The hydrogen isotope composition (δ2H) exhibits specific features that report the water conditions of a wheat crop, as well as the photosynthetic characteristics of the plant part considered.
Abstract
The stable carbon (δ13C) and oxygen (δ18O) isotope compositions in plant matter reflect photosynthetic and transpirative conditions in plants, respectively. However, the nature of hydrogen isotope composition (δ2H) and what it reflects of plant performance is poorly understood. Using durum wheat (Triticum turgidum var durum), this study evaluated the effect of different water and nitrogen growing field conditions on transpiration and how this effect influenced the performance of δ2H in autotrophic (flag leaf), mixotrophic (ears), and heterotrophic (grains and roots) organs. Moreover, δ2H was compared with the δ13C and δ18O in the same organs. Isotope compositions were analyzed in dry matter, the water-soluble fraction, and in water from different tissues of a set of genotypes. Similar to δ13C, the δ2H correlated negatively with stomatal conductance, whereas no correlation was observed for δ18O. Moreover, δ2H was not only affected by changes in transpiration but also by photosynthetic reactions, probably as a consequence of NADPH formation in autotrophic organs. Compared with the δ2H of stem water, plant δ2H was strongly diminished in photosynthetic organs such as the flag leaves, whereas it strongly increased in heterotrophic organs such as grains and roots. In heterotrophic organs, δ2H was associated with postphotosynthetic effects because there are several processes that lead to 2H-enrichment of carbohydrates. In summary, δ2H exhibited specific features that inform about the water conditions of the wheat crop, together with the photosynthetic characteristics of the plant part considered. Moreover, correlations of δ2H with grain yield illustrate that this isotope can be used to assess plant performance under different growing conditions.
Analyses of the stable isotope ratios of carbon and oxygen in plant material have been applied in time-integrated approaches for climatological, ecological, or biochemical research in plant science (Dawson et al., 2002; Barbour, 2007; Gessler et al., 2014), including the evaluation of crop performance under different environmental conditions (Richards, 1996; Farquhar et al., 1998, 2007; Barbour and Farquhar, 2000; Araus et al., 2003, 2013; Cabrera-Bosquet et al., 2009a, 2011). The stable isotope ratio of hydrogen in plant material has also been examined in different areas of plant research (Dawson et al., 2002). However, it has not been exploited in crop research to the same degree.
The carbon isotope composition (δ13C) of plant dry matter (DM), frequently expressed as a discrimination from surrounding air (Δ13C), has been used for decades as a tool for screening plants with high water use efficiency during the assimilate deposition period, due to the well-established link between Δ13C and the intercellular versus the atmospheric partial pressure of CO2 (Farquhar and Richards, 1984; Farquhar et al., 1989; Richards et al., 2002). In C3 plants, 13C discrimination mainly occurs during two steps of CO2 uptake: (1) CO2 diffusion from the air to the intercellular air space through the boundary layer and stomata, and (2) the carboxylation reaction by Rubisco (Farquhar et al., 1982). In addition, the water regime strongly affects the carbon isotope signature of the plant, with drought increasing δ13C due to low stomatal conductance-driven CO2 diffusion (Araus et al., 2003; Condon et al., 2004). However, the effects of other growing factors such as nitrogen (N) availability on δ13C remain unclear, and contradictory results have been reported. Thus, the δ13C in wheat (Triticum spp.) has been observed either to decrease (Shangguan et al., 2000; Zhao et al., 2007), increase (Zhao et al., 2007; Serret et al., 2008; Cabrera-Bosquet et al., 2009a), or be unaffected (Hubick et al., 1990) as N supply increases. Furthermore, the interaction of nitrogen fertilization and water regime may affect δ13C (Araus et al., 2013).
During recent years, interest has grown in using oxygen isotope composition (δ18O) in plant matter because it integrates evaporative conditions during the crop cycle (Barbour et al., 2000; Barbour, 2007). It is known that the δ18O of leaf water (and organic matter that carries leaf water signal) becomes isotopically enriched during transpiration (Barbour and Farquhar, 2000). Indeed, under common environmental conditions (where the δ18O of ambient vapor, ambient moisture content, and source water do not vary across different plants), the interest in δ18O is motivated by the concept that δ18O may be affected by transpiration, which simultaneously depends on stomatal conductance (gs; Barbour and Farquhar, 2000; Helliker and Ehleringer, 2002). Similar to δ18O, the effect of environment on transpiration and evaporation also drives leaf water evaporative 2H-enrichment in the plant (Smith and Freeman, 2006; Feakins and Sessions, 2010; Kahmen et al., 2013; Cernusak et al., 2016). Therefore, the plant δ2H in organic matter is not only influenced by gs, but also by the effects of climate on transpiration (Sternberg et al., 1984; Cernusak et al., 2016). Thus, a high correlation between δ18O and δ2H in organic matter may indicate the source (i.e. water) and environmental effects (Epstein et al., 1977), whereas a lack of correlation would suggest either an additional hydrogen (Sternberg et al., 1986) or oxygen (Barbour, 2007) isotope fractionation effect.
Theoretically, as driving factors, gs and leaf temperature can influence several parameters of the δ18O and δ2H leaf water enrichment model, either directly or indirectly (Flanagan et al., 1991; Farquhar and Lloyd, 1993). The model relates the enrichment of δ18O and δ2H in leaf water above the source of water during evaporation to (1) the kinetic fractionation during diffusion through the stomata such as ea/ei (through its influence on leaf temperature; Farquhar et al., 2007), (2) the Péclet number (through its influence on transpiration; Cuntz et al., 2007), (3) the leaf boundary layer, εk (the kinetic fractionation that occurs during diffusion and through the pores of the stomata in the leaf layer), and (4) ε+ (the proportional depression of water vapor pressure by the heavier H218O molecule), which is dependent on temperature.
As indicated previously, 18O is enriched in leaves or other transpiring organs relative to the source water (Gonfiantini et al., 1965; Farquhar et al., 1989; Pande et al., 1995). Even so, diverse factors can affect the use of δ18O to assess plant performance (Barbour and Farquhar, 2000; Sánchez-Bragado et al., 2016). Thus, the δ18O of photoassimilates may be affected by the isotopic composition of the water source available to the plant (Yakir et al., 1990a; Roden et al., 2000; Williams et al., 2005), by the plant height and leaf length (Helliker and Ehleringer, 2000, 2002) or by fractionation during postphotosynthetic processes due to biochemical reactions involved in the synthesis of organic matter (Farquhar and Lloyd, 1993) and its subsequent transport within the plant (Offermann et al., 2011). However, some studies have observed that there is no fractionation during Suc transport (Cernusak et al., 2005), although biochemical fractionation can be impacted by physiological processes such as the carbon turnover rate, which may affect the δ18O of organic matter (Song et al., 2014). Nonetheless, δ18O has been used to evaluate plant responses to different water regimes in cereals such as maize (Zea mays) and wheat (Barbour et al., 2000; Barbour, 2007; Cabrera-Bosquet et al., 2009b; Araus et al., 2013). However, studies combining the effects of N supply and water regime on δ18O are still scarce, and the results are contradictory (Cernusak et al., 2007; Cabrera-Bosquet et al., 2009a, 2011; Araus et al., 2013).
Similar to δ18O, δ2H in plant organic compounds is affected by the water source (Epstein et al., 1977; Sternberg et al., 1984; Chikaraishi and Naraoka, 2003; Sachse et al., 2006; Schwendenmann et al., 2015). However, an important factor that determines the δ2H but not the δ18O in plant organic compounds is related to the biochemical processes between organic compounds and cellular water, which may cause biosynthetic fractionation of 2H (Ziegler et al., 1976; Sternberg et al., 1984; Ziegler, 1989; Yakir and Deniro, 1990; Luo and Sternberg, 1991; Yakir, 1992). Unlike δ18O, the δ2H of organic matter is also affected by carbon metabolism; and it has been proposed, for example, as a proxy to assess crassulacean acid metabolism (CAM) metabolism in plants (Sternberg et al., 1984). Thus, photosynthesis has a major impact on the δ2H of plant organic matter (Ziegler et al., 1976; Luo et al., 1991; Yakir, 1992; Schmidt et al., 2003; Sachse et al., 2012). Although the mechanisms related to the effects of photosynthetic metabolism on plant δ2H are insufficiently understood (Sachse et al., 2012), these mechanisms seem clearly different from those determining δ13C and δ18O. Thus, the photosynthetic H fractionation processes that occur during NADPH formation in the photosynthetic light reactions and triose phosphate primary assimilation may also contribute to determining the δ2H in plant organic compounds (Roden et al., 2000). In fact, the NADPH produced during photosynthesis has been observed as being extremely depleted in 2H (Luo et al., 1991; Schmidt et al., 2003). Moreover, it has been reported that recently produced autotrophic cellulose in leaves might be depleted in 2H compared with available water (Yakir et al., 1990a; Luo et al., 1991). The reason for such depletion might be related to reduction reactions, whereby the NADPH-derived hydrogen that is added to carbon skeletons seems strongly depleted (on average) relative to water (Sachse et al., 2012). Conversely, during heterotrophic metabolism, all other reactions following the primary assimilation of triose phosphate may enrich the 2H of plant organic matter (Roden et al., 2000) due to the exchange of a large proportion of hydrogen atoms with surrounding water (Ziegler, 1989). In addition, postphotosynthetic 2H-fractionation processes may also occur via the oxidative pentose phosphate pathway during sugar metabolism. Thus, the NADPH produced may be more enriched (i.e. less depleted) in 2H (Yakir and Deniro, 1990; Schmidt et al., 2003). Hence, photosynthesis depletes the 2H of the carbon-bound hydrogen carbohydrates (the fractionation factor is around −200‰), whereas postphotosynthetic metabolism has the opposite effect (+150‰; Yakir, 1992; Sachse et al., 2012). Nonetheless, until now there has not been a clear understanding of the photosynthetic and postphotosynthetic biochemical processes that determine δ2H fractionation during plant organic biosynthesis. In fact, there have been fewer applications of hydrogen isotope ratios compared with the other stable light isotopes in studies of plant organic matter. The underlying reason is related to the presence of isotopically exchangeable atoms of hydrogen in the organic compounds (oxygen in the DM can also exchange with moisture, although such an effect is predicted to be much smaller than for δ2H; Yousfi et al., 2013). The percentage of hydrogen atoms of cellulose that are exchangeable can reach 30% (isotopes of hydrogen bound to oxygen in hydroxyl groups), whereas the remaining 70% are nonexchangeable hydrogen atoms bound to carbon (Filot et al., 2006). Therefore, the hydroxyl hydrogen group can easily exchange with environmental water sources, complicating the interpretation of this isotope in plant organic matter. Nevertheless, developments in isotope-ratio mass spectrometry for compound-specific analyses have promoted the use of H isotopes.
In summary, δ2H in plants may share some commonalties in terms of factors affecting its signature, with δ18O (affected by transpiration and the signature of the source water) and even with δ13C (through gs), which are both triggered by environmental factors (for example, the availability of water). However, δ2H may be further strongly affected by the trophic (photoautotrophic versus heterotrophic) nature of the plant part considered. In the case of a leaf (or another photosynthetic organ), the δ2H in carbohydrates will be a balance between autotrophic and heterotrophic processes (Yakir et al., 1990b). Therefore, although there is no evidence that the fractionation effect of δ2H is associated with environmental stress, the effect of any environmental stress on the photosynthetic activity might eventually affect the final δ2H in the carbohydrates of the plant.
The objective of this study was to evaluate the influence of growing conditions on transpiration and how these circumstances affect the δ2H of autotrophic (leaves), mixotrophic (ears), and heterotrophic (roots and mature kernels) organs compared with the δ13C and δ18O in DM and the water-soluble fraction (WSF) in the same organs. For this case study, durum wheat (Triticum turgidum var durum) was chosen due to its frequent exposure to the vagaries of abiotic stress. Durum wheat is among the main crops cultivated in the Mediterranean basin (Food and Agriculture Organization of the United Nations (FAOSTAT), 2017) where production areas are often simultaneously exposed to water stress (Lobell et al., 2008) and low N availability (Oweis et al., 1998; Sadras, 2004). Moreover, there is increasing evidence that ongoing climate change is already stagnating productivity (Moore and Lobell, 2015; Ceglar et al., 2016) by decreasing precipitation while increasing evapotranspiration. Thus, a panel of modern cultivars and landraces of durum wheat were grown in the field during two consecutive years under different combinations of water and nitrogen fertilization. To the best of our knowledge there have been no field studies in crop species reporting on the variation in δ2H within different organs and among genotypes under a combination of different water and nitrogen conditions; and therefore, comparisons among these three stable light isotopes (13C, 2H, and 18O) as ecophysiological indicators of plant performance are absent.
RESULTS
Average grain yield (GY), including all growing conditions, was higher in 2011 (3.1 Mg·ha−1) compared with that in 2010 (1.7 Mg·ha−1; data not shown). Similarly, cultivars showed higher GY (1.9 Mg·ha−1 and 3.1 Mg·ha−1 for 2010 and 2011, respectively) compared with that in landraces (1.5 Mg·ha−1 and 1.6 Mg·ha−1 for 2010 and 2011, respectively) during both growing seasons. Moreover, the GY of both landraces and cultivars was higher under support irrigation (SI) than rainfed (RF) conditions (Tables 1 and 2). Furthermore, whereas gs was much higher under SI compared with that under RF conditions in 2010 and 2011 (Tables 1 and 2), no significant differences were observed between landraces and cultivars in 2010 (Table 1). In contrast, in 2010, gs decreased in response to nitrogen fertilization (Table 1).
Table 1. Mean values of GY, gs, and stable isotope compositions (‰) of δ2H, δ18O, and δ13C.
