OsAAP5 might mediate basic amino acids (Lys and Arg) and neutral amino acids (Val and Ala) transport to affect cytokinin pathway.
Abstract
As fundamental nutrients, amino acids are important for rice (Oryza sativa) growth and development. Here, we identified the amino acid permease 5 (OsAAP5), that regulates tiller number and grain yield in rice. The OsAAP5 promoter sequence differed between indica and japonica rice varieties. Lower expression of OsAAP5 in the young leaf blade in indica varieties than in japonica varieties was associated with more tillers in indica than in japonica. Down-regulation of OsAAP5 expression in japonica using RNA interference (RNAi) and clustered regularly interspaced short palindromic repeats led to increases in tiller number and grain yield, whereas OsAAP5 overexpression (OE) had the opposite effect. Both a protoplast amino acid uptake assay and HPLC analysis indicated that more basic (Lys, Arg) and neutral (Val, Ala) amino acids were transported and accumulated in the OE lines than in the wild type, but the opposite was observed in the RNAi lines. Furthermore, exogenous application of Lys, Arg, Val, and Ala in the OE lines substantially inhibited tiller bud elongation, but the effect was lost in the RNAi lines. Notably, concentrations of the cytokinins cis-zeatin and dihydrozeatin were much lower in the OE lines than in the wild type, whereas concentrations in the RNAi lines were higher. Thus, OsAAP5 could regulate tiller bud outgrowth by affecting cytokinin levels, and knockout of OsAAP5 could be valuable for japonica breeding programs seeking high yield and grain quality.
Rice (Oryza sativa) is the staple food for more than half of the world’s population (Fairhurst and Dobermann, 2002). According to geographic distribution and genetic variation, Asian rice is classified into two subspecies, indica and japonica (Liu et al., 2018). Generally, japonica rice has a significantly higher head rice rate, lower degree of chalkiness, lower amylose content, and higher gel consistency than indica rice; all these characteristics of japonica contribute to improved grain quality (Feng et al., 2017). In addition, japonica cultivars are more cold tolerant than indica cultivars (Lu et al., 2014; Liu et al., 2018). However, the grain yield of japonica is much lower than that of indica due to the lower tiller number of japonica rice (Hu et al., 2015). In recent years, rice varieties with high tiller numbers have been pursued by many rice breeders, as these high-tillering varieties theoretically could produce more effective panicles. The effective panicle number per plant, grain number per panicle, and grain weight per panicle determine the grain yield of a rice plant (Xing and Zhang, 2010). Thus, increasing japonica rice tiller number is urgent for breeding programs to produce japonica cultivars with high yield and good grain quality.
Application of nitrogen (N) fertilizer has been reported as one of the most effective ways to increase tiller number, because it increases the cytokinin (CK) content within tiller nodes and further enhances tiller bud outgrowth (Sakakibara et al., 2006; Liu et al., 2011; Wang et al., 2017). CK content is positively correlated with soil N content (Takei et al., 2001, 2004), perhaps because the primary product of CK synthesis is the N6-(Δ2-isopentenyl) adenine nucleotide, which is formed by adenosine phosphate-isopentenyltransferase (IPT). There are seven IPT genes in Arabidopsis (Arabidopsis thaliana), of which IPT3 is upregulated by nitrate (Sakakibara et al., 2006). Another reason may be that nitric oxide, which is produced as part of N metabolism, is one of the most extensively used signaling molecules in living organisms (Schmidt and Walter, 1994). It has been reported that nitric oxide could directly interact with transzeatin (tZ) in vivo, creating nitrated CK species and thereby regulating the CK signaling pathway (Liu et al., 2013).
Nitrate and ammonium, the two main inorganic N forms, as well as peptides and amino acids (organic N forms), can be used by plants in soils (Tegeder and Rentsch, 2010; Moran-Zuloaga et al., 2015). Plants have evolved multiple efficient N uptake and transport systems to support their growth and development in environments with different forms and amounts of available N. In the rice inorganic N transporter family, the functions of NPF (Nitrate transporter1 [NRT1]/Peptide transporter [PTR] Family) members have been extensively studied. OsPTR9 (OsNPF8.20; Fang et al., 2013), NRT1.1B (OsNPF6.5; Hu et al., 2015), OsNPF7.2 (Wang et al., 2018), and OsNPF7.7 (Huang et al., 2018) could influence rice tiller number by regulating N content. In addition, Gln synthetase1;2, a key enzyme of ammonium assimilation that converts inorganic ammonium into Gln in plants, regulates tiller number in rice by affecting CK level (Funayama et al., 2013; Ohashi et al., 2015, 2017).
In addition to the inorganic N transporters mentioned above, organic N transporters also play important roles in plant growth and development (Tegeder and Rentsch, 2010). Amino acid permeases (AAPs), members of the amino acid transporter family, have been extensively functionally studied in plants. In Arabidopsis, seven AAP transporters play important roles in translocating different amino acids from source to sink organs (Zhao et al., 2012). AtAAP1 functions in root amino acid acquisition and seed yield in Arabidopsis (Lee et al., 2007; Sanders et al., 2009). AtAAP2 is important for amino acid transport from the xylem to the phloem (Zhang et al., 2010), whereas AtAAP3 mediates the uptake of neutral and basic amino acids (Okumoto et al., 2004). AtAAP4 has significant involvement in Val transport (Fischer et al., 1995), AtAAP5 transports anionic, neutral, and cationic amino acids (Boorer and Fischer, 1997; Svennerstam et al., 2008), and AtAAP6 affects the contents of Lys, Phe, Leu, and Asp and regulates rosette width and seed volume in Arabidopsis (Hunt et al., 2010). In addition, AtAAP8 transports acidic amino acids into the endosperm and is important for seed yield (Schmidt et al., 2007; Santiago and Tegeder, 2016).
Among the 19 AAP transporters (OsAAP1-OsAAP19) in rice, OsAAP3 (Lu et al., 2018) and OsAAP6 (Peng et al., 2014) have been reported to influence rice grain yield and quality, respectively, and OsAAP3 mainly transports Lys and Arg (Taylor et al., 2015). However, whether other OsAAPs are responsible for rice growth and development is unclear. In this study, we found that the OsAAP5 promoter sequence was divergent between indica and japonica, resulting in higher expression of OsAAP5 in japonica cultivars, which in turn caused fewer tillers than in indica. Moreover, the down-regulation of OsAAP5 significantly increased tiller number by decreasing the contents of basic amino acids (Lys and Arg) and neutral amino acids (Val and Ala) to maintain higher CK levels in rice plants.
