CsSUT1 downregulation induces male sterility in cucumber by altering carbohydrate supply, and potentially auxin signaling, suggesting a strategy for bioengineering male sterility in crop plants.
Abstract
In plants, male sterility is an important agronomic trait, especially in hybrid crop production. Many factors are known to affect crop male sterility, but it remains unclear whether Suc transporters (SUTs) participate directly in this process. Here, we identified and functionally characterized the cucumber (Cucumis sativus) CsSUT1, a typical plasma membrane-localized energy-dependent high-affinity Suc-H+ symporter. CsSUT1 is expressed in male flowers and encodes a protein that is localized primarily in the tapetum, pollen, and companion cells of the phloem of sepals, petals, filaments, and pedicel. The male flowers of CsSUT1-RNA interference (RNAi) lines exhibited a decrease in Suc, hexose, and starch content, relative to those of the wild type, during the later stages of male flower development, a finding that was highly associated with male sterility. Transcriptomic analysis revealed that numerous genes associated with sugar metabolism, transport, and signaling, as well as with auxin signaling, were down-regulated, whereas most myeloblastosis (MYB) transcription factor genes were up-regulated in these CsSUT1-RNAi lines relative to wild type. Our findings demonstrate that male sterility can be induced by RNAi-mediated down-regulation of CsSUT1 expression, through the resultant perturbation in carbohydrate delivery and subsequent alteration in sugar and hormone signaling and up-regulation of specific MYB transcription factors. This knowledge provides a new approach for bioengineering male sterility in crop plants.
The utilization of male sterility is of importance for hybrid seed production. Many mutations leading to male gametophyte sterility have been shown to interfere with tapetal programmed cell death, meiosis, and mitosis, etc., supporting pivotal roles for these processes in the production of functional pollen grains (Niu et al., 2013; Ko et al., 2014; Han et al., 2018). Abnormal meiosis in the naked oat (Avena nuda) mutant zbs1 also resulted in male sterility by forming defective callose walls and cell plates (Shi et al., 2016; Yang et al., 2016). The knockout of the Magnesium Transporter 5 resulted in Mg2+ deficiency, which led to defects in male gametophytes in Arabidopsis (Arabidopsis thaliana; Xu et al., 2015).
Carbohydrates also play critical roles in male gametophyte development by providing nutrients for normal growth, and may also influence development as signaling molecules during this process (Clement and Audran, 1995). Numerous male sterile lines have been shown to involve perturbations in carbohydrate metabolism. For example, in wheat, a decline in invertase activity alters carbohydrate metabolism, resulting in reduced starch accumulation within the pollen, leading to pollen abortion (Dorion et al., 1996). The rice (Oryza sativa) carbon starved anther (csa) mutant is also male sterile through a perturbation in sugar partitioning (Zhang et al., 2010). Overexpression of GhCK1, which encodes a cotton (Gossypium hirsutum) casein kinase, exhibits starch synthase kinase activity, perturbs Glc homeostasis, and triggers abscisic acid accumulation in cotton flower buds, leading to anther abortion (Min et al., 2013).
Suc, hexoses, and trehalose also function as signal molecules that participate in control of nutrient homeostasis, stress response, and organ development (Paul et al., 2008; Lastdrager et al., 2014). In addition, hexokinase 1 (HXK1) and Suc non-fermenting-1-related protein kinase (SnRK1) were reported to be related to sugar signaling, as two dependent pathways (Moore et al., 2003; Broeckx et al., 2016; Sakr et al., 2018). Although the relationship between trehalose-6-phosphate and Suc signaling in plant remains to be fully resolved, it is known that trehalose-phosphate synthase (TPS) plays an important role in controlling the ratio of trehalose-6-phosphate and Suc (Figueroa and Lunn, 2016; Sakr et al., 2018).
Auxin signaling is also closely related to the development of male floral organs and has crosstalk with sugar signaling (Eveland and Jackson, 2012; Lin et al., 2016). Overexpression of the strawberry (Fragaria x ananassa) auxin biosynthesis gene, FvYUC6, delays flowering and leads to male sterility (Liu et al., 2014). It was also reported that auxin could reverse male sterility caused by high temperature in Arabidopsis (Sakata et al., 2010).
Generally, Suc transporters (SUTs)/Suc carriers (SUCs) belong to the major facilitator superfamily, and are divided into four distinct clades (Sauer, 2007; Kühn and Grof, 2010). The high-affinity SUT1/SUC2 clade members are targeted to the plasma membrane and function in phloem loading, long-distance transport, unloading, and stress resistance (Williams et al., 2000; Patrick and Offler, 2001; Sivitz et al., 2008; Srivastava et al., 2008; Jia et al., 2015). By contrast, the low-affinity SUT2/SUC3 and SUT4/SUC4 clade members are thought to function in sink organ development, seed germination, stress response, and so forth (Hackel et al., 2006; Chincinska et al., 2008; Frost et al., 2012; Li et al., 2012). Finally, the overall importance of these SUTs in mediating Suc transport has been amply demonstrated.
As pollen grains are symplasmically isolated from the anther (Clement and Audran, 1995; Lemoine et al., 1999; Zhang et al., 2010), Suc is delivered to the microspore, from the phloem, via a SUT/SUC, or by a monosaccharide transporter when Suc is hydrolyzed into hexoses by an extracellular invertase (Goetz et al., 2001; Schneidereit et al., 2005). Some SUTs from tomato (Solanum lycopersicum; Hackel et al., 2006), Arabidopsis (Sivitz et al., 2008), and rice (Hirose et al., 2010) are expressed in pollen and support a link between Suc transport and pollen development and viability. SlSUT2 in tomato plays a role in pollen tube growth (Hackel et al., 2006), and disruption of OsSUT1 and AtSUC1 in rice and Arabidopsis, respectively, impairs pollen germination (Sivitz et al., 2008; Hirose et al., 2010).
Expression of SUT genes is regulated by transcription factors. For example, the apple (Malus domestica) ABA-responsive transcription factor, MdAREB2, directly binds to the promoters of MdSUT2 and amylase genes that are involved in soluble sugar accumulation (Ma et al., 2017). In addition, several transcription factors, such as MYB21, MYB24, MYB57, and MYB108, are involved in the regulation of male fertility in Arabidopsis (Cheng et al., 2009; Mandaokar and Browse, 2009; Song et al., 2011).
Presently, the question of whether SUTs directly affect male flower development and/or pollen formation remains to be established. Here, we identified a cucumber (Cucumis sativus) Suc transporter gene, CsSUT1, which was expressed primarily in the male flower; the gene encoded a plasma membrane-targeted protein that was localized on the tapetum, pollen, and companion cells (CCs) in the phloem of sepals, petals, filaments, and the pedicel of the male flower. Down-regulation of CsSUT1 expression resulted in a decrease in Suc, hexose, total soluble sugars, and starch levels in the male flower. In addition, this reduction in CsSUT1 expression gave rise to a decline in sugar metabolism, a reduction in expression of transport-related genes, and altered sugar and auxin signaling, and the up-regulation of the expression of several MYB transcription factors. These findings support the hypothesis that CsSUT1 plays a central role in male flower development, and that a block in this transport step can lead to male sterility.
RESULTS
CsSUT1 Encodes a Typical Suc Transporter and Is Expressed Primarily in Male Flowers
The gene encoding CsSUT1 was isolated from cucumber stage 12 male flowers (Accession no. MG324290), and its 1,488-bp open reading frame encoded a protein of 495 amino acid residues, with a predicted molecular mass of 46 kD (Supplemental Fig. S1). The predicted CsSUT1 amino acid sequence exhibited a modular structure typical for major facilitator superfamily members, comprising 12 transmembrane-spanning regions (Supplemental Fig. S2). A phylogenetic analysis indicated that CsSUT1 belongs to the SUT1 subfamily (Aoki et al., 2003; Kühn and Grof, 2010) of high-affinity Suc transporters (Supplemental Fig. S3; Supplemental Table S1). Another two cucumber Suc transporters, CsSUT2 and CsSUT4 (http://cucumber.genomics.org.cn/page/cucumber/index.jsp), belong to the SUT2 subfamily and the SUT4 subfamily, respectively (Supplemental Fig. S3). These findings established CsSUT1 as a classic SUT1-clade Suc transporter.