Mean values of GY, gs, and stable isotope composition (‰) of δ2H, δ18O, and δ13C of DM and the WSF of different plant parts (flag leaves, ears, and roots) sampled at mid-grain-filling plus mature kernels (grains) and in the water from the basal part of the stem [stem water (stem W)] under SI, RF, N fertilized (HN), and nonfertilized (LN) conditions, as examined in modern cultivars (cultivars) and old landraces (landraces) We measured δ13C DM and δ13C WSF, and δ18O WSF and δ2H WSF in 108 plots (five cultivars and four landraces, four growing conditions and three replicates); whereas we measured δ18O DM and δ2H DM in 48 plots (two cultivars and two landraces, four growing conditions and three replicates) during the 2010 crop cycle. Each value represents the mean ±sd. Mean values across plant tissues with different letters (a and b) are significantly different from SI versus RF and HN versus LN, and landraces versus cultivars, according to Tukey’s Honest Significant Difference Test (P < 0.05).
| Isotope/Organ/Fraction | Cultivars | Landraces | SI | RF | HN | LN |
|---|---|---|---|---|---|---|
| Hydrogen | ||||||
| δ2Hroots DM | −67.1 ± 18.6a | −73.2 ± 20.6a | −64.3 ± 23.5a | −75.8 ± 13.3b | −72.5 ± 17.7a | −68.0 ± 21.5a |
| δ2Hstem W | −46.4 ± 7.2a | −45.3 ± 5.7a | −46.3 ± 7.6a | −45.6 ± 5.6a | −47.1 ± 7.3a | −44.8 ± 5.7a |
| δ2Hflag DM | −114.9 ± 8.7a | −115.5 ± 8.8a | −120.6 ± 6.7a | −109.6 ± 6.8b | −111.5 ± 9.6a | −119.1 ± 5.5b |
| δ2Hflag WSF | −100.9 ± 6.9a | −104.4 ± 7.4b | −104.1 ± 7.8a | −100.9 ± 6.4b | −101.8 ± 8.1a | −103.2 ± 6.4a |
| δ2Hear DM | −90.0 ± 8.6a | −95.3 ± 10.6a | −99.0 ± 7.1a | −86.4 ± 8.1b | −88.9 ± 10.0a | −96.4 ± 8.4b |
| δ2Hear WSF | −65.7 ± 7.0a | −71.4 ± 9.2b | −72.5 ± 6.7a | −64.3 ± 8.0b | −65.4 ± 9.5a | −71.2 ± 6.4b |
| δ2Hgrain | −33.6 ± 8.2a | −30.9 ± 9.4a | −36.6 ± 6.6a | −28.2 ± 8.9b | −27.4 ± 7.7a | −35.8 ± 6.9b |
| Oxygen | ||||||
| δ18Oroots DM | 28.1 ± 7.5a | 30.8 ± 8.8a | 29.6 ± 9.0a | 29.3 ± 7.6a | 32.3 ± 9.4a | 26.7 ± 5.9b |
| δ18Ostem W | −6.3 ± 1.0a | −6.1 ± 0.6a | −6.4 ± 0.9a | −6.0 ± 0.8a | −6.4 ± 0.9a | −6.0 ± 0.7a |
| δ18Oflag DM | 0.8 ± 1.7a | 31.0 ± 1.1a | 30.3 ± 1.0a | 31.4 ± 1.6b | 31.1 ± 1.6a | 30.7 ± 1.3a |
| δ18Oflag WSF | 30.7 ± 2.2a | 30.0 ± 2.3a | 28.9 ± 1.9a | 32.3 ± 0.9b | 29.6 ± 2.7a | 31.2 ± 1.3a |
| δ18Oear DM | 26.6 ± 2.4a | 26.1 ± 2.6a | 26.5 ± 2.0a | 26.2 ± 2.9a | 26.7 ± 2.2a | 26.0 ± 2.7a |
| δ18Oear WSF | 30.9 ± 1.1a | 29.9 ± 1.0a | 30.2 ± 0.9a | 30.7 ± 1.3a | 30.3 ± 1.2a | 30.7 ± 1.1a |
| δ18Ograin | 30.4 ± 0.7a | 30.6 ± 0.9b | 30.4 ± 0.7a | 30.6 ± 0.9a | 30.3 ± 0.6a | 30.7 ± 0.9a |
| Carbon | ||||||
| δ13Cflag DM | −25.9 ± 0.9a | −25.5 ± 0.9b | −26.2 ± 0.7a | −25.2 ± 0.8b | −25.3 ± 0.8a | −26.2 ± 0.8b |
| δ13Cflag WSF | −27.0 ± 1.1a | −27.2 ± 1.1a | −27.9 ± 0.9a | −26.3 ± 0.7b | −27.2 ± 1.4a | −26.9 ± 0.7a |
| δ13Cear DM | −24.5 ± 1.0a | −25.0 ± 0.9b | −25.4 ± 0.7a | −24.1 ± 0.8b | −24.5 ± 1.0a | −25.0 ± 0.9b |
| δ13Cear WSF | −23.3 ± 0.9a | −24.4 ± 1.0b | −24.5 ± 0.9a | −23.1 ± 0.8b | −23.6 ± 1.1a | −24.0 ± 1.1a |
| δ13Cgrain | −24.6 ± 0.9a | −23.9 ± 1.0b | −25.0 ± 0.6a | −23.5 ± 0.7b | −24.0 ± 1.1a | −24.6 ± 0.8b |
| gs (mmol·H2O·m−2s−1) | 184.7 ± 78.1a | 170.2 ± 59.0a | 222.8 ± 56.2a | 133.8 ± 52.8b | 163.6 ± 84.5a | 193.1 ± 49.0b |
| GY (Mg·ha−1) | 1.9 ± 0.1a | 1.5 ± 0.1b | 2.1 ± 0.1a | 1.3 ± 0.1b | 1.8 ± 0.1 a | 1.6 ± 0.1 a |
Table 2. Mean values of GY, gs, and carbon, oxygen, and hydrogen stable isotope compositions.
Hydrogen isotope composition (‰) was analyzed in the flag leaf water (δ2Hflag W), grain water (δ2Hgrain W), stem water (δ2Hstem W), and irrigation water (δ2Hsource W) in SI plots. Oxygen isotope composition (‰) was analyzed in the flag leaf water (δ18Oflag W), grain water (δ18Ograin W), stem water (δ18Ostem W), irrigation water in SI (δ18Osource W), and the DM of the flag leaves (δ18Oflag DM) and mature kernels (δ13Cgrain). Carbon isotope composition (‰) was analyzed in the DM in the flag leaves (δ13Cflag DM) and mature kernels (δ13Cgrain). We performed δ13C analysis on nine durum wheat genotypes and three replicates grown under two different water conditions (SI versus RF, including all levels of N), accounting for a total of 54 plots. For water extracted from flag leaves, δ18O and δ2H were measured in a subset of two cultivars and two landraces (with three replicates) under fertilized conditions and two water regimes (18 plots). We measured δ18O and δ2H in extracted water from stems, developing grains and DM in a subset of five cultivars and five landraces (with three replicates) under fertilized conditions and two water regimes (45 plots) during the 2011 crop cycle (landraces were discarded due to lodging under SI conditions; see the “Materials and Methods” section). Each value represents the mean ±sd. Mean values across plant tissues with different letters (a and b) are significantly different according to Tukey’s Honest Significant Difference Test (P < 0.05). Dashes indicate no data.
| Isotope/Water Organ | SI | RF |
|---|---|---|
| Hydrogen | ||
| δ2Hflag W | −8.1 ± 0.4a | 17.9 ± 2.4b |
| δ2Hgrain W | −26.2 ± 0.9a | −9.7 ± 2.3b |
| δ2Hstem W | −43.0 ± 0.6a | −43.8 ± 0.6a |
| δ2Hsource W | −45.0 | – |
| Oxygen | ||
| δ18Oflag W | 3.0 ± 0.5a | 15.5 ± 1.0b |
| δ18Ograin W | 2.8 ± 0.2a | 9.3 ± 0.7b |
| δ18Ostem W | −5.6 ± 0.1a | −5.6 ± 0.1a |
| δ18Osource W | −6.1 | – |
| δ18Oflag DM | 27.7 ± 0.1a | 33.3 ± 0.2b |
| δ18Ograin | 30.2 ± 0.1a | 32.3 ± 0.1b |
| Carbon | ||
| δ13Cflag DM | −28.8 ± 0.1a | −26.5 ± 0.1b |
| δ13Cgrain | −26.3 ± 0.1a | −24.4 ± 0.1b |
| gs (mmol·H2O·m−2s−1) | 110.0 ± 36.3 | 29.1 ± 22.5 |
| GY (Mg·ha−1) | 4.5 ± 0.1a | 1.7 ± 0.1b |
Hydrogen, Oxygen, and Carbon Isotope Composition across Tissues
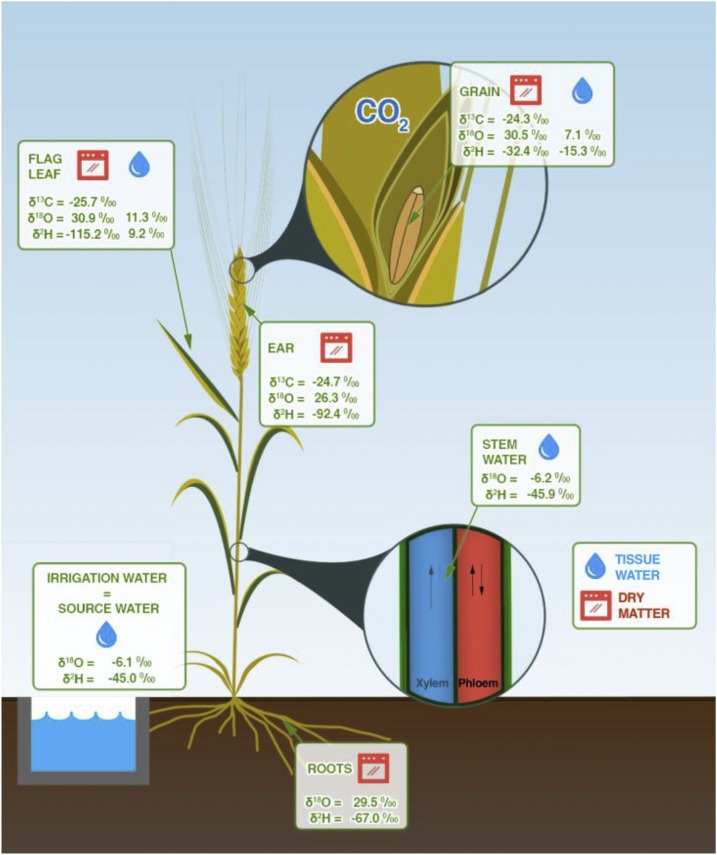
Mean values averaged across genotypes of stable hydrogen (δ2H), oxygen (δ18O), and carbon (δ13C) isotope composition within different tissues (Fig. 1). Hydrogen isotopic composition in mature grains showed the most enriched (less negative) values (δ2Hgrain = −32.4‰) compared with that in the ears (δ2Hear DM = −92.4‰), flag leaves (δ2HflagDM = −115.2‰), and roots (δ2Hroots DM = −67.0‰). For the δ13C, the ears (δ13Cear DM = −24.7‰) and mature grains (δ13Cgrain = −24.3‰) showed the most enriched values compared with that in the flag leaves (δ13Cflag DM = −25.7‰; Fig. 1). In the case of δ18O, the most enriched tissue was the flag leaf (δ18Oflag DM = 30.6‰).
Figure 1.
Illustration of a wheat plant and the stable isotope composition (‰) of δ2H, δ18O, and δ13C of different plant parts (flag leaves, ears, and roots) sampled at mid-grain-filling, plus mature kernels (grains) and the water (blue drops) from the basal part of the stems, flag leaves, and developing grains. Values presented are means from the DM of five representative plants per plot and including all treatments. We measured δ13C DM in 108 plots (five cultivars and four landraces, four growing conditions, and three replicates); whereas we measured δ18O DM and δ2H DM in 48 plots (two cultivars and two landraces, four growing conditions, and three replicates) during the 2010 crop cycle. The δ18O and δ2H of the water extracted from the flag leaves were analyzed in a subset of two cultivars and two landraces (with three replicates) under fertilized conditions and two water regimes (18 plots; landraces in SI conditions were discarded due to lodging). We measured δ18O and δ2H in water from the stems, developing grains and DM in a subset of five cultivars and five landraces (with three replicates) under fertilized conditions and two water regimes (45 plots; landraces were discarded due to lodging under SI conditions; see “Materials and Methods” section). Analyses of water extracted from different tissues were performed in samples from the 2011 crop season.
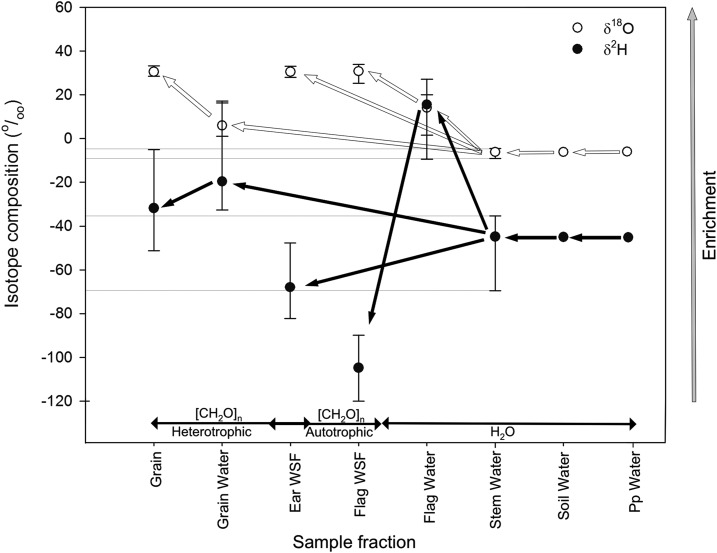
Hydrogen isotope composition of stem water (δ2Hstem W = −45.9‰) was depleted compared to that of the grain DM, but enriched compared with that of the flag leaves (WSF), the ears (WSF), and the roots (DM; Fig. 2). In contrast, the δ18O of stem water (δ18Ostem W = −5.6‰) displayed the most depleted value regardless of the tissues and fractions (DM, WSF) analyzed (Fig. 2). Moreover, the δ2H and δ18O of stem water were more depleted compared with that of grain water (δ2Hgrain W = −15.3‰ and δ18Ograin W = 7.0‰) and flag leaf water (δ2Hflag W = 9.2‰ and δ18Oflag W = 11.3‰; Fig. 2).
Figure 2.
Schematic representation of the major steps in the development of the ratios of oxygen (δ18O) and hydrogen (δ2H) isotope composition (‰) in plant carbohydrates and tissue water. Data was obtained from the WSF of the flag leaves and ears (flag WSF, ears WSF) and water extracted from different plant tissues (grain water, flag leaf water, and stem water) at mid-grain-filling, plus mature kernels (grains) in nine durum wheat genotypes and three replicates during the 2010 crop cycle. Each value represents the mean ± sd. Arrows represent the change in δ2H and δ18O from water sources (including irrigation water, soil water, precipitation water [Pp]) to carbohydrates in autotrophic or heterotrophic tissues. White circles and arrows represent δ18O, black circles and arrows represent δ2H.
Fractionation of Hydrogen, Oxygen, and Carbon Isotope Composition across Plant Tissues
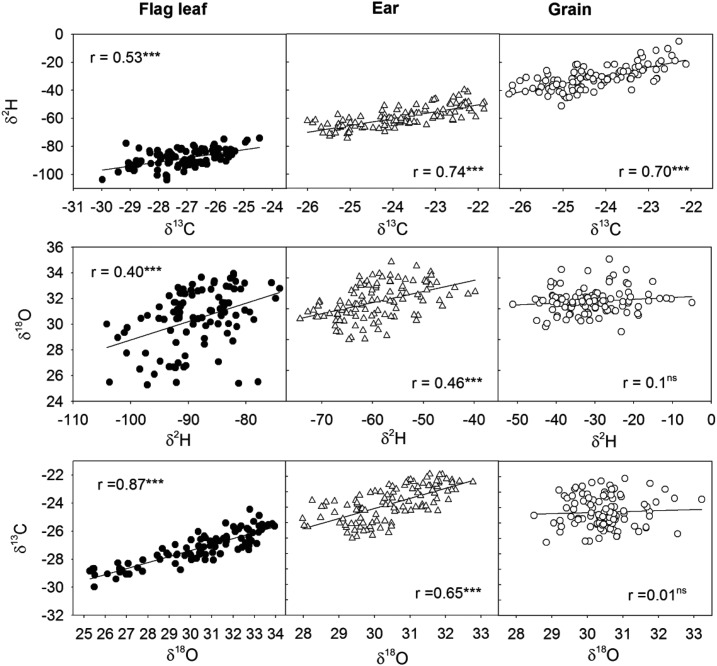
In order to further assess whether similar fractionation processes affected isotopic composition of hydrogen, oxygen, and carbon within the plant, a correlation analysis was performed between different isotope compositions (δ18O, δ13C, and δ2H) in the WSF of the same plant tissue (mature kernels, ears, and flag leaves; Fig. 3). The strongest relationship in the flag leaf WSF (left columns of Fig. 3) was observed between δ18O and δ13C (r = 0.87, P < 0.001); whereas in the WSF of the ears, the strongest correlation was found between δ2H and δ13C (r = 0.74, P < 0.001), followed by δ18O and δ13C (r = 0.65, P < 0.001). In mature kernels, δ2H and δ13C were highly correlated (r = 0.70, P < 0.001), whereas δ18O did not correlate with either δ2H or δ13C.
