Abscisic acid signaling can be exploited for generating plants with improved water use efficiency, resilient to water availability and light intensity but sensitive to heat.
Abstract
Improving the water use efficiency (WUE) of crop plants without trade-offs in growth and yield is considered a utopic goal. However, recent studies on model plants show that partial restriction of transpiration can occur without a reduction in CO2 uptake and photosynthesis. In this study, we analyzed the potentials and constraints of improving WUE in Arabidopsis (Arabidopsis thaliana) and in wheat (Triticum aestivum). We show that the analyzed Arabidopsis wild-type plants consume more water than is required for unrestricted growth. WUE was enhanced without a growth penalty by modulating abscisic acid (ABA) responses either by using overexpression of specific ABA receptors or deficiency of ABA coreceptors. Hence, the plants showed higher water productivity compared with the wild-type plants; that is, equal growth with less water. The high WUE trait was resilient to changes in light intensity and water availability, but it was sensitive to the ambient temperature. ABA application to plants generated a partial phenocopy of the water-productivity trait. ABA application, however, was never as effective as genetic modification in enhancing water productivity, probably because ABA indiscriminately targets all ABA receptors. ABA agonists selective for individual ABA receptors might offer an approach to phenocopy the water-productivity trait of the high WUE lines. ABA application to wheat grown under near-field conditions improved WUE without detectable growth trade-offs. Wheat yields are heavily impacted by water deficit, and our identification of this crop as a promising target for WUE improvement may help contribute to greater food security.
Plant growth requires the fixation of CO2, which diffuses from the atmosphere into leaf chloroplasts. The influx of CO2 shares the stomatal diffusion pathway with the efflux of water vapor (Farquhar et al., 1989; Franks et al., 2013), which results in an inevitable water loss during CO2 uptake. Terrestrial gross photosynthesis captures approximately 440 gigatons annually. Conversely, an estimated 62 teratons of water are annually released into the atmosphere by plants, mostly via stomatal transpiration (Hetherington and Woodward, 2003). The plant-driven mobilization of water from the soil to the atmosphere accounts for up to 80% of continental evapotranspiration (Jasechko et al., 2013). The large demand of plants for water imposes a world-wide challenge on fresh water resources for food and feed production. Current use of groundwater by agriculture is not sustainable and exacerbates the gradual depletion of groundwater tables (Wada et al., 2010). Hence, agriculture is facing a major challenge to provide yield stability and food security under limiting and depleted fresh water resources. Crops more resilient against water deficit and more efficient in their water use are urgently needed (Blum, 2005; Morison et al., 2008; Hall and Richards, 2013). However, progress toward these goals has been slow, even though there are numerous claims of engineered drought resistance in plants (Nuccio et al., 2018).
The phytohormone abscisic acid (ABA) is a key signaling molecule that mediates acclimation of plants to water deficit by reducing transpiration (Munemasa et al., 2015), protecting photosynthesis (Yang et al., 2006), and by triggering other metabolic adjustments, including the induction of stress proteins and osmolytes (Finkelstein, 2013). As a consequence, fine-tuning and modulating ABA responses has the promise to preadjust plants to drought by changes in both short-term and long-term physiology.
ABA regulates the protein phosphatase activity of receptor complexes consisting of the ABA-BINDING REGULATORY COMPONENT (RCAR)/PYRABACTIN RESISTANCE 1-LIKE (PYR1/PYL), sensu stricto the ABA receptor, and an associated clade A protein phosphatase of type 2C (PP2C) whose interaction is stabilized by ABA (Ma et al., 2009; Melcher et al., 2009; Miyazono et al., 2009; Park et al., 2009; Moreno-Alvero et al., 2017). In Arabidopsis (Arabidopsis thaliana) there are fourteen RCARs and nine clade A PP2C proteins, which are able to form more than 100 different functional binary receptor complexes (Fuchs et al., 2014; Tischer et al., 2017). ABA- and RCAR-mediated inactivation of the PP2C allows activation of OPEN STOMATA1/SUCROSE NONFERMENTING 1-RELATED PROTEIN KINASE 2 (OST1/SnRK2E/SnRK2.6) and other related SnRK2 protein kinases to phosphorylate downstream targets (Cutler et al., 2010; Wang et al., 2013). Primary targets are ion channels involved in stomatal closure (Munemasa et al., 2015), aquaporin (Grondin et al., 2015), and ABA-responsive bZip transcription factors, which are master regulators in the transcriptional ABA response network (Lumba et al., 2014; Yoshida et al., 2014; Song et al., 2016).
Already, decades ago, attempts were undertaken to enhance WUE and to confer drought resistance by activating ABA responses. Foliar administration of ABA and ABA agonists resulted in higher WUE of barley (Hordeum vulgare) and wheat (Triticum sp; Mizrahi et al., 1974; Rademacher et al., 1987), and increased ABA sensitivity improved the drought resistance of Arabidopsis and rapeseed (Brassica napus) by down-regulation of a farnesyltransferase (Pei et al., 1998; Wang et al., 2005). More recently, application of ABA and ABA agonists (Okamoto et al., 2013; Park et al., 2015; Cao et al., 2017), ectopic expression of ABA receptors (Santiago et al., 2009; Mosquna et al., 2011; Pizzio et al., 2013; González-Guzmán et al., 2014; Yang et al., 2016; Zhao et al., 2016), and reduced expression of ABA coreceptors (Rubio et al., 2009; Antoni et al., 2012) have been shown to minimize plant transpiration, and in several cases to boost survival rates under severe water deficit. A plethora of ABA agonists (Wilen et al., 1993; Benson et al., 2015; Vaidya et al., 2017; Frackenpohl et al., 2018a, 2018b; Nemoto et al., 2018) and antagonists (Takeuchi et al., 2014; Ito et al., 2015; Rajagopalan et al., 2016; Ye et al., 2017) were developed that allow the modulation of ABA responses.
Reducing stomatal aperture affects plant’s gas exchange and ultimately limits CO2 influx for photosynthesis and growth. The diffusion of CO2 and water vapor across the stomatal pore is driven by the differences in partial gas pressures. The intercellular CO2 concentration (Ci) of plants is under homeostatic control via regulation of stomatal conductance (gs) according to photosynthetic demand (Franks et al., 2013). Hence, the ratio of net photosynthesis (An) to gs, referred to as intrinsic WUE (iWUE) and equivalent to the CO2 gradient between the ambient CO2 level (Ca) and Ci at a given leaf-to-air water vapor-pressure deficit (VPD), is fairly constant for a plant at different light intensities and under well-watered conditions (Wong et al., 1979; Ubierna and Farquhar, 2014). Facing water deficit, plants are able to sustain photosynthesis at lower Ci values and increase iWUE, i.e. CO2 influx per water unit becomes more efficient at a given VPD. Lowering gs and Ci in response to water deficit imposes constraints on the CO2 level in the chloroplasts and potentially increases photorespiration and reduces net photosynthesis (Franks et al., 2013). The association of elevated iWUE and whole plant WUE with trade-offs in growth and yield potential is well known (Blum, 2005).
In different natural accessions of Arabidopsis, iWUE (An/gs) varied and higher iWUEs were associated with reduced rates of net photosynthesis (Easlon et al., 2014). However, several reports show increased iWUE in tomato (Solanum lycopersicum), barley, and Arabidopsis without a reduction in photosynthesis and growth by reducing stomatal density (Yoo et al., 2011; Franks et al., 2015; Hughes et al., 2017), and aperture via stimulating ABA biosynthesis (Thompson et al., 2007) or signaling (Yang et al., 2016). These plants have a moderately reduced gs and Ci without reduction in An and growth. The physiological basis of the compensatory adjustments that sustain higher iWUE and growth is unknown but might involve enhanced refixation of CO2 in roots and translocation to leaves (Hibberd and Quick, 2002), and induced C4-metabolic enzymes in Arabidopsis under CO2 limitation (Li et al., 2014). Such plants consume less water per biomass gain but the trait might inflict trade-offs including compromised evaporative cooling and reduced thermo-tolerance, and growth penalties under higher light intensities. These potential limitations are not investigated yet for those plant lines. In the ecosystem, reduced water consumption of a high iWUE plant would save water and provide it to neighboring plants without a major advantage for the water-efficient plant (Nicotra and Davidson, 2010). However, such a trait is expected to be beneficial in crop fields by saving soil moisture and mitigating yield limitations by water deficit (Yang et al., 2016). Our previous study demonstrated that overexpression of ABA receptor members from subfamily II, like RCAR6 and RCAR10, resulted in plants growing without trade-offs in the water-efficient mode, which is normally induced by water deficit. These plants had increased WUE, higher water productivity (increased WUE per time), and produced more biomass per unit of water under progressive drought (Yang et al., 2016). In the current study, we compared transgenic Arabidopsis RCAR-overexpressing lines with Arabidopsis accessions for their efficiency of water use, and we examined the effect of temperature and higher photosynthetic irradiance on the WUE of a RCAR6-overexpressing line (RCAR6 line). Arabidopsis mutants with a deficiency in ABA coreceptors, single and multiple, were analyzed to identify which PP2Cs are potential targets for iWUE improvement. In addition, foliar application of ABA was examined to increase WUE of Arabidopsis and wheat, and to explore the possibility of conferring the iWUE trait of RCAR6 plants to a crop species.