The molecular function of CsSUT1 was verified by heterologous expression in a Suc uptake-deficient yeast mutant, SUSY7/ura3 (Riesmeier et al., 1992). The drop test revealed that CsSUT1-SUSY7/ura3 transformants could rescue mutant yeast growth on Suc medium (Supplemental Fig. S4A). Yeast strains harboring the full-length CsSUT1 displayed linear 14C-Suc uptake characteristics (Supplemental Fig. S4B); the optimum pH for transport was 3.5 (Supplemental Figure S4C) and the K0.5 value was 75.6 μm (Supplemental Figure S4D).
Assay involved in testing the specificity of CsSUT1 for its substrate indicated that only Suc caused an inhibition of 14C-Suc uptake (Supplemental Fig. S4E). Furthermore, Suc transport was strongly inhibited by p-chloromercuribenzene sulfonate (SH group inhibitor), and the protonophore carbonyl cyanide m-chlorophenylhydrazone (an uncoupler of transmembrane proton gradients; Supplemental Fig. S4E). Additional 14C-Suc transport assays also showed that CsSUT1 can mediate Suc uptake when expressed in Xenopus oocytes (Supplemental Fig. S4F). Taken together, these findings indicated that CsSUT1 functions as a high-affinity, energy-dependent Suc-H+ symporter.
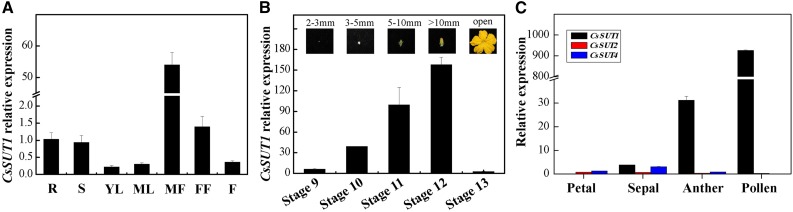
Expression of CsSUT1 occurred mainly in cucumber male flowers, but also in roots, stems, and female flowers, to some extent, with low expression in leaves and fruits (Fig. 1A). CsSUT1 expression increased gradually with male flower development, reaching a peak at stage 12, and then decreased sharply at the time of flower opening (Fig. 1B; Table 1). At stage 12, CsSUT1 was highly expressed in the anther, especially in pollen, with lower levels in sepals and petals, whereas expression levels of CsSUT2 and CsSUT4 were very low in all of these tissues (Fig. 1C).
Figure 1.
Spatio-temporal expression of CsSUT1 in cucumber. A, RT-qPCR analysis of CsSUT1 in different plant organs. B, RT-qPCR analysis of CsSUT1 at various stages of male flower development. Images in (B) were obtained from the male flower at various developmental stages, illustrated by different floral bud lengths. C, Expression analysis of three cucumber SUTs in sepals, petals, anthers, and pollen of stage 12 male flowers. Error bars represent ± sem (n = 3). Abbreviations: R, root; S, stem; YL, young leaf; ML, mature leaf; MF, male flower; FF, female flower; F, fruit.
Table 1. Characteristics used to define the developmental stages of cucumber male flowers.
| Stage | Length of Floral Bud (mm) | Morphological Indications |
|---|---|---|
| 1–7 | <1 | Anther expands |
| 8 | 1–1.5 | Locules differentiate morphologically |
| 9 | 2–3 | Microsporocytes initiate |
| 10 | 3–4 | Meiosis in anthers |
| 11 | 5–10 | Uninuclear pollen appears |
| 12 | >11 | Mature pollen is formed |
| 13 | Open | Pollen release |
CsSUT1 Is a Plasma Membrane Protein Detected Primarily in Tapetum, Pollen, and CCs in the Phloem of Male Flowers
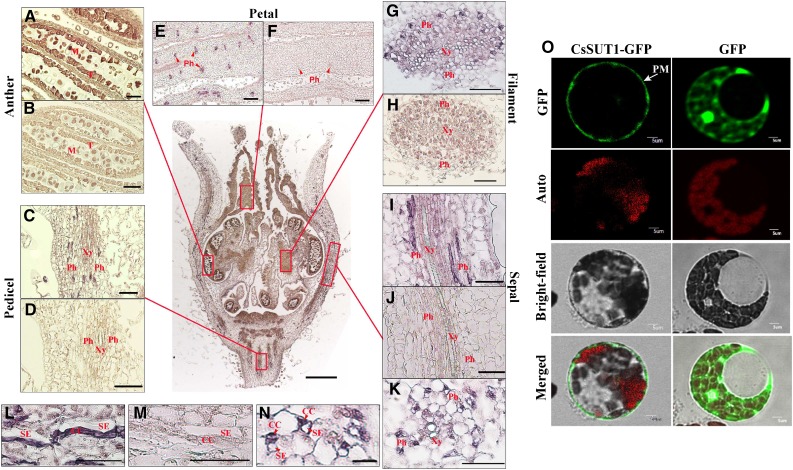
Immunolocalization studies indicated that CsSUT1 was primarily detected in the tapetum, pollen in the anther, and CCs of the phloem located in the pedicle, sepals, petals, and filaments of stage 10 male flowers (Fig. 2, A–N). Similar results were obtained in other stages of male flower development (Supplemental Fig. S5). Transient expression of a CsSUT1-green fluorescent protein (GFP) fusion protein construct in cucumber protoplasts (Fig. 2O) and tobacco (Nicotiana tabacum) epidermal cells (Supplemental Fig. S6) demonstrated that it was targeted to the plasma membrane. These findings are consistent with previous SUT1 localization studies (Kühn et al., 1997; Knop et al., 2004; Schmitt et al., 2008). Taken together, our results established that CsSUT1 is a plasma membrane protein that is located primarily in the tapetum, pollen, and CCs of the phloem of the male flower, implicating a potential role in cucumber male flower development.
Figure 2.
Histological and cellular localization of CsSUT1. A–N, Immunolocal detection of CsSUT1 in stage 10 male flowers using a CsSUT1-antiserum. A, C, E, G, I, K, L, N, Immunolabel detected in the tapetum and microspores in anthers, and phloem cells in sepals, petals, filaments, and the pedicel. B, D, F, H, J, M, Control sections incubated with preimmune serum were devoid of label. (G), (H), (K), and (N) are transverse sections, and the others are longitudinal sections. (L), (M), and (N), are the close-ups of (I), (J), and (K), respectively. O, Plasma membrane localization of CsSUT1-GFP fusion protein in protoplasts derived from cucumber cotyledons. GFP served as a control. Abbreviations: T, tapetum; M, microspore; Ph, phloem; Xy, xylem; SE, sieve element; PM, plasma membrane; Auto, chlorophyll autofluorescence. Scale bars = 50 μm in (A–N) and 5 μm in (O).