Figure 3.
Linear regression of the relationship among the carbon (δ13C), oxygen (δ18O), and hydrogen (δ2H) isotope compositions of the WSF within the flag leaves (left column, closed circles), ears (middle column, white triangles), and mature kernels (right column, white circles). Nine genotypes and three replicates per genotype were considered, accounting for a total of 108 plot values under all growing conditions of the 2010 crop season. Level of significance: ns, not significant, P > 0.05; ***, P < 0.001.
In order to estimate whether the fractionation processes affecting δ2H and δ18O were similar in the water transported by different plant tissues, a correlation analysis was performed between the oxygen and hydrogen isotope compositions of the water extracted from different tissues (Table 3). The result showed that δ2Hflag W was positively correlated with δ2Hgrain W (r = 0.66, P < 0.001), whereas no correlation was observed with δ2Hstem W. Similarly, δ18Oflag W was positively correlated with δ18Ograin W (r = 0.67, P < 0.001) but not with δ18Ostem W. In addition, in order to estimate whether the same fractionation processes affected δ2H and δ18O in the water of tissues, correlation analyses between the δ2H and δ18O of the water in the same tissues were performed (Table 3). It was observed that the δ2H and δ18O in flag leaf water were strongly correlated (r = 0.99, P < 0.001). Similarly, δ2H and δ18O were strongly correlated in the grain water (r = 0.99, P < 0.001) and stem water (r = 0.81, P < 0.001).
Table 3. Linear regressions of the relationships between hydrogen (δ2H) and oxygen (δ18O ) isotope compositions of water from different organs and GY .
This table shows the linear regression of the relationship between hydrogen isotope composition of the flag leaf water (δ2Hflag W), grain water (δ2Hgrain W) and stem water (δ2Hstem W) oxygen isotope composition of the flag leaf water (δ18Oflag W), grain water (δ18Ograin W), stem water (δ18Ostem W), and the DM of the flag leaves (δ18Oflag DM), mature kernels (δ18Ograin) and GY. Carbon isotope composition is shown for the DM in the flag leaves (δ13Cflag DM), mature kernels (δ13Cgrain), and GY. The δ18O and δ2H of the water extracted from the flag leaves were analyzed in a subset of two cultivars and two landraces (with three replicates) under fertilized conditions and two water regimes (18 plots; landraces in SI conditions were discarded due to lodging). The δ18O and δ2H were measured in water from the stems, developing grains, and DM in a subset of five cultivars and five landraces (with three replicates) under fertilized conditions and two water regimes (45 plots; landraces were discarded due to lodging under SI conditions; see the “Materials and Methods” section). Analyses were performed in samples from the 2011 crop season. Level of significance: ns, not significant; ***, P < 0.001; **, P < 0.01; *, P < 0.05. Dashes indicate no data.
| Isotope/Organ/Fraction | δ2Hflag W | δ2Hgrain W | δ2Hstem W | δ18Oflag W | δ18Ograin W | δ18Ostem W | GY |
|---|---|---|---|---|---|---|---|
| Hydrogen | |||||||
| δ2Hflag W | – | 0.66** | −0.13ns | 0.99*** | 0.70** | 0.01ns | −0.78*** |
| δ2Hgrain W | 0.65** | – | −0.26ns | 0.61** | 0.99*** | −0.21ns | −0.54*** |
| δ2HstemW | −0.13ns | −0.26ns | – | −0.12ns | −0.26ns | 0.81*** | 0.12ns |
| Oxygen | |||||||
| δ18Oflag W | 0.99*** | 0.61** | −0.12ns | – | 0.67** | 0.04ns | −0.82*** |
| δ18Ograin W | 0.70** | 0.99*** | 0.27ns | 0.67** | – | −0.20ns | −0.62*** |
| δ18Ostem W | −0.00ns | −0.21ns | −0.81*** | 0.04ns | −0.20ns | – | −0.07ns |
| δ18Oflag DM | 0.96*** | 0.72*** | −0.31ns | 0.97*** | 0.82*** | 0.11ns | −0.93*** |
| δ18Ograin | 0.90*** | 0.73*** | −0.43* | 0.90*** | 0.80*** | 0.07ns | −0.83*** |
| Carbon | |||||||
| δ13Cflag DM | 0.89*** | 0.69*** | −0.28ns | 0.92*** | 0.78*** | 0.16ns | −0.86*** |
| δ13Cgrain | 0.65** | 0.49** | −0.08ns | 0.71** | 0.59*** | 0.17ns | −0.93*** |
Water and Nitrogen Effects on Carbon, Oxygen, and Hydrogen Isotope Composition
Significant differences within SI and RF conditions (Table 1) were mainly observed in δ2H and δ13C in 2010. Concerning δ18O, only the flag leaf DM or the flag leaf WSF showed significant differences between the two water regimes in 2010. Overall, water stress tended to increase δ2H, δ18O, and δ13C irrespective of the tissue or fraction analyzed, with the exception of δ2Hroots DM (Table 1). Furthermore, fertilized plants (HN) showed higher δ2H and δ13C compared with that in to nonfertilized plants (LN), although no significant differences were observed in δ2Hroots DM, δ2Hstem W, and δ13CflagWSF. By contrast, δ18O did not exhibit significant differences among fertilization conditions, with the exception of in the roots (δ2Hroots DM).
Carbon, Oxygen, and Hydrogen Isotope Composition in Cultivars and Landraces
Overall, the δ2H in 2010 was lower in landraces compared with cultivars (Table 1), although significant differences were only observed in the δ2H of the WSF of the flag leaf and ear. A similar trend was exhibited by δ13C, with landraces having lower δ13C compared with cultivars, with the exception of δ13Cflag DM (Table 1). Conversely, δ13Cgrain and δ2Hgrain were less enriched in landraces compared with cultivars. There were no significant differences in δ18O, regardless of the organs and fractions considered, with the exception of in the grains (Table 1).
Correlations of δ2H, δ18O, and δ13C with GY, gs, and N Content
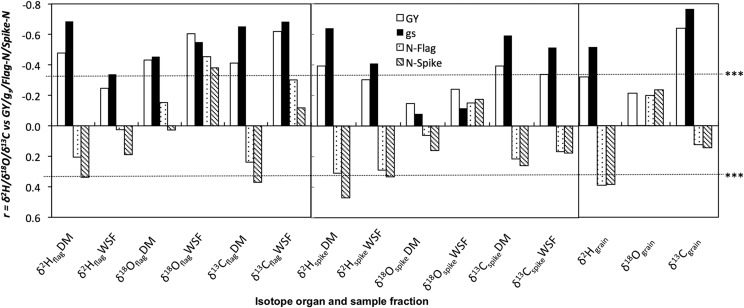
The δ2H, δ18O, and δ13C in DM and WSF from different tissues plus mature kernels were correlated with GY, gs, and N content in the flag leaves (N-Flag) and the ears (N-Ear). Correlations were calculated including all genotypes and either (1) across the whole set of growing conditions in 2010 (Fig. 4), or (2) across different water regimes for a given nitrogen fertilization level (HN and LN), or (3) across nitrogen regimes within each water condition (SI and RF; Table 4). In general, δ2H and δ13C in the different tissues and water were negatively correlated with GY (P < 0.05) and gs (P < 0.01) when all genotypes and growing conditions were combined (Fig. 4) and across the four different combinations of water and nitrogen regimes (SI, RF, HN, and LN; Table 4), with δ13C in mature grains showing the highest correlation against GY when all growing conditions were included (Fig. 4). Conversely, δ2H and δ13C were positively correlated with N-Flag (P < 0.01) and N-Ear (P < 0.01) under SI and RF conditions (Table 4); whereas under HN conditions, this correlation was negative (Table 4). With regard to δ18O, it was marginally correlated with GY, gs, N-Flag, and N-Ear in 2010 (Fig. 4; Table 4). However, when all growing conditions were combined in 2011 (Table 3), GY was negatively and strongly correlated with δ18Oflag DM (P < 0.001) as well as with δ18Oflag W (P < 0.001). Additionally, correlations of δ18Ograin W with GY were also observed (P < 0.001; Table 3).
Figure 4.
Linear regression of the relationship among the carbon (δ13C), oxygen (δ18O), and hydrogen (δ2H) isotope compositions in mature kernels (grains) and in the DM and WSF of the flag leaves and the ears with the grain yield (GY), the stomatal conductance (gs), and the total N concentration of the flag leaves (N-Flag) and ears (N-Ear). We measured the δ13C DM and δ13C WSF, δ18O WSF and δ2H WSF in 108 plots (five cultivars and four landraces, four growing conditions and three replicates per genotype and condition), whereas we measured δ18O DM and δ2H DM in 48 plots (two cultivars and two landraces, four growing conditions and three replicates) during the 2010 crop cycle. Analyses were performed in samples from the 2010 crop season. Level of significance: ***, P < 0.001.
Table 4. Linear regression of the relationship of the δ13C, δ18O, δ2H isotope compositions and GY, gs, and total N concentration.
Linear regression of the relationship of the δ13C, δ18O, and δ2H isotope compositions in the WSF and DM of the flag leaves, ears, and mature kernels (grains) with the GY, gs, and total N concentration of the flag leaves (N-Flag) and ears (N-Ear)Nine genotypes and three replicates per genotype were considered, accounting for a total of 54 values under RF (water stress) conditions, including fertilized and nonfertilized conditions and under fertilized conditions (HN), including the two water conditions. For the δ18O and δ2H of DM (flag leaves, ears, and roots), only two cultivars and two landraces were considered (24 plots). Analyses were performed on samples from the 2010 crop season. Level of significance: not significant, ns, P > 0.05; ***, P < 0.001; **, P < 0.01; *, P < 0.05.
| Isotope/Organ/ Fraction | gs | GY | N-Flag | N-Ear | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SI | RF | HN | LN | SI | RF | HN | LN | SI | RF | HN | LN | SI | RF | HN | LN | |
| Hydrogen | ||||||||||||||||
| δ2Hroots DM | 0.2ns | 0.2ns | 0.4ns | 0.4ns | −0.2ns | −0.1ns | 0.2ns | 0.4ns | −0.1ns | −0.1ns | 0.5* | −0.1ns | −0.2ns | −0.2ns | 0.5* | −0.1ns |
| δ2Hstem water | −0.3* | 0.4** | −0.1ns | 0.1ns | −0.3ns | −0.1ns | −0.2ns | 0.1ns | −0.1* | 0.3* | −0.2ns | −0.1ns | 0.2ns | 0.1ns | 0.3* | −0.0ns |
| δ2Hflag DM | −0.2ns | −0.7*** | −0.8*** | −0.3ns | 0.1ns | −0.5* | −0.6** | −0.3ns | 0.3ns | 0.5* | −0.6** | 0.0ns | 0.3ns | 0.7*** | −0.1ns | 0.1ns |
| δ2Hflag WSF | −0.2* | −0.6** | −0.4** | −0.1ns | −0.1ns | −0.3* | −0.4** | −0.1ns | −0.1* | 0.6** | −0.4** | 0.2ns | 0.1ns | 0.7*** | 0.2ns | 0.2ns |
| δ2Hear DM | −0.2ns | −0.4** | −0.8*** | −0.4ns | 0.3ns | −0.4* | −0.6** | −0.4ns | 0.5ns | 0.4** | −0.3ns | 0.4ns | 0.5* | 0.9*** | 0.2ns | 0.5* |
| δ2Hear WSF | 0.2ns | −0.6*** | −0.4** | −0.3* | 0.4** | −0.5*** | −0.3* | −0.3* | 0.4ns | 0.7*** | −0.1ns | 0.1ns | 0.3* | 0.5*** | 0.1ns | 0.1ns |
| δ2Hgrain | 0.1ns | −0.6*** | −0.5*** | −0.5*** | 0.3* | −0.4** | −0.5*** | −0.5*** | 0.7ns | 0.4** | −0.5*** | 0.1ns | 0.6*** | 0.4** | −0.2ns | −0.0ns |
| Oxygen | ||||||||||||||||
| δ18Oroots DM | 0.1ns | −0.1ns | 0.1ns | 0.3ns | 0.1ns | −0.2ns | 0.1ns | 0.3ns | 0.5ns | −0.1ns | −0.1ns | −0.0ns | −0.3ns | 0.1ns | −0.1ns | −0.0ns |
| δ18Oxylem water | 0.3* | 0.3* | 0.3* | −0.0ns | 0.3ns | −0.2ns | −0.3* | 0.1ns | −0.2* | −0.3* | −0.2ns | −0.1ns | 0.2ns | 0.2ns | 0.4** | 0.1ns |
| δ18Oflag DM | 0.1ns | −0.2ns | −0.4** | −0.3ns | 0.1ns | −0.2ns | −0.3* | −0.3ns | −0.2ns | 0.0ns | −0.5*** | −0.1ns | −0.1ns | 0.2ns | −0.2ns | 0.2ns |
| δ18Oflag WSF | −0.3* | 0.0ns | −0.8*** | −0.2ns | −0.2ns | −0.5** | −0.6*** | −0.2ns | −0.7* | −0.3ns | −0.5*** | −0.1ns | −0.6*** | −0.2ns | −0.3* | 0.1ns |
| δ18Oear DM | −0.1ns | 0.2ns | −0.2ns | 0.1ns | 0.1ns | −0.3ns | −0.1ns | 0.1ns | −0.1ns | −0.1ns | 0.1ns | −0.2ns | −0.0ns | −0.0ns | −0.0ns | −0.1ns |
| δ18Oear WSF | −0.1ns | 0.2ns | −0.3* | −0.3* | 0.1ns | −0.4** | −0.1ns | −0.3* | −0.1ns | −0.2ns | −0.1ns | −0.1ns | −0.2ns | −0.1ns | −0.1ns | −0.1ns |
| δ18Ograin | 0.1ns | 0.1ns | −0.0ns | −0.0ns | −0.2ns | −0.2ns | −0.2ns | 0.1ns | −0.2ns | −0.2ns | −0.1ns | 0.1ns | −0.3* | −0.2ns | −0.1ns | −0.2ns |
| Carbon | ||||||||||||||||
| δ13Cflag DM | −0.2ns | −0.7*** | −0.7*** | −0.59*** | 0.1ns | −0.3* | −0.6*** | −0.6*** | 0.4s | 0.3* | −0.6*** | 0.1ns | 0. 5*** | 0.4** | −0.1ns | 0.1ns |
| δ13Cflag WSF | −0.4** | −0.4** | −0.8*** | −0.6*** | −0.3ns | −0.3* | −0.6*** | −0.6*** | −0.5** | 0.1ns | −0.6*** | −0.1ns | −0.4** | 0.2ns | −0.1ns | 0.1ns |
| δ13Cear DM | −0.1ns | −0.5*** | −0. 7*** | −0.5*** | 0.4** | −0.4** | −0.4** | −0.5*** | 0.5ns | 0.4** | −0.3* | 0.1ns | 0.5*** | 0.4** | −0.1ns | 0.2ns |
| δ13Cear WSF | −0.1ns | −0.3* | −0.6*** | −0.42** | 0.4** | −0.5*** | −0.4** | −0.4** | 0.4ns | 0.3* | −0.2ns | 0.1ns | 0.2ns | 0.3* | −0.1ns | 0.2ns |
| δ13Cgrain | −0. 4** | −0.7*** | −0.8*** | −0.29* | −0.3* | −0.3* | −0.8*** | −0.3* | 0.21** | 0.47*** | −0.57*** | 0.3* | 0.16ns | 0.4** | −0.3* | 0.3ns |
Furthermore, in order to test which isotope, tissue, and fraction better explained yield, a stepwise regression analysis was performed in the 2010 trials between the analyzed signatures of the different isotopes, tissues, and fractions (either DM and WSF) as independent variables and with GY as the dependent variable (Table 5). The stepwise analysis was performed combining all treatments together (global) for each of the water regimes and both fertilization levels together (SI, RF) and for each N fertilization level and both water regimes combined (HN, LN). In the global and HN analyses, the first independent variable chosen by the model was δ13Cgrain, whereas in the LN analysis, it was the δ18Oflag WSF. Conversely, in the SI and RF analyses, δ2Hear WSF and δ2Hgrain were the first variables chosen by the model, respectively.