RESULTS
Growth and Leaf Surface Temperatures Among Arabidopsis Accessions and ABA Receptor Lines
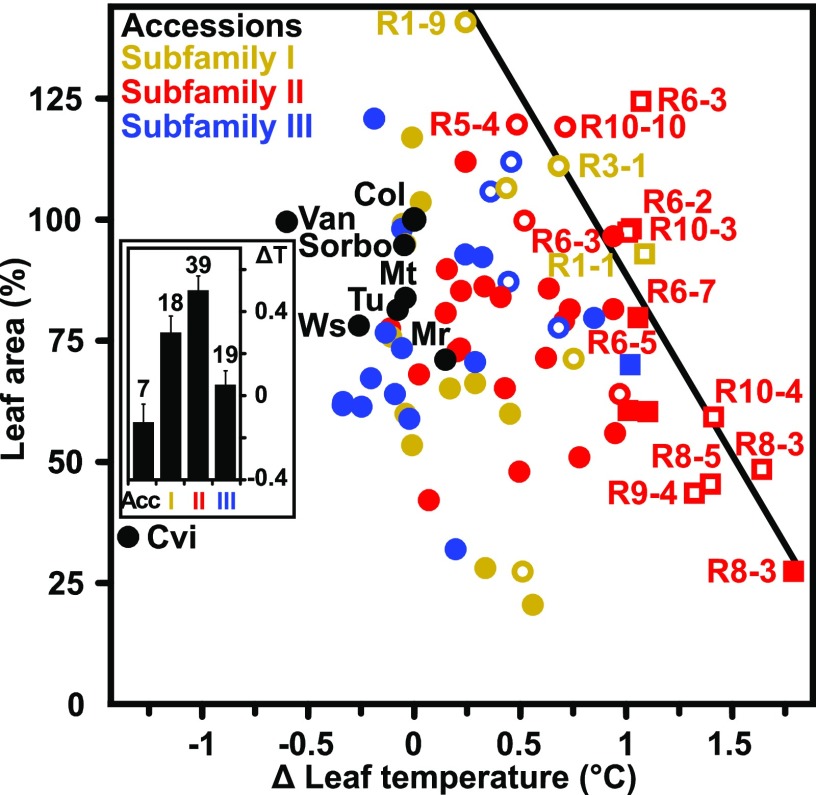
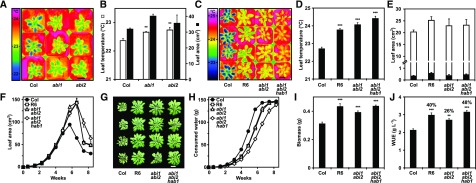
In natural Arabidopsis accessions, higher iWUE was associated with lower An (Easlon et al., 2014). Reduction of An negatively impacts growth and biomass accumulation; however, several ABA receptor-overexpressing Arabidopsis lines (ABA receptor lines) had higher iWUE without reduced An and growth (Yang et al., 2016). These lines revealed a strong positive correlation of growth capacity at elevated leaf surface temperature, i.e. reduced transpiration, with WUE at the intrinsic, integrated (based on 13C discrimination), or whole plant levels. We examined a limited number of Arabidopsis accessions to see if variation in growth and transpiration occurs frequently by analyzing leaf temperature and increases in leaf area as approximations for transpiration and biomass accumulation, respectively. Growth was assessed over four weeks under well-watered conditions with a relative soil water content (SWC, v/v) ≥60% and a soil water potential Ψ ≥ −0.08 MPa (megapascal; Supplemental Fig. S1). Columbia (Col-0) and five other natural accessions (Mr-0, Mt-0, Sorbo, Tu-0, and Ws-0) had similar leaf temperatures (22.7°C ± 0.1°C) with the exceptions of Cvi-0 and Van-0, which had lower leaf temperatures by 1.3°C ± 0.1°C and 0.6°C ± 0.1°C, respectively, compared with Col-0 (Fig. 1). Leaf rosette sizes of the natural accessions were similar or somewhat smaller than Col-0, except for the outlier Cvi-0, which was severely impaired in growth. Extending the analysis to 46 independently generated ABA receptor lines showed substantial variation in growth and leaf temperature compared with the parental Col-0 plant (Fig. 1). Leaf temperatures significantly higher than Col-0 (>0.5°C; P < 0.01) were primarily found in plants overexpressing ABA receptors of subfamily II. The average temperature increase was approximately 0.5°C across all 39 analyzed subfamily II lines (inset in Fig. 1, representing 22 independent lines), while the mean leaf temperature of subfamily III lines was close to the reference (+0.05°C). Several subfamily I lines showed significantly higher leaf temperatures, with an average increase of 0.3°C above Col-0.
Figure 1.
Variation in rosette leaf area and leaf surface temperatures in Arabidopsis accessions and Arabidopsis lines ectopically expressing different ABA receptors. The Arabidopsis accessions analyzed are Col-0, Cvi-0, Mr-0, Mt-0, Sorbo, Tu-0, Van-0, and Ws-0, and the data are expressed relative to Col-0 as reference. The wild-type plants (black symbols) and Col-0 lines with ectopic expression of single subfamily I receptors including the members RCAR1-RCAR4 (I, yellow symbols), subfamily II receptors RCAR5-RCAR10 (II, red), and subfamily III RCAR11-RCAR14 (III, blue) were analyzed with 2 to 5 independent lines per receptor. The trendline for the border function (correlation coefficient [R] = 0.8, P < 0.001, linear regression analysis) between maximum growth versus reduced transpiration is depicted as a dotted line by using data points with more than 1°C higher leaf temperature than Col-0 (P < 0.001, one-way ANOVA), shown as square symbols. The leaf area of 18-d-old plantlets at the onset of the experiment was 0.6 ± 0.1 cm2, and Col-0 plants increased the leaf area to 30.6 cm2 ± 2.1 cm2 after 22 d. Data from this study (filled symbols) were combined with data (open symbols) from Yang et al. (2016) . The inset displays the leaf temperature difference (ΔT in °C) to Col-0 (22.7°C) as the average value for Arabidopsis accessions without the outlier Cvi-0, and for RCAR subfamilies including the numbers of independent lines on the top of the columns. Plants were grown under well-watered (Ψ ≥ −0.08 MPa) conditions with a light-humidity regime of 8-h light per day at 0.15 mmol m−2 s−1 photosynthetically active radiation (PAR), 21.5°C, 50% relative humidity in daytime, and 17°C, 60% relative humidity at night. Inset mean ±sem; n = 4 biological replicates per data point; sem ±7% for leaf area; sem ±0.08°C for leaf temperature.
The analysis indicates a boundary function between high leaf temperature and trade-offs in maximum attainable growth. Close to or at the boundary line were RCAR8 lines, with 1.8°C higher leaf temperatures associated with major growth reduction; as well as RCAR6 and RCAR10 lines, with leaf rosette areas similar to Col-0 and about 1°C elevated leaf temperatures. Among these plants were the previously characterized RCAR6-3 and RCAR10-4 water-productive lines, i.e. combining high WUE with high growth performance (Yang et al., 2016). Crossing RCAR6-3 and RCAR10-4 and analyzing homozygous plants revealed that pyramiding of the two RCAR expression cassettes did not further improve WUE efficiency consistent with the boundary function (Supplemental Fig. S2). We conclude from the experiments that none of the analyzed natural accessions grew at the optimal water use delineated by the boundary function. Hence, the examined wild-type accessions probably can be improved in their iWUE, WUE, and water productivity, similar to RCAR6 and RCAR10 lines compared with Col-0.
WUE of ABA-Receptor Lines under Various Environmental Conditions
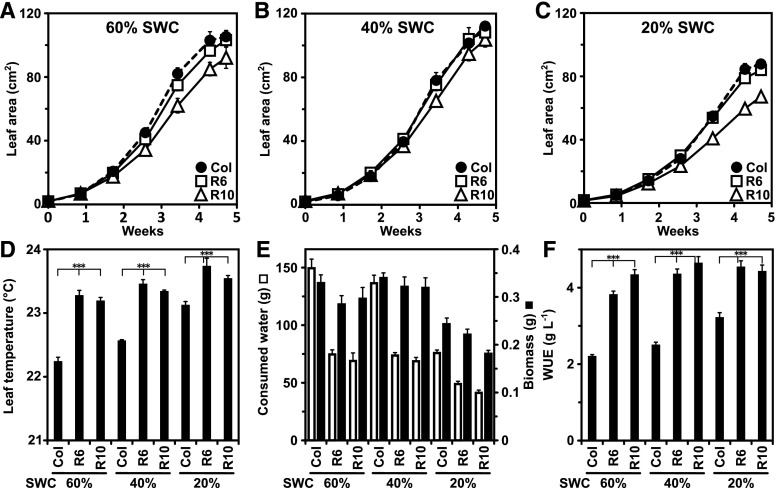
The enhanced transpiration efficiency of RCAR6-3 and RCAR10-4 lines was associated with An comparable to Col-0 at well-watered conditions (Yang et al., 2016). To examine the robustness of the improved iWUE trait at water limitations, plants were grown at different SWCs of approximately 60%, 40% and 20% corresponding to no (Ψ ≥ −0.08 MPa), mild (−0.1 MPa ≤ Ψ ≤ −0.08 MPa) and moderate (−0.21 MPa ≤ Ψ ≤ −0.1 MPa) water deficit, respectively (Fig. 2, A to C; Supplemental Fig. S1). The growth of the RCAR6-3 lines, as indicated by the increase in leaf area, was comparable to Col-0; while the growth of RCAR10-4 was somewhat reduced, notably at 20% SWC (Fig. 2C). The plants responded to the imposed water restriction at mild and moderate water deficit by increasing the leaf temperature (Fig. 2D). At 20% SWC, leaf temperature was 0.9°C and approximately 0.4°C higher for Col-0 and the ABA receptor lines, respectively, compared with the well-watered condition. The iWUE-improved lines had significantly higher leaf temperatures compared with the wild-type plants at all SWCs, indicating a reduced transpiration (Fig. 2D). After five weeks of growth, water consumption of the RCAR6-3 and RCAR10-4 lines was about half that of Col-0 at 60% and 40% SWC, and it was reduced by 35% and 50% compared with Col-0 at 20% SWC, respectively (Fig. 2E). The above-ground biomass of all lines was reduced at 20% SWC compared with the well-watered and mild deficit conditions (Fig. 2E). The RCAR lines showed a tendency toward a growth trade-off at 60% SWC and at 20% SWC, notably the RCAR10-4 line (Fig. 2E). The WUE of the ABA receptor lines were, however, clearly higher in comparison to Col-0 by up to 90% at no or mild water restriction (Fig. 2F). Hence, the RCAR lines produced more biomass per unit of transpired water and time, i.e. were more water-productive than the parental plant, irrespective of the soil water availability.
Figure 2.
Growth and water productivity of RCAR6- and RCAR10-overexpressing Arabidopsis under controlled soil water levels. Plant growth was assessed by the increase in leaf area at different water regimes with (A) 60% (Ψ = −0.08 MPa), (B) 40% (Ψ = −0.10 MPa), and (C) 20% (Ψ = −0.21 MPa) relative soil water content (v/v, SWC) for the wild-type Col-0 (Col), RCAR6-3 lines (R6), and RCAR10-4 (R10). D, Leaf temperature of plants at day 18. E, Consumed water and final above-ground dry matter. F, Water use efficiency (WUE). Plantlets were grown for 25 d under short-day conditions and pots were allowed to reach the designated SWC by evaporation prior to the onset of the experiment. At this stage, the plants had a leaf area of 1.7 ± 0.1 cm2, 1.8 ± 0.1 cm2, 2.0 ± 0.1 cm2 for Col, R6, and R10, respectively. Water was administrated in intervals of 3 days to adjust the water content to the target SWCs. The lowest SWC values reached during the experiment were approximately 45%, 25%, and 15% SWC for the water regime of 60%, 40%, and 20% SWC, respectively. Mean ±sem; n = 4 biological replicates for each data point; ***P < 0.001 (one-way ANOVA) compared with the wild-type Col-0.