RESULTS
OsAAP5 Promoter Sequence was Divergent between indica and japonica Cultivars
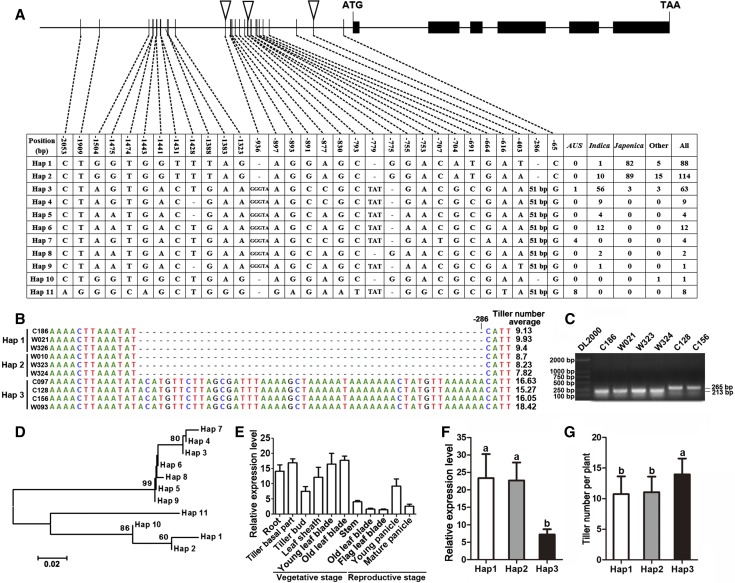
We used Rice Variation Map v2.0, a database for rice genome variation (Chen et al., 2014), to investigate the sequences of the OsAAP5 promoter region in 306 cultivars from around the world with different tiller numbers. Among these 306 cultivars, 26 single nucleotide polymorphisms (SNPs) and 5 insertion/deletions (indels) were detected in 11 variant types of the OsAAP5 promoter, which were named haplotypes 1 to 11 (Hap1-Hap11; Fig. 1A). Interestingly, Hap1 and Hap2 were mainly found in japonica cultivars, and Hap3 was mainly found in indica cultivars. A 51-bp insertion was validated in Hap3 by agarose gel electrophoresis (Fig. 1, B and C), which might be useful for distinguishing the indica and japonica promoter types. Additionally, phylogenetic analysis showed that two clusters existed among the 11 haplotypes, an indica cluster containing Hap3 and a japonica cluster containing Hap1 and Hap2 (Fig. 1D). These results demonstrated divergence in the OsAAP5 promoter sequence between indica and japonica cultivars.
Figure 1.
Sequence divergence in the OsAAP5 promoter regions in 306 rice accessions collected worldwide. A, SNP divergence in the promoters of OsAAP5. B, Distribution of the 51-bp indel and the average tiller number of some cultivars in rice Hap1-Hap3. C, Detection of the 51-bp indel using agarose gel electrophoresis. D, The phylogeny tree of OsAAP5 promoters was constructed using the neighbor-joining method with MEGA software (version 5.1). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown above the branches. Scale bar = number of base substitutions per site. E, The expression pattern of OsAAP5 in different tissues of ZH11. F, The expression level of OsAAP5 in the young leaf blade of rice Hap1-Hap3. G, The average tiller number of all cultivars in rice Hap1-Hap3. Values are means ± sd, and three replications were performed in each analysis. The letters above the error bars are ranked by Duncan’s multiple range test. Different letters indicate significant differences at P < 0.05.
The expression pattern of OsAAP5 in japonica cultivar ZH11 (Hap1) showed that OsAAP5 was expressed in various tissues, including the root, tiller basal part, leaf sheath, leaf blade, and young panicle (Fig. 1E). We chose the young leaf blade at the vegetative stage to compare the expression of OsAAP5 in Hap1, Hap2, and Hap3. The results showed that the expression of OsAAP5 in japonica cultivars (Hap1 and Hap2) was significantly higher than that in indica cultivars (Hap3; Fig. 1F). We also detected the expression pattern of OsAAP5 among Hap1, Hap2, and Hap3 and found that japonica haplotypes (Hap1 and Hap2) exhibited different expression patterns from indica haplotypes (Hap3), especially in the root, tiller basal part, and leaf sheath (Supplemental Fig. S1). Furthermore, we compared the tiller numbers of indica haplotypes and japonica haplotypes and found that indica haplotypes produced more tillers than japonica haplotypes (Fig. 1G). These results demonstrated that japonica accessions with Hap1 and Hap2 had higher expression of OsAAP5 but fewer tillers than indica accessions with Hap3, indicating a negative association between OsAAP5 expression and tiller number.
OsAAP5 Expression Mainly Occurred in Vascular Aboveground Tissues, and Its Protein Localized to the Plasma Membrane
To further investigate the expression pattern of OsAAP5, POsAAP5::GUS transgenic plants were produced in the ZH11 background. GUS staining showed that the GUS signal was particularly strong in the root (Fig. 2A), tiller basal part (Fig. 2B), stem (Fig. 2C), leaf sheath (Fig. 2D), and young leaf blade (Fig. 2E) at the vegetative stage and in the young panicle at the reproductive stage (Fig. 2F). Additionally, GUS activity was abundant in all cells in the transverse section of the root (Fig. 2, G and H) and in the vascular parenchyma cells in the leaf sheath (Fig. 2, I and J) and young panicle (Fig. 2K). These results demonstrated that OsAAP5 might absorb nutrients through the roots and transport nutrients through the vasculature of aboveground tissues.
Figure 2.
GUS staining and subcellular localization of OsAAP5. GUS staining of the root tip (A), tiller basal part (B), stem (C), leaf sheath (D), young leaf blade (E), and young panicle (F) from the POsAAP5::GUS transgenic plants. The following are also shown: transverse section (G) and its vascular enlargement (H) of a root from the POsAAP5::GUS transgenic plants; transverse section (I) and its vascular enlargement (J) of a leaf sheath from the POsAAP5::GUS transgenic plants; and transverse section of a young panicle (K) from the POsAAP5::GUS transgenic plants. VT, vascular tissue; Ep, epidermis; VPC, vascular parenchyma cells. L, Free GFP expression in rice protoplasts. M, The expression of OsAAP5-GFP, which was coexpressed with plasma membrane protein OsMCA1 fused with mCherry. Scale bars = 5 mm (A–F), 200 μm (G, I, and K), 50 μm (H and J), and 10 μm (L and M).
To investigate the roles of OsAAP5 in response to various forms of N, ZH11 seedlings were cultured in nutrient solution with inorganic N (NO3−, NH4+, and NH4NO3) as the sole N source, and the expression of OsAAP5 was detected in roots and tiller buds. The results showed that OsAAP5 expression levels were up-regulated with increasing N concentrations, except under NH4NO3 treatment (Supplemental Fig. S2, A and B). These results indicated that the expression of OsAAP5 could be regulated in response to various N treatments, especially in the root.
OsAAP5 was predicted to be an amino acid permease with 11 transmembrane domains (Supplemental Fig. S3) and possibly localized to the plasma membrane. To validate this hypothesis, we fused GFP to the OsAAP5 C terminus and transiently expressed this fusion protein in rice protoplasts. The green fluorescence signal of 35S::GFP was enriched in the cytoplasm and nucleus (Fig. 2L), but coexpression of OsAAP5-GFP and a plasma membrane mCherry marker showed that OsAAP5 clearly localized to the plasma membrane (Fig. 2M). These results indicated that OsAAP5 might play a key role in membrane transport.