Table 5. Stepwise analysis between GY, and δ13C, δ18O, and δ2H isotope compositions.
Stepwise analysis for the whole set of nine genotypes per three replicates in 2010, under SI, RF, N fertilization (HN), and without N fertilization (LN), including all growing conditions (global) and a combination of SI and N fertilization (SI + HN), SI without N fertilization (SI-LN), RF conditions and N fertilization (RF + HN), and RF conditions without N fertilization (RF-LN), with GY as a dependent variable, and δ13C, δ18O, and δ2H isotope composition of mature kernels (grains), soluble organic matter of flag leaves (leaf WSF), and ears (ear WSF), and oxygen and hydrogen isotope composition of stem water (stem W) as independent variables. The “global” stepwise analysis represents values obtained from the average of the three replicates per genotype under each growing condition (108 plots, n = 36). The “SI” and “RF” stepwise analyses represent values obtained from the average of three replicates per genotype, including HN and LN conditions (n = 18). The “HN” and “LN” stepwise analyses represent values obtained from the average of three replicates per genotype, including SI and RF conditions (n = 18).
| Treatment | Variable Chosen | r | R2 | Significance |
|---|---|---|---|---|
| Global | δ13Cgrain | 0.71 | 0.51 | 0.000 |
| δ13Cgrain, δ18Oflag WSF | 0.83 | 0.70 | 0.000 | |
| δ13Cgrain, δ18Oflag WSF, δ13Cflag WSF | 0.87 | 0.75 | 0.000 | |
| δ13Cgrain, δ18Oflag WSF, δ13Cflag WSF, δ13Cear WSF | 0.89 | 0.80 | 0.000 | |
| SI | δ2Hear WSF | 0.75 | 0.57 | 0.000 |
| δ2Hear WSF, δ2Hstem W | 0.85 | 0.71 | 0.000 | |
| RF | δ2Hgrain | 0.61 | 0.38 | 0.006 |
| δ2Hgrain, δ18Oflag WSF | 0.84 | 0.70 | 0.000 | |
| HN | δ13Cgrain | 0.89 | 0.80 | 0.000 |
| δ13Cgrain, δ18Oflag WSF | 0.94 | 0.82 | 0.000 | |
| δ13Cgrain, δ18Oflag WSF, δ18Ograin | 0.96 | 0.92 | 0.000 | |
| LN | δ18Oflag WSF | 0.76 | 0.58 | 0.000 |
| δ18Oflag WSF, δ13Cgrain | 0.84 | 0.70 | 0.000 |
Experimental Estimation of the Electron Transport Rate’s Association with δ2H Depletion
The δ2H and δ13C, together with gs and the electron transport rate (ETR), were assessed in the flag leaves of the same durum wheat variety growing under controlled conditions under two different relative humidity (RH) conditions (40% and 80% RH). Plants growing under 80% RH showed depleted δ13C values and higher gs in the flag leaves compared to that in plants grown under 40% RH. Accordingly, flag leaves exhibited more depleted δ2H values and higher ETR under 80% RH, compared with that under 40% RH (Supplemental Table S1).
DISCUSSION
Photosynthetic Fractionation and Autotrophic Effects
Similar to the effect on δ13C, photosynthesis could have a major impact on δ2H (Sternberg et al., 1984). In our study, the δ2H from the flag leaf water showed enriched values compared with that of δ2Hflag (either DM or WSF), indicating that depleted values of the δ2Hflag in plant matter or the WSF may not originate from evaporative processes, but are mainly due to photosynthetic reactions. In fact, δ2Hflag WSF versus δ13Cflag WSF were better correlated than δ2Hflag WSF versus δ18Oflag WSF (Fig. 3), suggesting that leaf δ2H is not only affected by changes in transpiration and gs, alongside the evaporative conditions (Cernusak et al., 2016), but also by photosynthetic reactions (Yakir et al., 1990b) and carbon metabolism in plants (Cormier et al., 2018). Recent autotrophically produced cellulose, lipids (Sternberg, 1988), or starch (Hayes, 2001) in leaves might be depleted in 2H compared with the available water (Yakir et al., 1990a; Fig. 2). Although the hydrogen isotope composition in the leaf plant water may be imprinted in sugars and metabolites and thus also retained in organic compounds (Cernusak et al., 2016), the isotopic composition of the H transferred from NADPH to biosynthetic substrates might be one of the most important factors controlling the hydrogen-isotopic composition of organic matter in photosynthetic organs (Hayes, 2001). This evidence was supported and quantified by a study performed by Yakir and Deniro (1990) in Lemma gibba grown under autotrophic conditions. In this study, the negative fractionation factor between the water and photosynthates caused a strong depletion (−171‰). Such a low delta value was postulated to be the consequence of the extremely deuterium-depleted protons (Luo et al., 1991; Hoganson and Babcock, 1997) used (from a water molecule within the cell) for the reduction of NADP+ to NADPH. However, in a study performed by Cormier et al. (2018) in different vascular plant species, the increase in light intensity (above 115 μM m−2s−1) and consequently the photosynthetic rate, did not clearly deplete the δ2H in the studied organic compounds. In spite of that, H pools that are strongly depleted relative to leaf water have been observed to result from photosynthetic 2H fractionation in the chloroplast during light reactions where ferredoxin-NADP+ reductase produces NADPH with reduced H (Luo et al., 1991). The results observed in the growth chamber experiment, with plants grown under two different RH conditions (40% and 80% RH), agree with the findings of Luo et al. (1991); (Supplemental Table S1). Plants growing under 80% RH showed depleted δ13C values and higher gs in the flag leaf, suggesting that these plants were exposed to less water-limiting conditions compared with plants grown under 40% RH. Accordingly, flag leaves exhibited more depleted δ2H values and higher ETRs under 80% RH compared with that under 40% RH (Supplemental Table S1). Because the ETR is associated with a reduction of NADP+ to NADPH during the light reaction of photosynthesis (Foyer et al., 2012) under less water-limiting conditions (80% RH), it is worth considering that there is a causal association of the ETR with the contribution of δ2H-depleted NADPH to organic δ2H. In contrast, under more water-limiting conditions (40% RH), the ETR was lower, causing a reduction in NADPH levels and consequently a decrease in the contribution of δ2H-depleted NADPH to organic δ2H, resulting in enriched plant organic δ2H compared with 80% RH. Indeed, the depleted values of δ2H observed in our experiment in the flag leaves and ears compared with that in the grains would agree with the autotrophic activity of the former organs (Yakir and Deniro, 1990).
Evaporative Fractionation: Transpirative Effects
The δ2H and δ18O of water from the flag leaves, ears, and grains were enriched compared with the source of water (water collected from the base of the stem). Indeed, the isotope signature of the water from different plant tissues might influence the isotope signature in the DM and WSF. In our study, the increase in the δ2H and the δ18O of the DM and WSF from the flag leaves to the apical part of the plant (flag leaves compared with the ears and grains) may be due in part to the effect of a progressive enrichment in δ2H and δ18O of the plant water associated with evaporative demand (Helliker and Ehleringer, 2002). Likewise, δ18OflagWSF and δ13CflagWSF (and δ18OearWSF and δ13CearWSF) were strongly correlated, suggesting that in autotrophic organs (leaves and to some extent ears), both isotopes are probably governed by changes in transpiration and gs, as previously reported in durum wheat by Cabrera-Bosquet et al. (2009a).
However, in spite of the previously discussed evaporation-driven effect from the bottom to the top of the aerial parts of the plant, the grain water was less enriched in δ2H and δ18O compared with that in leaf water, although the grain water was enriched compared with that in the source water. In fact, leaf water (and organic matter that carries the leaf water signal) becomes more 2H enriched than the grains as a result of the evaporative process during transpiration (Craig and Gordon, 1965; Gonfiantini et al., 1965). In the case of water in the grains, different mechanisms may apply. Even if the grains have the photosynthetic “green layer” of aleurone (Caley et al., 1990), few stomata are present on the pericarp (Barlow et al., 1980). In addition, grains are surrounded by the ear bracts, which may therefore minimize transpirative losses (Bort et al., 1996). Moreover, transpiration of the culm, including leaf sheaths, is smaller than in the leaf blades, which may also cause less isotopic enrichment of the water if it goes straight from the base of the stem to the growing grains—rather than, for example, from the base of the stem to the leaf blades (Araus and Tapia, 1987). In addition, there is a xylem discontinuity in the floral axis (Cochrane and Duffus, 1979) and therefore in the longer-term water transport from the stem base to the growing grains. Taking into consideration that transpirative water losses in the grain are low, the acropetal transport of leaf water in the phloem to the developing grains may contribute to enrichment of the δ2H and δ18O in the grains relative to the base of the stem as a result of mixing of the phloem water from the leaves with the source water from the base of the stem (Cernusak et al., 2016). In addition, the biphasic enrichment in the grains linked to developmental metabolism of the grain and rapid loss of water during the last part of the grain filling might have enriched the 2H in the grain water compared with that in the source of water (Pande et al., 1994). Indeed, invoking variation in the δ2H of water among tissues as an explanation for among-tissue (leaf versus grain) variation in DM and the WSF does not seem a convincing conclusion. Further, the “progressive enrichment” concept (Helliker and Ehleringer, 2002) does not appear to hold at the whole-plant level (or at least when comparing leaves with grains).
Moreover, evaporative 2H enrichment of leaf water was markedly higher compared with 18O enrichment. The contrast between the 2H and 18O may be related to the relative magnitude of the equilibrium and kinetic fractionation in the Craig and Gordon model of evaporative site enrichment (Cernusak et al., 2016). The 18O in the leaf water is mainly triggered by kinetic fractionation, whereas equilibrium fractionation mainly dominates 2H (Cernusak et al., 2016). Kinetic effects are closely dependent on stomata and boundary layer resistance (Farquhar et al., 1989), whereas the equilibrium effect varies as a function of temperature (Bottinga and Craig, 1968). In a study performed in Australia by Kahmen et al. (2013) over a large-scale environmental gradient, the effect of disequilibrium between the source water and atmospheric vapor was stronger for 2H than that for 18O. (Correlations between the leaf water isotope signature and air RH were stronger for δ18O than that for δ2H.) Moreover, under nonequilibrium conditions, evaporative processes tend to cause greater (relative) enrichment of 18O than 2H; and as a consequence, the slope between δ2H and δ18O of water gets flatter (Dansgaard, 1964). However, plants were exposed under steady-state conditions because leaves exhibit relatively open stomata during the day (considering that leaves were not succulent; Cernusak et al., 2008) and therefore these could be assumed as equilibrium conditions. Under such conditions, isotopic enrichment is likely to be stronger for 2H in the leaf water compared with 18O, causing proportionally higher values in 2H than 18O in the leaf water.
Postphotosynthetic Fractionation and Heterotrophic Effects
During heterotrophic metabolism, a substantial fractionation of hydrogen isotopes from leaf water to organic matter has been described in Sternberg et al. (1986), a situation that may lead to 2H enrichment in organic matter (Ziegler, 1989). Postphotosynthetic 2H enrichment starts in the rapid reciprocal exchange between the triose phosphate pool and the hexosephosphate pool during carbohydrates synthesis (Buchanan et al., 2015; Cormier et al., 2018). Subsequently, there are four processes that lead to 2H-enrichment of carbohydrates: (1) the synthesis of glyceraldehyde-3-phosphate in the Calvin cycle, which enables exchange with cellular (2H-enriched) water (Rieder and Rose, 1959); (2) the C-bound H in newly photosynthesized glyceraldehyde-3-phosphate derives from an 2H-enriched precursor molecule, 3-phosphoglyceraldehyde (due to previous exchange with cellular water); (3) the formation of Fru 1,6-biphosphate from two triose phosphates during hexosephosphate production leads to loss of one of the four C-bound H atoms to the nearby water (Rieder and Rose, 1959), and this action results in an 2H-enrichment in the glyceraldehyde-3-phosphate pool due to the more rapid reaction of the lighter isotopologues during this process (Schmidt et al., 2015); and (4) the interconversion of Glc 6-phosphate and Fru 6-phosphate, which is performed by the enzyme phosphoglucose isomerase, may also 2H-enrich glyceraldehyde-3-phosphate pools (Schleucher et al., 1999).
According to our results, the δ2H of mature grains was enriched compared with that in other analyzed plant parts (including the ear; Fig. 2), and it was also enriched in roots compared with that in flag leaves (Table 1). Only 15% of C-bound H atoms present in carbohydrates in heterotrophic tissues originate from the 2H-depleted NADPH that is produced during the light reactions of photosynthesis in the chloroplast (Cormier et al., 2018), and this result leads to 2H-enriched values in heterotrophic organs such as grains, and specific compounds such as starch or cellulose (Epstein et al., 1976; Sternberg et al., 1984). These findings suggest that δ2H was exposed to postphotosynthetic enrichment (as explained above) in most heterotrophic organs (such as the grains and roots) in comparison with the more depleted δ2H in autotrophic organs such as the leaves (Yakir et al., 1990b).
If we follow the same reasoning used for the leaves but with respect to the ear, the δ2H in the WSF of the ears was depleted compared with mature grains, but enriched compared with the flag leaves (Fig. 2). On one hand, such increases from the flag leaves to the ears could be a consequence of lower gs in the latter organ (Araus et al., 1993; Tambussi et al., 2005) as well as the result of the mixotrophic nature of the ear bracts (combining large portions of heterotrophic areas with photosynthetic tissues) compared with the leaves (Blum, 1985; Knoppik et al., 1986; Araus et al., 1993; Bort et al., 1994; Li et al., 2006). Furthermore, enriched δ2H values of the ears in the WSF compared with that in the flag leaves could be a consequence of a degree of CAM metabolism in the ears (Sternberg and Deniro, 1983; Sternberg et al., 1984; Winter et al., 2008; Feakins and Sessions, 2010; Sachse et al., 2012; Winter and Holtum, 2014). Recent studies in wheat glumes and lemmas have shown that the activity of the RuBP carboxylase enzyme decreases significantly in response to water stress, whereas the activity of phosphoenolpyruvate carboxylase increases along with that of NADP-malate dehydrogenase (Jia et al., 2015).
On the other hand, such a decrease in the δ2H in the DM of the ears with respect to the grains (besides the fact that the grains are subjected to heterotrophic metabolism) could be due to the presence of epicuticular waxes in the ears alongside the support tissues (Araus et al., 1993). In fact, it has been reported in a deciduous conifer that lipids derived from epicuticular waxes and support tissues (e.g. collenchyma and sclerenchyma) are highly depleted in deuterium (Sessions et al., 1999; Chikaraishi et al., 2004; Hou et al., 2007; Yang and Leng, 2009; Zhou et al., 2011) because 2H-depleted NADPH is a critical source of H in lipid biosynthesis (Cormier et al., 2018). Consequently, the presence of lipids derived from cuticular waxes in the DM of the ears might have depleted the δ2H compared with that in the grains.