Down-regulating CsSUT1 Expression Leads to Male Sterility
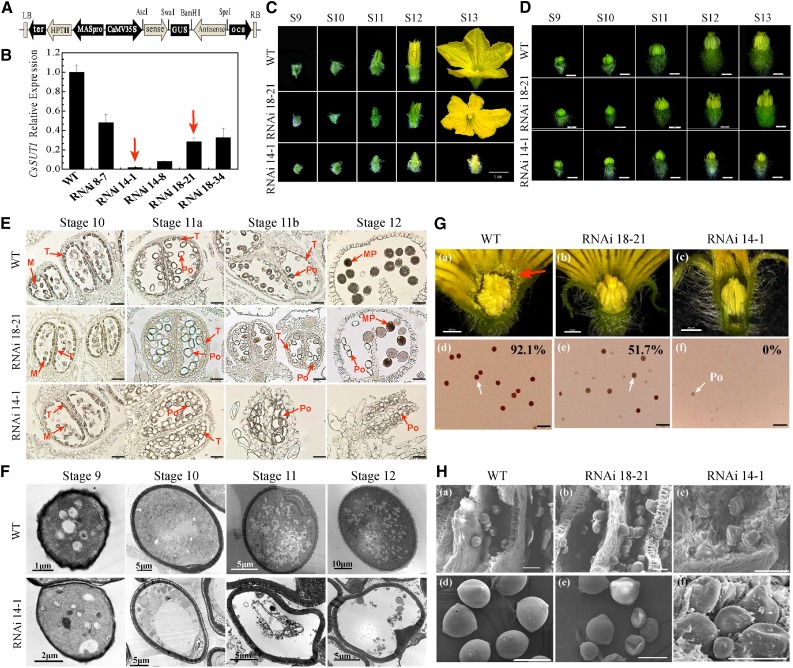
To further study the function of CsSUT1, we constructed an RNAi expression vector (Fig. 3A) that was used for Agrobacterium-mediated transformation of cucumber cotyledons (Cheng et al., 2015; Li et al., 2017a). Five of 30 transgenic lines were variously down-regulated, and pollen germination in these lines was impacted (Supplemental Fig. S7). In T1 plants, CsSUT1-RNAi 14-1 and 18-21 lines were selected for further study (Fig. 3B). There were no visible differences in general plant morphology between wild type and these CsSUT1-RNAi lines with respect to growth, stem diameter, and leaf size, with the exception of the male flowers (Supplemental Fig. S8).
Figure 3.
Morphological and structural characterization of CsSUT1-RNAi male flowers. A, Schematic illustration of the CsSUT1-RNAi expression vector used in this study. B, RT-qPCR analysis of CsSUT1 transcripts present in stage 12 male flowers of wild-type (WT) and CsSUT1-RNAi lines. Error bars = ± sem (n = 3). Two lines were selected for further research (red arrows). C, Morphological features of male flowers at different developmental stages in wild type and CsSUT1-RNAi lines. D, Anther development in wild type and CsSUT1-RNAi male flowers. E, Transverse sections illustrating anther and pollen development in wild type and CsSUT1-RNAi lines. F, Transmission electron micrographs of developing pollen from wild type and CsSUT1-RNAi 14-1 plants. G, Phenotype of male flowers at anthesis stage (a–c), and triphenyltetrazolium chloride stained pollen grains (d–f, white arrows), with percentage viability (%) of wild type and CsSUT1-RNAi lines. Red arrow in (a) indicates abundant pollen. H, Scanning electron micrographs of anthers (a–c) and pollen grains (d–f) from stage 12 male flowers of wild type and CsSUT1-RNAi lines. Abbreviations: M, microspore; MP, mature pollen; Po, pollen; T, tapetum. Scale bars = 1 cm in (C), 200 μm in (D) and (G), and 50 μm in (E) and (H).
Immunolocalization studies indicated that the CsSUT1 signal in male flowers of the CsSUT1-RNAi lines was very weak compared with wild-type male flowers (Supplemental Fig. S9). In these CsSUT1-RNAi lines, male flower development was perturbed; these flowers grew more slowly and were smaller than wild type, at all stages of development, and did not open at anthesis when CsSUT1 was strongly down-regulated (Fig. 3, C and D).
Transverse sections taken from stages 10 to 12 male flowers indicted that the cell walls of anther cells developed abnormally, the microspores became enlarged and accumulated less storage material, and hollow and fragmented pollen grains were extruded into a narrow anther space. In addition, the tapetum layer was thinner, more irregular, and disappeared earlier in CsSUT1-RNAi line anthers than in the wild type, especially in CsSUT1-RNAi 14-1 (Fig. 3E).
Ultrastructural observations revealed that, in the final stage of development, the microsporocyte of CsSUT1-RNAi 14-1 line developed abnormally and formed empty and shriveled pollen, whereas in wild-type male flowers the microsporocyte formed normal pollen grains (Fig. 3F). Moreover, in CsSUT1-RNAi lines pollen viability was greatly reduced (Fig. 3G). At the scanning electron microscope level, relative to the regular anther wall and mature pollen grains of wild type, in the CsSUT1-RNAi lines, the anther walls appeared distorted, and pollen grains were shriveled, displayed abnormal shapes, and adhered together with the contorted anther wall; the stronger the knockdown the more shrunken the pollen grains, and in the most severe case, there was no normal pollen (Fig. 3H).
Taken together, these findings provided support for the hypothesis that CsSUT1 plays a crucial role in cucumber male flower development, and down-regulating CsSUT1 causes male sterility in cucumber.
Carbohydrate Metabolism in Male Flowers of CsSUT1-RNAi Lines Is Perturbed
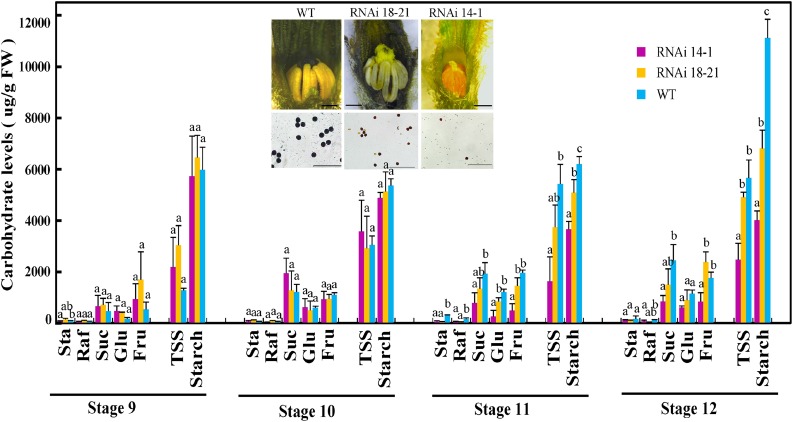
Carbohydrate analyses revealed that Suc, Fru, Glc, and starch decreased gradually in the CsSUT1-RNAi lines, relative to wild-type plants, from stages 10 to 12. Interestingly, the levels of stachyose and raffinose were low, with little difference between CsSUT1-RNAi and wild-type male flowers (Fig. 4). Based on iodine–potassium iodide (I2-KI) staining, starch accumulation in petals, anthers, and pollen of the CsSUT1-RNAi lines was lower compared with these same wild-type floral tissues (Fig. 4).
Figure 4.
Carbohydrate levels analysis in male flowers from wild-type (WT) and CsSUT1-RNAi lines. Data presented are means ± sem (n = 3) with units of µg/g fresh weight (FW). An ANOVA was undertaken using Tukey Honestly Significant Difference test, n = 3; the letters above the bars indicate significant differences (P < 0.05). Inset: Starch levels in male flowers and pollen of wild-type and CsSUT1-RNAi lines, detected by I2-KI staining. Abbreviations: Sta, stachyose; Raf, raffinose; TSS, total soluble sugar. Scale bars = 1 mm in inset figure.