OsAAP5 Regulated Rice Tiller Number and Grain Yield by Influencing Tiller Bud Outgrowth
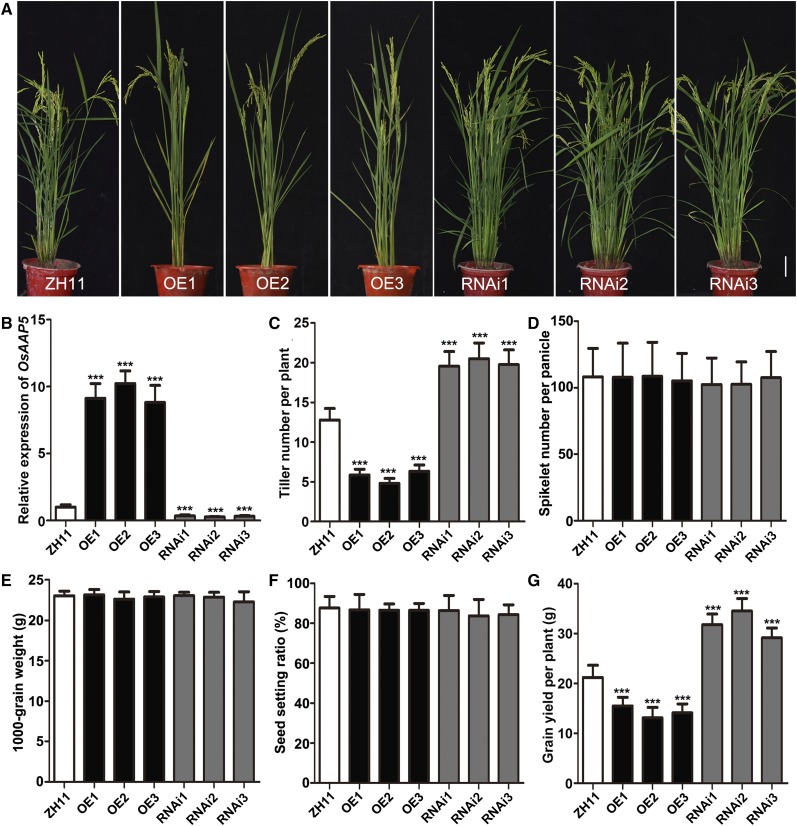
To uncover the function of OsAAP5 in rice growth and development, we generated OsAAP5 overexpression (OE) lines and RNA interference (RNAi) lines in the japonica ZH11 background and validated OsAAP5 expression by reverse transcription-quantitative PCR (RT-qPCR) in the young leaf blades of T2 transgenic lines (Fig. 3, A and B). We also detected the expression of the 18 other OsAAPs in the young leaf blades of ZH11, the OE lines, and the RNAi lines. The results showed that alteration of OsAAP5 expression also affected the expression levels of OsAAP1, OsAAP11, and OsAAP17 (Supplemental Fig. S4), which indicated that OsAAP5 might coordinate with OsAAP1, OsAAP11, and OsAAP17 to mediate amino acid transport. In addition, altered expression of OsAAP5 changed the plant architecture, especially tiller number (Fig. 3A). When compared with that of ZH11, the tiller number of the RNAi lines increased significantly, but the tiller number of the OE lines decreased (Fig. 3C). Because rice grain yield is also determined by two other factors, grain number per panicle and grain weight per panicle (Xing and Zhang, 2010), we investigated additional yield traits of ZH11, the OE lines, and the RNAi lines. No significant differences in spikelet number per panicle, 1000-grain weight, and seed set ratio were observed among the differential expression lines of OsAAP5 (Fig. 3, D–F). When compared with that in ZH11, grain yield per plant in paddy significantly increased in the RNAi lines but decreased in the OE lines (Fig. 3G). These results indicated that reduced expression of OsAAP5 could significantly increase tiller number, thus improving grain yield in ZH11.
Figure 3.
Phenotype analysis of OsAAP5 transgenic plants grown in paddy field. Whole plant phenotype (A), relative expression levels of OsAAP5 (B), tiller number per plant (n = 30; C), spikelet number per panicle (n = 30; D), 1000-grain weight (n = 30; E), seed setting rate (n = 30; F), and grain yield per plant (n = 30; G) of transgenic plants are shown. OE1-OE3 indicate OsAAP5 overexpressing lines, and RNAi1-RNAi3 represent OsAAP5-RNAi lines. Scale bar = 10 cm (A). Values are means ± sd, and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), *** P < 0.001.
Rice tiller bud outgrowth is an important factor determining tiller number (Li et al., 2003). To examine tiller bud development across the differential expression lines, seedlings at 7 d after germination (DAG) were cultured with 2.0 mm NH4NO3 under hydroponic conditions for 3 weeks until the tiller buds began to elongate. At 27 DAG, the seedlings of the RNAi lines were larger than those of ZH11, and the opposite result was found in the OE lines (Supplemental Fig. S5A). In addition, repressing the expression of OsAAP5 led to significantly increased biomass per plant, including fresh weight and dry weight, compared with that of ZH11 (Supplemental Fig. S5C). The tiller buds, especially the second tiller bud, in the RNAi lines were longer than that in ZH11, but no significant difference in bud length was found between the OE lines and ZH11 (Supplemental Fig. S5, B and D). These results demonstrated that OsAAP5 affected tiller bud outgrowth and thereby tiller number.
OsAAP5 Might Transport Basic Amino Acids and Neutral Amino Acids in Rice Plants
Because OsAAP5 is an amino acid permease, we detected the free amino acid concentrations in ZH11, the OE lines, and the RNAi lines to investigate the effect of OsAAP5 expression level on amino acid transport. The analysis of total amino acid concentration indicated that OsAAP5 OE significantly increased the total free amino acid concentration in the tiller basal part, leaf sheath, and leaf blade, whereas OsAAP5 repression exhibited the opposite result (Fig. 4, A–C). We then measured the concentration of each individual amino acid in the tiller basal part, leaf sheath, and leaf blade of ZH11, the OE lines, and the RNAi lines using HPLC. The concentrations of basic amino acids (Lys and Arg) and neutral amino acids (Thr, Ala, and Val) in the tiller basal part, leaf sheath, and leaf blade of the OE lines were higher than those of ZH11, but the opposite result occurred in the RNAi lines (Fig. 4, D–F). In addition, the elevated expression of OsAAP5 also caused the accumulation of Ser in the leaf blade but not in the tiller basal part and leaf sheath (Fig. 4, D–F). γ-Aminobutyric acid (GABA) functions as a signal that modulates plant growth, development, and stress response (Ramesh et al., 2017); therefore, we also detected the content of GABA and found no significant difference in GABA content in lines with different OsAAP5 expression levels (Supplemental Fig. S6). These results indicated that OsAAP5 might mediate basic and neutral amino acid transport in rice plants.
Figure 4.
Effects of OsAAP5 on amino acid concentration among ZH11, OE, and RNAi lines. Total concentration of amino acids in tiller basal part (A), leaf sheath (B), and leaf blade (C) of ZH11, OE lines, and RNAi lines. Single concentration of amino acids in tiller basal part (D), leaf sheath (E), and leaf blade (F) of ZH11, OE lines, and RNAi lines are also shown. OE-M indicates OE1-OE3 mixed, and RNAi-M indicates RNAi1-RNAi3 mixed. DW, dry weight. Values are means ± sd, and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), * P < 0.05, ** P < 0.01, *** P < 0.001.
A protoplast-esculin assay is a method to assay plant Suc transporters (Rottmann et al., 2018). To further confirm that OsAAP5 mediates basic and neutral amino acid transport, we performed a protoplast amino acid uptake assay. Protoplasts were cultured with a fluorescein isothiocyanate–labeled amino acid (Lys-FITC, Arg-FITC, Val-FITC, Ala-FITC, Ser-FITC, or Pro-FITC) at 1 mm. To examine OsAAP5 transport function, we detected the fluorescent signal after 1 h of culture. We detected higher fluorescent cell ratio and higher fluorescence signal intensity in the protoplasts of the OE lines cultured with Lys-FITC and Arg-FITC than in ZH11 protoplasts, and the FITC signal was weaker in the RNAi lines than in ZH11 (Fig. 5, A, B, E, and F). The fluorescent signals of protoplasts cultured with Val-FITC and Ala-FITC for 1 h showed similar results to those with the basic amino acids (Fig. 5, C–F). In addition, protoplasts cultured with Ser-FITC (Supplemental Fig. S7, A, C, and D) and Pro-FITC (Supplemental Fig. S7, B, E, and F) showed no significant differences in either fluorescent cell ratio or fluorescence signal intensity among ZH11, the OE lines, and the RNAi lines. These results indicated that OsAAP5 might play a crucial role in transporting basic amino acids (Lys and Arg) and neutral amino acids (Val and Ala) into rice plant cells.