Water and Nitrogen Effects on δ13C, δ18O, and δ2H
GY recorded during 2010 and 2011 was in the range reported in earlier work in the Mediterranean basin under dry RF and low supplementary irrigation conditions (Araus et al., 1998, 2003, 2013). SI significantly increased yield, whereas no effect was observed on yield under N fertilization conditions (Table 1).
In our study, N fertilization increased δ13C and δ2H in the leaf DM, whereas δ18O showed a similar trend, although no significant differences were observed. Likewise, an increase in δ13C due to N fertilization has been reported (Farquhar, 1989) as a consequence of a reduction in the Ci/Ca ratio, due to either an increase in photosynthetic capacity or a decrease in gs (Farquhar and Richards, 1984; Condon et al., 2004). Such findings agree with our results, where fertilized plots showed lower gs compared with that of nonfertilized plots (Table 1). Moreover, the higher δ2H values in the leaf DM under N fertilization can be explained by the evaporative 2H enrichment of leaf water. In the modified Péclet effect model (Farquhar and Lloyd, 1993), transpiration has been observed to reduce 2H-enrichment of leaf water due to a mixture of leaf water and source of water (Cernusak et al., 2016). Therefore, the increase in δ2H in the leaf DM under N fertilization observed in our experiments can be explained by reduced gs and a subsequent decrease in transpiration resulting in a decreased Péclet effect (Cernusak et al., 2016). In addition, δ13C and δ2H were positively correlated with N-Flag (nitrogen content of the flag leaves DM) and negatively correlated with gs under RF and SI conditions (both including HN and LN treatments; Table 4). Such results indicate that biomass is increased with increases in nitrogen supply, forcing the plants to compete for water resources and exacerbating water stress (therefore resulting in lower gs and higher δ13C and δ2H). However, under SI conditions, N fertilization (which may have increased growth and consequently biomass and yield) did not increase N-Flag as a consequence of a growth dilution effect in the leaf due to leaf expansion (Salazar-Tortosa et al., 2018). This result is supported by the negative correlation between GY and N-Flag (r = −0.426, P < 0.01, data not shown) under SI conditions, whereas under RF conditions the correlation was positive (r = 0.285, P < 0.05, data not shown). Thus, N fertilization under SI conditions showed positive correlation between δ13C and δ2H and N-Flag, supporting the idea that plants with lower N-Flag showed higher GY (i.e. biomass), which, as mentioned before, may have caused a temporary mild water stress during plant growth.
However, under HN conditions (but including the two water regimes), correlations of δ13C and δ2H with N-Flag were in some cases negative (Table 4). These results suggest that under HN conditions (including SI and RF regimes), N fertilization does not necessarily have a negative effect as water stress increases, but rather, the opposite is observed. It has been reported that providing N when there is water available in the soil (i.e. under irrigation conditions) may improve not only growth but also the water status of the crop by contributing to better root growth (Jensen et al., 1990). Overall, these findings suggest that δ2H and δ13C are subject, at least in part, to a similar source of variation; meaning that both isotopes responded with an increase in isotope signature as a result of N fertilization for a given level of water regime (SI, RF) or with a decrease in isotope signature in response to water supply under nitrogen fertilization conditions (HN).
Differences in δ13C, δ18O, and δ2H between Cultivars and Landraces
GY was higher in cultivars compared with that in landraces for both growing years of the study. Although landraces in this study were chosen on the basis of their close phenology to modern cultivars, the latter on average still reached heading 5 d earlier (data not shown). Cultivars have been observed as having a shorter duration to heading compared with landraces (Araus et al., 2002, 2013), escaping from water stress produced during the reproductive stage (Araus et al., 2007). In fact, more enriched values of δ13C, δ2H, and δ18O in grains were observed in landraces compared with that in cultivars (Table 1), evidencing that landraces were exposed to an extended stress episode contributing to a lower GY compared with cultivars (Araus et al., 2007, 2013).
Applicability of δ13C and δ18O for Assessing Plant Performance
In agreement with previous studies (Condon et al., 1987; Araus et al., 1998, 2003; Fischer et al., 1998; Monneveux et al., 2005; Lopes and Reynolds, 2010), δ13C was negatively correlated with GY when all growing conditions were combined (Fig. 4). Conversely, some correlations of δ13C with GY under SI conditions (including HN and LN conditions) were positive for the flag leaves and the ears, and negative for the grains (Table 4). The positive slope between δ13C (in the flag leaves and ear) and GY under SI may be the consequence of N fertilization causing water stress, as discussed previously. Moreover, negative genotypic correlations with GY were only observed with δ13Cgrain (Supplemental Table S2) and were weaker in 2010 than in 2011. Indeed, correlations between GY and δ13Cgrain decreased under poor growing conditions (Supplemental Fig. S1), whereas the δ18Oflag WSF showed the opposite trend (Supplemental Fig. S1). According to our results, in trials under drought conditions with mean yields below 2 Mg·ha−1 (as was the case for 2010), nonsignificant (Araus et al., 2003) or even positive relationships between GY and δ13Cgrain (Voltas et al., 1999) have been reported, suggesting that higher plant water-use efficiency (and thus higher δ13C) increases yield under stress (Farquhar and Richards, 1984; Araus et al., 2003, 2013; Condon et al., 2004). Thus, δ13Cgrain could be a good indicator of the water strategy that plants are following.
Concerning δ18O in 2011, the isotope composition in the grain (δ18Ograin) was strongly associated with GY (r = −0.83, P < 0.000) under all water regimes and nitrogen levels combined (Table 3). However, the correlation was weaker in 2010 (r = −0.21, P = 0.027, Fig. 4). The lack of consistency between the 2010 and 2011 crop seasons may be related to differences in environmental conditions. GY was much lower in 2010 (1.7 Mg·ha−1 on average) compared with that in 2011 (3.1 Mg·ha−1 on average). In fact, in a study on wheat by Barbour et al. (2000), correlations of δ18Ograin with GY and gs were also not constant among the three seasons they analyzed. In the same study, δ18Ograin was only correlated with GY during one season, and it was the season with the highest precipitation and lowest solar radiation. Such results indicate that δ18Ograin might not reflect evaporative conditions under narrow environmental ranges and with moderate to severe drought. Therefore, as a consequence of high levels of remobilization under more severe water conditions, preservation of evaporative conditions imprinted in the δ18O of grains might be low or even nonexistent (Barbour et al., 2000; Ferrio et al., 2007). Likewise, the disparity observed in our study between the two growing seasons regarding the relationship between δ18Ograin and GY could be due to the relative proportions of remobilized photo-assimilates (Barbour et al., 2000). Triose phosphates formed from photosynthesis during the day are converted to Suc for transport (Barbour and Farquhar, 2000). Thus, the main exchange of water with carbonyl oxygen occurs in the leaves during the formation of triose phosphate molecules, because two of the three oxygen atoms present in the molecule belong to carbonyl groups (Sternberg et al., 1986; Barbour et al., 2000). Indeed, correlations of GY with δ18Oflag in the WSF and DM were higher than with δ18Ograin (Fig. 4; Supplemental Fig. S2), indicating that signals of the evaporative conditions are still preserved in leaf assimilates but not in other organs because no correlations of GY with the δ18Oear (either DM or WSF), δ18Ograin, or δ18Oroots were observed. On one hand, as mentioned previously, such a lack of correlation may be related to the δ18O fractionation associated with biochemical reactions during the synthesis of organic matter (Farquhar and Lloyd, 1993) and its subsequent transport (Offermann et al., 2011). On the other hand, the δ18O of organic matter may also be influenced by the source of water (Figs. 1 and 2; Epstein et al., 1977; Yakir et al., 1990b; Roden et al., 2000; Williams et al., 2005; Barbour, 2007). This is not straightforward because the δ18O of source water (water from the base of the stem) is also subjected to evaporative enrichment in the leaf during transpiration (Farquhar and Lloyd, 1993) and during grain formation (Fig. 2; Pande et al., 1994). In fact, it has been reported that the δ18O of water in developing grains exhibits a biphasic enrichment compared with stem water (Pande et al., 1994). The biphasic enrichment may be linked to developmental metabolism of the grain and rapid loss of water, together with oxidative metabolism during later stages of maturation (Pande et al., 1995). Such biphasic enrichment in the grains could therefore affect δ18O, which consequently might have enriched the δ18O of water from the developing grains compared with that in stem water (Table 2). Accordingly, the enrichment of water in the grain could be an additional factor that may hinder the registration of environmental conditions in the δ18Ograin DM. Moreover, the δ18O of water in the flag leaves was enriched compared with that in water from developing grains and stems (Fig. 2; Table 2), an idea that also agrees with the widely reported strong evaporation processes taking place in the leaf (Farquhar and Gan, 2003; Barbour et al., 2004). Besides, the δ18O from the water of photosynthetic and transpiring organs such as the flag leaves was strongly correlated with GY (Table 3) when all growing conditions were included. In short, the strong correlation between GY and δ18O from water in leaves suggests that leaf water mainly reflects evaporative enrichment and thus environmental conditions, with the additional advantage (at least in the case of δ18O) of avoiding the fractionation associated with biochemical reactions during the synthesis of organic matter (Farquhar and Lloyd, 1993).
Applicability of Plant δ2H to Assess Plant Performance
Strong correlations between δ18O and δ2H in the cellulose of leaves have been reported in the literature, which suggests similar sources of variation for plant isotopic signals (Epstein et al., 1977), whereas the absence of a correlation would indicate additional biochemical effects (Sternberg et al., 1986). Keeping this in mind, in the 2010 season, the absence of significant correlations between δ18Ograin and δ2Hgrain (Fig. 3), together with the lack of any relationship between δ18Ograin and GY, suggests that δ18Ograin is more sensitive to biochemical reactions than δ2Hgrain during grain formation (Farquhar and Lloyd, 1993) or is more likely to undergo exchange with the (18O) of source water (Barbour, 2007).
Although exchange of hydrogen isotopes with water within the cytosol can affect the δ2H of organic compounds and the contribution to NADPH (Zhou et al., 2018), the analysis of δ2H depletion in plant organs could be used as an indicator of the net isotopic effect associated with NADPH synthesis in the chloroplast (Hayes, 2001; Zhou et al., 2010). Therefore, if NADPH is not regenerated continuously, reduction power can be strictly limited, and distinct metabolic processes such as photosynthetic electron transport or nitrate reduction (Bloom, 2015) may inhibit carbon fixation (Foyer et al., 2012). In agreement with our results (Fig. 3), data from a study in mature kernels of wheat (Liu et al., 2015) also showed a strong positive correlation between δ13C and δ2H, supporting the idea that both isotopes’s composition (even in heterotrophic organs) are affected by photosynthetic activity even if the source of variation is different. Thus, for example, whereas a decrease in photosynthetic activity caused by water stress is the consequence of a lower CO2 availability (which increases δ13C), the decreased activity diminishes the synthesis of NADPH produced in the chloroplast (and then δ2H decreases less).
However, as discussed previously for δ18O, the δ2H of organic matter may also be affected by the source water δ2H (Fig. 2) and be subjected to evaporative enrichment in the leaves and to biphasic enrichment in grains. Nevertheless, in spite of these fractionation processes, there were good correlations of the δ2H from the flag leaves, ears, and grains with gs and GY (Fig. 4). In fact, the only isotope composition that correlated with GY (as a dependent variable) in the stepwise analysis in 2010 was the δ2H under SI and RF conditions (SI r = 0.75, P = 0.000; RF r = 0.62, P = 0.006). In contrast to δ18O, these results suggest that δ2H was not hindered by fractionation processes within different organs, either during transport of assimilates to the grains or during heterotrophic metabolism within the grains. Therefore, δ2H may provide simultaneous time-integrated records of the photosynthetic and evaporative performance of the plant during crop development based on, among other aspects, its tighter association with δ 13C than with δ 18O.
CONCLUSIONS
In autotrophic organs, such as the flag leaf, δ2H was not only affected by changes in transpiration and gs but also by photosynthetic carbon metabolism because the net isotopic effect (δ2H depletion) was negatively associated with ETR. Contrastingly, δ2H enrichment in heterotrophic organs such as the grains and roots was associated with postphotosynthetic effects because there are several processes that lead to 2H-enrichment of carbohydrates. In the case of the ears, their intermediate δ2H values (lying between the flag leaves and grains) may be the consequence of different factors—such as lower transpiration compared with that in the leaves, the mixotrophic nature of the bracts, or some degree of CAM metabolism.
The significant correlations between δ2H and GY and the existence of genotypic variability in plant δ2H are encouraging when considering this isotope for assessing plant performance under different growing conditions.
MATERIALS AND METHODS
Germplasm Used and Experimental Conditions
Ten durum wheat (Triticum turgidum ssp durum [Desf.] Husn.) genotypes were sown: five historical Spanish landraces (Blanqueta, Griego de Baleares, Negro, Jerez 37, and Forment de Artes) and five modern Spanish commercial varieties released after 1990 (Anton, Bolo, Don Pedro, Regallo, and Sula). Landraces were chosen based on their similarity to the phenology of modern cultivars. Field experiments were conducted during the 2010 and 2011 growing seasons at the experimental station of the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA) of Aranjuez (40°03′N, 3°31′E, 500 m asl), with experimental conditions explained in Sanchez-Bragado et al. (2014). Two water treatments (SI and RF) combined with two nitrogen regimes (fertilized, HN; and nonfertilized, LN) were assayed. The trials were planted on December 30, 2010 and November 18, 2011 in plots with six rows 0.20 m apart, covering a total area of 6 m2 (5 m length and 1.2 m width) per plot. Total accumulated precipitation during the 2010 and 2011 seasons was 275.4 and 126.1 mm, respectively. For both years, sprinkler irrigation was applied to irrigated plots around GS41 (beginning of April; Zadoks et al., 1974) and GS71 (around May 15th and 30th) with approximately 60 mm supplied on each date. Environmental conditions during growth are detailed in Figure 5. Prior to sowing, all trials received 60 kg ha−1 of phosphorous as superphosphate (18%), and 60 kg ha−1 potassium as potassium chloride (60%). Further, the HN plants were dressed with nitrogen applied at the beginning of tillering (January 27th in 2010 and December 29th in 2011) and jointing (March 20th in 2010 and February 20th in 2011) using a dose of 45 kg ha−1 and 105 kg ha−1 of urea (46%), respectively. The LN plants were not N fertilized, relying exclusively on the N available in the soil before sowing. Water and nitrogen treatments were arranged according to a split-split plot design with three replicates. Experimental plots were kept free of weeds, insects, pests, and diseases by recommended chemical measures (Sanchez-Bragado et al., 2014).