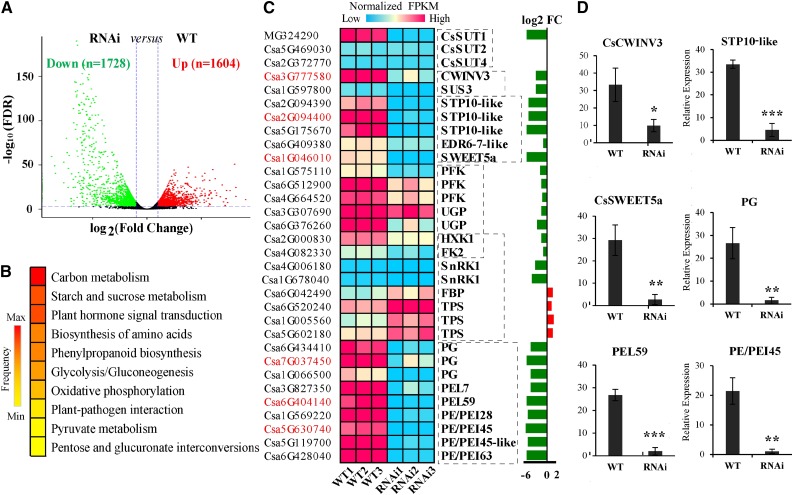
To further explore the cause of cucumber male sterility, we sequenced the mutant transcriptome (wild type as the control). RNA sequencing (RNA-seq) data revealed that, in 12th stage male flowers of CsSUT1-RNAi lines, relative to wild-type plants, of the total of expressed genes, 1604 genes were up-regulated and 1728 were down-regulated (Fig. 5A). In CsSUT1-RNAi lines, of the differentially expressed genes (DEGs), most were enriched in biological processes of carbon metabolism, starch and Suc metabolism, and plant hormone signal transduction (Fig. 5B). Further analysis indicated that most of the genes related to sugar and carbon metabolism and sugar transport were down-regulated, such as cell wall invertase (CWINV3), Suc synthetase3 (SUS3), sugar transport protein10 (STP10), sugar will eventually be exported transporter5a (SWEET5a), HXK1, polygalacturonase (PG), and pectate lyase (PEL; Fig. 5C). This result was also confirmed by reverse transcription-quantitative PCR (RT-qPCR; Fig. 5D). In addition, genes related to sugar signaling, such as HXK1, fructokinase2 (FK2), and SnRK1 underwent a reduction in expression, whereas expression of Fru-1,6-bisphosphatase (FBP) and TPS was increased (Fig. 5C).
Figure 5.
Identification of genes related to carbohydrate metabolism in the cucumber male flower. A, Volcano plot of down-regulated (Down) and up-regulated (Up) DEGs developed based on RNA-Seq data from CsSUT1-RNAi lines versus wild-type (WT) lines. B, Functional categories of DEGs in RNA-Seq data identified by Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis; the top ten categories are shown. ‘Frequency’ stands for the percentage of genes in a certain category to the total DEGs. C, The DEGs involved in carbohydrate metabolism, transport, and signaling. D, Expression of several genes related to carbohydrate metabolism in (C), verified by RT-qPCR. α-Tubulin (AJ715498) was used as the normalization control. Error bars = ± sem (n = 3). Student’s t test, *P < 0.05; **P < 0.01; ***P < 0.001. Abbreviations: FC, fold change; SWEET, sugar will eventually be exported transporter; PFK, phosphofructokinase; UGP, UTP-Glc-1-phosphate uridylyltransferase; HXK1, hexokinase 1; FK2, fructokinase 2; SnRK1, SNF1-related protein kinase; FBP, Fru-1,6-bisphosphatase; TPS, alpha-trehalose-phosphate synthase; PG, polygalacturonase; PEL, pectate lyase; PE/PEI, pectinesterase/pectinesterase inhibitor; FPKM, fragments per kilobase of transcript per million fragments mapped; Min, minimum; Max, maximum.
Auxin Signaling and MYB Transcription Factors May Contribute to Male Sterility
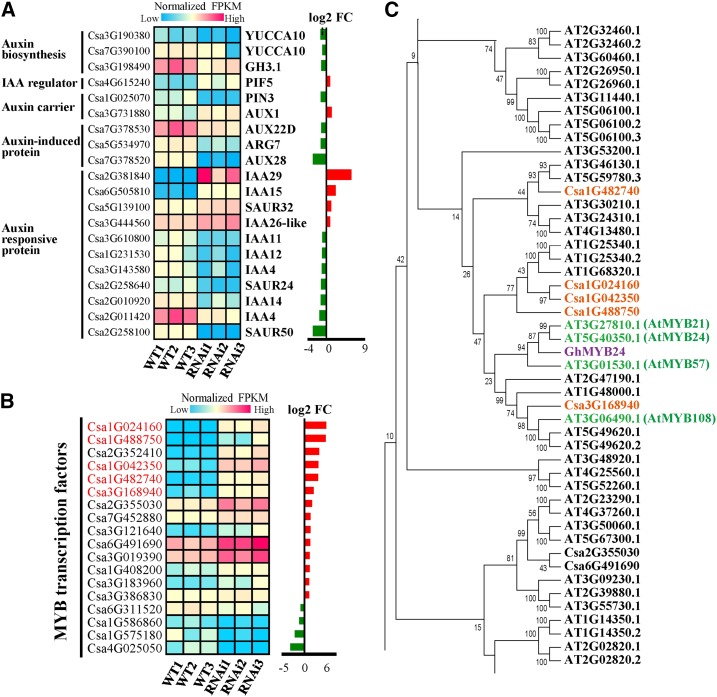
In the CsSUT1-RNAi lines, genes encoding proteins involved in auxin biosynthesis, auxin-induced genes, and genes of numerous auxin response proteins (except for IAA29 and IAA15) were down-regulated; expression of an auxin carrier gene and an IAA regulator were up-regulated (Fig. 6A). Interestingly, two MYB-binding sites are located in the CsSUT1 promoter, and our transcriptomic analysis indicated that numerous MYB transcription factors were up-regulated (except for a few) in the CsSUT1-RNAi lines, especially Csa1G024160, Csa1G488750, Csa1G042350, Csa1G482740, and Csa3G168940 (Fig. 6B). Proteins encoded by these genes shared high sequence similarity with GhMYB24 (Li et al., 2013b) and AtMYB24 (Yang et al., 2007), respectively (Fig. 6C), for which overexpression was previously reported to cause male sterility in Arabidopsis. Thus, our findings are consistent with the hypothesis that both auxin signaling and MYB transcription factors participate in the development of male sterility in the CsSUT1-RNAi lines.
Figure 6.
Identification of auxin signaling and MYB transcription factor genes whose expression patterns were changed in flowers of CsSUT1-RNAi compared to wild-type (WT) plants. A, Heat map of DEGs involved in auxin metabolic pathways, including auxin biosynthesis, transport, and response. B, Heat map showing normalized FRKM values of MYB transcription factors; five genes that changed significantly are highlighted in red. C, Phylogenetic analysis of MYB transcription factors. The five cucumber proteins highlighted in red font shared high sequence similarity with Arabidopsis AtMYB21, 24, 57, 108 (green font), and cotton GhMYB24 (purple font). The genes presented in (A) and (B) were selected with an FPKM value ≥ 10 before down-regulation or after up-regulation. Abbreviations: FC, fold change; PIN3, PIN-formed 3; PIF5, phytochrome-interacting factor 5; AUX1, auxin transporter-like protein; FPKM, fragments per kilobase of transcript per million fragments mapped.
DISCUSSION
CsSUT1 Encodes a Plasma Membrane Protein and Is Expressed Primarily in Male Flowers
Suc transporters in various plants have been widely shown to play important roles in phloem loading in source organs (Hackel et al., 2006; Srivastava et al., 2008), unloading in sink organs, as well as influencing sink organ development (Lemoine et al., 1999; Kühn et al., 2003; Chincinska et al., 2008). In this regard, SUT1 of many crops has been shown to be targeted to the plasma membrane (Riesmeier et al., 1992; Sauer and Stolz, 1994; Kühn et al., 1997; Knop et al., 2004; Schmitt et al., 2008) and most of them are pH dependent with higher affinity at more acidic external pH, except for a ZmSUT1 from maize (Zea mays), which was reported as being pH independent (Chandran et al., 2003; Knop et al., 2004; Baker et al., 2016).
