ABSTRACT
PAK1 and PAK4 are members of the p-21 activated kinase family of serine/threonine kinases. PAK1 has previously been implicated in both the formation and disassembly of invasive cell protrusions, termed invadopodia. We recently reported a novel role for PAK4 during invadopodia maturation and confirmed a specific role for PAK1 in invadopodia formation; findings we will review here. Moreover, we found that PAK4 induction of maturation is delivered via interaction with the RhoA regulator PDZ-RhoGEF. We can now reveal that loss of PAK4 expression leads to changes in invadopodia dynamics. Ultimately we propose that PAK4 but not PAK1 is a key mediator of RhoA activity and provide further evidence that modulation of PAK4 expression levels leads to changes in RhoA activity.
KEYWORDS: invadopodia, PAK, RhoA
As melanoma cells move through complex tissue there is a regulated rearrangement of the actin cytoskeleton, a process thought to be coordinated by Rho family GTPases.1 Moreover, during tissue migration cancer cells need to navigate the 3D architecture of the densely packed stroma. To achieve efficient migration these cells are thought to employ protease-secreting invasive protrusions rich in actin, termed invadopodia.2 These invadopodial structures are now widely accepted to occur both in vitro and in vivo and are also thought to be critical for intravasation of the vasculature.3,4
Kinase activity forms a vital part of cytoskeletal dynamics including the regulation of invadopodia.5 The p-21 activated kinase (PAK) family of serine/threonine kinases are known effectors of Rho GTPases Rac and Cdc42 that control cytoskeletal dynamics and cell movement.6 The human PAK family consists of six isoforms, which are separated into two groups according to their sequence and structural homology: group I, containing PAKs 1–3; and group II, containing PAKs 4–6.6
The overexpression of PAKs is found in a wide variety of cancer tissue types and is often associated with an increase in invasive potential and poor prognosis.6 PAK1 has been shown to localize to invadopodia,7 however, studies investigating the specific function of this protein in invadopodia formation/function have yielded conflicting results. Previous studies in melanoma provide evidence that PAK1 is important for the formation of invadopodia via the phosphorylation of cortactin at Ser113.8 However, a more recent study in breast cancer suggests that PAK1 (once again via the phosphorylation of cortactin at Ser113), promotes the disassembly of invadopodia protrusions; with a depletion of PAK1 leading to an increase in matrix degradation.7 Additionally, several investigations have been conducted into the function of PAK1 in the invadopodia-like protrusion, podosomes. These studies agree that reduced PAK1 activity results in a decrease in podosome formation9 and increased PAK1 expression enhanced the formation of this protrusion.10 Despite the controversy PAK1 is clearly associated with invadopodia dynamics, in contrast, the role of related family member PAK4 had not been previously explored. Although, it had been shown that PAK4 was localized to podosomes and that depletion of PAK4 reduced podosome number.11 We were therefore interested to test if PAK4 also played a functional role in invadopodia.