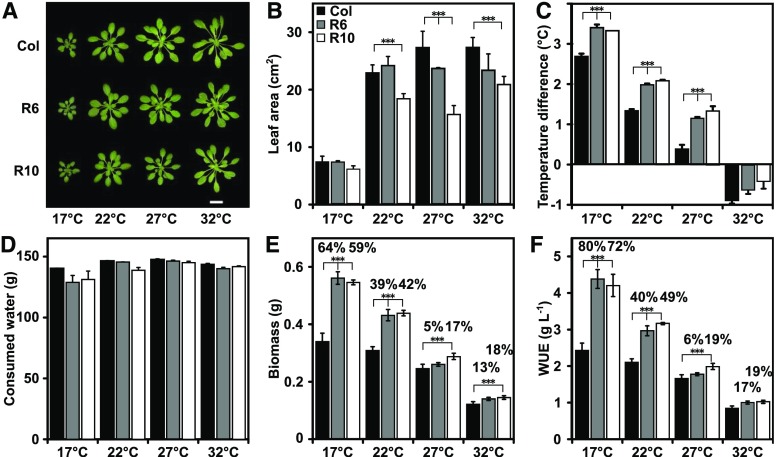
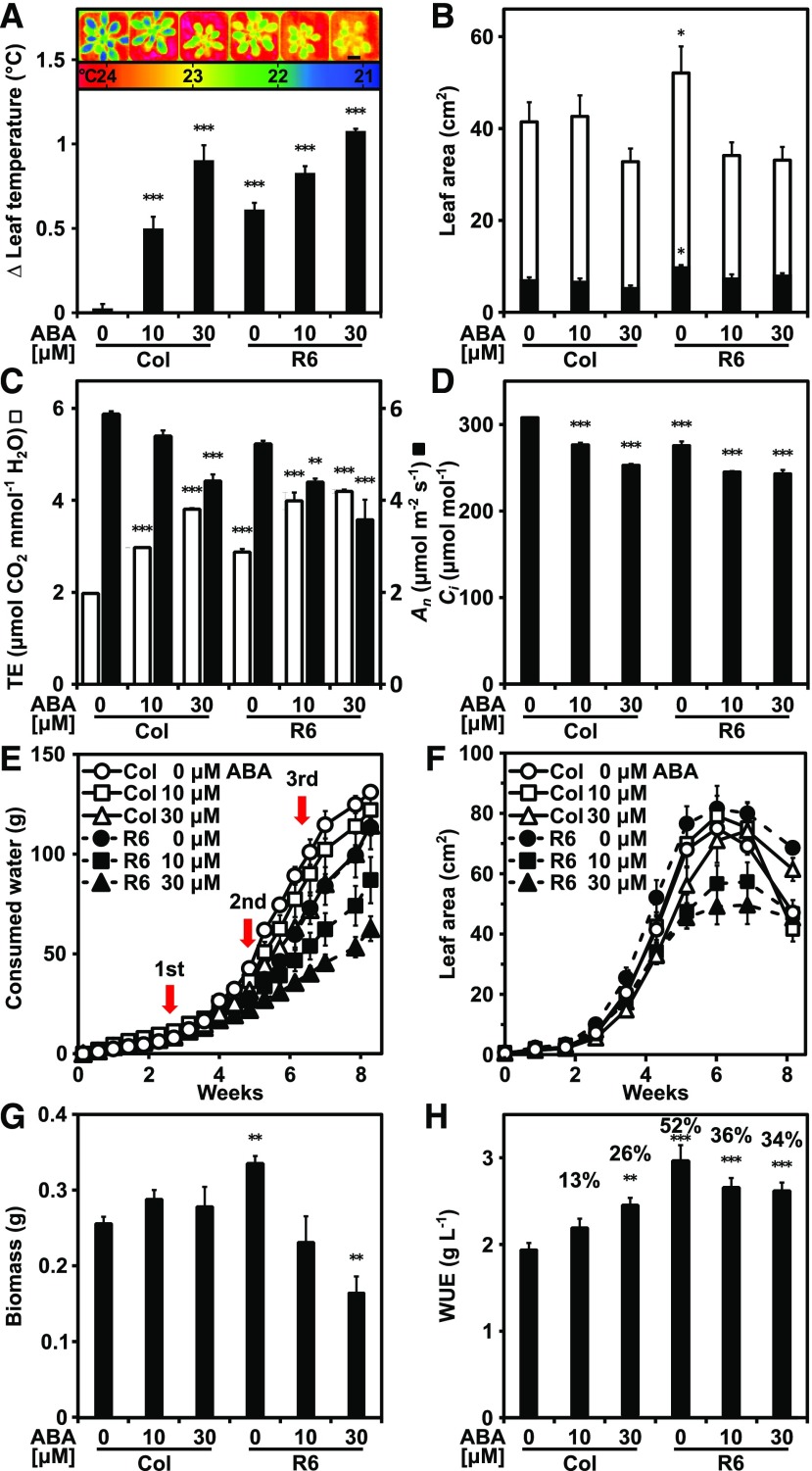
Drought frequently occurs in combination with heat (Zandalinas et al., 2018). The combined effects of drought and high ambient temperatures may offset the increased biomass and enhanced WUE found for the ABA-receptor lines. Temperature affects gas diffusion and VPD (Urban et al., 2017), and the reduced transpirational cooling of RCAR lines might impair their performance under heat. To explore the impact of temperature on the WUE and growth, the plants were grown at different ambient temperatures from 17°C to 32°C in 5°C increments. Plantlets were exposed to a slowly increasing water deficit starting from well-watered to terminal drought, which required 40 to 70 d depending on the temperature (Fig. 3). The plants were kept in a vegetative state by short-day light conditions. At 17°C, plant development was retarded compared with plants grown at higher temperatures, clearly visible after 18 d of growth (Fig. 3, A and B). Leaf temperature of Col-0 at 17°C was almost 3°C higher than the ambient temperature; but this difference decreased with increasing ambient temperature and became negative at 32°C (Fig. 3C), indicating the demand for enhanced transpirational cooling under heat. Both ABA receptor lines showed higher leaf temperatures than Col-0 at all temperature regimes. At the end of the progressive drought experiment, the available water was consumed (Fig. 3D), and the RCAR6-3 and RCAR10-4 lines acquired approximately 40% more biomass than Col-0 grown at 22°C (Fig. 3E). The gains were lower at 27°C and 32°C. Both temperatures led to a reduction in total biomass in all plants, and the WUE of Col-0 dropped from 2.1 ± 0.1 g/L at 22°C to 0.9 ± 0.1 g/L at 32°C (Fig. 3F). The WUE benefit of the RCAR6-3 and RCAR10-4 lines was lowered to 17% and 19% at 32°C. At the cooler growth temperature of 17°C, however, the advantage in WUE of both RCAR lines was increased to 70% to 80%, and more shoot biomass was harvested compared with Col-0 (Fig. 3F). This analysis supports the notion that the ambient temperature is a critical parameter for the iWUE trait of ABA receptor lines. The combination of drought and heat compromises the WUE benefit of the RCAR lines.
Figure 3.
High ambient temperature reduces WUE and the WUE advantage of RCAR lines. A, Representative pictures. The scale bar = 2 cm. B, Rosette leaf areas. C, Leaf temperature differences to ambient target temperature of 36-d-old Col-0, RCAR6-3 (R6), and RCAR10-4 (R10) grown at different temperatures and under well-watered conditions. The growth condition was as described in Fig. 1 except that temperature during the day was 22°C. Both day- and nighttime temperatures were shifted in 5°C increments from this condition, keeping the relative humidity values constant. D, Consumed water. E, Above-ground biomass. F, WUE at the end of the terminal drought, which took 70 d, 60 d, 50 d, and 40 d for 17°C, 22°C, 27°C, and 32°C, respectively. Single plantlets were 18 d old and had a leaf area of 0.7 cm2 ± 0.1 cm2 at the beginning of the drought experiments. E and F, The numbers above the columns indicate the difference to Col-0. In (A) to (E), mean ±sem; n = 5 biological replicates; ***P < 0.001 (one-way ANOVA) compared to Col-0.
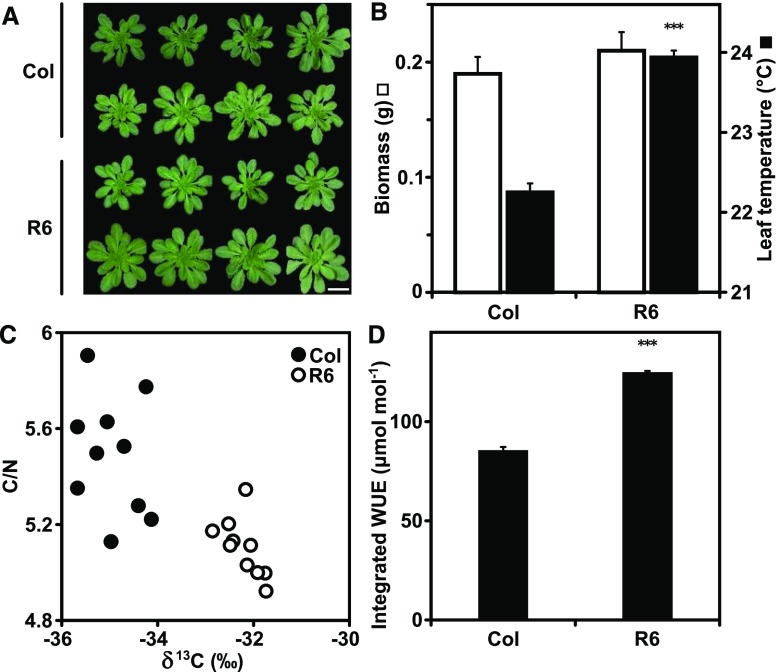
As a next step, the photosynthetic CO2 demand was stimulated by increasing the photosynthetically active radiation (PAR) from 0.15 to 0.5 mmol m−2 s−1, and growth of Col-0 and the RCAR6-3 line were compared under well-watered conditions and at a 21°C ambient temperature. There was no significant difference in accumulated biomass after 30 d of growth (Fig. 4, A and B). The leaf surface temperature was clearly elevated in the ABA receptor line (Fig. 4B), and analysis of leaf material for carbon (C) isotope discrimination and C/nitrogen (C/N) ratios (Fig. 4C) corroborated a reduced transpiration by showing a 43% higher integrated WUE for the RCAR6 line (Fig. 4D). Interestingly, there was the tendency of reduced C/N ratios in the RCAR6 plants compared with Col-0 (Fig. 4C).
Figure 4.
Differences in water productivity and C/N ratios between the ABA receptor line RCAR6-3 and the wild-type Col-0 at higher PAR. A, Representative wild-type Columbia (Col) plants and RCAR6-overexpressing line RCAR6-3 (R6) grown at PAR of 0.5 mM m−2 s−1 and under well-watered conditions for 30 d. The scale bar = 2 cm. B, Above-ground dry biomass (white columns) of 30-d-old plants and leaf temperature (black columns) of 25-d-old plants. C, Association of δ13C composition in above-ground biomass with the ratio C/N. D, Integrative WUE based on δ13C shown in (C). Growth conditions as in Fig. 1, with the exception of light intensity and 21°C temperature. Mean ±sem; n = 10 biological replicates; ***P < 0.001 (one-way ANOVA) compared with Col-0.
ABA Coreceptors as Targets for Enhanced WUE and Water Productivity
The ABA receptors inhibit the PP2C activity of ABA coreceptors in concert with ABA. The PP2Cs targeted by RCAR6 and RCAR10 and conferring the WUE trait are unknown. A systematic analysis of ABA receptors revealed that both receptors are able to regulate all coreceptors, with the exception of ABA HYPERSENSITIVE GERMINATION1 (AHG1; Tischer et al., 2017). Multiple deficiencies in ABA coreceptors can reduce leaf transpiration, and triple mutants deficient in the PP2Cs HYPERSENSITIVE TO ABA 1 (HAB1), ABA INSENSITIVE 1 (ABI1), and ABI2 or/and PP2CA/AHG3 were impaired in growth performance (Rubio et al., 2009; Antoni et al., 2012). To elucidate potential PP2C targets of RCAR6 and RCAR10 contributing to enhanced WUE under well-watered conditions, Arabidopsis lines deficient in single ABA coreceptors were analyzed for growth and leaf temperature. Leaf temperatures were only significantly (P < 0.01) higher for plants deficient in ABI1 (abi1) or ABI2 (abi2; Fig. 5, A and B; Supplemental Fig. S3A). Both mutants showed no reduction in rosette leaf area compared with Col-0 after 48 d of growth (Fig. 5, A and B), while pp2ca/ahg3, ahg1, or HIGHLY ABA-INDUCED PP2C GENE 1 (hai1) to hai3 mutants were smaller than the parental line and produced less above-ground biomass (Supplemental Fig. S3, B to D). The double mutant abi1abi2 showed a leaf temperature similar to that of the RCAR6 line and higher order combinations with hab1 or pp2ca (including the quadruple mutant abi1abi2hab1pp2ca) resulted in even higher leaf surface temperatures (Fig. 5, C and D; Supplemental Fig. S4A). Other combinations of PP2C deficiency such as hab1hab2 or abi1hab1pp2ca mutants resulted in no or increased leaf temperatures compared with Col-0, respectively (Supplemental Fig. S4A). Growth of abi1abi2 and abi1abi2hab1 mutants was comparable to that of the wild type (Fig. 5E); however, growth of the other double and triple mutants was reduced (Supplemental Fig. S4, B and C).