Figure 5.
Protoplast amino acid uptake assay in transgenic plants. Fluorescence was detected after culturing protoplasts with FITC-labeled amino acids for an hour. Green fluorescence images of ZH11, OE, and RNAi lines under Lys-FITC (A), Arg-FITC (B), Ala-FITC (C), and Val-FITC (D) are shown. E, Statistical analysis of the proportion of fluorescence cells. A total of 400 cells were statistically analyzed. F, Detection of cell fluorescence signal intensity. Fluorescence intensities were normalized to the area of the respective cell by ImageJ software, and a total of 100 cells were statistically analyzed. Scale bars = 50 μm. Values are means ± sd, and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), * P < 0.05, ** P < 0.01, *** P < 0.001.
Exogenous High-Concentration Amino Acids Inhibited Tiller Bud Outgrowth
Maintenance of internal amino acid homeostasis in plants is crucial in plant growth and development (Lu et al., 2018). Nutrient solutions with added Lys, Arg, Val, and Ala at concentrations of 1.0 mm, 0.2 mm, 1.0 mm , and 1.0 mm, respectively, promote tiller bud outgrowth in ZH11, and higher concentrations of these amino acids than those mentioned above inhibit tiller bud outgrowth (Lu et al., 2018). To further validate the effects of these amino acids on tiller bud outgrowth across different OsAAP5 expression lines, an exogenous amino acid assay was performed. The tiller bud length exhibited no significant difference between the OE lines and ZH11. However, the second tiller bud length significantly increased in the RNAi lines compared with that in ZH11 when the Lys concentration in the nutrient solution was increased to 1.5 mm (Fig. 6, A and C). When the Arg concentration in the nutrient solution was elevated to 0.3 mm, a similar result was observed (Fig. 6, B and D). Additionally, when the concentrations of the neutral amino acids Ala and Val were increased to 2.0 mm, the tiller bud length, especially that of the second tiller bud, significantly increased in the RNAi lines compared with ZH11, but no differences in tiller bud length were found between the OE lines and ZH11 (Supplemental Fig. S8, A and B). These results demonstrated that OsAAP5 might mediate the uptake of Lys, Arg, Val, and Ala and that excessive amino acid accumulation in plant cells retards rice tiller bud elongation.
Figure 6.
Effects of Lys and Arg on bud elongation of seedlings grown in hydroponic culture. Phenotypes (A) and bud length (C) of ZH11, OE1-OE3, and RNAi1-RNAi3 lines with 1.0 mm NH4NO3 containing 1.5 mm Lys are shown. Phenotypes (B) and bud length (D) of ZH11, OE1-OE3, and RNAi1-RNAi3 lines with 1.0 mm NH4NO3 containing 0.3 mm Arg are also shown. The tiller buds were digitally extracted for comparison. White arrows indicate the first tiller bud, and red arrows indicate the second tiller bud. Scale bars = 3 mm. Values are means ± sd (n > 15), and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), * P < 0.05, ** P < 0.01, *** P < 0.001.
Altered Expression of OsAAP5 Regulated CK Levels in Rice
Shoot branching, which is regulated by the plant hormones CK, auxin, and strigolactone (SL), is important for generating diverse plant forms (Gomez-Roldan et al., 2008; Umehara et al., 2008). CK promotes bud outgrowth, whereas auxin and SLs inhibit it (Gomez-Roldan et al., 2008; Domagalska and Leyser, 2011). Recently, both OsGln synthetase1;2 and the nitrate transporter OsNPF7.2 have been reported to affect tiller bud outgrowth through the CK pathway (Ohashi et al., 2017; Wang et al., 2018). This study indicated that reduced expression of OsAAP5 promoted tiller bud outgrowth. Based on these results, we speculated that alterations in OsAAP5 expression might affect CK levels in plant cells. To validate this hypothesis, we measured CK-related gene expression in the leaf sheath and leaf blade across lines with differential expression levels of OsAAP5. In this study, we detected two genes of the cytokinin oxidase/dehydrogenase (CKX) family, OsCKX2 and OsCKX4, which were reported to regulate tiller number in rice (Gao et al., 2014; Yeh et al., 2015). When compared with that in ZH11, leaf sheath expression of OsCKX2 significantly increased in the OE lines, whereas it significantly decreased in the RNAi lines. However, no obvious differences in OsCKX4 expression levels occurred among ZH11, the OE lines, and the RNAi lines (Fig. 7C). Additionally, the expression of the type-A response regulator (RR) OsRR1 in response to CKs increased in the RNAi lines, whereas it decreased in the OE lines, compared with ZH11 (Fig. 7C). These results indicated that the suppression of OsAAP5 might lead to increased CK content. Subsequently, we measured the contents of individual CKs in the leaf sheath and leaf blade in the differential expression lines. CK contents, especially tZ, cis-zeatin (cZ), and dihydrozeatin (DZ) contents, were higher in the leaf sheaths of the RNAi lines than in those of ZH11, but only tZ and DZ contents decreased in the OE lines compared with ZH11 (Fig. 7A). In the leaf blade, cZ and DZ contents were higher in the RNAi lines and lower in the OE lines than in ZH11 (Fig. 7B). Additionally, the indole-3-acetic acid (IAA) and methyl indole-3-acetate (ME-IAA) levels in the leaf shealths of OE and RNAi lines were both decreased, but the indole-3-carboxaldehyde (ICA) levels increased, compared with ZH11, respectively (Supplemental Fig. S9A). In leaf blade, only ME-IAA content was decreased in RNAi lines, and ICA contents were both increased in OE and RNAi lines, compared with ZH11, respectively (Supplemental Fig. S9B). These results indicated that the auxin levels in the leaf sheath and leaf blade exhibited no obvious trends with altered OsAAP5 expression. Moreover, we detected the expression of SL biosynthetic genes (OsD27, OsD17, and OsD10), a perception gene (OsD14), signaling genes (OsD3 and OsD53), and another important tiller-related gene OsFC1 (Ishikawa et al., 2005; Zou et al., 2005; Arite et al., 2007, 2009; Lin et al., 2009; Minakuchi et al., 2010; Yoshida et al., 2012; Jiang et al., 2013; Zhou et al., 2013) in ZH11, the OE lines, and the RNAi lines. However, no significant difference in the expression levels of these genes was observed, except that OsD3 expression was found to be lower in OE lines and OsD53 expression levels were both higher in the OE and RNAi lines than in ZH11 (Supplemental Fig. S10). These results indicated that OsAAP5 might regulate tiller bud outgrowth through the CK pathway.
Figure 7.
Effects of OsAAP5 on CK concentrations. Four CK concentrations were detected in the leaf sheath (A) and leaf blade (B) of ZH11, OE lines, and RNAi lines grown in hydroponic culture. OE-M indicates OE1-OE3 mixed, and RNAi-M indicates RNAi1-RNAi3 mixed. IP, isopentenyladenine. The expressions of OsCKX2, OsCKX4, and OsRR1 in tiller basal part (C) of seedlings in hydroponic culture are shown. Values are means ± sd, and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), * P < 0.05, ** P < 0.01, *** P < 0.001. DW, dry weight.