In this study, we isolated a CsSUT1 from the cucumber male flower, which encodes a plasma membrane-localized Suc-H+ symporter (Fig. 2O; Supplemental Fig. S4 and S6). CsSUT1 was expressed primarily in the later stages of male flower development (Fig. 1), which is similar to that of Brassica napus BnSUT1c and rice OsbHLH142 ( Li et al., 2013a; Ko et al., 2014). CsSUT1 shared high sequence similarity with Arabidopsis Suc transporter AtSUC1 (Supplemental Figs. S2 and S3), which is observed mainly in pollen grains of ecotype Columbia-0 (Sivitz et al., 2008; Feuerstein et al., 2010). Various SUT genes are also expressed in pollen grains and have been reported to affect pollen germination and/or tube elongation (Hackel et al., 2006; Sivitz et al., 2008). It is worth mentioning that another cucumber Suc transporter gene, CsSUT2, is expressed in mature leaves and participates in the phloem loading (Ma et al., 2019).
Previous studies have established that many Suc transporters are localized to the phloem sieve elements and/or CCs where they function in the loading of Suc into the sieve tube system (Chandran et al., 2003; Kühn et al., 2003; Knop et al., 2004), whereas some SUTs are localized to xylem parenchyma or pollen grains (Decourteix et al., 2006; Schmitt et al., 2008; Sivitz et al., 2008). In this study, strong CsSUT1 signals were primarily detected in the pollen, tapetum, and CCs of the phloem located in the pedicel, sepals, petals, and filaments (Fig. 2, A–N; Supplemental Fig. S5). These locations indicate that CsSUT1 likely plays a role in regulation Suc delivery from the phloem into the tapetum and pollen. Collectively, our findings establish that CsSUT1 is a plasma membrane-localized high-affinity and energy-dependent Suc-H+ symporter that appears to performs a nonredundant function in male flower development.
Down-regulating CsSUT1 Expression Induced Male Sterility
Developing floral organs act as strong sinks; however, both the tapetum and pollen are symplasmically isolated. Hence, these male organs must obtain nutrients, via the apoplasm, to fuel their development (Clement and Audran, 1995; Lemoine et al., 1999; Xu et al., 2015), and here, Suc uptake into the cytoplasm of these cells requires the presence of functional SUT proteins. Indeed, several studies have shown that SUTs are expressed in pollen, and play a role in pollen germination (Lemoine et al., 1999; Hackel et al., 2006; Sivitz et al., 2008). For example, antisense inhibition of the tomato SlSUT2 reduced pollen tube growth (Hackel et al., 2006) and the Arabidopsis Atsuc1 mutant had impaired pollen germination (Sivitz et al., 2008). In addition, the growth and development of male floral organs, such as sepals, petals, filaments, and pollen, are entirely dependent on sugar unloading from the phloem, and in this context, CsSUT1 would appear to be essential to this process.
It is important to note, however, that in contrast with our findings for CsSUT1, suppression of other plant SUTs has not been reported to give rise to pollen abortion or induction of male sterility. Thus, male sterility obtained by suppression of CsSUT1 and the consequential reduction in nutrient delivery to the tapetum and pollen and other male floral tissues may offer an effective strategy for cucumber hybrid seed production. This would greatly facilitate cucumber, as well as other crops, breeding efficiency by obviating the need for artificial emasculation and also remove the challenges associated with preventing self-crossing caused by insect pollination.
Carbon Undersupply Is the Main Cause for Male Sterility
The mechanisms underlying crop male sterility can be very complex. Many genetic factors, as well as hormones, photoperiod, temperature, and various forms of stress (Takeno et al., 1996; Kobayasi and Atsuta, 2010; Han et al., 2018), along with a perturbation to carbohydrate supply, can affect anther development, thereby leading to male sterility. As an example, we previously mapped a male sterility gene, ms-3, in a cucumber mutant that is defective in microsporogenesis (Han et al., 2018).
Many studies have shown that genes associated with either sugar metabolism or transport are related to male sterility. For example, alteration in invertase activity affected pollen development, pollen tube growth, and pollination through alterations in sugar signals (Goetz et al., 2001; Wang and Ruan, 2016). In cucumber and Arabidopsis, both CsHT1 and AtSTP10, two homologous monosaccharide transporters, play key roles in pollen tube growth (Cheng et al., 2015; Rottmann et al., 2016). Silencing OsUgp2, a gene encoding a rice UDP-Glc pyrophosphorylase that is expressed preferentially in pollen, can result in failure to accumulate starch, leading to sterile pollen (Mu et al., 2009).
Our study established that down-regulating CsSUT1 caused a significant reduction in sugar levels within male flowers (Fig. 4). Meanwhile, the expression of many genes related to carbohydrate metabolism and sugar transport were also down-regulated, such as CWINV3, SUS3, STP10, UTP-Glc-1-phosphate uridylyltransferase, PG, and PEL. In addition to the up-regulation of FBP and TPS, numerous genes related to sugar signaling, such as HXK1, FK2, and SnRK1, also underwent a reduction in expression (Fig. 5C). These genes participate in many physiological and biochemical processes, such as AtHXK1, a gene encoding a well-known Glc sensor, that is involved in regulation of nutrient, light, hormone, and sugar signaling networks in plant development and growth (Moore et al., 2003; Cho et al., 2006). Antisense inhibition of SnRK1 causes abnormal pollen development and male sterility in transgenic barley (Zhang et al., 2001). Taken together, these studies suggest that both the carbohydrate supply and sugar signaling, likely acting together, may regulate the growth and development of male flowers.
Auxin Signaling and MYB Transcription Factors May Underlie Male Sterility
Auxin is closely related to male sterility (Yang et al., 2012; Liu et al., 2014). It has been shown that, in strawberry, overexpressing FvYUC6, an auxin biosynthesis gene, can lead to male sterility (Liu et al., 2014). In Arabidopsis, the AUXIN RESPONSE FACTOR17 is essential for pollen development and the arf17 mutant exhibited a male-sterile phenotype (Yang et al., 2013). In our study, knockdown of CsSUT1 caused not only carbon undersupply, but also changes in genes associated with hormone signaling, notably auxin signaling. In this regard, we demonstrated that, for CsSUT1-RNAi relative to wild-type plants, at the 12th stage of male flower development expression of genes related to auxin biosynthesis (YUCCA10 and GH3.1), auxin transport (PIN-formed 3 [PIN3]), auxin-induced proteins, and auxin response proteins (except for IAA29 and IAA15, etc.), were down-regulated, whereas expression of an IAA regulator gene (phytochrome-interacting factor 5, PIF5) and an auxin carrier gene (auxin transporter-like protein1) were up-regulated (Fig. 6A). Our results are similar to the cotton male sterility induced by high temperature, in that both sugar levels and the expression of genes related to auxin signaling were significantly altered (Min et al., 2014).
MYB transcription factors can influence anther and pollen development (Yang et al., 2007; Zhang et al., 2010; Song et al., 2011), and are involved in sugar-regulated gene expression (Lu et al., 2002) or sugar signaling (Chen et al., 2017). In our study, a MYB-binding site was identified in the CsSUT1 promoter, and most MYB transcription factor genes in the DEGs were up-regulated in the CsSUT1-RNAi lines. The five most substantially up-regulated genes encode proteins that shared high sequence similarity with homologous to Arabidopsis MYB21, MYB24, MYB57, MYB108, and cotton GhMYB24 (Fig. 6, B and C). These Arabidopsis and cotton genes have been reported to participate in the development of male floral organs (Cheng et al., 2009; Huang et al., 2017). For example, in Arabidopsis, overexpression of AtMYB24 causes aberrant anther development (Yang et al., 2007); AtMYB108 acts together with AtMYB24 to regulate stamen maturation (Mandaokar and Browse, 2009); overexpression of the cotton gene GhMYB24 leads to Arabidopsis flower malformation and male sterility, and it is able to recover, partially, male fertility in the myb21 myb24 double mutant (Li et al., 2013b). Thus, misexpression of these MYB transcription factor genes in the developing flowers of our CsSUT1 RNAi lines may have contributed to the observed male sterility phenotype.
