosynthesis in water-stressed leaves. Plant Physiol 177: 911–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264: 1452–1455 [DOI] [PubMed] [Google Scholar]
- Mizoguchi M, Umezawa T, Nakashima K, Kidokoro S, Takasaki H, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K (2010) Two closely related subclass II SnRK2 protein kinases cooperatively regulate drought-inducible gene expression. Plant Cell Physiol 51: 842–847 [DOI] [PubMed] [Google Scholar]
- Negin B, Moshelion M (2017) The advantages of functional phenotyping in pre-field screening for drought-tolerant crops. Funct Plant Biol 44: 107–118 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126: 467–475 [DOI] [PubMed] [Google Scholar]
- Pantin F, Monnet F, Jannaud D, Costa JM, Renaud J, Muller B, Simonneau T, Genty B (2013) The dual effect of abscisic acid on stomata. New Phytol 197: 65–72 [DOI] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Tsz-fung FC (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidkowich MS, Klenz JE, Haughn GW (1999) The making of a flower: Control of floral meristem identity in Arabidopsis. Trends Plant Sci 4: 64–70 [DOI] [PubMed] [Google Scholar]
- Popova LP, Tsonev TD, Lazova GN, Stoinova ZG (1996) Drought‐and ABA‐induced changes in photosynthesis of barley plants. Physiol Plant 96: 623–629 [Google Scholar]
- Qin X, Zeevaart JAD (2002) Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol 128: 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K, Hedrich R (1985) Simultaneous and independent effects of abscisic acid on stomata and the photosynthetic apparatus in whole leaves. Planta 163: 105–118 [DOI] [PubMed] [Google Scholar]
- Riboni M, Galbiati M, Tonelli C, Conti L (2013) GIGANTEA enables drought escape response via abscisic acid-dependent activation of the florigens and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS. Plant Physiol 162: 1706–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA. (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51: 447–458 [DOI] [PubMed] [Google Scholar]
- Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc Natl Acad Sci USA 104: 19631–19636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán M, Gómez-Mena C, Ruiz-García L, Salinas J, Martínez-Zapater JM (1999) Sucrose availability on the aerial part of the plant promotes morphogenesis and flowering of Arabidopsis in the dark. Plant J 20: 581–590 [DOI] [PubMed] [Google Scholar]
- Saab IN, Sharp RE, Pritchard J, Voetberg GS (1990) Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol 93: 1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs RM, Hackett WP (1969) Control of vegetative and reproductive development in seed plants. HortScience 4: 103–107 [Google Scholar]
- Sade N, Moshelion M (2017) Plant aquaporins and abiotic stress. In Chaumont F and Tyerman S, eds, Plant Aquaporins. Signaling and Communication in Plants. Springer, Cham, Switzerland, pp 185–206 [Google Scholar]
- Sade N, Gallé A, Flexas J, Lerner S, Peleg G, Yaaran A, Moshelion M (2014a) Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 239: 357–366 [DOI] [PubMed] [Google Scholar]
- Sade N, Shatil-Cohen A, Attia Z, Maurel C, Boursiac Y, Kelly G, Granot D, Yaaran A, Lerner S, Moshelion M (2014b) The role of plasma membrane aquaporins in regulating the bundle sheath-mesophyll continuum and leaf hydraulics. Plant Physiol 166: 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Scazzocchio C, Fluhr R (2002) The absence of molybdenum cofactor sulfuration is the primary cause of the flacca phenotype in tomato plants. Plant J 31: 305–317 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001) Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature 410: 327–330 [DOI] [PubMed] [Google Scholar]