Knockout of OsAAP5 in ZH11 Significantly Increased Tiller Number and Grain Yield
Because OsAAP5 negatively regulated tiller number, we knocked out the OsAAP5 sequence of japonica ZH11 using CRISPR technology to verify the application value of this gene in rice breeding programs. The target site of OsAAP5 is shown in Fig. 8A. The sequencing results revealed 2-bp and 1-bp deletions in the sixth exon of OsAAP5, which caused frameshifts of OsAAP5 in the OsAAP5-CRISPR lines (OsAAP5-C1 and OsAAP5-C2). In the mature stage, OsAAP5-C1 and OsAAP5-C2 produced more tillers than ZH11 (Fig. 8, B–D). Accordingly, the grain number per plant of OsAAP5-C1 and OsAAP5-C2 were both higher than that of ZH11 (Fig. 8, E–G). Statistical analysis revealed that both tiller number and grain number per plant in OsAAP5-C1 and OsAAP5-C2 significantly increased compared with those in ZH11 (Fig. 8, H and I), resulting in a significant improvement in rice grain yield per plant in paddy (Fig. 8J). These results demonstrate that knockout of OsAAP5 is valuable for high-yield rice breeding programs and that the application of CRISPR technology is especially important for japonica improvement.
Figure 8.
Knockout of OsAAP5 using CRISPR technology significantly improved grain yield in ZH11. A, Sequencing of base deletion of OsAAP5-CRISPR lines. Phenotype of ZH11 (B) and OsAAP5-CRISPR lines (C and D) are shown. Grain yield per plant of ZH11 (E) and OsAAP5-CRISPR lines (F and G) are also shown. Tiller number per plant (H), filled grain number per plant (I), and grain yield per plant (J) were analyzed between ZH11 and OsAAP5-CRISPR lines. Scale bars = 20 cm (B–D) and 5 cm (E–G). Values are means ± sd (n > 20), and three replications were performed in each analysis. Student’s t test (each transgenic line vs. ZH11), ** P < 0.01.
DISCUSSION
OsAAP5 Knockout Is Valuable for japonica Rice Breeding
Indica and japonica, two subspecies of Asian-cultivated rice, are divergent at the physiological, molecular, and biochemical levels (Dan et al., 2014). In the N metabolic pathway, OsNPF6.5 (Hu et al., 2015) and OsAAP3 (Lu et al., 2018) show obvious differentiation between indica and japonica rice, and the indica alleles of both OsNPF6.5 and OsAAP3 are beneficial to the improvement of tiller and grain yield in rice. In this study, OsAAP5, a gene found to be highly differentiated between indica and japonica rice, could negatively regulate rice tiller number and grain yield and might, together with OsAAP3 and OsNPF6.5, control plant type differences between indica and japonica rice by affecting N metabolism. Because of the high homology between the OsAAP3 and OsAAP5 sequences in rice, we detected the expression of OsAAP3 in lines with different OsAAP5 expression levels and found that changes in the expression level of OsAAP5 do not affect OsAAP3 gene expression levels. In addition, there was no significant difference in the expression of OsAAP5 among OsAAP3 lines with different expression levels (Supplemental Fig. S11). These results indicated that the effect of OsAAP5 on tiller number might be independent of OsAAP3. Furthermore, knockout of OsAAP5 in the ZH11 background using CRISPR technology enhanced tiller formation and significantly increased grain yield (Fig. 8). Based on these findings, we conclude that OsAAP5 plays a role in the difference between indica and japonica rice plant types. Plant membrane transporter genes can be incorporated into plants to enhance crop yields (Schroeder et al., 2013). Therefore, knockout of OsAAP5 might be of value in breeding japonica rice with high yield and grain quality.
OsAAP5 Might Negatively Regulate Rice Tiller Bud Outgrowth by Mediating Lys, Arg, Val, and Ala Transport
In this study, the contents of basic amino acids (Lys and Arg) and neutral amino acids (Val and Ala) in the OsAAP5 OE lines significantly increased according to HPLC and protoplast amino acid uptake assays (Figs. 4 and 5). To further explore the effects of Lys, Arg, Val, and Ala on rice growth, we performed an exogenous amino acid assay with ZH11, the OE lines, and the RNAi lines. The results revealed that reduced expression of OsAAP5 significantly promoted second tiller bud outgrowth, which might be attributed to the accumulation of Lys, Arg, Val, and Ala within the appropriate concentration range in the RNAi lines. However, the excessive accumulation of Lys, Arg, Val, and Ala in the OE lines and ZH11 retarded tiller bud outgrowth (Fig. 6; Supplemental Fig. S8).
The accumulation of excessive amino acids in plant cells retards plant growth and development (Lee et al., 2007), and specifically, Lys can inhibit the length of the main root and the tiller bud in Arabidopsis (Yang et al., 2014) and in rice (Lu et al., 2018). Moreover, the root system of the Ataap3 mutant is highly developed and shows a larger number of long main roots and a higher density of lateral roots (Marella et al., 2013). Similarly, the Arabidopsis mutant aap2 demonstrates reduced neutral amino acid transport but increased branch number and seed yield (Zhang et al., 2010). Our findings with respect to OsAAP5 were consistent with these studies. Based on these results, we demonstrated that OE of OsAAP5 could inhibit bud outgrowth by enhancing the absorption and accumulation of some amino acids, especially basic (Lys and Arg) and neutral amino acids (Val and Ala).
CK Accelerated Tiller Bud Outgrowth Due to Reduced Expression of OsAAP5
Rice tiller bud outgrowth is an intricate developmental process, and it is regulated by phytohormones, such as CK, auxin, and SL (Leyser, 2003; Ferguson and Beveridge, 2009). Elevated CK levels promote tiller bud outgrowth, and CK activity in plants is closely related to N availability (Kamada-Nobusada et al., 2013). This study found that decreased expression of OsAAP5 significantly increased CK levels in the leaf sheath and leaf blade, whereas OsAAP5 OE significantly decreased CK levels (Fig. 7). This study also detected the level of auxin, which inhibits tiller bud outgrowth; however, the auxin levels in plants with different OsAAP5 expression levels exhibited no obvious trends (Supplemental Fig. S9). These results indicated that OsAAP5 might regulate rice tiller formation by affecting CK levels rather than auxin levels in plant cells.
SL inhibits tiller bud outgrowth (Domagalska and Leyser, 2011). This study also detected the expression levels of some genes in the SL pathway in ZH11, the OE lines, and the RNAi lines to evaluate the effect of OsAAP5 on SL metabolism. The results demonstrated that the expression of SL metabolic genes (OsD27, OsD17, OsD10, OsD14, and OsFC1) was not significantly affected by OsAAP5 expression level. Although the expression of the OsD3 was significantly decreased in OE lines, and OsD53 significantly increased in OE and RNAi lines, no obvious trends were found with altered OsAAP5 expression (Supplemental Fig. S10). Based on the results above, we conclude that OsAAP5 could influence rice tiller bud outgrowth mainly by regulating CK levels rather than auxin and SL levels, resulting in the observed effects on rice tiller number and grain yield. However, the mechanism by which the overaccumulation of amino acids, especially Lys, Arg, Val, and Ala, in plants decreases the CK level remains to be studied. Future experiments could explore the relationship between the metabolism of these amino acids and the CK pathway.
MATERIALS AND METHODS
Sequence Variation in the OsAAP5 Promoter
SNP and indel information on the OsAAP5 promoter of rice (Oryza sativa) cultivars is available on RiceVarMap v2.0 (http://ricevarmap.ncpgr.cn/v2/) according to Zhao et al. (2015). The main haplotypes of the OsAAP5 promoter, Hap1, Hap2, and Hap3, were validated by PCR in corresponding cultivars. Haplotypes with a frequency of 5% or higher (at least fifteen accessions) were used for an association analysis of tiller number via one-way ANOVA in SPSS. Duncan’s multiple range test was used for multiple comparisons. The primers are listed in Supplemental Table S1.