Figure 5.
Water use efficiency conferred by deficiency in ABA coreceptors. A, Thermogram of 43-d-old wild-type Col-0 and Arabidopsis mutants deficient of ABI1 (abi1) and ABI2 (abi2). Scale bar = 2 cm. B, The averaged leaf temperature from (A) and the leaf area of the plants at day 48 grown under well-watered conditions. C to J, Analysis of the double mutant abi1abi2 and the triple mutant abi1abi2hab1 in comparison to Col-0 (Col) and the RCAR6-3 line (R6) under progressive drought. C, Thermogram. Scale bar = 2 cm. D, Leaf temperature. E, Leaf area at day 22 (open column) of the progressive drought experiment. At day 22, the plants were grown 40 d at well-watered conditions (SWC ≥ 60%, Ψ ≥ −0.08 MPa). The size of the plantlets at day 0 of the experiment is indicated by filled columns in (E). F, Growth of the plants expressed as an increase in the projected leaf area. Note the decrease in leaf area caused by wilting and starting for Col-0 from day 40. G, Wilted Col-0 and turgescent rosettes of other plant lines at day 46. Scale bar = 4 cm. H, Consumed water. I) Above-ground dry matter. The values above the columns indicate the percentage gain in WUE compared with Col-0. J, WUE at the end of the terminal drought at day 58. In (A) to (I), mean ±sem, n = four biological replicates per line, **P < 0.01, ***P < 0.001 (one-way ANOVA) compared with Col-0.
The two higher-order PP2C-deficient mutants abi1abi2 and abi1abi2hab1, which combined unimpaired growth with elevated leaf temperatures, were subjected to progressive drought to examine their growth performance and WUE under water deficit. Growth of both PP2C-deficient lines was similar to the RCAR6 line as indicated by the congruent increase in leaf area over 6 weeks (Fig. 5F) and outperformed Col-0 resulting in larger leaf areas when water became growth-limiting for Col-0. The improved efficiency of water use was evident in week 6 of the drought experiment; at this point in time the leaves of Col-0 plants were wilted and rosettes were shrunken, while the PP2C-deficient and the RCAR6 lines had turgescent, large rosettes (Fig. 5G). The rate of water use in these lines was clearly lower in relation to the wild type (Fig. 5H), and biomass and WUE were higher at the end of the drought experiment when the plant-available water was consumed (Fig. 5, I and J). In terms of WUE, the triple-deficient mutant abi1abi2hab1 was even better than the RCAR6 line, with an increase in WUE of 48% compared with 40% over the wild type, respectively. The abi1abi2 double mutant was less water-use efficient but clearly more than Col-0. Although other PP2C deficiencies did not appear to affect water use under well-watered conditions, they might contribute to minimize water use under drought as indicated by the analysis of hab1hab2, abi1hab1pp2ca, and abi1abi2hab1pp2ca mutants under progressive drought (Supplemental Fig. S4, D to F). The pp2ca allele further increased the WUE of the abi1abi2hab1 mutant from 3.2 ± 0.1 g/L to 3.9 ± 0.1 g/L in the quadruple mutant abi1abi2hab1pp2ca (Fig. 5J, Supplemental Fig. S4F). Taken together, the single deficiency in ABI1 and ABI2 led to increased leaf temperatures, and combining the deficiencies with HAB1 resulted in plants that fully mimic the RCAR6 line by providing similarly enhanced WUE without trade-offs in growth performance.
Transpiration Efficiency Affected by ABA Administration
Our results indicate that inactivation of unique PP2Cs can result in water-use–efficient plants, similar to RCAR6 overexpression. The higher water-productivity of these plants can be attributed to a subtle activation of ABA signaling (Rubio et al., 2009; Yang et al., 2016). It is not known to what extent ABA is sufficient to mimic the water-use–efficient trait of the Arabidopsis lines and whether the WUE of the RCAR6 line can be further optimized by higher endogenous ABA levels. Pioneering work on tomato has revealed that enhanced expression of an ABA biosynthesis enzyme can lead to higher ABA levels and improved iWUE, suggesting that ABA might be sufficient to confer a water-use–efficient trait (Thompson et al., 2007). However, the requirement for specific RCARs or PP2C deficiencies for generating the improved iWUE trait in Arabidopsis argues against it. Previous analysis in wheat and barley using exogenous ABA application revealed an increase in WUE (Mizrahi et al., 1974; Rademacher et al., 1987). We used administration of aqueous ABA solutions to Arabidopsis leaves to analyze the increase in leaf surface temperature. Foliar ABA treatment at different concentrations starting from 0.1 µM ABA raised the leaf temperature, and increases above 1°C were observed at 10 µM and higher ABA levels (Supplemental Fig. S5, A and B). The rise in leaf temperature continued for several hours after ABA administration, and the maximum effect lasted for several days (Supplemental Fig. S5C). We decided to use 10 and 30 μM ABA for leaf application, because of the effect on the wild type in which Col-0 reached similar leaf temperatures as observed in untreated RCAR6 plants (Fig. 6A). Administration of these ABA concentrations also increased leaf temperatures of the RCAR6 line and had a moderate negative effect on growth (Fig. 6, A and B). The transpiration efficiency of Col-0 and the RCAR6 lines was enhanced by ABA application and resulted in a concomitant reduction of An (Fig. 6C) and Ci (Fig. 6D). The lowered Ci leads to an enhanced iWUE based on the direct relationship of Ca – Ci and iWUE at constant temperature and VPD (Farquhar et al., 1989), while a lowered An would impinge on growth. The differently ABA-treated plants were subjected to progressive drought. The water consumption of the wild-type plants challenged with 30 µM ABA matched the water use of mock-treated RCAR6 plants over the course of the drought experiment (Fig. 6E). ABA application to RCAR6-overexpressing plants further minimized transpiration but also impaired growth, while Col-0 was only significantly affected in growth at 30 µM ABA (Fig. 6, E and F). The wild-type plants had increased biomass and WUE in response to ABA administration, which were up to 26% higher than the mock-challenged Col-0 (Fig. 6, G and H). The RCAR6 line had an approximately 50% higher WUE compared with Col-0 without ABA treatment, but provision of exogenous ABA curtailed the benefit. The results indicated that ABA application to the wild-type plants improved WUE, though it was associated with a reduction in An and growth at the higher ABA level. ABA application to the RCAR6 line further elevated the leaf temperature but negatively affected growth and WUE, consistent with a supra-optimal activation of ABA signaling.
Figure 6.
Foliar application of ABA to the wild-type Arabidopsis provides a partial phenocopy of the water-use efficient trait of the RCAR6-3 line. A, Increase of leaf surface temperatures in response to mock treatment or ABA application to 36-d-old Col-0 and RCAR6-3 (R6) plants. The panel above depicts representative thermograms of leaf rosettes treated as indicated below. The analysis was performed 24 h after treatment, and changes are expressed as the difference of the leaf surface temperature relative to Col-0 without exogenous ABA application, 22.5°C ± 0.1°C. Scale bar = 2 cm. B, Growth is indicated by the leaf area increase of 36-d-old (black columns) to 50-d-old (white columns) plants in the well-watered phase (Ψ ≥ −0.08 MPa) of the progressive drought experiment. C, Transpiration efficiency (TE; white columns); net C assimilation rate (An, black columns). D, intercellular CO2 concentration (Ci) of Col-0 and R6 were assessed using whole rosettes of 31 ± 1-d-old plants. E, Water consumption. F, Leaf area of Col-0 (solid line) and R6 (interrupted line) plants over the 60 d of the progressive drought experiment. The time points of the mock and ABA treatments are indicated by red arrows. G, Final above-ground biomass as dry matter. H, WUE; the percentage increase of WUE relative to mock-treated Col-0 is shown by the values above the columns. In (A), (B), (E), and (G), mean ±sem; n = 4 biological replicates. In (C) and (D), n = 3 biological replicates, *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA) compared with Col-0 without exogenous ABA.
ABA-Mediated Increase of WUE and Water Productivity in Wheat
The above experiments showed that foliar administration of ABA can improve WUE in Arabidopsis. However, in our hands this chemical treatment was not as efficient as genetic modulation of ABA signaling using ectopic RCAR expression or PP2C deficiencies. Whether these genetic approaches can be successfully translated into a C3 crop such as wheat is a tempting speculation. To explore the potential for improving WUE in wheat, we analyzed the change in growth and water use of the crop in response to ABA application under near-field conditions.