CONCLUSION
In summary, down-regulating CsSUT1 expression causes consequential reduction in nutrient delivery, with a subsequent alteration in sugar and auxin signaling, along with misexpression of specific MYB transcription factors, thereby leading to male sterility in cucumber.
MATERIALS AND METHODS
Cloning of CsSUT1
Wild-type cucumber (Cucumis sativus cv ‘Xintaimici’) plants and CsSUT1-RNAi lines were grown under greenhouse conditions. Total RNA was isolated from cucumber stage 12 male flowers, using a Plant RNA extract kit (Huayueyang), and subsequently reverse transcribed to complementary DNA (cDNA) using an oligo(dT) primer (Tiangen), according to the manufacturer’s instructions. Stage division of male flowers was as described by Bai et al., 2004, with slight modification (Table 1). Full-length CsSUT1 coding sequence was amplified from the cDNA, using the primer pairs CsSUT1F and CsSUT1R (Supplemental Table S2). The resultant PCR product was cloned into pMD19-T (TaKaRa), and confirmed by sequencing.
Heterologous CsSUT1 Expression in Yeast and Xenopus Oocytes
The CsSUT1 open reading frame was subcloned into the yeast expression vector pDR196 (Cheng et al., 2015) and then transformed into yeast strain SUSY7/ura3 (Riesmeier et al., 1992). Yeast carrying the pDR196 empty vector served as the control. Drop test and Suc uptake assays were preformed, as previously described (Cheng et al., 2015). The medium for drop tests was supplemented with 2% (w/v) Glc or Suc, as the sole carbon source; for Suc uptake assays the medium contained 0.1 μCi 14C-Suc (26 GBq mmol−1; PerkinElmer). The CsSUT1 coding region was also subcloned into the Xenopus oocyte expression vector, pCS107, linearized with AscI (New England Biolabs) and 1 mg then used as template for complementary RNA (cRNA) synthesis. Suc uptake assays were then performed, as previously described (Chen et al., 2010).
RT-qPCR Analysis
Total RNA was extracted from the specified tissues of wild type or CsSUT1-RNAi lines, respectively, and reverse transcribed into cDNA. Gene-specific primers and internal control (tubulin mRNA) primers (Supplemental Table S2) were used to amplify PCR products on an ABI 7500 system (Bio-Rad). Three biological replicates (samples from three individual plants) were performed and relative amounts of mRNA were calculated using the 2−△△CT method (Livak and Schmittgen, 2001).
Immunolocalization Assays
CsSUT1-specific antiserum was produced in rabbits (two specific peptide fragments MEHGGVVSKGMASDPSSC and CSENQFDPLEIDEEATPF, derived from the CsSUT1 protein sequence, were chosen to synthesize polypeptides), by the Beijing Biotech Company, and antiserum specificity was confirmed by immunoblot, using yeast proteins extracted from SUSY7/ura3 yeast cells (Supplemental Fig. S1). Male flowers were fixed (Wang et al., 2014), and paraffin sections treated with alkaline phosphatase immunoglobulin G-horse radish peroxidase (AP IgG-HRP) and examined with an Olympus BX53 microscope.
Subcellular Localization Assays
Full-length CsSUT1 was subcloned into PUC-SPYNE and pCAMBIA 1300 vectors to produce a CsSUT1-GFP fusion protein and then expressed, respectively, in cucumber mesophyll protoplasts (Huang et al., 2013), or transformed into Agrobacterium and then introduced into tobacco epidermal cells (Li et al., 2017b). GFP fluorescence was visualized at 488 nm wavelength, and red chlorophyll auto-fluorescence was visualized at 546 nm wavelength using an Olympus confocal laser scanning microscope.
Construction of a CsSUT1-RNAi Vector and Cucumber Transformation
Two fragments of CsSUT1 were generated through PCR amplification, using specific primers containing AscI (5′ end) and SwaI (3′ end) sites, and SpeI (5′ end) and BamHI (3′ end) sites. These fragments were inserted, in reverse orientation, into the pFGC1008 vector (Lü et al., 2017) to form the construct 35S-CsSUT1-RNAi. Agrobacterium-mediated transformation of Cucumis sativus cv Xintaimici was then performed, as previously described (Cheng et al., 2015; Li et al., 2017a).
Pollen and Soluble Sugar and Starch Assays
Pollen germination assays (Cheng et al., 2015) were observed under an Olympus BX53 microscope. Pollen viability was assessed using a 2% (w/v) triphenyltetrazolium chloride staining solution. Sugar and starch measurements were performed, as previously described (Lü et al., 2017). Male flowers, at stage 12, were harvested and immersed in the I2-KI staining solution for several minutes, and then washed in distilled water and photographed to assay for starch.
Scanning and Transmission Electron Microscopy Observations
Stage 12 anthers were collected and processed, as previously described (Cheng et al., 2015), and observed under a HITACHI SU8010 scanning electron microscope. Anthers were also fixed, washed, embedded, and stained, as previously described (Sui et al., 2017), and examined under a HITACHI-7500 transmission electron microscope.
RNA-seq
Stage 12 male flowers of wild type and CsSUT1-RNAi lines were sampled for total RNA extraction. RNA library construction and sequencing were performed on an Illunima HiSeq X-ten platform (Biomarker Biotechnology Co.). Each sample yielded more than 6 gigabytes of data. Clean data were aligned to the reference cucumber genome Chinese Long v2.0 via HISAT2. Quantification of gene expression levels were estimated by fragments per kilobase of transcript per million fragments mapped (FPKM). A corrected P value < 0.01 and fold change > 2 were set as the threshold for significantly differential expression.
Statistical Analysis
Student’s t test and Tukey’s Honestly Significant Difference test were used to evaluate the significant differences based on the IBM_SPSS_Statistics software. Data presented are means ± se of three independent experimental replicates.
Accession Numbers
Accession numbers of the major genes/proteins mentioned in this paper can be seen in Fig. 5, Fig. 6, and Supplemental Table S1.
Supplemental Data
The following supplemental information is available:
Supplemental Figure S1. Immunoblot of proteins extracted from SUSY7/ura3 yeast strains probed with CsSUT1 antiserum.
Supplemental Figure S2. Alignment of CsSUT1 and selected Suc transport proteins from Arabidopsis.
Supplemental Figure S3. Suc transporter (SUT) phylogenetic tree analysis.
Supplemental Figure S4. Suc transport characteristics of CsSUT1 assayed in yeast and Xenopus oocytes.
Supplemental Figure S5. Immunological detection of CsSUT1 in different developmental stages of cucumber male flowers using CsSUT1-antiserum.
Supplemental Figure S6. Plasma membrane localization of CsSUT1-GFP fusion protein in tobacco leaf epidermal cells.
Supplemental Figure S7. Analysis of CsSUT1-RNAi lines in T0 cucumber plants.
Supplemental Figure S8. Phenotype comparison between wild-type (WT) and CsSUT1-RNAi cucumber lines.
Supplemental Figure S9. Immunological detection of CsSUT1 protein in male flowers of wild-type (WT) and CsSUT1-RNAi lines.
Supplemental Table S1. GenBank accession numbers of the genes/proteins used for phylogenetic analysis.
Supplemental Table S2. Primers used in this study.
Acknowledgments
We thank Drs. Qinghua Tao and Renbo Tan (Tsinghua University) for assistance in Xenopus oocyte uptake assays.
Footnotes
This work was supported by the National Natural Science Foundation of China (NSFC) (31471876 to Z.Z.), Beijing Innovation Consortium of Agriculture Research System (BAIC01 to X.S.), China Agriculture Research System (CAS-23), and 111 Project of Ministry of Education of P.R.C. (B17043).
Articles can be viewed without a subscription.