Plant Materials and Agronomic Trait Analysis
The 1398-bp OsAAP5 (LOC_Os01g65660) complementary DNA (cDNA) was amplified from ZH11 and then inserted downstream of the 35S promoter in the pCAM1306 vector using Kpn I and Xba I to construct the p35S::OsAAP5 plasmid. To construct the OsAAP5-RNAi vector, two 290-bp fragments of OsAAP5 cDNA were obtained from ZH11 and inserted downstream of the Ubi-1 promoter in the vector pTCK303 (Wang et al., 2004) using BamH I/Kpn I and Spe I/Sac I. All the constructed plasmids were transformed into japonica rice variety ZH11 by the Agrobacterium tumefaciens–mediated transformation method. Homozygous T2 generation transgenic lines were chosen for further study. The primers are listed in Supplemental Table S1.
For the basic agronomic trait analysis, rice plants were grown in the paddy field at the rice experimental station of the Wuhan Institute of Bioengineering. At the mature stage, 20 plants of each line were randomly chosen for further detection. The number of spikelets per panicle was counted. The 1000-grain weight and grain yield per plant were measured. The seed set ratio was equal to the number of grains per plant divided by the number of spikelets per plant.
GUS Activity Analysis and Subcellular Localization of OsAAP5
For GUS activity analysis, a 2455-bp OsAAP5 promoter fragment was generated from ZH11 and inserted upstream of the GUS coding region using Hind III and BamH I in pCAMBIA1391Z to generate the POsAAP5::GUS plasmid. Then, this plasmid was transformed into japonica rice variety ZH11 to produce POsAAP5::GUS lines. Histochemical GUS assays were conducted as previously described (Sieburth and Meyerowitz, 1997). First, the samples for GUS staining were vacuum infiltrated for 15 min and gently fixed in formalin-acetic acid (70% [v/v] ethanol [1:1:18]) at 4°C for 20–30 min. Then, the samples were incubated in the staining buffer at 37°C overnight. After incubation in a solution of 80% (v/v) ethanol was cleared through, the stained samples were observed using a stereomicroscope.
To determine the subcellular localization of OsAAP5, the OsAAP5 ORF was amplified and fused with GFP using Bgl II and Spe I in the pCAM1302 vector to generate the P35S::OsAAP5-GFP fusion plasmid. Then, the generated plasmid was transiently expressed in rice protoplasts prepared from etiolated seedlings of ZH11. The plasma membrane colocalized marker was the rice homolog of putative Ca2+-permeable mechanosensitive channels in Arabidopsis (Arabidopsis thaliana), (OsMCA1) fused with mCherry (Kurusu et al., 2012). The fluorescence was observed using a confocal laser scanning microscope. The primers are listed in Supplemental Table S1.
Bud Outgrowth and Amino Acid Analysis
To explore the effects of altered OsAAP5 expression on seedling growth and bud outgrowth, the seedlings of ZH11 and transgenic lines at 7 DAG were cultured with basic rice nutrient solution (Yoshida et al., 1976). The nutrient solution was renewed every 3 d. At 30 DAG (the fifth leaf stage), biomass, including fresh weight and dry weight, and tiller bud length in ZH11, the OE lines, and the RNAi lines were measured. The leaf sheath and leaf blade of ZH11 and the transgenic lines were prepared for amino acid analysis, phytohormone detection, and RNA extraction.
For the amino acid analysis, seedlings of ZH11 and the transgenic lines at 30 DAG were used. The total free amino acid concentration was measured by the ninhydrin method (Fang et al., 2013). Single amino acid concentrations in the leaf sheath and leaf blade were measured using HPLC (Lu et al., 2018). Rice tissues were extracted with 10 mL 80% (v/v) ethanol at 80°C. A 1-mL aliquot of each sample was evaporated to remove the ethanol, redissolved in 1 mL 0.02 m HCl, and subsequently analyzed with HPLC.
The determination of GABA content was performed according to Sansenya et al. (2017) with appropriate modification. One gram dry powder was taken from each sample, dissolved in 5 mL deionized water, and extracted by oscillation for 1 h. Then, the supernatant was centrifuged at 12000 rpm for 15 min, and the supernatant was removed and filtered through a 0.45-μm filter membrane. Next, 0.5 mL of the filtered sample was taken, and 0.2 mL 0.2 m borate buffer (pH 9.0), 1 mL 6% (v/v) phenol reagent, and 0.4 mL 9% (w/v) NaClO were added successively. The mixture was shaken thoroughly, boiled for 10 min, and then cooled in the cooling bath until a blue color appeared. The content of GABA was determined by spectrophotometry with a wavelength of 645 nm. A standard GABA content curve was prepared according to Sansenya et al. (2017).
To investigate the effects of single amino acids on tiller bud outgrowth in ZH11 and OsAAP5 transgenic lines, seedlings cultured with rice basic nutrient solution for 14 d were grown in a solution containing 1.5 mm Lys, 0.3 mm Arg, 2.0 mm Val, 2.0 mm Ala, and 2.0 mm Thr with the original N decreased to half. The tiller bud length of seedlings was measured from 21 DAG among the OsAAP5 differential expression lines.
Protoplast Amino Acid Uptake Assay
Amino acids with FITC markers (Lys-FITC, Arg-FITC, Val-FITC, Ala-FITC, Pro-FITC, and Ser-FITC) were synthesized by Yuan Peptide Biotechnology Company. A protoplast amino acid uptake assay was performed as described previously (Rottmann et al., 2018). Rice protoplasts prepared from etiolated seedlings of ZH11, and the transgenic lines were kept in 1 mL W5 buffer (154 mm NaCl, 125 mm CaCl2, 5 mm KCl, 5 mm Glc, and 2 mm MES, pH 5.8) supplemented with each FITC-labeled amino acid to a final concentration of 1 mm at room temperature in the dark. At 1 h after culture, the protoplasts were centrifuged at 100g for 5 min. After the supernatant was removed carefully with a pipette, the protoplasts were suspended in 1 mL W5 solution and centrifuged again at 100g for 5 min. To fully remove free FITC-marked amino acids from the solution, the protoplasts were resuspended three times and then observed using a confocal laser scanning microscope.
RNA Extraction and RT-qPCR
Total RNA was isolated from different tissues with TRIzol reagent (Invitrogen), and cDNA was synthesized using M-MLV reverse transcriptase (Promega). RT-qPCR was performed in the 7500 RT qPCR system (Applied Biosystems) according to the manufacturer’s instructions. The rice ACTIN gene (LOC_Os03g50885), UBIQUITIN (LOC_Os05g06770), and GAPDH (LOC_Os04g40950) were used as internal references, and the gene expression levels were normalized to the geometric average of these internal reference genes. Three biological replicates were performed for each sample. The primers for RT-qPCR are listed in Supplemental Table S1.
Detection of Phytohormones
The leaf sheath and leaf blade of ZH11 and transgenic seedlings at 30 DAG were obtained and reduced to dry powder. Then, CK (isopentenyladenine, tZ, cZ and DZ) and auxin (IAA, ME-IAA and ICA) contents were detected by MetWare (http://www.metware.cn/) based on the AB Sciex QTRAP4500 LC-MS/MS platform. Three replicates of each assay were performed.