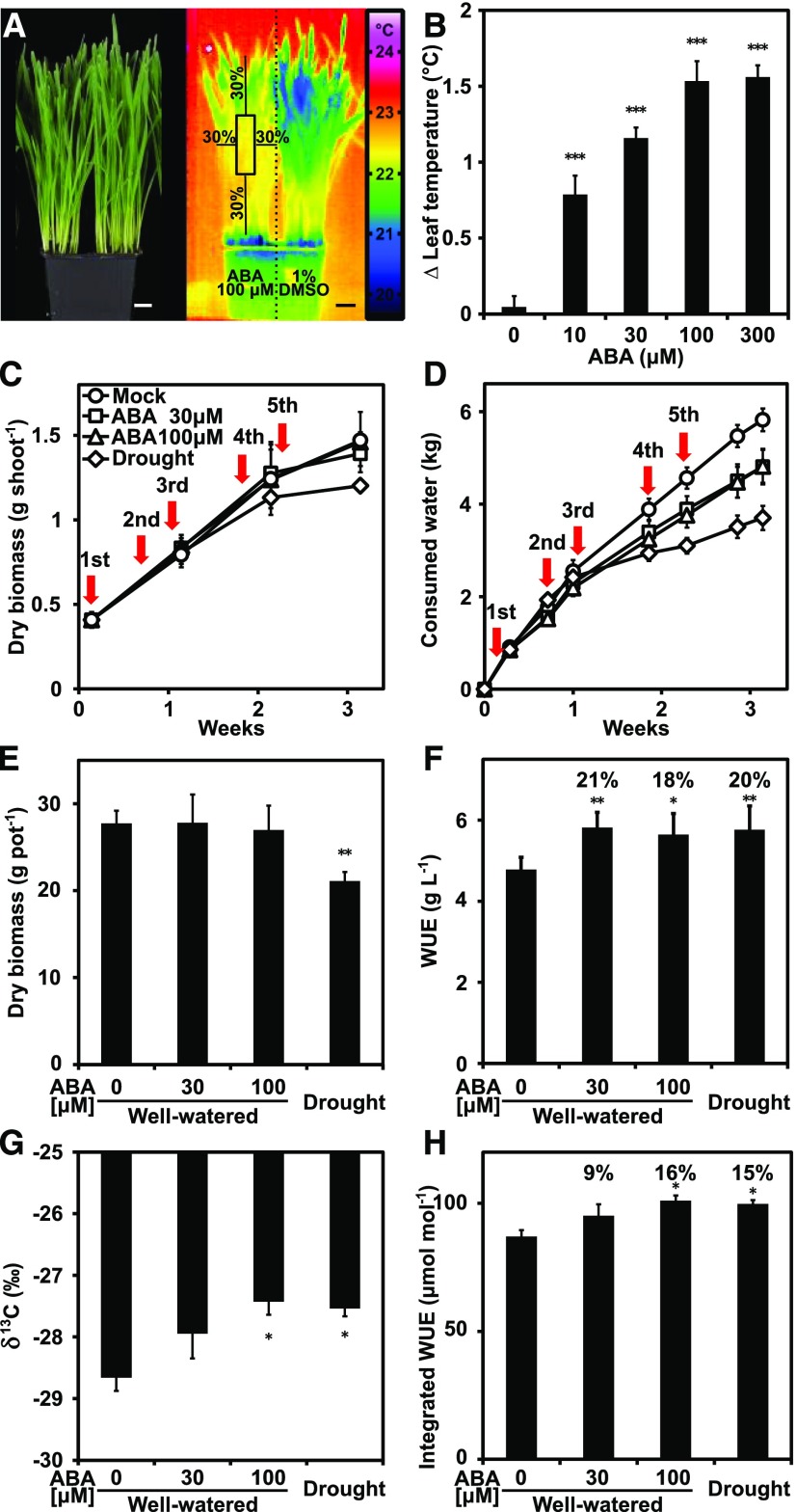
Application of different ABA concentrations to wheat plantlets increased the leaf surface temperatures in a dose-dependent manner (Fig. 7, A and B). Administration of 30 µM ABA enhanced the leaf temperature of wheat by approximately 1°C, similar to the response of Arabidopsis toward 10 µM ABA (Fig. 7B, Supplemental Fig. S5B). We decided to use 30 and 100 µM concentrations of ABA for reducing transpiration of wheat grown under near-field conditions. The plants were grown in large pots and were exposed to full sunlight and vapor pressure deficits similar to the nearby field (Supplemental Fig. S6). The wheat seedlings were allowed to develop their root system in 1.1 m long cylinders containing 19 L of soil for 33 d prior to ABA administration, mock treatment, or drought onset. ABA was repeatedly provided by foliar spraying, and both biomass increase and water consumption were gravimetrically monitored over the course of the experiment (Fig. 7, C and D). Growth among the well-watered control and ABA-treated plants did not statistically differ, while the drought-exposed wheat plants showed an approximately 25% reduced biomass in the tillers after 23 d (P < 0.01, one-way ANOVA). The rate of water consumption, i.e. evapotranspiration, was reduced in ABA-challenged plants and even more diminished under drought compared with the control. However, total harvested above-ground biomass was similar between the control and ABA-treated plants but significantly differed for plants exposed to drought (Fig. 7E). The ABA application resulted in approximately 20% higher WUE values similar to the effect of drought, albeit without the negative impact on biomass (Fig. 7F). The enhanced WUE induced by ABA administration was corroborated by C isotope analysis of the same wheat plants and determination of 13C-derived integrated WUE values (Fig. 7G,H). Hence, wheat plants can respond positively to ABA administration by enhancing WUE without detectable growth trade-offs when cultivated under near-field conditions. The results indicate the suitability of this crop for iWUE improvement.
Figure 7.
Foliar application of ABA enhances the water productivity of wheat in near-field conditions. A to B, Changes of leaf temperature in response to ABA application. A, Wheat plants are shown 24 h after ABA application (left panel) and in a thermogram (right panel). Half of the plants grown for 30 d in single pots (n = 3) were exposed to 100 µM ABA solution or mock-treated with aqueous solution containing 1% (v/v) dimethyl sulfoxide. The inner center of the treated plants as indicated by the box in the thermogram was used to determine the leaf surface temperature, and temperature differences were expressed in comparison to the mock-treated plants of the same pot. Scale bar = 2 cm. B, Leaf temperature increase in response to different ABA concentrations was assessed 24 h after treatment. C to H, Wheat plants were grown under near-field conditions and treated with ABA or exposed to drought. The well-watered plants (33 d old) were treated with either mock or ABA solutions (30 and 100 µM) at time points indicated by red arrows. Eight to nine plants were cultivated in cylinders (n = 4 per treatment), with a diameter of 0.15 m, length of 1.1 m, and soil volume of 19 L. Three, two or three, and three plants were harvested in the first, the second, and the third week, respectively. C, Dry biomass per shoot. D, Water consumption by evapotranspiration. E, Total dry biomass. F, WUE (biomass increase per evapotranspired water) during 23 d. G, Shows 13C composition. H, Shows 13C-derived integrative WUE of wheat plants conferred by exogenous ABA application or drought. Three 40-d-old wheat plants per cylinder analyzed in (C) and (D) were harvested for each treatment to determine the C isotope composition. Mean ±sem; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA) compared to plants without exogenous ABA.
DISCUSSION
The homeostasis of a plant’s water status is a delicate balance between water acquisition and water loss during CO2 uptake for C assimilation. Terrestrial plants evolved ABA-regulated stomata for optimizing and fine-tuning gas exchange of leaves (Hetherington and Woodward, 2003; Negin and Moshelion, 2016). Plants have evolved strategies to avoid or tolerate water deficit. These methods include deep rooting systems, succulent organs, or true drought tolerance. However, at the level of gas exchange in leaves, the strategies are limited. The net flux of CO2 and water vapor across the stomatal pore are driven by the respective partial pressure gradients (Franks et al., 2013). As a consequence, plants can only improve the exchange rate of CO2 for water vapor by favorably changing the stomatal gradients of the two gases, i.e. lowering Ci and lowering VPD (Cernusak et al., 2018) at a given ambient condition.
The water use-efficient RCAR lines have lowered Ci and unchanged An hallmarks of improved plant water productivity (Yang et al., 2016). The analysis also showed that differences in leaf temperature and rosette area among Arabidopsis lines grown under well-watered conditions correlated closely with changes in iWUE and WUE. Using differences in leaf temperature and rosette area as approximations for differences in transpiration and growth, our study indicates that the Col-0 accession and other analyzed wild-type accessions of Arabidopsis do not grow with the minimal transpiration required to sustain full growth (Fig. 1). Seven accessions including Col-0 examined had similar growth performances and transpirational cooling, with the exception of the clear outlier Cvi-0. The accession Cvi-0 is from the Cape Verde islands close to the equator and is known to have larger stomata and higher transpiration caused by a unique allele of the mitogen-activated protein kinase 12 (Koornneef et al., 2004; Des Marais et al., 2014). No natural accession was found to be more water-use efficient than Col-0 in our study, which does not exclude the existence of natural Arabidopsis accessions with high WUE. In fact, a considerable variation in WUE among Arabidopsis natural accessions was reported, and a positive correlation between WUE and reduced An was observed (Easlon et al., 2014). Several accessions such as Kas-1 and Et-0 exhibited higher integrated WUE (McKay et al., 2003, 2008; Easlon et al., 2014). However, data on growth performance and biomass acquisition were not provided and, hence, the studies do not allow us to conclude that these plants are improved in water productivity. In our comparative analysis with ABA receptor lines, we found that the leaf temperature could be shifted by at least 1°C to higher temperatures compared with the wild-type accessions without growth penalties. A higher leaf temperature means a lower transpiration rate and, in combination with unimpaired growth, indicates an improved iWUE and WUE as observed in certain ABA receptor lines. The dissociation of WUE and trade-offs in growth is in agreement with the results of a large-scale study in which wheat plants grown under variable environmental conditions achieved considerable variations in WUE and showed a boundary function for optimized water consumption and yield (Sadras et al., 2010).
The less-efficient water use of the wild-type Arabidopsis in our experiment is not surprising given that water was not the limiting factor for growth. More transpiration means more cooling, higher Ci values at a given An, and probably fewer constraints on photorespiration. Moreover, more water flux through plants results in more water uptake and possibly in better acquisition of dissolved nutrients. The iWUE trait of RCAR6 plants was strongly dependent on the ambient temperature and was minimized at 27°C and 32°C; whereas at 17°C, iWUE and WUE were clearly higher than at 22°C. The relative humidity in this analysis was kept constant; however, VPD increases with temperature and was more than doubled between 17°C and 32°C (Farquhar and Sharkey, 1982). The enhanced demand for transpirational cooling during heat is evident from the lowering of leaf surface temperatures below the ambient temperature at 32°C, which caused a reduction of WUE for Col-0 by 65%. Nevertheless, the RCAR lines had approximately 15% higher WUE and biomass. Heat stress seems to partly override the restriction in gas exchange of the ABA receptor lines similar to the response in pine (Pinus taeda), in which heat induced stomatal opening even in the presence of water deficit (Urban et al., 2017). In regards to gene expression, the response of Arabidopsis to drought differs largely from the response to the combined stress of heat and drought (Rizhsky et al., 2004; Prasch and Sonnewald, 2013).
The water-use–efficient trait of RCAR6 plants was not negatively affected by increasing the light intensity from 0.15 to 0.5 mmol m−2 s−1. The higher radiation is closer to the light saturation point of 0.9 mmol m−2 s−1 for Arabidopsis (Flexas et al., 2007), and it supports higher photosynthesis rates and therefore an enhanced CO2 demand compared with our standard light conditions. The RCAR6 plants still showed the iWUE trait without growth penalty and outperformed the wild type with respect to biomass and WUE under high PAR (Fig. 4). Surprisingly, there was a tendency toward lower C/N ratios of the RCAR6 line in the shoot biomass, indicating that the plants combined reduced transpiration with efficient uptake of N sources. A higher N acquisition may contribute to increased CO2 uptake and increased WUE by the beneficial effect of nitrate reduction involved in the photorespiratory pathway (Busch et al., 2018). The increased N content might be caused by changes in root architecture or in the up-regulation of N acquisition. Grafting experiments indicated that the root system of RCAR lines contributes to WUE (Yang et al., 2016). Interestingly, the ABA coreceptors ABI1 and ABI2 were identified as regulators of the C/N response (Léran et al., 2015; Lu et al., 2015). Both PP2Cs are controlled by RCAR6 and RCAR10 (Tischer et al., 2017) and might specifically affect N acquisition because water-use–efficient Arabidopsis plants with reduced stomatal density were not changed in nutrient uptake (Hepworth et al., 2015).
The ABA receptor lines RCAR6 and RCAR10 outperformed the parental genotype Col-0 in terms of WUE and water productivity, irrespective of the water potential (Fig. 2), though growth of the RCAR10 line was somewhat impaired. The water content of the soil was maintained at conditions of moderate water deficit, and in this respect the analysis resembled deficit irrigation practices in which a growth-limiting supply of water maximizes WUE (Monaghan et al., 2013). The wild-type plants showed a 46% increase in WUE and a growth reduction of approximately 26% at 20% SWC compared with the well-watered conditions. Water consumption of the ABA receptor lines at 40% SWC was similar to that of Col-0 at 20% SWC, however, with only a marginal growth reduction. As a consequence, WUE was almost doubled compared with Col-0 at well-watered conditions. Close homologs of RCAR6 and RCAR10 are OsPYL4 to OsPYL6 from rice (Oryza sativa; Umezawa et al., 2010) and deficiency in these ABA receptors resulted in higher transpiration and higher yielding rice under non-water–limited conditions (Miao et al., 2018), supporting the notion that these ABA receptors are important for regulation of stomatal conductance. Taken together, the analyses of the water-use–efficient Arabidopsis lines revealed a robust increase in WUE that was resilient to changes in water availability and light intensity but negatively affected by heat. The temperature is a major factor influencing WUE, and variations in temperature together with VPD may explain largely the variation of WUE observed in field trials (Sadras et al., 2010). RCAR6 consistently combined high WUE with no or marginal growth penalty relative to the wild type. As a consequence, the physiological features of RCAR6 plants provide a model for improvement of WUE in C3 plants.