References
- Aoki N, Hirose T, Scofield GN, Whitfeld PR, Furbank RT (2003) The sucrose transporter gene family in rice. Plant Cell Physiol 44: 223–232 [DOI] [PubMed] [Google Scholar]
- Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L.). Planta 220: 230–240 [DOI] [PubMed] [Google Scholar]
- Baker RF, Leach KA, Boyer NR, Swyers MJ, Benitez-Alfonso Y, Skopelitis T, Luo A, Sylvester A, Jackson D, Braun DM (2016) Sucrose transporter ZmSut1 expression and localization uncover new insights into sucrose phloem loading. Plant Physiol 172: 1876–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckx T, Hulsmans S, Rolland F (2016) The plant energy sensor: Evolutionary conservation and divergence of SnRK1 structure, regulation, and function. J Exp Bot 67: 6215–6252 [DOI] [PubMed] [Google Scholar]
- Chandran D, Reinders A, Ward JM (2003) Substrate specificity of the Arabidopsis thaliana sucrose transporter AtSUC2. J Biol Chem 278: 44320–44325 [DOI] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, et al. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YS, Chao YC, Tseng TW, Huang CK, Lo PC, Lu CA (2017) Two MYB-related transcription factors play opposite roles in sugar signaling in Arabidopsis. Plant Mol Biol 93: 299–311 [DOI] [PubMed] [Google Scholar]
- Cheng H, Song S, Xiao L, Soo HM, Cheng Z, Xie D, Peng J (2009) Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet 5: e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Wang Z, Yao F, Gao L, Ma S, Sui X, Zhang Z (2015) Down-regulating CsHT1, a cucumber pollen-specific hexose transporter, inhibits pollen germination, tube growth, and seed development. Plant Physiol 168: 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chincinska IA, Liesche J, Krügel U, Michalska J, Geigenberger P, Grimm B, Kühn C (2008) Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiol 146: 515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Yoo SD, Sheen J (2006) Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 127: 579–589 [DOI] [PubMed] [Google Scholar]
- Clement C, Audran JC (1995) Anther wall layers control pollen sugar nutrition in Lilium. Protoplasma 187: 172–181 [Google Scholar]
- Decourteix M, Alves G, Brunel N, Améglio T, Guillio A, Lemoine R, Pétel G, Sakr S (2006) JrSUT1, a putative xylem sucrose transporter, could mediate sucrose influx into xylem parenchyma cells and be up-regulated by freeze-thaw cycles over the autumn-winter period in walnut tree (Juglans regia L.). Plant Cell Environ 29: 36–47 [DOI] [PubMed] [Google Scholar]
- Dorion S, Lalonde S, Saini HS (1996) Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol 111: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveland AL, Jackson DP (2012) Sugars, signalling, and plant development. J Exp Bot 63: 3367–3377 [DOI] [PubMed] [Google Scholar]
- Feuerstein A, Niedermeier M, Bauer K, Engelmann S, Hoth S, Stadler R, Sauer N (2010) Expression of the AtSUC1 gene in the female gametophyte, and ecotype-specific expression differences in male reproductive organs. Plant Biol (Stuttg) 12(Suppl 1): 105–114 [DOI] [PubMed] [Google Scholar]
- Figueroa CM, Lunn JE (2016) A tale of two sugars: Trehalose 6-phosphate and sucrose. Plant Physiol 172: 7–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost CJ, Nyamdari B, Tsai CJ, Harding SA (2012) The tonoplast-localized sucrose transporter in Populus (PtaSUT4) regulates whole-plant water relations, responses to water stress, and photosynthesis. PLoS One 7: e44467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Godt DE, Guivarc’h A, Kahmann U, Chriqui D, Roitsch T (2001) Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc Natl Acad Sci USA 98: 6522–6527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel A, Schauer N, Carrari F, Fernie AR, Grimm B, Kühn C (2006) Sucrose transporter LeSUT1 and LeSUT2 inhibition affects tomato fruit development in different ways. Plant J 45: 180–192 [DOI] [PubMed] [Google Scholar]
- Han Y, Zhao F, Gao S, Wang X, Wei A, Chen Z, Liu N, Tong X, Fu X, Wen C, et al. (2018) Fine mapping of a male sterility gene ms-3 in a novel cucumber (Cucumis sativus L.) mutant. Theor Appl Genet 131: 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Zhang Z, Miyao A, Hirochika H, Ohsugi R, Terao T (2010) Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. J Exp Bot 61: 3639–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HY, Wang ZY, Cheng JT, Zhao WC, Li X, Wang HY, Zhang ZX, Sui XL (2013) An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci Hortic (Amsterdam) 150: 206–212 [Google Scholar]
- Huang H, Gao H, Liu B, Qi T, Tong J, Xiao L, Xie D, Song S (2017) Arabidopsis MYB24 regulates jasmonate-mediated stamen development. Front Plant Sci 8: 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Zhang L, Wu D, Liu S, Gong X, Cui Z, Cui N, Cao H, Rao L, Wang C (2015) Sucrose transporter AtSUC9 mediated by a low sucrose level is involved in Arabidopsis abiotic stress resistance by regulating sucrose distribution and ABA accumulation. Plant Cell Physiol 56: 1574–1587 [DOI] [PubMed] [Google Scholar]
- Knop C, Stadler R, Sauer N, Lohaus G (2004) AmSUT1, a sucrose transporter in collection and transport phloem of the putative symplastic phloem loader Alonsoa meridionalis. Plant Physiol 134: 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SS, Li MJ, Sun-Ben Ku M, Ho YC, Lin YJ, Chuang MH, Hsing HX, Lien YC, Yang HT, Chang HC, Chan MT (2014) The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice. Plant Cell 26: 2486–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayasi K, Atsuta Y (2010) Sterility and poor pollination due to early flower opening induced by methyl jasmonate. Plant Prod Sci 13: 29–36 [Google Scholar]
- Kühn C, Grof CPL (2010) Sucrose transporters of higher plants. Curr Opin Plant Biol 13: 288–298 [DOI] [PubMed] [Google Scholar]
- Kühn C, Franceschi VR, Schulz A, Lemoine R, Frommer WB (1997) Macromolecular trafficking indicated by localization and turnover of sucrose transporters in enucleate sieve elements. Science 275: 1298–1300 [DOI] [PubMed] [Google Scholar]
- Kühn C, Hajirezaei MR, Fernie AR, Roessner-Tunali U, Czechowski T, Hirner B, Frommer WB (2003) The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiol 131: 102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastdrager J, Hanson J, Smeekens S (2014) Sugar signals and the control of plant growth and development. J Exp Bot 65: 799–807 [DOI] [PubMed] [Google Scholar]
- Lemoine R, Bürkle L, Barker L, Sakr S, Kühn C, Regnacq M, Gaillard C, Delrot S, Frommer WB (1999) Identification of a pollen-specific sucrose transporter-like protein NtSUT3 from tobacco. FEBS Lett 454: 325–330 [DOI] [PubMed] [Google Scholar]
- Li F, Yan L, Lai J, Ma C, Gautam M, Fu T (2013a) Molecular cloning and mRNA expression profile of sucrose transporter gene BnSUT1C from Brassica napus L. Indian J Exp Biol 51: 1130–1136 [PubMed] [Google Scholar]
- Li X, Ma S, Shan N, Zhang X, Sui X, Zhang Z (2017a) A protocol for Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) from cotyledon explants. Protoc Exch. 10.1038/protex.2017.107 [DOI] [Google Scholar]