While PAK1 and PAK4 are structurally distinct and activated differently6 they share a plethora of overlapping substrates and the unique signaling pathways that may drive invasive potential during tumourigenesis have not been clearly defined.12 Research into these potential differences could help guide the further development of therapeutic drugs. Currently, pharmaceutical companies are focused on developing group or isoform specific inhibitors.13-15 Therefore, data indicating whether both groups contribute to invasion and metastasis in the same way, may determine whether the use of pan-selective inhibitors is more beneficial than isoform selective inhibitors in treating some cancer types. Despite the difficulties in separating PAK1 and PAK4 functions mouse knockout (KO) phenotypes suggest that at least for PAK4 there are isoform specific functions as PAK4 KO mice are embryonically lethal while PAK1 KO mice remain viable and fertile.6
We sought to address this issue of isoform specificity by performing the same quantitative invasion assays on cells depleted of either PAK1 or PAK4 expression.16 From this work we were able to provide further evidence that the predominant role of PAK1, at least in melanoma, is to drive invadopodia formation rather than disassembly. Using a gelatin degradation assay we were able to show that cells depleted of PAK1 expression do not initiate actin puncta; indicative of invadopodia formation and there is no subsequent degradation of the underlying matrix. However we observed a different phenotype in PAK4 depleted cells. These cells were able to form actin puncta but were then unable to degrade the underlying matrix. Thus by systematically testing PAK1 and PAK4 depletion in the same cell lines, using the same functional assay, we were able to identify a differential function. Where PAK1 activity is focused toward the early stages of invadopodia formation and PAK4 activity restricted to the later maturation stages (Fig. 1A). The formation of puncta but loss of degradation placed PAK4 at the maturation phase of the invadopodia lifecycle and we detected a loss of membrane type-1-matrix metalloproteinase (MT1-MMP) localization. To further explore the difference between the two PAK isoforms we performed live cell imaging to ascertain the lifetime of invadopodia formation in the depleted cells. To directly image invadopodial dynamics stable control shRNA, PAK1shRNA and PAK4shRNA A-375M2 cells were infected with lentivirus to stably express LifeAct-mRFP. These cells were plated onto gelatin and immediately treated with GM6001 to inhibit invadopodia formation. The inhibitor was then removed 1h prior to imaging to synchronise the population and maximise data acquisition. Invadopodia assembly and disassembly rates were calculated by measuring the fluorescence intensity of the actin rich invadopodia. As expected the actin puncta that we could detect in PAK1 depleted cells were extremely unstable and accurate quantification of lifetimes was not achievable. However, in contrast to PAK1 depleted cells the actin puncta in PAK4 depleted cells exhibited a longer lifetime than control cells (Fig. 1B and C). Thus, the invadopodia formed in PAK4 depleted cells were more persistent and failed to turnover at the expected rate (Fig. 1B and C). These data, further support a role for PAK4 activity at the later stages where disassembly is considered the final requirement during the invadopodia lifecycle.
Figure 1.
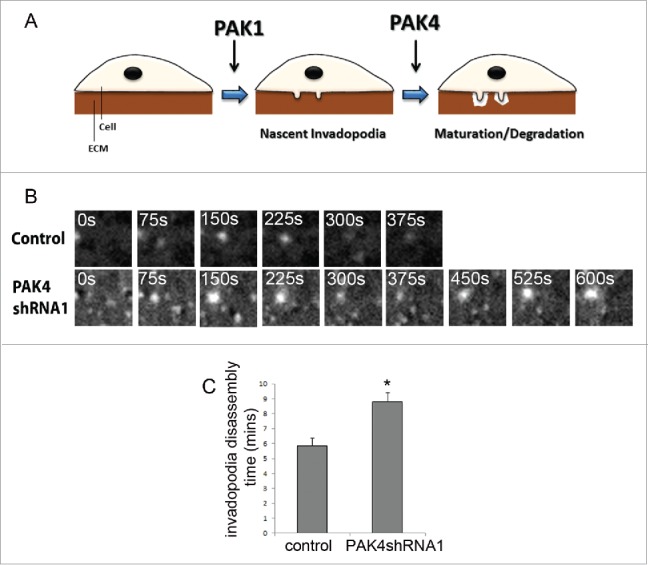
PAK4 activity is restricted to the maturation phase of invadopodia lifecycle. (A) Possible functions for PAK1 and PAK4 in the invadopodia lifecycle. PAK1 plays a role in the formation of nascent invadopodia. PAK4 functions in the maturation/degradation stage of the invadopodia lifecyle. (B) and (C) Lifetime imaging of A-375M2 cells with depleted PAK4 expression. Lifeact-mRFP transfected cells were plated on gelatin and images were taken at 15 second intervals over 15 mins. Representative images are shown in 75 second intervals for each condition. Significance was calculated to wildtype cells. Data are mean values ± SEM of 15 cells, over 3 independent experiments; * = P < 0.05.