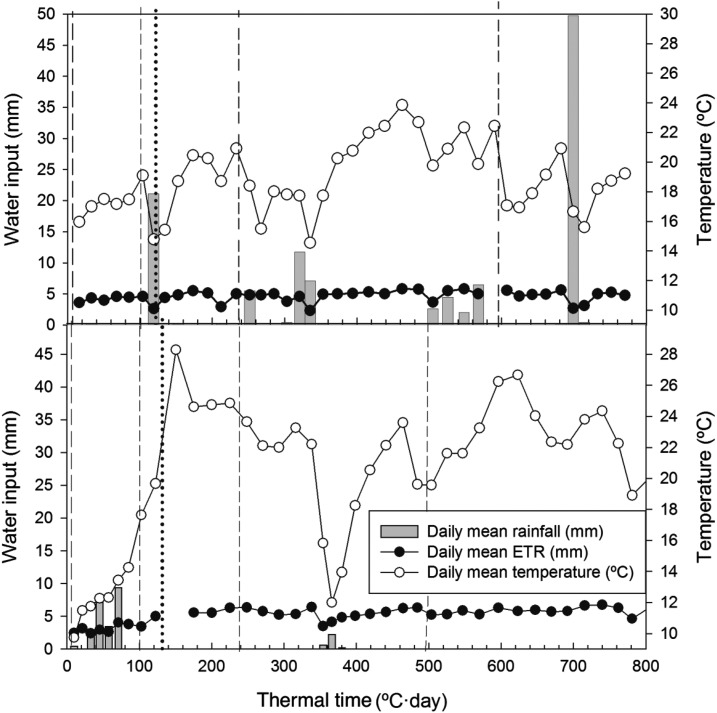
Figure 5.
Daily mean precipitation (mm), evapotranspiration (mm), and air temperature (°C) during the growing season from flowering to physiological maturity expressed as thermal time (°C·day) during the 2010 (top panel) and 2011 crop seasons (bottom panel). ETR = electron transport rate. Vertical dashed lines = dates of irrigation. Vertical dotted lines = sampling dates.
Sampling was performed around 7 d after anthesis, corresponding to the GS71-75 Zadoks stages (Zadoks et al., 1974) in 2010 and two weeks after anthesis (stages GS75-81) in 2011. In 2010, the genotype Foment de Artes was discarded due to late phenology. Also in 2011, all five landraces under SI were discarded due to lodging. In 2010, roots were collected from the upper layer (0–10cm) with a split tube sampler (Ref. 04.17.01.C, Eijkelkamp Soil & Water), rinsed with distilled water, and then placed inside a paper envelope. Thereafter, five representative flag leaves and ears were collected per plot, then oven dried together with collected roots at 70°C for 48 h, then weighed and finely ground for hydrogen, oxygen, and carbon isotope analyses (in total DM). In 2011, flag leaves and developing grains from five representative tillers were collected and immediately frozen for subsequent water extraction. Stomatal conductance (gs) was measured with a leaf porometer (Decagon; http://www.decagon.com) in one leaf per plot. At maturity, the central four rows of each plot were harvested and the GY was recorded. Subsequently, mature kernels were processed for isotope analysis. Harvest was performed manually and by machine in 2010 and 2011, respectively.
Carbon Isotope Analyses
Carbon isotope analyses of mature grains as well as the total DM and WSF of the flag leaf blades and ears from the field trials, together with the DM of the flag leaves from the growth chamber experiment, were performed using an Elemental Analyzer (EA; Flash 1112 EA, Thermo Fisher Scientific) coupled with an isotope ratio-mass spectrometer (IRMS; Delta C IRMS, Thermo Fisher Scientific) operating in continuous flow mode in order to determine the stable carbon (13C/12C) isotope ratios of the same samples. Samples of approximately 1 mg of total DM for mature grains, 0.7 mg for flag leaves and ears, and reference materials were weighed into tin capsules, sealed, and then loaded into an automatic sampler (Thermo Fisher Scientific) before EA-IRMS analysis. The 13C/12C ratios of plant material were expressed in δ notation (Coplen, 1988): δ13C = (13C/12C) sample / (13C/12C) standard −1. “Sample” refers to plant material and “standard” to international secondary standards of known 13C/12C ratios [International Atomic Energy Agency (IAEA) CH7 polyethylene foil, IAEA CH6 Suc, and United States Geological Survey (USGS) 40 l-Glu] calibrated against Vienna Pee Dee Belemnite calcium carbonate with an analytical precision (SD) of 0.10‰.
Measurements were carried out at the Scientific Facilities of the University of Barcelona. The δ13C of flag leaves (DM), ears (DM), roots, and mature kernels are referred to as δ13Cflag DM, δ13Cear DM, δ13Croots DM, and δ13Cgrain, respectively.
Oxygen Isotope Analyses
The 18O/16O ratios of the same mature grains (as well as the total DM and WSF of flag leaf blades and ears) were determined by an online pyrolysis technique using a Thermo-Chemical Elemental Analyzer (TC/EA; Thermo Fisher Scientific) coupled with an IRMS (Delta C Finnigan MAT). Samples of 1 mg of total DM for mature grains, flag leaves, ears, roots, and reference materials were weighed into silver capsules, sealed, and oven-dried at 60°C for no less than 72 h to remove moisture, and then loaded into an automatic sampler. Results were expressed as δ18O values, using two secondary standards (IAEA 601 and IAEA 602) calibrated against Vienna Standard Mean Oceanic Water (VSMOW), and the analytical precision was ∼0.25‰. Analyses were conducted at Iso-Analytical Limited (Crewe). The δ18O of flag leaves (DM), ears (DM), roots, and mature kernels are referred to as δ18Oflag DM, δ18Oear DM, δ18Oroots DM, and δ18Ograin, respectively.
Hydrogen Isotope Analyses
The 2H/1H ratios of the same mature grains, as well as the total DM and WSF of the flag leaf blades and ears (and only leaves DM in the growing chamber experiment) were determined by an online pyrolysis technique using a TC/EA (Thermo Fisher Scientific) coupled with an IRMS (Delta plus xp). Samples of 0.15 mg of total DM for mature grains, flag leaves, ears, roots, and reference materials were weighed into silver capsules, sealed, and oven-dried at 60°C for not less than 72 h to remove moisture, and then loaded into an automatic sampler. In addition, samples were always kept under free moisture conditions with silica gel in a desiccator. Results were expressed as δ2H values, using international secondary standards (for calibration and checking precision and accuracy) of known 2H/1H ratios (IAEA CH7 polyethylene foil, 5α-androstane, coumarin, and eicosanoic acid methyl ester) calibrated against VSMOW, and the analytical precision was ∼0.5‰. In addition, a secondary internal standard (IAEA 601, δ2H = −85.1‰) was selected to provide at least a two-point calibration (normalization) of the hydrogen isotope delta scale anchored by VSMOW. Measurements were carried out at the Scientific Facilities of the University of Barcelona. The δ2H of flag leaves (DM), ears (DM), roots, and mature kernels are referred to as δ2Hflag DM, δ2Hear DM, δ2Hroots DM, and δ2Hgrain, respectively.
Water-Soluble Fraction
The protein-free WSFs of the flag leaves and ears were extracted from the same dry samples tested for carbon, hydrogen, and oxygen isotopes, as described previously in Cabrera-Bosquet et al. (2011) and Yousfi et al. (2013). Aliquots of 40 µL (carbon), 20 µL (hydrogen), and 100 µL (oxygen) of supernatant containing protein-free WSF were transferred into tin capsules for carbon analysis, and into silver capsules for hydrogen and oxygen analyses. The capsules containing the aliquots were oven dried at 60°C. The WSFs of the δ13C, δ2H, and δ18O of flag leaves and ears are referred to as δ13Cflag WSF, δ13Cear WSF, δ2Hflag WSF, δ2Hear WSF, δ18Oflag WSF, and δ18Oear WSF, respectively. Additionally, in order to estimate any possible exchange between the samples and the water used to extract the protein-free WSF, the powdered samples were suspended using three water reference sources with different δ2H (snow water, δ2H = −77.5‰; deuterated water, δ2H = −94.4‰, and seawater, δ2H = −3.3‰). In fact, using extraction water sources with different δ2H signatures does not significantly affect the δ2H of the soluble fraction, and the absolute differences in δ2H between soluble fractions extracted from the DM with the different water sources were minor (Supplemental Table S3).
Hydrogen and Oxygen Composition in Plant Water
To determine source water variations in the 2010 and 2011 field experiments, a portion of the stem base was harvested in the field. In 2010, variations in source water were determined from pressed stem juice. Stem base segments were pressed with a high-pressure press in order to obtain a liquid extract. Subsequently, extracted liquid was transferred to 2-mL glass vials with crimp caps. Glass vials were sealed and sterilized in a water bath at 100°C for 2 h to prevent fermentation processes, and then kept cool until isotope analysis. In 2011, a portion of the stem base was placed into sealed tubes immediately after sampling and subsequently frozen in a freezer at −20°C. Thereafter, water was extracted from the stem base using a cryogenic vacuum distillation line (Dawson and Ehleringer, 1993). The δ2H and δ18O of water extracted from the stem are referred to as δ2Hstem W and δ18Ostem W, respectively.
In 2011, flag leaves and developing grains were collected and placed into sealed tubes and then frozen immediately after sampling. Thereafter, water was extracted from the developing grains and flag leaves using a cryogenic vacuum distillation line (Dawson and Ehleringer, 1993) and measured together with stem water samples. The δ2H and δ18O of water extracted from flag leaves and developing grains are referred to as δ2Hflag W, δ 2Hgrain W, δ18Oflag W, and δ18Ograin W, respectively.
Oxygen and hydrogen compositions (δ18O, δ2H) in water distilled from stem bases, flag leaves, and developing grains (experiment 2011) and stem juice extracts (stem water, experiment 2010) were determined by laser spectroscopy at the Serveis Científico-Tècnics of the Universitat de Lleida using a Picarro L2120i (Picarro, Inc.) coupled to a high-precision vaporizer A0211. All samples were centrifuged at 12,000 g in order to remove any suspended solid, and the supernatants were transferred to glass vials with a 250-mL insert. In the case of stem juice, because large amounts of sugars reduce the performance of the vaporizer, juice samples were diluted to 50% with distilled water of known isotopic composition prior to injection as explained in Sánchez-Bragado et al. (2016). At the same time, the potential presence of organic contaminants was checked using the postprocessing software ChemCorrect 1.2.0 (Picarro), giving in some cases positive results. Consequently, the data were thereafter corrected for consistency across all samples (to avoid undesired effects of organic contaminants) as described by Martín-Gómez et al. (2015). Nevertheless, we found very strong correlations between corrected and uncorrected values (r2 = 0.998 for δ18O; r2 = 0.992 for δ2H; n = 106), with 83% of the samples showing differences lower than 0.4‰ for δ18O, and lower than 4‰ for δ2H.
Isotopes were expressed in delta (δ) notation (‰) relative to VSMOW (i.e. isotopic composition of oxygen, δ18O, and hydrogen, δ2H). Raw values were calibrated against three internal laboratory references [calibrated against IAEA standards VSMOW2, Standard Light Antarctic Precipitation2 (SLAP2), and Greenland Ice Sheet Precipitation]. Overall uncertainty, determined as the SE of repeated analyses, (n = 20) of a reference material not included in the calibration, was 0.05‰ and 0.17‰, for δ18O and δ2H, respectively.
Dual-Water Equilibration Method to Quantify the Fraction of Exchangeable H
A dual-water equilibration method was performed in order to quantify the fraction of exchangeable H and to determine the δ2H in the nonexchangeable H fraction (Schimmelmann et al., 2001; Sauer et al., 2009; Qi and Coplen, 2011). Dry leaf material and the WSF of the same sample, plus standards, were used for the dual-water equilibration method. A set of three aliquots extracted from the same sample plus the standards were weighed and loaded into individual silver capsules, and then each capsule was held in a plastic tray. Each plastic tray was placed in a glass desiccator for equilibration with water sources with different δ2H. One set was equilibrated in a glass desiccator with water depleted in 2H (ambient snow water, δ2H = −77.5‰), and the second set was equilibrated with seawater (ambient seawater, δ2H = −3.3‰). In each glass desiccator, a set of standards was also included. Samples were equilibrated in each desiccator for 7 d at ambient temperature (25°C). In order to remove the moisture prior the equilibration period, the desiccators were purged with helium for 5 minutes at 120 mL min−1. Subsequently, samples from light and heavy water were dried in separate desiccators filled with Sicapent (P2O5) for at least 7 d. In parallel, a set of identical samples used for water equilibration was oven dried at 50°C for 7 d. After 7 d of oven and Sicapent drying, δ2H measurements were performed with an online pyrolysis technique using a TC/EA (Thermo Fisher Scientific) coupled with an IRMS (Delta plus xp) as described previously. In addition, the samples in the TC/EA carousel were purged with helium (120 mL min−1). The helium purged gas was fed from the top of the TC/EA reactor. All results were normalized to the VSMOW‐SLAP isotope scale (Coplen, 1988) using the Laboratory Information Management System for Light Stable Isotope, so that the δ2H VSMOW of SLAP was −428‰ relative to VSMOW (Gonfiantini, 1978). Results were expressed as δ2H values, using the same international secondary standards of known 2H/1H ratios indicated before, and the analytical precision was ∼0.5‰. Finally, the fraction of total hydrogen that is exchangeable ( ) was calculated as described by Schimmelmann et al. (2001):
) was calculated as described by Schimmelmann et al. (2001):
where δ2HTA and δ2HTB are the δ2H values of the total hydrogen of the same samples equilibrated with water standards wA and wB, respectively; and where δ2HWA and δ2HWB are the δ2H values of water vapor A and B, respectively. Then the isotopic composition of nonexchangeable hydrogens (δ2Hn) can be calculated as follows:
where  is the fractionation effect between exchangeable organic hydrogen and water hydrogen. Although
is the fractionation effect between exchangeable organic hydrogen and water hydrogen. Although 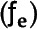 might range from 30% to 110%, depending on the molecule structure containing organic exchangeable H (Schimmelmann, 1991), typical values for most material of interest in environmental studies (proteins, cellulose, humic acids) presented a fractionation effect of 80‰ ± 20‰ (Wassenaar and Hobson, 2000). Therefore the
might range from 30% to 110%, depending on the molecule structure containing organic exchangeable H (Schimmelmann, 1991), typical values for most material of interest in environmental studies (proteins, cellulose, humic acids) presented a fractionation effect of 80‰ ± 20‰ (Wassenaar and Hobson, 2000). Therefore the 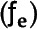 factor used in the calculation was 80‰, as has been previously determined experimentally for well-defined cellulose (Schimmelmann, 1991). Then the true δ2Hn values of the WSF and DM were calculated (Supplemental Table S4); and the δ2H of the WSF and DM in the flag leaves, the ears, and the grains were corrected using the fraction of total hydrogen that is exchangeable, obtained within the different equilibration conditions. Thus the δ2H of the WSF and DM presented in the results is the δ2H of nonexchangeable H of the organics. However, the DM fraction did not show any significant exchange with surrounding moisture (Supplemental Fig. S2). Dry organic material is likely to be in a temporary hydrophobic state, and thus the exchange is much slower than in the hydrated form (A. Schimmelmann, personal communication; Wassenaar and Hobson, 2000). Moreover, leaf samples were exposed to the same kind of atmospheric moisture, so the exchangeable hydrogen was equilibrated to a common background of water. This method ensures that relative differences among samples in the DM can be interpreted in terms of parameters that are environmental (i.e. growing conditions) and biological (i.e. biochemical composition of leaf material). Nevertheless, the δ2H values of the DM presented in the results were also corrected using the fraction of total hydrogen that is exchangeable, obtained within the different equilibration conditions.
factor used in the calculation was 80‰, as has been previously determined experimentally for well-defined cellulose (Schimmelmann, 1991). Then the true δ2Hn values of the WSF and DM were calculated (Supplemental Table S4); and the δ2H of the WSF and DM in the flag leaves, the ears, and the grains were corrected using the fraction of total hydrogen that is exchangeable, obtained within the different equilibration conditions. Thus the δ2H of the WSF and DM presented in the results is the δ2H of nonexchangeable H of the organics. However, the DM fraction did not show any significant exchange with surrounding moisture (Supplemental Fig. S2). Dry organic material is likely to be in a temporary hydrophobic state, and thus the exchange is much slower than in the hydrated form (A. Schimmelmann, personal communication; Wassenaar and Hobson, 2000). Moreover, leaf samples were exposed to the same kind of atmospheric moisture, so the exchangeable hydrogen was equilibrated to a common background of water. This method ensures that relative differences among samples in the DM can be interpreted in terms of parameters that are environmental (i.e. growing conditions) and biological (i.e. biochemical composition of leaf material). Nevertheless, the δ2H values of the DM presented in the results were also corrected using the fraction of total hydrogen that is exchangeable, obtained within the different equilibration conditions.