Statistical Analysis
A two-tailed t test (each transgenic line vs. ZH11; N treatment N2 or N3 vs. N1; N treatment N5 or N6 vs. N4; N treatment N8 or N9 vs. N7) was performed using SPSS software (IBM, Inc.) with *, **, and *** indicating significant differences at P < 0.05, P < 0.01, and P < 0.001, respectively, in Figures 3–8 and Supplemental Figures. S2–S10. For multiple comparisons, one-way ANOVA with Duncan’s multiple range test (comparison of all lines) was performed using SPSS software; different letters indicated significant differences at P < 0.05 in Fig. 1 and Supplemental Fig. S1.
Accession Numbers
Sequence data from this article can be found in the Rice Genome Annotation Project or GenBank under the following accession numbers: OsAAP5, LOC_Os01g65660; OsACTIN, LOC_Os03g50885; UBIQUITIN, LOC_Os05g06770; and GAPDH, LOC_Os04g40950.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The expression pattern analysis of OsAAP5 in different promoter types.
Supplemental Figure S2. Analysis of OsAAP5 expression under different nitrogen treatments.
Supplemental Figure S3. Prediction of the transmembrane domain of OsAAP5.
Supplemental Figure S4. The expression levels of OsAAP5 homologous genes in ZH11, OE, and RNAi lines.
Supplemental Figure S5. Altered expression of OsAAP5 influenced seedling growth and bud outgrowth.
Supplemental Figure S6. Detection of GABA contents in OsAAP5 lines with different expression levels.
Supplemental Figure S7. Protoplast amino acid uptake assay in transgenic plants.
Supplemental Figure S8. Effects of neutral amino acids on bud elongation of ZH11, OE, and RNAi lines.
Supplemental Figure S9. Effects of OsAAP5 on auxin concentrations.
Supplemental Figure S10. The expression levels of genes related to the SL pathway in ZH11, OE, and RNAi lines.
Supplemental Figure S11. The expression of OsAAP5 in OsAAP3 differential expression lines.
Supplemental Table S1. List of primers used in this study.
Footnotes
This work was supported by the Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology) the National Key Research and 516 Development Program (2016YFD0100700) and the National Natural Science Foundation of China (NSFC) (31301250/31701990).
References
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J (2009) d14, A strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50: 1416–1424 [DOI] [PubMed] [Google Scholar]
- Boorer KJ, Fischer WN (1997) Specificity and stoichiometry of the Arabidopsis H+/amino acid transporter AAP5. J Biol Chem 272: 13040–13046 [DOI] [PubMed] [Google Scholar]
- Chen W, Gao Y, Xie W, Gong L, Lu K, Wang W, Li Y, Liu X, Zhang H, Dong H, et al. (2014) Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet 46: 714–721 [DOI] [PubMed] [Google Scholar]
- Dan Z, Liu P, Huang W, Zhou W, Yao G, Hu J, Zhu R, Lu B, Zhu Y (2014) Balance between a higher degree of heterosis and increased reproductive isolation: A strategic design for breeding inter-subspecific hybrid rice. PLoS One 9: e93122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Fairhurst T, Dobermann A (2002) Rice in the global food supply. Bett Crop Int 16: 3–6 [Google Scholar]
- Fang Z, Xia K, Yang X, Grotemeyer MS, Meier S, Rentsch D, Xu X, Zhang M (2013) Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol J 11: 446–458 [DOI] [PubMed] [Google Scholar]
- Feng F, Li Y, Qin X, Liao Y, Siddique KHM (2017) Changes in rice grain quality of Indica and Japonica type varieties released in China from 2000 to 2014. Front Plant Sci 8: 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Beveridge CA (2009) Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol 149: 1929–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer WN, Kwart M, Hummel S, Frommer WB (1995) Substrate specificity and expression profile of amino acid transporters (AAPs) in Arabidopsis. J Biol Chem 270: 16315–16320 [DOI] [PubMed] [Google Scholar]
- Funayama K, Kojima S, Tabuchi-Kobayashi M, Sawa Y, Nakayama Y, Hayakawa T, Yamaya T (2013) Cytosolic glutamine synthetase1;2 is responsible for the primary assimilation of ammonium in rice roots. Plant Cell Physiol 54: 934–943 [DOI] [PubMed] [Google Scholar]
- Gao S, Fang J, Xu F, Wang W, Sun X, Chu J, Cai B, Feng Y, Chu C (2014) CYTOKININ OXIDASE/DEHYDROGENASE4 integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol 165: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Hu B, Wang W, Ou S, Tang J, Li H, Che R, Zhang Z, Chai X, Wang H, Wang Y, Liang C, Liu L, et al. (2015) Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat Genet 47: 834–838 [DOI] [PubMed] [Google Scholar]
- Huang W, Bai G, Wang J, Zhu W, Zeng Q, Lu K, Sun S, Fang Z (2018) Two splicing variants of OsNPF7.7 regulate shoot branching and nitrogen utilization efficiency in rice. Front Plant Sci 9: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt E, Gattolin S, Newbury HJ, Bale JS, Tseng HM, Barrett DA, Pritchard J (2010) A mutation in amino acid permease AAP6 reduces the amino acid content of the Arabidopsis sieve elements but leaves aphid herbivores unaffected. J Exp Bot 61: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa S, Maekawa M, Arite T, Onishi K, Takamure I, Kyozuka J (2005) Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol 46: 79–86 [DOI] [PubMed] [Google Scholar]
- Jiang L, Liu X, Xiong G, Liu H, Chen F, Wang L, Meng X, Liu G, Yu H, Yuan Y, et al. (2013) DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504: 401–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Makita N, Kojima M, Sakakibara H (2013) Nitrogen-dependent regulation of de novo cytokinin biosynthesis in rice: The role of glutamine metabolism as an additional signal. Plant Cell Physiol 54: 1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H, Yamanaka T, Iida K, Nakagawa Y, Saji H, et al. (2012) Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol 12: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Foster J, Chen J, Voll LM, Weber AP, Tegeder M (2007) AAP1 transports uncharged amino acids into roots of Arabidopsis. Plant J 50: 305–319 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2003) Regulation of shoot branching by auxin. Trends Plant Sci 8: 541–545 [DOI] [PubMed] [Google Scholar]
- Li X, Qian Q, Fu Z, Wang Y, Xiong G, Zeng D, Wang X, Liu X, Teng S, Hiroshi F, et al. (2003) Control of tillering in rice. Nature 422: 618–621 [DOI] [PubMed] [Google Scholar]
- Lin H, Wang R, Qian Q, Yan M, Meng X, Fu Z, Yan C, Jiang B, Su Z, Li J, Wang Y (2009) DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21: 1512–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ou S, Mao B, Tang J, Wang W, Wang H, Cao S, Schläppi MR, Zhao B, Xiao G, et al. (2018) Early selection of bZIP73 facilitated adaptation of japonica rice to cold climates. Nat Commun 9: 3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WZ, Kong DD, Gu XX, Gao HB, Wang JZ, Xia M, Gao Q, Tian LL, Xu ZH, Bao F, et al. (2013) Cytokinins can act as suppressors of nitric oxide in Arabidopsis. Proc Natl Acad Sci USA 110: 1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ding YF, Wang QS, Meng DX, Wang SH (2011) Effects of nitrogen and 6-benzylaminopurine on rice tiller bud growth and changes in endogenous hormones and nitrogen. Crop Sci 51: 786–792 [Google Scholar]
- Lu G, Wu FQ, Wu W, Wang HJ, Zheng XM, Zhang Y, Chen X, Zhou K, Jin M, Cheng Z, et al. (2014) Rice LTG1 is involved in adaptive growth and fitness under low ambient temperature. Plant J 78: 468–480 [DOI] [PubMed] [Google Scholar]