On the molecular level, the question remains as to the identity of the ABA coreceptors regulated by RCAR6 and contributing to the water-productive phenotype. Analysis of all nine ABA coreceptors revealed that a single deficiency in ABI1 or ABI2 resulted in elevated leaf temperatures without a negative impact on growth (Fig. 5, A and B; Supplemental Fig. S3, A, C, and D). Clade A PP2Cs are negative regulators of ABA signaling, and their deficiency is associated with ABA hypersensitivity (Saez et al., 2006; Rubio et al., 2009; Antoni et al., 2012; Bhaskara et al., 2012; Fuchs et al., 2013). Deficiency in HAI1 to HAI3 and PP2CA did not detectably affect transpiration at well-watered growth conditions but resulted in minor growth trade-offs, pointing to a role of these PP2Cs in growth maintenance under nonstress conditions (Supplemental Fig. S3). HAI1 to HAI3 are upregulated in plants at low water potential (Bhaskara et al., 2012) and may affect WUE under water deficit. Pyramiding loss-of-function alleles of ABI1 and ABI2 with PP2CA/AHG3 and HAB1 showed that the triple abi1abi2hab1 mutant best reduced transpiration, with efficient growth similar to the RCAR6 line (Fig. 4). Several PP2C deficiency combinations, including pp2ca, revealed improved WUE but were impaired in growth, consistent with previous results (Rubio et al., 2009). Our data support the idea that the ABA coreceptors ABI1, ABI2, and HAB1 are major negative players in controlling iWUE under well-watered conditions in accordance with the gain-of-function mutants abi1-1 and abi2-1, which have a wilty phenotype (Koornneef et al., 1984). ABI1, ABI2, and HAB1 are moderately inhibited by RCAR6 at basal ABA levels (Tischer et al., 2017) and, hence, could be the key targets for generating the iWUE and WUE trait.
The specificity of ABA receptors and coreceptors required for generating the improved water productivity in Arabidopsis implies that exogenous ABA application would not be selective enough to specifically target ABA receptors such as RCAR6 and the downstream acting PP2Cs. Nevertheless, foliar application of ABA has a long history of being used to improve WUE of plants, though with negative impact on growth performance (Mizrahi et al., 1974; Rademacher et al., 1987). In our study, we applied exogenous ABA to Arabidopsis with the objectives of assessing to what extent this application can phenocopy the iWUE trait of the RCAR6 line and whether the RCAR6 plants can be further improved in their water use. We also explored the potential of improving WUE in an elite wheat variety by activating ABA responses. Repeated administration of ABA to Arabidopsis and wheat elevated leaf temperature, reduced water consumption, and enhanced WUE. Arabidopsis wild-type plants responded positively to ABA administration by increasing transpiration efficiency and iWUE, thereby partially phenocopying the iWUE trait of the RCAR6 line, but the growth was reduced and the WUE was less enhanced compared with the RCAR6 line under drought. The RCAR6 line responded negatively toward ABA administration consistent with the supra-optimal activation of ABA signaling. In response to exogenous ABA, wheat increased WUE by approximately 20% without a detectable growth penalty similar to or even better than the Arabidopsis wild type. The wheat analysis under near-field conditions supports the conclusion that this C3 crop is also amenable to improvement of iWUE and WUE. However, in other studies, selection of a high WUE trait in wheat was associated with reduced growth and yield in favorable environments (Morgan et al., 1993; Fischer et al., 1998; Condon et al., 2004), though introgression of a genomic region conferred high WUE and increased biomass production in water-limited environments (Rebetzke et al., 2002). Considerable genetic variations in WUE were reported in other C3 crops, such as rice (Laza et al., 2006), cotton (Gossypium sp.; Saranga et al., 2004), tomato (Martin et al., 1999), and rapeseed (Pater et al., 2017). The studies show substantial variation in WUE and yield with no strict correlation, indicating the potential to increase water productivity of crops. The physiological response of C3 plants to increase WUE under water deficit, for example durum wheat (Triticum turgidum var. durum; Rizza et al., 2012), has not yet been explored for the generation of crops with a constitutive water-use–efficient trait comparable to the RCAR6 line. The C4 crop maize (Zea mays) seems to be less promising for such an approach because in this C4 plant, a reduction of the Ci level, which is much lower compared with that in C3 plants, sensitively affects photosynthesis (Blankenagel et al., 2018). The improvement of iWUE in C3 crops such as wheat could be achieved either by genetic means or by using ABA agonists selective for distinct ABA receptors.
Some ABA agonists have been identified with preferential binding to distinct ABA receptors (Benson et al., 2015; Helander et al., 2016; Vaidya et al., 2017; Frackenpohl et al., 2018a, 2018b). Engineering of ABA receptors for novel ligands offers another approach to activate a unique ABA receptor (Park et al., 2015) or the modulated expression of ABA receptor components as shown for Arabidopsis. The challenge will be to identify the key ABA receptors or coreceptors of crops (Gordon et al., 2016) that need to be targeted for enhancing water use without impinging on growth potential.
CONCLUSION
Our study shows that the Arabidopsis wild-type plants analyzed can grow optimal with less water. Hence, water use could be minimized without growth penalty by modulating ABA responses using specific ABA receptors and coreceptors. The more efficient water use of Arabidopsis conferred by enhanced ABA receptor expression was resilient to changes in light intensity and water availability but was reduced by high ambient temperatures. ABA application to the wild-type plants partially mimicked the water-efficient trait generated by modifying the expression of ABA signaling components. However, ABA administration to plants was never as effective as the genetic approach, probably because ABA targets all ABA receptors. ABA agonists selective for the receptors that are positive regulators of water productivity might allow us to phenocopy the genetically engineered water-productive trait. ABA application to wheat under near-field conditions showed that wheat is amenable to both iWUE and WUE improvement. Considering the importance of wheat in global caloric supply and the high yield-loss imposed by water deficit, this crop is a promising target for an enhancement of water productivity.
MATERIALS AND METHODS
Plant Materials and Chemicals
Chemicals were obtained from SigmaAldrich (www.sigmaaldrich.com), J. T. Baker (www.avantormaterials.com), and (S)-ABA from CHEMOS (www.chemos.de). The Arabidopsis (Arabidopsis thaliana) accessions Cvi-0 (N902), Mr-0 (N6795), Mt-0 (N6799), Sorbo (N931), Tu-0 (N1566), Van-0 (N6884), and Ws-0 (N6891) and the PP2C T-DNA lines were received from the Nottingham Arabidopsis Stock Center. The T-DNA lines and the primers used to identify homozygous knockout plants are listed in Supplemental Table S1. Homozygous lines with ectopic expression of specific RCARs were established by transferring an expression cassette for RCARs under the control of the cauliflower mosaic virus 35S promoter into the Arabidopsis wild-type Col-0 as reported (Yang et al., 2016). RCARs-overexpressing lines of T3 and T4 generations were used in this study. Transgenic lines harboring both RCAR6 and RCAR10 expression cassettes, double, and quadruple mutants of PP2Cs were generated by crossing and screening for homozygosity. The triple mutants of abi1,abi2,hab1 and abi1,hab1,pp2ca were provided by Pedro Luis Rodriguez (Rubio et al., 2009). The wheat (Triticum aestivum) cultivar, Westonia (W3900) is an elite wheat line (El-Hendawy et al., 2005).
Plant Growth Conditions
Arabidopsis seeds were allowed to germinate (day 0) and to grow for 7 days on agar plates with solidified 0.5x MS (Murashige and Skoog) medium and at 0.06 mmol m−2 s−1 PAR prior to transfer of single seedlings to 0.2 L pots filled with soil (Classic Profi Substrate Einheitserde Werkverband) containing 3 mg N fertilizer per gram of dry soil. Unless otherwise described, plants were grown under an 8-h light regime, 0.15 mmol m−2 s−1 PAR, 22°C and 50% relative humidity during the day, and 17°C and 60% at night in plant growth cabinets (Conviron E15) or at 0.5 mmol m−2 s−1 PAR, 21°C at the TUM model EcoSystem Analyzer (TUMmesa) facility with LED light equipment. The ambient CO2 concentration was approximately 0.42 mmol mol−1 and 0.5 mmol mol−1 in the Conviron growth chamber and at TUMmesa, respectively. Wheat plants were grown in a rainout-shelter facility of TUM in Dürnast in cylinders of 1.1 m × 0.15 m filled with 19 L of sandy-loamy soil, with online recording of the climatic conditions (temperature, PAR, relative humidity).
Water Deficit Assays
The progressive drought assay was performed as described by Yang et al. (2016). Briefly, established Arabidopsis plantlets (day 18) were exposed to a slowly increasing water deficit by preventing evaporation and withholding watering. SWC levels of 60% and higher provided well-watered conditions with a water potential Ψ ≥ −0.08 MPa (Supplemental Fig. S1). The soil water potential was determined by using a psychrometer (Wescor). Repetitions of the experiment were conducted independently over the course of 16 months. The projected leaf area of Arabidopsis rosettes was analyzed by using Photoshop Elements software (Adobe). Above-ground material was harvested for determination of biomass after drying the material for 3 d at 60°C to achieve constant weight. For the analysis of Arabidopsis at different ambient temperatures, the plants were established at standard conditions until day 18, then temperatures were stepwise changed to reach the target temperature between 17°C and 32°C (±0.1°C) within four days. The relative humidity and night/day differences were kept constant. Calculation of VPD was according to VPD = (100 − RH)/100) × SVP, in which RH is the relative humidity to saturation vapor pressure (SVP) at a given ambient temperature; vapor pressure inside the leaf is assumed as SVP (Farquhar and Sharkey, 1982) which is calculated according to the Goff–Gratch formula (List, 1984).
Foliar Administration of ABA and Thermo-Imaging
An aqueous solution of 1% (v/v) dimethyl sulfoxide and 0.01% (v/v) Tween 20 with or without ABA was applied to leaves by spraying until the run-off of droplets. The leaf temperature was recorded one day after administration. Thermal imaging was carried out as mentioned in Yang et al. (2016) using the InfraTec instrument (Dresden, Germany). Plants were grown in trays containing 24 pots at randomized positions, and the trays were analyzed within the environmentally controlled plant growth cabinet.
Gas Exchange Analysis
Gas exchange measurements were used to determine An, Ci, gs, and transpiration efficiency (TE) in the whole plant configuration using the GFS-3000 gas exchange system equipped with custom-built cuvettes (Heinz Walz, Effeltrich) routinely at 150 μmol m−2 s−1 PAR, 400 μmol mol−1 external CO2, and a VPD of 19 ± 1 Pa kPa−1, using the software of the instrument supplier.