- Seiler C, Harshavardhan VT, Reddy PS, Hensel G, Kumlehn J, Eschen-Lippold L, Rajesh K, Korzun V, Wobus U, Lee J, et al. (2014) Abscisic acid flux alterations result in differential abscisic acid signaling responses and impact assimilation efficiency in barley under terminal drought stress. Plant Physiol 164: 1677–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Ishida J, Narusaka M, Fujita M, Nanjo T, Umezawa T, Kamiya A, Nakajima M, Enju A, Sakurai T, et al. (2002) Monitoring the expression pattern of around 7,000 Arabidopsis genes under ABA treatments using a full-length cDNA microarray. Funct Integr Genomics 2: 282–291 [DOI] [PubMed] [Google Scholar]
- Shatil-Cohen A, Attia Z, Moshelion M (2011) Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne ABA? Plant J 67: 72–80 [DOI] [PubMed] [Google Scholar]
- Sinclair TR, Tanner CB, Bennett JM (1984) Water-use efficiency in crop production. Bioscience 34: 36–40 [Google Scholar]
- Sreenivasulu N, Harshavardhan VT, Govind G, Seiler C, Kohli A (2012) Contrapuntal role of ABA: Does it mediate stress tolerance or plant growth retardation under long-term drought stress? Gene 506: 265–273 [DOI] [PubMed] [Google Scholar]
- Su W-R, Huang K-L, Shen R-S, Chen W-S (2002) Abscisic acid affects floral initiation in Polianthes tuberosa. J Plant Physiol 159: 557–559 [Google Scholar]
- Su Z, Ma X, Guo H, Sukiran NL, Guo B, Assmann SM, Ma H (2013) Flower development under drought stress: Morphological and transcriptomic analyses reveal acute responses and long-term acclimation in Arabidopsis. Plant Cell 25: 3785–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E (2006) Plant Physiology, Ed 4. Sinauer Associates, Sundeland, Massachusetts [Google Scholar]
- Takahashi Y, Kinoshita T, Matsumoto M, Shimazaki K (2016) Inhibition of the Arabidopsis bHLH transcription factor by monomerization through abscisic acid-induced phosphorylation. Plant J 87: 559–567 [DOI] [PubMed] [Google Scholar]
- Takezawa D, Komatsu K, Sakata Y (2011) ABA in bryophytes: How a universal growth regulator in life became a plant hormone? J Plant Res 124: 437–453 [DOI] [PubMed] [Google Scholar]
- Tanner CB, Sinclair TR (1983) Efficient water use in crop production: research or re-search? In Taylor HM, Jordan WR, Sinclair TR, eds, Limitations to Efficient Water Use in Crop Production. American Society of Agronomy, Wisconsin, pp 1–27 [Google Scholar]
- Travaglia C, Reinoso H, Cohen A, Luna C, Tommasino E, Castillo C, Bottini R (2010) Exogenous ABA increases yield in field-grown wheat with moderate water restriction. J Plant Growth Regul 29: 366–374 [Google Scholar]
- Tung SA, Smeeton R, White CA, Black CR, Taylor IB, Hilton HW, Thompson AJ (2008) Over-expression of LeNCED1 in tomato (Solanum lycopersicum L.) with the rbcS3C promoter allows recovery of lines that accumulate very high levels of abscisic acid and exhibit severe phenotypes. Plant Cell Environ 31: 968–981 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol 51: 1821–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Sakurai N, Kuraishi S (1989) Effects of ABA on synthesis of cell-wall polysaccharides in segments of etiolated squash [Cucurbita maxima] hypocotyl I. Changes in incorporation of glucose and myo-inositol into cell-wall components. Plant Cell Physiol 30: 99–105. [Google Scholar]
- Wang RS, Pandey S, Li S, Gookin TE, Zhao Z, Albert R, Assmann SM (2011) Common and unique elements of the ABA-regulated transcriptome of Arabidopsis guard cells. BMC Genomics 12: 21621554708 [Google Scholar]
- Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, McCourt P, Huang Y (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. Plant J 43: 413–424 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang F, Hong Y, Yao J, Ren Z, Shi H, Zhu J-K (2018) The flowering repressor SVP confers drought resistance in Arabidopsis by regulating abscisic acid catabolism. Mol Plant 11: 1184–1197 [Google Scholar]
- Yang JC, Zhang JH, Wang ZQ, Zhu QS, Liu LJ (2003) Involvement of abscisic acid and cytokinins in the senescence and remobilization of carbon reserves in wheat subjected to water stress during grain filling. Plant Cell Environ 26: 1621–1631 [DOI] [PubMed] [Google Scholar]