- Lu K, Wu B, Wang J, Zhu W, Nie H, Qian J, Huang W, Fang Z (2018) Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol J 16: 1710–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella HH, Nielsen E, Schachtman DP, Taylor CG (2013) The amino acid permeases AAP3 and AAP6 are involved in root-knot nematode parasitism of Arabidopsis. Mol Plant Microbe Interact 26: 44–54 [DOI] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, Umehara M, Luo L, Kobayashi K, Hanada A, Ueno K, Asami T, Yamaguchi S, Kyozuka J (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol 51: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran-Zuloaga D, Dippold M, Glaser B, Kuzyakov Y (2015) Organic nitrogen uptake by plants: Reevaluation by position-specifc labeling of amino acids. Biogeochemistry 125: 359–374 [Google Scholar]
- Ohashi M, Ishiyama K, Kusano M, Fukushima A, Kojima S, Hanada A, Kanno K, Hayakawa T, Seto Y, Kyozuka J (2015) Lack of cytosolic glutamine synthetase1;2 in vascular tissues of axillary buds causes severe reduction in their outgrowth and disorder of metabolic balance in rice seedlings. Plant J 81: 347–356 [DOI] [PubMed] [Google Scholar]
- Ohashi M, Ishiyama K, Kojima S, Kojima M, Sakakibara H, Yamaya T, Hayakawa T (2017) Lack of cytosolic glutamine synthetase1;2 activity reduces nitrogen-dependent biosynthesis of cytokinin required for axillary bud outgrowth in rice seedlings. Plant Cell Physiol 58: 679–690 [DOI] [PubMed] [Google Scholar]
- Okumoto S, Koch W, Tegeder M, Fischer WN, Biehl A, Leister D, Stierhof YD, Frommer WB (2004) Root phloem-specific expression of the plasma membrane amino acid proton co-transporter AAP3. J Exp Bot 55: 2155–2168 [DOI] [PubMed] [Google Scholar]
- Peng B, Kong H, Li Y, Wang L, Zhong M, Sun L, Gao G, Zhang Q, Luo L, Wang G, et al. (2014) OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat Commun 5: 4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh SA, Tyerman SD, Gilliham M, Xu B (2017) γ-Aminobutyric acid (GABA) signalling in plants. Cell Mol Life Sci 74: 1577–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann TM, Fritz C, Lauter A, Schneider S, Fischer C, Danzberger N, Dietrich P, Sauer N, Stadler R (2018) Protoplast-esculin assay as a new method to assay plant sucrose transporters: Characterization of AtSUC6 and AtSUC7 sucrose uptake activity in Arabidopsis Col-0 ecotype. Front Plant Sci 9: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11: 440–448 [DOI] [PubMed] [Google Scholar]
- Sanders A, Collier R, Trethewy A, Gould G, Sieker R, Tegeder M (2009) AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J 59: 540–552 [DOI] [PubMed] [Google Scholar]
- Sansenya S, Hua Y, Chumanee S, Phasai K, Sricheewin C (2017) Effect of gamma irradiation on 2-Acetyl-1-pyrroline content, GABA content and volatile compounds of germinated rice (Thai upland rice). Plants (Basel) 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JP, Tegeder M (2016) Connecting source with sink: The role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol 171: 508–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HH, Walter U (1994) NO at work. Cell 78: 919–925 [DOI] [PubMed] [Google Scholar]
- Schmidt R, Stransky H, Koch W (2007) The amino acid permease AAP8 is important for early seed development in Arabidopsis thaliana. Planta 226: 805–813 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Delhaize E, Frommer WB, Guerinot ML, Harrison MJ, Herrera-Estrella L, Horie T, Kochian LV, Munns R, Nishizawa NK, et al. (2013) Using membrane transporters to improve crops for sustainable food production. Nature 497: 60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieburth LE, Meyerowitz EM (1997) Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9: 355–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerstam H, Ganeteg U, Näsholm T (2008) Root uptake of cationic amino acids by Arabidopsis depends on functional expression of amino acid permease 5. New Phytol 180: 620–630 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Taniguchi M, Sugiyama T (2001) Nitrogen-dependent accumulation of cytokinins in root and the translocation to leaf: Implication of cytokinin species that induces gene expression of maize response regulator. Plant Cell Physiol 42: 85–93 [DOI] [PubMed] [Google Scholar]
- Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H (2004) AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant Cell Physiol 45: 1053–1062 [DOI] [PubMed] [Google Scholar]
- Taylor MR, Reinders A, Ward JM (2015) Transport function of rice amino acid permeases (AAPs). Plant Cell Physiol 56: 1355–1363 [DOI] [PubMed] [Google Scholar]
- Tegeder M, Rentsch D (2010) Uptake and partitioning of amino acids and peptides. Mol Plant 3: 997–1011 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Wang J, Lu K, Nie H, Zeng Q, Wu B, Qian J, Fang Z (2018) Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice (N Y) 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu J, Ren T, Hussain S, Guo C, Wang S, Cong R, Li X (2017) Effects of nitrogen and tiller type on grain yield and physiological responses in rice. AoB Plants 9: plx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen C, Xu Y, Jiang R, Han Y, Xu Z, Chong K (2004) A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol Biol Report 22: 409–417 [Google Scholar]
- Xing Y, Zhang Q (2010) Genetic and molecular bases of rice yield. Annu Rev Plant Biol 61: 421–442 [DOI] [PubMed] [Google Scholar]
- Yang H, Postel S, Kemmerling B, Ludewig U (2014) Altered growth and improved resistance of Arabidopsis against Pseudomonas syringae by overexpression of the basic amino acid transporter AtCAT1. Plant Cell Environ 37: 1404–1414 [DOI] [PubMed] [Google Scholar]
- Yeh SY, Chen HW, Ng CY, Lin CY, Tseng TH, Li WH, Ku MS (2015) Down-regulation of Cytokinin Oxidase 2 expression increases tiller number and improves rice yield. Rice (N Y) 8: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Forno DA, Cook JH, Gomez KA (1976) Routine procedures for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice. International Rice Research Institute, Los Baños, Philippines, pp 61–66 [Google Scholar]
- Yoshida S, Kameoka H, Tempo M, Akiyama K, Umehara M, Yamaguchi S, Hayashi H, Kyozuka J, Shirasu K (2012) The D3 F-box protein is a key component in host strigolactone responses essential for arbuscular mycorrhizal symbiosis. New Phytol 196: 1208–1216 [DOI] [PubMed] [Google Scholar]
- Zhang L, Tan Q, Lee R, Trethewy A, Lee YH, Tegeder M (2010) Altered xylem-phloem transfer of amino acids affects metabolism and leads to increased seed yield and oil content in Arabidopsis. Plant Cell 22: 3603–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ma H, Yu L, Wang X, Zhao J (2012) Genome-wide survey and expression analysis of amino acid transporter gene family in rice (Oryza sativa L.). PLoS One 7: e49210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yao W, Ouyang Y, Yang W, Wang G, Lian X, Xing Y, Chen L, Xie W (2015) RiceVarMap: A comprehensive database of rice genomic variations. Nucleic Acids Res 43: D1018–D1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, Wu F, Mao H, Dong W, Gan L, et al. (2013) D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 504: 406–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Chen Z, Zhang S, Zhang W, Jiang G, Zhao X, Zhai W, Pan X, Zhu L (2005) Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L.). Planta 222: 604–612 [DOI] [PubMed] [Google Scholar]