13Carbon Isotope Analysis and Integrated WUE
C isotope composition (δ13C) of whole-shoot biomass was analyzed as described in Yang et al. (2016). Then 13C discrimination (Δ13C, in ‰) was calculated as Δ13C = (δ13Cair − δ13Cplant)/(1 + δ13Cplant/1000) (Farquhar et al., 1989). Intrinsic WUE has been defined as iWUE = An/gs = 0.625 (Ca − Ci) = 0.625Ca (1 − Ci/Ca), where An is the net CO2 assimilation rate and gs is the stomatal conductance (Franks et al., 2013). The factor 0.625 gives the ratio of the diffusivities of CO2 and water in air, and Ca and Ci designate the CO2 concentrations in ambient air and intercellular space. In the growth cabinet, Ca was 500 ± 54 μmol mol−1, with an approximated δ13C air value of −14.5‰. In the rainout-shelter, Ca was 400 μmol mol−1, with an approximated δ13C air value of −9.7 ‰. The “simplified” Farquhar model based on nonlimiting mesophyll conductance was applied to estimate Ci/Ca: Δ13C = a + (b − a) Ci/Ca. The term a (4.4‰) denotes the fractionation of 13CO2 relative to 12CO2 during diffusion through the stomatal pores; and b (27.6‰) denotes the net fractionation during carboxylation reactions.
Statistical Analysis
Data were analyzed by using one-way ANOVA and linear regression analysis with the SPSS version 16.0 software for Windows. The linear regression in Figure 1 was conducted on the data points with more than 1°C higher leaf temperature than Col-0 (P < 0.001).
Accession Numbers
The Arabidopsis Genome Initiative locus identifiers for RCAR6, RCAR10, ABI1, ABI2, HAB1, HAB2, AHG1, PP2CA, HAI1, HAI2, and HAI3 are AT5G45870, AT2G38310, AT4G26080, AT5G57050, AT1G72770, AT1G17550, AT5G51760, AT3G11410, AT5G59220, AT1G07430, and AT2G29380, respectively.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. The dependence of soil water potential Ψ on soil water content.
Supplemental Figure S2. No further improvement of water productivity by combining the water-use–efficient traits of R6-3 and R10-4.
Supplemental Figure S3. Leaf temperature and growth of PP2C-deficient mutants.
Supplemental Figure S4. Enhanced WUE at terminal drought of PP2C-deficient mutants and growth trade-offs under well-watered conditions.
Supplemental Figure S5. Changes in evaporative cooling of Arabidopsis leaves in response to foliar administration of ABA.
Supplemental Figure S6. Wheat plants grown in a rainout shelter under near-field conditions.
Supplemental Table S1. List of primers used for analysis of RCAR-overexpressing lines and T-DNA insertion lines.
Acknowledgments
We thank Johanna Berger and Claudia Buchhart for technical assistance and Farhah Assaad and Michael Papacek for comments on the article. We thank Pedro Luis Rodriguez for providing triple PP2C-deficient Arabidopsis mutants and the Nottingham Arabidopsis Stock Center for Arabidopsis material. We also thank Sharon Zytynska and Roman Meier for their help in using the TUMmesa plant growth facility.
Footnotes
This work was supported by the German Science Foundation (Deutsche Forschungsgemeinschaft) grants INST 95/1184-1 FUGG, EG938, and SFB924.
Articles can be viewed without a subscription.
References
- Antoni R, Gonzalez-Guzman M, Rodriguez L, Rodrigues A, Pizzio GA, Rodriguez PL (2012) Selective inhibition of clade A phosphatases type 2C by PYR/PYL/RCAR abscisic acid receptors. Plant Physiol 158: 970–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CL, Kepka M, Wunschel C, Rajagopalan N, Nelson KM, Christmann A, Abrams SR, Grill E, Loewen MC (2015) Abscisic acid analogs as chemical probes for dissection of abscisic acid responses in Arabidopsis thaliana. Phytochemistry 113: 96–107 [DOI] [PubMed] [Google Scholar]
- Bhaskara GB, Nguyen TT, Verslues PE (2012) Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160: 379–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenagel S, Yang Z, Avramova V, Schön C-C, Grill E (2018) Generating plants with improved water use efficiency. Agronomy (Basel) 8: 194 [Google Scholar]
- Blum A. (2005) Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust J Agric Res 56: 1159–1168 [Google Scholar]
- Busch FA, Sage RF, Farquhar GD (2018) Plants increase CO2 uptake by assimilating nitrogen via the photorespiratory pathway. Nat Plants 4: 46–54 [DOI] [PubMed] [Google Scholar]
- Cao M-J, Zhang Y-L, Liu X, Huang H, Zhou XE, Wang W-L, Zeng A, Zhao C-Z, Si T, Du J, et al. (2017) Combining chemical and genetic approaches to increase drought resistance in plants. Nat Commun 8: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernusak LA, Ubierna N, Jenkins MW, Garrity SR, Rahn T, Powers HH, Hanson DT, Sevanto S, Wong SC, McDowell NG, et al. (2018) Unsaturation of vapour pressure inside leaves of two conifer species. Sci Rep 8: 7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD (2004) Breeding for high water-use efficiency. J Exp Bot 55: 2447–2460 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR (2010) Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol 61: 651–679 [DOI] [PubMed] [Google Scholar]
- Des Marais DL, Auchincloss LC, Sukamtoh E, McKay JK, Logan T, Richards JH, Juenger TE (2014) Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proc Natl Acad Sci USA 111: 2836–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easlon HM, Nemali KS, Richards JH, Hanson DT, Juenger TE, McKay JK (2014) The physiological basis for genetic variation in water use efficiency and carbon isotope composition in Arabidopsis thaliana. Photosynth Res 119: 119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hendawy SE, Hu Y, Schmidhalter U (2005) Growth, ion content, gas exchange, and water relations of wheat genotypes differing in salt tolerances. Aust J Agric Res 56: 123–134 [Google Scholar]
- Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Finkelstein R. (2013) Abscisic acid synthesis and response. Arabidopsis Book 11: e0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RA, Rees D, Sayre KD, Lu Z-M, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467––1475. [Google Scholar]
- Flexas J, Ortuño MF, Ribas-Carbo M, Diaz-Espejo A, Flórez-Sarasa ID, Medrano H (2007) Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol 175: 501–511 [DOI] [PubMed] [Google Scholar]
- Frackenpohl J, Bojack G, Baltz R, Bickers U, Busch M, Dittgen J, Franke J, Freigang J, Grill E, Gonzalez S, et al. (2018a) Potent analogues of abscisic acid—Identifying cyano-cyclopropyl moieties as promising replacements for the cyclohexenone headgroup. Eur J Org Chem 2018: 1416–1425 [Google Scholar]
- Frackenpohl J, Grill E, Bojack G, Baltz R, Busch M, Dittgen J, Franke J, Freigang J, Gonzalez S, Heinemann I, et al. (2018b) Insights into the in vitro and in vivo SAR of abscisic acid—Exploring unprecedented variations of the side chain via cross-coupling-mediated syntheses. Eur J Org Chem 2018: 1403–1415 [Google Scholar]
- Franks PJ, Adams MA, Amthor JS, Barbour MM, Berry JA, Ellsworth DS, Farquhar GD, Ghannoum O, Lloyd J, McDowell N, et al. (2013) Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytol 197: 1077–1094 [DOI] [PubMed] [Google Scholar]
- Franks PJW, W Doheny-Adams T, Britton-Harper ZJ, Gray JE (2015) Increasing water-use efficiency directly through genetic manipulation of stomatal density. New Phytol 207: 188–195 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Grill E, Meskiene I, Schweighofer A (2013) Type 2C protein phosphatases in plants. FEBS J 280: 681–693 [DOI] [PubMed] [Google Scholar]
- Fuchs S, Tischer SV, Wunschel C, Christmann A, Grill E (2014) Abscisic acid sensor RCAR7/PYL13, specific regulator of protein phosphatase coreceptors. Proc Natl Acad Sci USA 111: 5741–5746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Guzmán M, Rodríguez L, Lorenzo-Orts L, Pons C, Sarrión-Perdigones A, Fernández MA, Peirats-Llobet M, Forment J, Moreno-Alvero M, Cutler SR, et al. (2014) Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J Exp Bot 65: 4451–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CS, Rajagopalan N, Risseeuw EP, Surpin M, Ball FJ, Barber CJ, Buhrow LM, Clark SM, Page JE, Todd CD, et al. (2016) Characterization of Triticum aestivum abscisic acid receptors and a possible role for these in mediating fusairum head blight susceptibility in wheat. PLoS One 11: e0164996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin A, Rodrigues O, Verdoucq L, Merlot S, Leonhardt N, Maurel C (2015) Aquaporins contribute to ABA-triggered stomatal closure through OST1-mediated phosphorylation. Plant Cell 27: 1945–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AJ, Richards RA (2013) Prognosis for genetic improvement of yield potential and water-limited yield of major grain crops. Field Crops Res 143: 18–33 [Google Scholar]
- Helander JDM, Vaidya AS, Cutler SR (2016) Chemical manipulation of plant water use. Bioorg Med Chem 24: 493–500 [DOI] [PubMed] [Google Scholar]
- Hepworth C, Doheny-Adams T, Hunt L, Cameron DD, Gray JE (2015) Manipulating stomatal density enhances drought tolerance without deleterious effect on nutrient uptake. New Phytol 208: 336–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Quick WP (2002) Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415: 451–454 [DOI] [PubMed] [Google Scholar]
- Hughes J, Hepworth C, Dutton C, Dunn JA, Hunt L, Stephens J, Waugh R, Cameron DD, Gray JE (2017) Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol 174: 776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kondoh Y, Yoshida K, Umezawa T, Shimizu T, Shinozaki K, Osada H (2015) Novel abscisic acid antagonists identified with chemical array screening. ChemBioChem 16: 2471–2478 [DOI] [PubMed] [Google Scholar]
- Jasechko S, Sharp ZD, Gibson JJ, Birks SJ, Yi Y, Fawcett PJ (2013) Terrestrial water fluxes dominated by transpiration. Nature 496: 347–350 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Reuling G, Karssen CM (1984) The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol Plant 61: 377–383 [Google Scholar]
- Koornneef M, Alonso-Blanco C, Vreugdenhil D (2004) Naturally occurring genetic variation in Arabidopsis thaliana. Annu Rev Plant Biol 55: 141–172 [DOI] [PubMed] [Google Scholar]
- Laza MR, Kondo M, Ideta O, Barlaan E, Imbe T (2006) Identification of quantitative trait loci for δC-13 and productivity in irrigated lowland rice. Crop Sci 46: 763––773. [Google Scholar]
- Léran S, Edel KH, Pervent M, Hashimoto K, Corratgé-Faillie C, Offenborn JN, Tillard P, Gojon A, Kudla J, Lacombe B (2015) Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci Signal 8: ra43. [DOI] [PubMed] [Google Scholar]
- Li Y, Xu J, Haq NU, Zhang H, Zhu X-G (2014) Was low CO2 a driving force of C4 evolution? Arabidopsis responses to long-term low CO2 stress. J Exp Bot 65: 3657–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- List RJ. (1984) Smithsonian Meteorological Tables, Ed. 6, Vol 114 Smithsonian Institution, Washington DC [Google Scholar]
- Lu Y, Sasaki Y, Li X, Mori IC, Matsuura T, Hirayama T, Sato T, Yamaguchi J (2015) ABI1 regulates carbon/nitrogen-nutrient signal transduction independent of ABA biosynthesis and canonical ABA signalling pathways in Arabidopsis. J Exp Bot 66: 2763–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumba S, Toh S, Handfield L-F, Swan M, Liu R, Youn J-Y, Cutler SR, Subramaniam R, Provart N, Moses A, (2014) A mesoscale abscisic acid hormone interactome reveals a dynamic signaling landscape in Arabidopsis. Dev Cell 29: 360–372 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Martin B, Tauer CG, Lin RK (1999) Carbon isotope discrimination as a tool to improve water-use efficiency in tomato. Crop Sci 39: 1775–1783 [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T (2003) Genetics of drought adaptation in Arabidopsis thaliana: I. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol 12: 1137–1151 [DOI] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Nemali KS, Sen S, Mitchell-Olds T, Boles S, Stahl EA, Wayne T, Juenger TE (2008) Genetics of drought adaptation in Arabidopsis thaliana II. QTL analysis of a new mapping population, KAS-1 × TSU-1. Evolution 62: 3014–3026 [DOI] [PubMed] [Google Scholar]