- Li Y, Li LL, Fan RC, Peng CC, Sun HL, Zhu SY, Wang XF, Zhang LY, Zhang DP (2012) Arabidopsis sucrose transporter SUT4 interacts with cytochrome b5-2 to regulate seed germination in response to sucrose and glucose. Mol Plant 5: 1029–1041 [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang J, Du ML, Li L, Wang XL, Li XB (2013b) A cotton gene encoding MYB-like transcription factor is specifically expressed in pollen and is involved in regulation of late anther/pollen development. Plant Cell Physiol 54: 893–906 [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Zhang H, Zhang Q, Zhai H, Liu Q, He S (2017b) The plasma membrane-localized sucrose transporter IbSWEET10 contributes to the resistance of sweet potato to Fusarium oxysporum. Front Plant Sci 8: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XY, Ye YQ, Fan SK, Jin CW, Zheng SJ (2016) Increased sucrose accumulation regulates iron-deficiency responses by promoting auxin signaling in Arabidopsis plants. Plant Physiol 170: 907–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Xie WF, Zhang L, Valpuesta V, Ye ZW, Gao QH, Duan K (2014) Auxin biosynthesis by the YUCCA6 flavin monooxygenase gene in woodland strawberry. J Integr Plant Biol 56: 350–363 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lu CA, Ho TH, Ho SL, Yu SM (2002) Three novel MYB proteins with one DNA binding repeat mediate sugar and hormone regulation of α-amylase gene expression. Plant Cell 14: 1963–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü J, Sui X, Ma S, Li X, Liu H, Zhang Z (2017) Suppression of cucumber stachyose synthase gene (CsSTS) inhibits phloem loading and reduces low temperature stress tolerance. Plant Mol Biol 95: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QJ, Sun MH, Lu J, Liu YJ, Hu DG, Hao YJ (2017) Transcription factor AREB2 is involved in soluble sugar accumulation by activating sugar transporter and amylase genes. Plant Physiol 174: 2348–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Sun L, Sui X, Li Y, Chang Y, Fan J, Zhang Z (2019) Phloem loading in cucumber: Combined symplastic and apoplastic strategies. Plant J. 98: 391–404 [DOI] [PubMed] [Google Scholar]
- Mandaokar A, Browse J (2009) MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol 149: 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Zhu L, Tu L, Deng F, Yuan D, Zhang X (2013) Cotton GhCKI disrupts normal male reproduction by delaying tapetum programmed cell death via inactivating starch synthase. Plant J 75: 823–835 [DOI] [PubMed] [Google Scholar]
- Min L, Li Y, Hu Q, Zhu L, Gao W, Wu Y, Ding Y, Liu S, Yang X, Zhang X (2014) Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol 164: 1293–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Mu H, Ke JH, Liu W, Zhuang CX, Yip WK (2009) UDP-glucose pyrophosphorylase 2 (OsUgp2), a pollen-preferential gene in rice, plays a critical role in starch accumulation during pollen maturation. Chin Sci Bull 54: 234–243 [Google Scholar]
- Niu N, Liang W, Yang X, Jin W, Wilson ZA, Hu J, Zhang D (2013) EAT1 promotes tapetal cell death by regulating aspartic proteases during male reproductive development in rice. Nat Commun 4: 1445. [DOI] [PubMed] [Google Scholar]
- Patrick JW, Offler CE (2001) Compartmentation of transport and transfer events in developing seeds. J Exp Bot 52: 551–564 [PubMed] [Google Scholar]
- Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59: 417–441 [DOI] [PubMed] [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1992) Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J 11: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann T, Zierer W, Subert C, Sauer N, Stadler R (2016) STP10 encodes a high-affinity monosaccharide transporter and is induced under low-glucose conditions in pollen tubes of Arabidopsis. J Exp Bot 67: 2387–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, Miyazawa Y, Takahashi H, Watanabe M, Higashitani A (2010) Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci USA 107: 8569–8574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr S, Wang M, Dédaldéchamp F, Perez-Garcia MD, Ogé L, Hamama L, Atanassova R (2018) The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int J Mol Sci 19: 2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Sauer N, Stolz J (1994) SUC1 and SUC2: Two sucrose transporters from Arabidopsis thaliana; expression and characterization in baker’s yeast and identification of the histidine-tagged protein. Plant J 6: 67–77 [DOI] [PubMed] [Google Scholar]
- Schmitt B, Stadler R, Sauer N (2008) Immunolocalization of solanaceous SUT1 proteins in companion cells and xylem parenchyma: new perspectives for phloem loading and transport. Plant Physiol 148: 187–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneidereit A, Scholz-Starke J, Sauer N, Büttner M (2005) AtSTP11, a pollen tube-specific monosaccharide transporter in Arabidopsis. Planta 221: 48–55 [DOI] [PubMed] [Google Scholar]
- Shi X, Wu JX, Zhou HT, Yang XH, Li TL, Zhang XJ, Yang C, Han X (2016) Defective callose walls and cell plates during abnormal meiosis cause male-sterility in the oat mutant zbs1. J Integr Agric 15: 241–248 [Google Scholar]
- Sivitz AB, Reinders A, Ward JM (2008) Arabidopsis sucrose transporter AtSUC1 is important for pollen germination and sucrose-induced anthocyanin accumulation. Plant Physiol 147: 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Qi T, Huang H, Ren Q, Wu D, Chang C, Peng W, Liu Y, Peng J, Xie D (2011) The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AC, Ganesan S, Ismail IO, Ayre BG (2008) Functional characterization of the Arabidopsis AtSUC2 Sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148: 200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X, Shan N, Hu L, Zhang C, Yu C, Ren H, Turgeon R, Zhang Z (2017) The complex character of photosynthesis in cucumber fruit. J Exp Bot 68: 1625–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeno K, Suyama T, Nishino E (1996) Influence of irradiance level on flowering and male sterility induced by short days in Salsola komarovii Iljin. J Plant Physiol 149: 703–706 [Google Scholar]
- Wang L, Ruan YL (2016) Critical roles of vacuolar invertase in floral organ development and male and female fertilities are revealed through characterization of GhVIN1-RNAi cotton plants. Plant Physiol 171: 405–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Sui X, Guo J, Wang Z, Cheng J, Ma S, Li X, Zhang Z (2014) Antisense suppression of cucumber (Cucumis sativus L.) sucrose synthase 3 (CsSUS3) reduces hypoxic stress tolerance. Plant Cell Environ 37: 795–810 [DOI] [PubMed] [Google Scholar]
- Williams LE, Lemoine R, Sauer N (2000) Sugar transporters in higher plants--a diversity of roles and complex regulation. Trends Plant Sci 5: 283–290 [DOI] [PubMed] [Google Scholar]
- Xu XF, Wang B, Lou Y, Han WJ, Lu JY, Li DD, Li LG, Zhu J, Yang ZN (2015) Magnesium Transporter 5 plays an important role in Mg transport for male gametophyte development in Arabidopsis. Plant J 84: 925–936 [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou HT, Shi X, Zhang XJ, Li TL, Yang XH, Zhang Q, Lu TG (2016) Identification and analysis of Zbs1, a dominant male-sterile mutant of naked oat (Avena nuda L.). Crop Sci 56: 1423–1428 [Google Scholar]
- Yang J, Tian L, Sun MX, Huang XY, Zhu J, Guan YF, Jia QS, Yang ZN (2013) AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol 162: 720–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Li JG, Pei M, Gu H, Chen ZL, Qu LJ (2007) Over-expression of a flower-specific transcription factor gene AtMYB24 causes aberrant anther development. Plant Cell Rep 26: 219–228 [DOI] [PubMed] [Google Scholar]
- Yang X, Liu X, Lv W, Li L, Shi Q, Yang J, Zhang M (2012) Reduced expression of BjRCE1 gene modulated by nuclear-cytoplasmic incompatibility alters auxin response in cytoplasmic male-sterile Brassica juncea. PLoS One 7: e38821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liang W, Yang X, Luo X, Jiang N, Ma H, Zhang D (2010) Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22: 672–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shewry PR, Jones H, Barcelo P, Lazzeri PA, Halford NG (2001) Expression of antisense SnRK1 protein kinase sequence causes abnormal pollen development and male sterility in transgenic barley. Plant J 28: 431–441 [DOI] [PubMed] [Google Scholar]