These observations coupled with our finding that both PAK1 and PAK4 kinase activity is required in the invadopodia, led us to explore the possibility that PAK1 and PAK4 may have different substrates in the invadopodia. While PAK1 phosphorylation of cortactin is established during formation,8 PAK4 activity had not been previously reported in invadopodia. We have now identified PDZ-RhoGEF as a PAK4 binding protein, already known to be phosphorylated and inactivated by PAK4,17 with functional significance in invadopodia dynamics. The novel function of this PAK4/PDZ-RhoGEF pathway in invadopodia dynamics was confirmed by the use of a dominant negative mutant of PDZ-RhoGEF which could bind, but not activate Rho.18,19 The expression of this mutant in cells with depleted PAK4 protein resulted in the rescue of invadopodia formation and degradation back to levels seen in wildtype cells. Furthermore, the expression of wildtype PDZ-RhoGEF mimicked a PAK4 depletion phenotype, reducing both the percentage of cells with invadopodia and the invadopodia induced matrix degradation. PDZ-RhoGEF, along with PAK4 was found to be localized to invadopodia supporting the suggestion that the proposed signaling pathway occurs within these protrusions. This localization also suggests that RhoA activation is likely required at some stage during the lifecycle of the protrusion (low levels of Rho activity in invadopodia can be easily achieved by the localization of PDZ-RhoGEF away from the protrusion). Our hypothesis is that PAK4 phosphorylates and inactivates PDZ-RhoGEF which in turn suppresses RhoA activity and allows the invadopodia to mature. PDZ-RhoGEF is known to be specific for Rho, with no binding to Cdc42 or Rac1. Moreover, this GEF preferentially activates RhoA, over RhoB and RhoC.20 Investigations into the function of RhoA in invadopodia have yielded contradictory results. Some studies have suggested that reduced RhoA expression decreases invadopodia formation and degradation,21 while others have found no effect on invadopodia degradation when RhoA activation is inhibited or activated.22 However, recent studies suppressing RhoA activity promoted invadopodia formation23 and work with podosomes have indicated that RhoA inactivation is required for podosome formation24 while the constitutive activation of this protein reduces podosome formation.24,25 It has been suggested that a balance of RhoA activation and inactivation is important for podosome function with both the constitutive activation and reduction of RhoA activity resulting in reduced podosome degradation.26 Our findings suggest that the same may be true for invadopodia function and provide additional evidence that the control of RhoA activity is important in invadopodia function. To date, the mechanism by which the PAK4/PDZ-RhoGEF/RhoA pathway functions in invadopodia is unknown. RhoA plays a key role in the actin cytoskeletal dynamics and actomyosin contractility which is important in the formation of protrusions such as invadopodia.27 Therefore, one potential mechanism by which the inhibition of RhoA brought about by the PAK4/PDZ-RhoGEF/RhoA pathway can function at invadopodia, is through a reduction in the contractile force exerted on the protrusion to allow for the extension of the membrane. Furthermore, with RhoA activation being important for focal adhesions (incorporation of stress fibers), which is often associated with invadopodia dissolution, RhoA inactivation at invadopodia may tip the balance away from invadopodia formation toward disassembly as RhoA activity is redirected toward the formation of focal adhesions. It will be interesting to explore further how levels of RhoA activity correlate with invadopodia dynamics.
We were able to show both in our recent melanoma work and previous work28 with prostate cancer cells that depletion of PAK4 leads to an increase in RhoA activity measured both by activity pulldown assays and FRET biosensors. There is a particular level of complexity surrounding the role of PAK1/PAK4 in regulation of RhoA activity. PAK4 is purported to contain a GEF interacting domain (GID)6 not found in PAK1, however both PAK1 and PAK4 have been reported to inhibit RhoA activator, GEF-H129-31. Phosphorylation of GEF-H1 by PAK1 or PAK4 at serine 885 initiates a 14-3-3 binding event that is thought to sequester GEF-H1 on microtubules and inhibit activation of RhoA. Indeed, our previous work demonstrated that PAK4 depletion can elevate the level of RhoA activity28 while concomitantly decreasing GEF-H1 S885 phosphorylation.32,33 In contrast, RhoA activation has not been observed in PAK1 depleted cells.34 Moreover, these PAK1:GEF-H1 studies were not conducted in melanoma cell lines and in our study we did not see modulation of GEF-H1 serine phosphorylation in PAK1 nor PAK4 depleted cells. It should be noted that PAK2 and PKA can also phosphorylate GEF-H1 at this site.35,36 In our recent study we also did not find a change in RhoA activity in PAK1 depleted cells using the FRET biosensors.