- Melcher K, Ng L-M, Zhou XE, Soon F-F, Xu Y, Suino-Powell KM, Park S-Y, Weiner JJ, Fujii H, Chinnusamy V, et al. (2009) A gate-latch-lock mechanism for hormone signalling by abscisic acid receptors. Nature 462: 602–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao C, Xiao L, Hua K, Zou C, Zhao Y, Bressan RA, Zhu J-K (2018) Mutations in a subfamily of abscisic acid receptor genes promote rice growth and productivity. Proc Natl Acad Sci USA 115: 6058–6063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Miyakawa T, Sawano Y, Kubota K, Kang H-J, Asano A, Miyauchi Y, Takahashi M, Zhi Y, Fujita Y, et al. (2009) Structural basis of abscisic acid signalling. Nature 462: 609–614 [DOI] [PubMed] [Google Scholar]
- Mizrahi Y, Scherings SG, Arad SM, Richmond AE (1974) Aspects of the effect of ABA on the water status of barley and wheat seedlings. Physiol Plant 31: 44–50 [Google Scholar]
- Monaghan JM, Daccache A, Vickers LH, Hess TM, Weatherhead EK, Grove IG, Knox JW (2013) More “crop per drop”: Constraints and opportunities for precision irrigation in European agriculture. J Sci Food Agric 93: 977–980 [DOI] [PubMed] [Google Scholar]
- Moreno-Alvero M, Yunta C, Gonzalez-Guzman M, Lozano-Juste J, Benavente JL, Arbona V, Menéndez M, Martinez-Ripoll M, Infantes L, Gomez-Cadenas A, et al. (2017) Structure of ligand-bound intermediates of crop ABA receptors highlights PP2C as necessary ABA co-receptor. Mol Plant 10: 1250–1253 [DOI] [PubMed] [Google Scholar]
- Morgan JA, LeCain DR, McCaig TN, Quick JS (1993) Gas exchange, carbon isotope discrimination, and productivity in winter wheat. Crop Sci 33: 178–186 [Google Scholar]
- Morison JIL, Baker NR, Mullineaux PM, Davies WJ (2008) Improving water use in crop production. Philos Trans R Soc Lond B Biol Sci 363: 639–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquna A, Peterson FC, Park S-Y, Lozano-Juste J, Volkman BF, Cutler SR (2011) Potent and selective activation of abscisic acid receptors in vivo by mutational stabilization of their agonist-bound conformation. Proc Natl Acad Sci USA 108: 20838–20843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Hauser F, Park J, Waadt R, Brandt B, Schroeder JI (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negin B, Moshelion M (2016) The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Science 251: 82–89 [DOI] [PubMed] [Google Scholar]
- Nemoto K, Kagawa M, Nozawa A, Hasegawa Y, Hayashi M, Imai K, Tomii K, Sawasaki T (2018) Identification of new abscisic acid receptor agonists using a wheat cell-free based drug screening system. Sci Rep 8: 4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra AB, Davidson A (2010) Adaptive phenotypic plasticity and plant water use. Funct Plant Biol 37: 117–127 [Google Scholar]
- Nuccio ML, Paul M, Bate NJ, Cohn J, Cutler SR (2018) Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Science 273: 110–119 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Peterson FC, Defries A, Park S-Y, Endo A, Nambara E, Volkman BF, Cutler SR (2013) Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc Natl Acad Sci USA 110: 12132–12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow T-FF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-Y, Peterson FC, Mosquna A, Yao J, Volkman BF, Cutler SR (2015) Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 520: 545–548 [DOI] [PubMed] [Google Scholar]
- Pater D, Mullen JL, McKay JK, Schroeder JI (2017) Screening for natural variation in water use efficiency traits in a diversity set of Brassica napus L. identifies candidate variants in photosynthetic assimilation. Plant Cell Physiol 58: 1700–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI (1998) Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282: 287–290 [DOI] [PubMed] [Google Scholar]
- Pizzio GA, Rodriguez L, Antoni R, Gonzalez-Guzman M, Yunta C, Merilo E, Kollist H, Albert A, Rodriguez PL (2013) The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol 163: 441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch CM, Sonnewald U (2013) Simultaneous application of heat, drought, and virus to Arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol 162: 1849–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher W, Maisch R, Liessegang J, Jung J (1987) Water consumption and yield formation in crop plants under the influence of synthetic analogues of abscisic acid. In Hawkins AF, Stead AD, Pinfield NJ, eds, Plant Growth Regulators for Agricultural and Amenity Use. BCPC Monograph 36. British Crop Protection Enterprises, Alton, United Kingdom, pp. 53–66 [Google Scholar]
- Rajagopalan N, Nelson KM, Douglas AF, Jheengut V, Alarcon IQ, McKenna SA, Surpin M, Loewen MC, Abrams SR (2016) Abscisic acid analogues that act as universal or selective antagonists of phytohormone receptors. Biochemistry 55: 5155–5164 [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD (2002) Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci 42: 739–745 [Google Scholar]
- Rizhsky L, Liang H, Shuman J, Shulaev V, Davletova S, Mittler R (2004) When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol 134: 1683–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza F, Ghashghaie J, Meyer S, Matteu L, Mastrangelo AM, Badeck F-W (2012) Constitutive differences in water use efficiency between two durum wheat cultivars. Field Crops Res 125: 49–60 [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim T-H, Santiago J, Flexas J, Schroeder JI, Rodriguez PL (2009) Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol 150: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadras VO, Grassini P, Steduto P (2010) Status of water use efficiency of main crops. SOLAW Background Thematic Report TR07. Food and Agriculture Organization of the United Nations, Rome: http://www.fao.org/fileadmin/templates/solaw/files/thematic_reports/TR_07_web.pdf [Google Scholar]
- Saez A, Robert N, Maktabi MH, Schroeder JI, Serrano R, Rodriguez PL (2006) Enhancement of abscisic acid sensitivity and reduction of water consumption in Arabidopsis by combined inactivation of the protein phosphatases type 2C ABI1 and HAB1. Plant Physiol 141: 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J, Rodrigues A, Saez A, Rubio S, Antoni R, Dupeux F, Park S-Y, Márquez JA, Cutler SR, Rodriguez PL (2009) Modulation of drought resistance by the abscisic acid receptor PYL5 through inhibition of clade A PP2Cs. The Plant Journal 60: 575–588 [DOI] [PubMed] [Google Scholar]
- Saranga Y, Jiang C-X, Wright RJ, Yakir D, Paterson AH (2004) Genetic dissection of cotton physiological responses to arid conditions and their inter-relationships with productivity. Plant Cell Environ 27: 263–277 [Google Scholar]
- Song L, Huang SC, Wise A, Castanon R, Nery JR, Chen H, Watanabe M, Thomas J, Bar-Joseph Z, Ecker JR (2016) A transcription factor hierarchy defines an environmental stress response network. Science 354: aag1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi J, Okamoto M, Akiyama T, Muto T, Yajima S, Sue M, Seo M, Kanno Y, Kamo T, Endo A, et al. (2014) Designed abscisic acid analogs as antagonists of PYL-PP2C receptor interactions. Nat Chem Biol 10: 477–482 [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Farquhar GD, Smeeton RC, Smillie IRA, Black CR, et al. (2007) Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol 143: 1905–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer SV, Wunschel C, Papacek M, Kleigrewe K, Hofmann T, Christmann A, Grill E (2017) Combinatorial interaction network of abscisic acid receptors and coreceptors from Arabidopsis thaliana. Proc Natl Acad Sci USA 114: 10280–10285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubierna N, Farquhar GD (2014) Advances in measurements and models of photosynthetic carbon isotope discrimination in C3 plants. Plant Cell Environ 37: 1494–1498 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Nakashima K, Miyakawa T, Kuromori T, Tanokura M, Shinozaki K, Yamaguchi-Shinozaki K (2010) Molecular basis of the core regulatory network in ABA responses: Sensing, signaling and transport. Plant Cell Physiol 51: 1821–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J, Ingwers MW, McGuire MA, Teskey RO (2017) Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J Exp Bot 68: 1757–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya AS, Peterson FC, Yarmolinsky D, Merilo E, Verstraeten I, Park S-Y, Elzinga D, Kaundal A, Helander J, Lozano-Juste J, et al. (2017) A rationally designed agonist defines subfamily IIIA abscisic acid receptors as critical targets for manipulating transpiration. ACS Chem Biol 12: 2842–2848 [DOI] [PubMed] [Google Scholar]
- Wada Y, van Beek LPH, van Kempen CM, Reckman JWTM, Vasak S, Bierkens MFP (2010) Global depletion of groundwater resources. Geophys Res Lett 37: L20402 [Google Scholar]
- Wang P, Xue L, Batelli G, Lee S, Hou Y-J, Van Oosten MJ, Zhang H, Tao WA, Zhu J-K (2013) Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci USA 110: 11205–11210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ying J, Kuzma M, Chalifoux M, Sample A, McArthur C, Uchacz T, Sarvas C, Wan J, Dennis DT, et al. (2005) Molecular tailoring of farnesylation for plant drought tolerance and yield protection. The Plant Journal 43: 413–424 [DOI] [PubMed] [Google Scholar]
- Wilen RW, Hays DB, Mandel RM, Abrams SR, Moloney MM (1993) Competitive inhibition of abscisic acid-regulated gene expression by stereoisomeric acetylenic analogs of abscisic acid. Plant Physiol 101: 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Yang Y, Sulpice R, Himmelbach A, Meinhard M, Christmann A, Grill E (2006) Fibrillin expression is regulated by abscisic acid response regulators and is involved in abscisic acid-mediated photoprotection. Proc Natl Acad Sci USA 103: 6061–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liu J, Tischer SV, Christmann A, Windisch W, Schnyder H, Grill E (2016) Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc Natl Acad Sci USA 113: 6791–6796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Zhou L, Liu X, Liu H, Li D, Cao M, Chen H, Xu L, Zhu J-K, Zhao Y (2017) A novel chemical inhibitor of ABA signaling targets all ABA receptors. Plant Physiol 173: 2356–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo CY, Hasegawa PM, Mickelbart MV (2011) Regulation of stomatal density by the GTL1 transcription factor for improving water use efficiency. Plant Signal Behav 6: 1069–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Mogami J, Yamaguchi-Shinozaki K (2014) ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr Opin Plant Biol 21: 133–139 [DOI] [PubMed] [Google Scholar]
- Zandalinas SI, Mittler R, Balfagón D, Arbona V, Gómez-Cadenas A (2018) Plant adaptations to the combination of drought and high temperatures. Physiol Plant 162: 2–12 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chan Z, Gao J, Xing L, Cao M, Yu C, Hu Y, You J, Shi H, Zhu Y, et al. (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Natl Acad Sci USA 113: 1949–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]