In addition, to prove that the extraction water had not influenced the δ2H of the WSF, further extractions were performed on aliquots of DM from representative samples (the same samples used in the dual-water equilibration method) using four sources of water with different δ2H: snow water (−77.5‰), deuterated water (+94.4‰), seawater (−3.3‰), and lab water (−43.2‰). Furthermore, the differences between the WSFs were compared. However, using water sources with different δ2H signatures for extraction did not significantly affect the δ2H of the soluble fraction; and the absolute differences in δ2H between soluble fractions extracted from the DM, with the different water sources were minor (Supplemental Table S4). All the measurements were carried out at the Scientific Facilities of the University of Barcelona.
Experimental Estimation of the ETR’s Association with δ2H Depletion
A modern Spanish durum wheat cultivar (Sula) was grown in 3-L pots (three replicates) filled with sand (one plant per pot). Plants were watered three times a week with Hoagland nutrient solution and were grown under controlled conditions in a growth chamber (Conviron E15, Controlled Environments Ltd., Winnipeg, Canada). Plants were supplied with a PPFD of about 400 µM m−2s−1 at plant level during the light period (14 h). Plants were grown in a constant RH of 40% or 80% within two different growth chambers, with a temperature of 23°C /17°C during the light and dark periods, respectively. Six flag leaves from the main tiller of different plants grown under different RH (40% or 80%); and the ETR, gs, and photosynthetic rate of the flag leaf blades were measured using a LI-6400XT portable gas exchange photosynthesis system (Li-COR, Lincoln, NE), approximately 2 weeks after anthesis. The ETR, gs, and photosynthetic rate were estimated at a saturating PPFD of 1500 μM m−2s−1 and 20°C.
Following the ETR measurements, the same six flag leaves from each of the RH conditions, the δ2H and δ13C in the DM of the leaves, were then analyzed as previously mentioned (see the hydrogen and carbon isotope analysis sections).
In order to support a causal association between the ETR and the effect that 2H-depleted NADPH has on δ2H of the organic matter, the δ2H and the ETR in the flag leaf were compared between the different RH treatments (Supplemental Table S1).
Statistical Analysis
Grain yield, agronomic components, and isotopic data were subjected to one-way analyses of variance (ANOVA) using the general linear model to calculate the effects of water regime, N supply, genotype, and their interactions with the studied parameters. Water regime, N supply, and genotype were included as fixed factors, including three blocks and three replicates per block. Means were compared by Tukey’s Honest Significant Difference Test and were performed on a combination of water treatments and N supply. Mean values across plant tissues with different letters (a and b) presented in the tables are significantly different from SI versus RF and HN versus LN, according to Tukey’s Honest Significant Difference Test (P < 0.05). A bivariate correlation procedure was constructed to analyze the relationships between the measured traits. Statistical analyses were performed using the SPSS 18.0 statistical package (SPSS Inc.). Figures were created using the Sigma-Plot 10.0 program (SPSS Inc.).
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. Polynomial regression between the mean grain yield of the trials and the regression coefficient of the relationship between grain yield carbon, oxygen and hydrogen isotope compositions of different plant components.
Supplemental Figure S2. Mean values of stable isotope composition (‰) of hydrogen (δ2H) in the water-soluble fraction (WSF) and dry matter (DM) obtained in the dual-water equilibration method.
Supplemental Table S1. Mean values of isotope composition (‰) of hydrogen(δ2H) and carbon (δ13C) in dry matter and the ETR, gs, and photosynthetic rate of the flag leaf of plants grown under two different relative humidity conditions in the growth chamber experiment
Supplemental Table S2. Linear regression of the relationship of the carbon (δ13C) oxygen (δ18O) and hydrogen (δ2H) isotope compositions in the water-soluble fraction (WSF) and dry matter (DM) of the flag leave, ears and mature grains with the grain yield (GY)
Supplemental Table S3. Mean values of stable isotope composition (‰) of hydrogen (δ2H) in the water-soluble fraction (WSF) using four sources of water with different δ2H for the extraction of the WSF
Supplemental Table S4. Mean values of stable isotope composition (‰) of hydrogen (δ2H) in the flag leaf water-soluble fraction (WSF) and dry matter (DM) obtained with the dual water equilibration method
Acknowledgments
Thank you to Kiko Girbés for designing Figure 1. We also thank Arndt Schimmelmann and Ian Begley for their valuable comments on how to quantify the fraction of exchangeable H.
Footnotes
This work was supported by the Ministry of Economy and Competitiveness (MINECO), Spain (project AGL2016-76527-R) and by the Catalan Institution for Research and Advanced Studies (ICREA) Academia, Government of Catalonia, Spain to J.L.A.
References
- Araus JL, Tapia L (1987) Photosynthetic gas exchange characteristics of wheat flag leaf blades and sheaths during grain filling: The case of a spring crop grown under Mediterranean climate conditions. Plant Physiol 85: 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Brown HR, Febrero A, Bort J, Serret MD (1993) Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant Cell Environ 16: 383–392 [Google Scholar]
- Araus JL, Amaro T, Casadesús J, Asbati A, Nachit MM (1998) Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Aust J Plant Physiol 25: 835–842 [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and drought in C3 cereals: What should we breed for? Ann Bot 89: 925–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Villegas D, Aparicio N, García del Moral LF, El Hani S, Rharrabti Y, Ferrio JP, Royo C (2003) Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Sci 43: 170–180 [Google Scholar]
- Araus JL, Ferrio JP, Buxó R, Voltas J (2007) The historical perspective of dryland agriculture: Lessons learned from 10,000 years of wheat cultivation. J Exp Bot 58: 131–145 [DOI] [PubMed] [Google Scholar]
- Araus JL, Cabrera-Bosquet L, Serret MD, Bort J, Nieto-Taladriz MT (2013) Comparative performance of δ13C, δ18O and δ15N for phenotyping durum wheat adaptation to a dryland environment. Funct Plant Biol 40: 595–608 [DOI] [PubMed] [Google Scholar]
- Barbour MM. (2007) Stable oxygen isotope composition of plant tissue: A review. Funct Plant Biol 34: 83–94 [DOI] [PubMed] [Google Scholar]
- Barbour MM, Farquhar GD (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant Cell Environ 23: 473–485 [Google Scholar]
- Barbour MM, Fischer RA, Sayre KD, Farquhar GD (2000) Oxygen isotope ratio of leaf and grain material correlates with stomatal conductance and grain yield in irrigated wheat. Aust J Plant Physiol 27: 625–637 [Google Scholar]
- Barbour MM, Roden JS, Farquhar GD, Ehleringer JR (2004) Expressing leaf water and cellulose oxygen isotope ratios as enrichment above source water reveals evidence of a Péclet effect. Oecologia 138: 426–435 [DOI] [PubMed] [Google Scholar]
- Barlow EWR, Lee JW, Munns R, Smart MG (1980) Water relations of the developing wheat grain. Aust J Plant Physiol 7: 519 [Google Scholar]
- Bloom AJ. (2015) Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth Res 123: 117–128 [DOI] [PubMed] [Google Scholar]
- Blum A. (1985) Photosynthesis and transpiration in leaves and ears of wheat and barley varieties. J Exp Bot 36: 432–440 [Google Scholar]
- Bort J, Febrero A, Amaro T, Araus J (1994) Role of awns in ear water-use efficiency and grain weight in barley. Agronomie 14: 133–139 [Google Scholar]
- Bort J, Brown RH, Araus JL (1996) Refixation of respiratory CO2 in the ears of C3 cereals. J Exp Bot 47: 1567–1575 [Google Scholar]
- Bottinga Y, Craig H (1968) Oxygen isotope fractionation between CO2 and water, and the isotopic composition of marine atmospheric CO2. Earth Planet Sci Lett 5: 285–295 [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL editors (2015) Biochemistry & Molecular Biology of Plants, Ed 2 Wiley Blackwell, Oxford UK [Google Scholar]
- Cabrera-Bosquet L, Molero G, Nogués S, Araus JL (2009a) Water and nitrogen conditions affect the relationships of Δ13C and Δ18O to gas exchange and growth in durum wheat. J Exp Bot 60: 1633–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Sánchez C, Araus JL (2009b) Oxygen isotope enrichment (Δ18O) reflects yield potential and drought resistance in maize. Plant Cell Environ 32: 1487–1499 [DOI] [PubMed] [Google Scholar]
- Cabrera-Bosquet L, Albrizio R, Nogués S, Araus JL (2011) Dual Δ13C/δ18O response to water and nitrogen availability and its relationship with yield in field-grown durum wheat. Plant Cell Environ 34: 418–433 [DOI] [PubMed] [Google Scholar]
- Caley CY, Duffus CM, Jeffcoat B (1990) Photosynthesis in the pericarp of developing wheat grains. J Exp Bot 41: 303–307 [Google Scholar]
- Ceglar A, Toreti A, Lecerf R, Van der Velde M, Dentener F (2016) Impact of meteorological drivers on regional inter-annual crop yield variability in France. Agricultural and Forest Meteorol 216: 58–67 [Google Scholar]
- Cernusak LA, Farquhar GD, Pate JS (2005) Environmental and physiological controls over oxygen and carbon isotope composition of Tasmanian blue gum, Eucalyptus globulus. Tree Physiol 25: 129–146 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58: 3549–3566 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Mejia-Chang M, Winter K, Griffiths H (2008) Oxygen isotope composition of CAM and C3Clusia species: Non-steady-state dynamics control leaf water 18O enrichment in succulent leaves. Plant Cell Environ 31: 1644–1662 [DOI] [PubMed] [Google Scholar]
- Cernusak LA, Barbour MM, Arndt SK, Cheesman AW, English NB, Feild TS, Helliker BR, Holloway-Phillips MM, Holtum JAM, Kahmen A, et al. (2016) Stable isotopes in leaf water of terrestrial plants. Plant Cell Environ 39: 1087–1102 [DOI] [PubMed] [Google Scholar]
- Chikaraishi Y, Naraoka H (2003) Compound-specific δD–δ13C analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry 63: 361–371 [DOI] [PubMed] [Google Scholar]
- Chikaraishi Y, Naraoka H, Poulson SR (2004) Carbon and hydrogen isotopic fractionation during lipid biosynthesis in a higher plant (Cryptomeria japonica). Phytochemistry 65: 323–330 [DOI] [PubMed] [Google Scholar]
- Cochrane MP, Duffus CM (1979) Morphology and ultrastructure of immature cereal grains in relation to transport. Ann Bot 44: 67–72 [Google Scholar]
- Condon AG, Richards RA, Farquhar GD (1987) Carbon isotope discrimination is positively correlated with grain yield and dry matter production in field-grown wheat. Crop Sci 27: 996–1001 [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55: 2447–2460 [DOI] [PubMed] [Google Scholar]
- Coplen TB. (1988) Normalization of oxygen and hydrogen isotope data. Chem Geol (Isot Geosci Sect) 72: 293–297 [Google Scholar]
- Cormier M-A, Werner RA, Sauer PE, Gröcke DR, Leuenberger MC, Wieloch T, Schleucher J, Kahmen A (2018) 2H-fractionations during the biosynthesis of carbohydrates and lipids imprint a metabolic signal on the δ2H values of plant organic compounds. New Phytol 218: 479–491 [DOI] [PubMed] [Google Scholar]
- Craig H, Gordon LI (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. In Tongiorgi, ed., Proceedings of a Conference on Stable Isotopes in Oceanographic Studies and Paleotemperatures, Lischi and Figli, Pisa, pp. 9–130 [Google Scholar]
- Cuntz M, Ogée J, Farquhar GD, Peylin P, Cernusak LA (2007) Modelling advection and diffusion of water isotopologues in leaves. Plant Cell Environ 30: 892–909 [DOI] [PubMed] [Google Scholar]
- Dansgaard W. (1964) Stable isotopes in precipitation. Tellus 16: 436–468 [Google Scholar]
- Dawson TE, Ehleringer JR (1993) Isotopic enrichment of water in the “woody” tissues of plants: Implications for plant water source, water uptake, and other studies which use the stable isotopic composition of cellulose. Geochim Cosmochim Acta 57: 3487–3492 [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33: 507–559 [Google Scholar]
- Epstein S, Yapp CJ, Hall JH (1976) The determination of the D/H ratio of non-exchangeable hydrogen in cellulose extracted from aquatic and land plants. Earth Planet Sci Lett 30: 241–251 [Google Scholar]
- Epstein S, Thompson P, Yapp CJ (1977) Oxygen and hydrogen isotopic ratios in plant cellulose. Science 198: 1209–1215 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Gan KS (2003) On the progressive enrichment of the oxygen isotopic composition of water along a leaf . Plant Cell Environ 26: 1579–1597 [PubMed] [Google Scholar]
- Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In Ehleringer A, Hall J, Farquhar G, eds, Stable Isotopes and Plant Carbon–Water Relations, Academic P., San Diego, pp 47–70 [Google Scholar]
- Farquhar G, Richards RA (1984) Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol 11: 539–552 [Google Scholar]
- Farquhar GD, Oleary MH, Berry JA (1982) On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust J Plant Physiol 9: 121–137 [Google Scholar]
- Farquhar G, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar G, Barbour MM, Henry BK (1998) Interpretation of oxygen isotope composition of leaf material. In.Griffiths HG, ed, Stable Isotopes: Integration of Biochemical, Ecological and Geochemical Processes, Bios Scien, Oxford, pp 27–62 [Google Scholar]
- Farquhar GD, Cernusak LA, Barnes B (2007) Heavy water fractionation during transpiration. Plant Physiol 143: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feakins SJ, Sessions AL (2010) Controls on the D/H ratios of plant leaf waxes in an arid ecosystem. Geochim Cosmochim Acta 74: 2128–2141 [Google Scholar]
- Ferrio JP, Mateo MA, Bort J, Abdalla O, Voltas J, Araus JL (2007) Relationships of grain δ13C and δ18O with wheat phenology and yield under water-limited conditions. Ann Appl Biol 150: 207–215 [Google Scholar]
- Filot MS, Leuenberger M, Pazdur A, Boettger T (2006) Rapid online equilibration method to determine the D/H ratios of non-exchangeable hydrogen in cellulose. Rapid Commun Mass Spectrom 20: 3337–3344 [DOI] [PubMed] [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Larqué-Saavedra A (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475 [Google Scholar]
- Flanagan C, Comstock JP, Ehleringer JR (1991) Comparison of modeled and observed environmental influences on the stable oxygen and hydrogen isotope composition of leaf water in Phaseolus vulgaris L. Plant Physiol 96: 588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAOSTAT) (2017) http://www.fao.org/home/en/ (July 25, 2017)
- Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63: 1637–1661 [DOI] [PubMed] [Google Scholar]
- Gessler A, Ferrio JP, Hommel R, Treydte K, Werner RA, Monson RK (2014) Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol 34: 796–818 [DOI] [PubMed] [Google Scholar]
- Gonfiantini R. (1978) Standards for stable isotope measurements in natural compounds. Nature 271: 534–536 [Google Scholar]
- Gonfiantini R, Gratziu S, Tongiorgi E (1965) Oxygen isotopic composition of water in leaves. Symposium of the Use of Isotopes and Radiation in Soil-Plant Nutrition Studies, International Atomic Energy Agency, Vienna, pp 405–410 [Google Scholar]
- Hayes JM. (2001) Fractionation of carbon and hydrogen isotopes in biosynthetic processes. Rev Mineral Geochem 43: 225–277 [Google Scholar]
- Helliker BR, Ehleringer JR (2000) Establishing a grassland signature in veins: 18O in the leaf water of C3 and C4 grasses. Proc Natl Acad Sci USA 97: 7894–7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliker BR, Ehleringer JR (2002) Differential 18O enrichment of leaf cellulose in C3 versus C4 grasses. Funct Plant Biol 29: 435–442 [DOI] [PubMed] [Google Scholar]
- Hoganson CW, Babcock GT (1997) A metalloradical mechanism for the generation of oxygen from water in photosynthesis. Science 277: 1953–1956 [DOI] [PubMed] [Google Scholar]
- Hou J, D’Andrea WJ, MacDonald D, Huang Y (2007) Hydrogen isotopic variability in leaf waxes among terrestrial and aquatic plants around Blood Pond, Massachusetts (USA). Org Geochem 38: 977–984 [Google Scholar]
- Hubick KT, Hammer GL, Farquhar GD, Wade LJ, von Caemmerer S, Henderson SA (1990) Carbon isotope discrimination varies genetically in c4 species. Plant Physiol 92: 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen A, Lorenzen B, Østergaard HS, Hvelplund EK (1990) Radiometric estimation of biomass and nitrogen content of barley grown at different nitrogen levels. Int J Remote Sens 11: 1809–1820 [Google Scholar]
- Jia S, Lv J, Jiang S, Liang T, Liu C, Jing Z (2015) Response of wheat ear photosynthesis and photosynthate carbon distribution to water deficit. Photosynthetica 53: 95–109 [Google Scholar]
- Kahmen A, Schefuß E, Sachse D (2013) Leaf water deuterium enrichment shapes leaf wax n-alkane δD values of angiosperm plants I: Experimental evidence and mechanistic insights. Geochim Cosmochim Acta 111: 39–49 [Google Scholar]
- Knoppik D, Selinger H, Ziegler-Jöns A (1986) Differences between the flag leaf and the ear of a spring wheat cultivar (Triticum aestivum cv. Arkas) with respect to the CO2 response of assimilation, respiration and stomatal conductance. Physiol Plant 68: 451–457 [Google Scholar]
- Li X, Wang H, Li H, Zhang L, Teng N, Lin Q, Wang J, Kuang T, Li Z, Li B, et al. (2006) Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiol Plant 127: 701–709 [Google Scholar]
- Liu H, Guo B, Wei Y, Wei S, Ma Y, Zhang W (2015) Effects of region, genotype, harvest year and their interactions on δ13C, δ15N and δD in wheat kernels. Food Chem 171: 56–61 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP, Naylor RL (2008) Prioritizing climate change adaptation needs for food security in 2030. Science 319: 607–610 [DOI] [PubMed] [Google Scholar]
- Lopes MS, Reynolds MP (2010) Dissecting drought adaptation into its phenotypic and genetic components in wheat. Asp Appl Biol 105: 7–11 [Google Scholar]
- Luo Y, Sternberg L (1991) Deuterium heterogeneity in starch and cellulose nitrate of cam and C3 plants. Phytochemistry 30: 1095–1098 [Google Scholar]
- Luo Y-H, Steinberg L, Suda S, Kumazawa S, Mitsui A (1991) Extremely low D/H ratios of photoproduced hydrogen by cyanobacteria. Plant Cell Physiol 32: 897–900 [Google Scholar]
- Martín-Gómez P, Barbeta A, Voltas J, Peñuelas J, Dennis K, Palacio S, Dawson TE, Ferrio JP (2015) Isotope-ratio infrared spectroscopy: A reliable tool for the investigation of plant-water sources? New Phytol 207: 914–927 [DOI] [PubMed] [Google Scholar]
- Monneveux P, Reynolds MP, Trethowan R, González-Santoyo H, Peña RJ, Zapata F (2005) Relationship between grain yield and carbon isotope discrimination in bread wheat under four water regimes. Eur J Agron 22: 231–242 [Google Scholar]
- Moore FC, Lobell DB (2015) The fingerprint of climate trends on European crop yields. Proc Natl Acad Sci USA 112: 2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann C, Ferrio JP, Holst J, Grote R, Siegwolf R, Kayler Z, Gessler A (2011) The long way down—Are carbon and oxygen isotope signals in the tree ring uncoupled from canopy physiological processes? Tree Physiol 31: 1088–1102 [DOI] [PubMed] [Google Scholar]
- Oweis T, Pala M, Ryan J (1998) Stabilizing rainfed wheat yields with supplemental irrigation and nitrogen in a Mediterranean climate. Agron J 90: 672–681 [Google Scholar]
- Pande PC, Datta PS, Bhattacharya SK (1994) Biphasic enrichment of H218O in developing wheat grain water. Indian J Plant Physiol 37: 30–31 [Google Scholar]
- Pande PC, Datta PS, Bhattacharya SK, Tyagi SK (1995) Post-anthesis metabolic-enrichment of H218O in wheat grain. Indian J Exp Biol 33: 394–396 [Google Scholar]
- Qi H, Coplen TB (2011) Investigation of preparation techniques for δ2H analysis of keratin materials and a proposed analytical protocol. Rapid Commun Mass Spectrom 25: 2209–2222 [DOI] [PubMed] [Google Scholar]
- Richards RA. (1996) Defining selection criteria to improve yield under drought. Plant Growth Regul 20: 157–166 [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF (2002) Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci 42: 111–121 [DOI] [PubMed] [Google Scholar]
- Rieder SV, Rose IA (1959) The mechanism of the triosephosphate isomerase reaction. J Biol Chem 234: 1007–1010 [PubMed] [Google Scholar]
- Roden JS, Lin G, Ehleringer JR (2000) A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim Cosmochim Acta 64: 21–35 [Google Scholar]
- Sachse D, Radke J, Gleixner G (2006) δD values of individual n-alkanes from terrestrial plants along a climatic gradient—Implications for the sedimentary biomarker record. Org Geochem 37: 469–483 [Google Scholar]
- Sachse D, Billault I, Bowen GJ, Chikaraishi Y, Dawson TE, Feakins SJ, Freeman KH, Magill CR, McInerney FA, Van der Meer MTJ, et al. (2012) Molecular paleohydrology: Interpreting the hydrogen-isotopic composition of lipid biomarkers from photosynthesizing organisms. Annu Rev Earth Planet Sci 40: 221–249 [Google Scholar]
- Sadras VO. (2004) Yield and water-use efficiency of water- and nitrogen-stressed wheat crops increase with degree of co-limitation. Eur J Agron 21: 455–464 [Google Scholar]
- Salazar-Tortosa D, Castro J, Villar-Salvador P, Viñegla B, Matías L, Michelsen A, Rubio de Casas R, Querejeta JI (2018) The “isohydric trap”: A proposed feedback between water shortage, stomatal regulation, and nutrient acquisition drives differential growth and survival of European pines under climatic dryness. Glob Chang Biol 24: 4069–4083 [DOI] [PubMed] [Google Scholar]
- Sanchez-Bragado R, Elazab A, Zhou B, Serret MD, Bort J, Nieto-Taladriz MT, Araus JL (2014) Contribution of the ear and the flag leaf to grain filling in durum wheat inferred from the carbon isotope signature: Genotypic and growing conditions effects. J Integr Plant Biol 56: 444–454 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bragado R, Araus JL, Scheerer U, Cairns JE, Rennenberg H, Ferrio JP (2016) Factors preventing the performance of oxygen isotope ratios as indicators of grain yield in maize. Planta 243: 355–368 [DOI] [PubMed] [Google Scholar]
- Sauer PE, Schimmelmann A, Sessions AL, Topalov K (2009) Simplified batch equilibration for D/H determination of non-exchangeable hydrogen in solid organic material. Rapid Commun Mass Spectrom 23: 949–956 [DOI] [PubMed] [Google Scholar]
- Schimmelmann A. (1991) Determination of the concentration and stable isotopic composition of nonexchangeable hydrogen in organic matter. Anal Chem 63: 2456–2459 [Google Scholar]
- Schimmelmann A, Lawrence M, Michael E (2001) Stable isotopes ratios of organic H, C and N in the Miocene Monterey Formation, California. In Isaac CM, Rullkötter J, eds, Monterey Formation: From Rocks to Molecules, Columbia University Press, New York, pp 86–106 [Google Scholar]
- Schleucher J, Vanderveer P, Markley JL, Sharkey TD (1999) Intramolecular deuterium distributions reveal disequilibrium of chloroplast phosphoglucose isomerase. Plant Cell Environ 22: 525–533 [Google Scholar]
- Schmidt HL, Werner RA, Eisenreich W (2003) Systematics of 2H patterns in natural compounds and its importance for the elucidation of biosynthetic pathways. Phytochem Rev 2: 61–85 [Google Scholar]
- Schmidt HL, Robins RJ, Werner RA (2015) Multi-factorial in vivo stable isotope fractionation: Causes, correlations, consequences and applications. Isotopes Environ Health Stud 51: 155–199 [DOI] [PubMed] [Google Scholar]
- Schwendenmann L, Pendall E, Sanchez-Bragado R, Kunert N, Hölscher D (2015) Tree water uptake in a tropical plantation varying in tree diversity: Interspecific differences, seasonal shifts and complementarity. Ecohydrology 8: 1–12 [Google Scholar]
- Serret MD, Ortiz-Monasterio I, Pardo A, Araus JL (2008) The effects of urea fertilisation and genotype on yield, nitrogen use efficiency, δ15N and δ13C in wheat. Ann Appl Biol 153: 243–257 [Google Scholar]
- Sessions AL, Burgoyne TW, Schimmelmann A, Hayes JM (1999) Fractionation of hydrogen isotopes in lipid biosynthesis. Org Geochem 30: 1193–1200 [Google Scholar]
- Shangguan ZP, Shao MA, Dyckmans J (2000) Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ Exp Bot 44: 141–149 [DOI] [PubMed] [Google Scholar]
- Smith FA, Freeman KH (2006) Influence of physiology and climate on δD of leaf wax n-alkanes from C3 and C4 grasses. Geochim Cosmochim Acta 70: 1172–118 [Google Scholar]
- Song X, Farquhar GD, Gessler A, Barbour MM (2014) Turnover time of the non-structural carbohydrate pool influences δ18O of leaf cellulose. Plant Cell Environ 37: 2500–2507 [DOI] [PubMed] [Google Scholar]
- Sternberg L. (1988) D/H ratios of environmental water recorded by D/H ratios of plant lipids. Nature 333: 59–61 [Google Scholar]
- Sternberg L, Deniro MJ (1983) Isotopic composition of cellulose from C3, C4, and CAM plants growing near one another. Science 220: 947–949 [DOI] [PubMed] [Google Scholar]
- Sternberg LO, Deniro MJ, Johnson HB (1984) Isotope ratios of cellulose from plants having different photosynthetic pathways. Plant Physiol 74: 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg LdaS, Deniro MJ, Savidge RA (1986) Oxygen isotope exchange between metabolites and water during biochemical reactions leading to cellulose synthesis. Plant Physiol 82: 423–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambussi EA, Nogués S, Araus JL (2005) Ear of durum wheat under water stress: Water relations and photosynthetic metabolism. Planta 221: 446–458 [DOI] [PubMed] [Google Scholar]
- Voltas J, Romagosa I, Lafarga A, Armesto AP, Araus JL, Sombrero A (1999) Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Aust J Agric Res 50: 1263–1271 [Google Scholar]
- Wassenaar LI, Hobson KA (2000) Improved method for determining the stable-hydrogen isotopic composition (δmproved method for determining the stable-hydrogen isotop. Envirn. Sci. Technol. 34: 2354–2360. [Google Scholar]
- Williams DG, Coltrain JB, Lott M, English NB, Ehleringer JR (2005) Oxygen isotopes in cellulose identify source water for archaeological maize in the American Southwest. J Archaeol Sci 32: 931–939 [Google Scholar]
- Winter K, Holtum JAM (2014) Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J Exp Bot 65: 3425–3441 [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM (2008) On the nature of facultative and constitutive CAM: Environmental and developmental control of CAM expression during early growth of Clusia, Kalanchöe, and Opuntia. J Exp Bot 59: 1829–1840 [DOI] [PubMed] [Google Scholar]
- Yakir D. (1992) Variations in the natural abundance of oxygen-18 and deuterium in plant carbohydrates. Plant Cell Environ 15: 1005–1020 [Google Scholar]
- Yakir D, Deniro MJ (1990) Oxygen and hydrogen isotope fractionation during cellulose metabolism in Lemna gibba L. Plant Physiol 93: 325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakir D, Deniro MJ, Ephrath JE (1990a) Effects of water stress on oxygen, hydrogen and carbon isotope ratios in two species of cotton plants. Plant Cell Environ 13: 949–955 [Google Scholar]
- Yakir D, Deniro MJ, Gat JR (1990b) Natural deuterium and oxygen-18 enrichment in leaf water of cotton plants grown under wet and dry conditions: Evidence for water compartmentation and its dynamics. Plant Cell Environ 13: 49–56 [Google Scholar]
- Yang H, Leng Q (2009) Molecular hydrogen isotope analysis of living and fossil plants—Metasequoia as an example. Prog Nat Sci 19: 901–912 [Google Scholar]
- Yousfi S, Serret MD, Araus JL (2013) Comparative response of δ13C, δ18O and δ15N in durum wheat exposed to salinity at the vegetative and reproductive stages. Plant Cell Environ 36: 1214–1227 [DOI] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14: 415–421 [Google Scholar]
- Zhao LJ, Xiao HL, Liu XH (2007) Relationships between carbon isotope discrimination and yield of spring wheat under different water and nitrogen levels. J Plant Nutr 30: 947–963 [Google Scholar]
- Zhou Y, Stuart-Williams H, Farquhar GD, Hocart CH (2010) The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 71: 982–993 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Grice K, Chikaraishi Y, Stuart-Williams H, Farquhar GD, Ohkouchi N (2011) Temperature effect on leaf water deuterium enrichment and isotopic fractionation during leaf lipid biosynthesis: Results from controlled growth of C3 and C4 land plants. Phytochemistry 72: 207–213 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhang B, Stuart-Williams H, Grice K, Hocart CH, Gessler A, Kayler ZE, Farquhar GD (2018) On the contributions of photorespiration and compartmentation to the contrasting intramolecular 2H profiles of C3 and C4 plant sugars. Phytochemistry 145: 197–206 [DOI] [PubMed] [Google Scholar]
- Ziegler H. (1989) Hydrogen isotope fractionation in plant tissues. In Stable Isotopes in Ecological Research. In Rundel P, Ehleringer JR, Nagy K, eds, Springer-Verlag, New York, pp 105–123 [Google Scholar]
- Ziegler H, Osmond CB, Stichler W, Trimborn P (1976) Hydrogen isotope discrimination in higher plants: Correlations with photosynthetic pathway and environment. Planta 128: 85–92 [DOI] [PubMed] [Google Scholar]