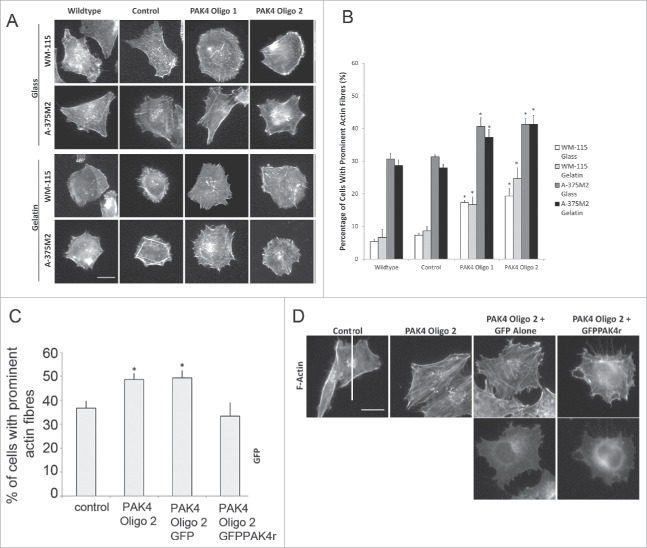
To further investigate the relationship between PAK4 and regulation of RhoA we have extended our monitoring of the prominence of actin stress fibers in our PAK4 depleted cells as a readout for RhoA activity. Control and PAK4 depleted WM-115 and A-375M2 cells were seeded onto glass or gelatin coated coverslips allowed to adhere for 3 h and then fixed and stained for F-actin. The cells were imaged (Fig. 2A) and the images quantified for the presence of prominent F-actin stress fibers. Interestingly, we found that on both glass and gelatin PAK4 depleted cells display an increase in prominent actin stress fibers (Fig. 2B). To confirm that this increase in actin stress fibers is specific to loss of PAK4 expression we also expressed GFP-alone or GFP-PAK4r (resistant to the PAK4 specific siRNA sequence) in PAK4siRNA treated cells prior to plating. All cells were fixed and stained for F-actin and the presence of prominent actin stress fibers quantified. Importantly, we found that PAK4 depleted cells and PAK4 depleted cells expressing GFP-alone exhibited higher levels of actin stress fibers compared to control but that PAK4 depleted cells expressing siRNA resistant GFP-PAK4r returned to control levels (Fig. 2C and D).
Figure 2.
PAK4 specifically influences RhoA activity cells were plated on glass or gelatin matrix coated coverslips 4 days post-transfection of siRNA oligonucleotides to reduce PAK4 expression. (A) Representative images of WM-115 and A-375M2 cells on glass and gelatin for each condition. Scale bar = 10μm (B) Quantification of the percentage of cells with prominent actin fibers. Significance was calculated to wildtype and control cells transfected with non-specific siRNA. Data are mean values ± SEM of 150 cells, over 3 independent experiments; * = P < 0.05. Control = cells transfected with non-specific siRNA. (C) Cells were plated in 6 well plates and transfected with siRNA oligonucleotides to reduce PAK4 expression. Two days post-transfection the cells were transfected with GFP alone or GFPPAK4r constructs. After 48 hrs, these cells were seeded on glass coverslips and incubated overnight, fixed and stained for F-actin. Quantification of the percentage of cells with prominent actin fibers. Significance was calculated to control cells transfected with non-specific siRNA. Data are mean values ± SEM of 150 cells, over 3 independent experiments; * = P < 0.05. Control = cells transfected with non-specific siRNA. (D) Representative images of A-375M2 cells for each condition. Scale bar = 10μm.
In conclusion, using our systematic approach we have demonstrated that both PAK1 and PAK4 play an important role in melanoma cell invasion (Fig. 3) but have distinct pathways in invadopodia function; with PAK4 promoting maturation and/or degradation through the localized inhibition of PDZ-RhoGEF. Taken together our recent study16 and previous work28 strongly points to a significant regulatory role for PAK4 in the RhoA pathway via its interaction with multiple RhoA GEFs. We believe that this is a distinction between PAK1 and PAK4 that is likely to play out in multiple cells and processes.
Figure 3.

PAK1 and PAK4 differential function in invadopodia dynamics. PAK4 localizes to the invadopodia and inhibits the function of PDZ-RhoGEF. This in turn prevents the activation of RhoA to promote invadopodia maturation and turnover. This may be through the inhibition of membrane contraction. Active RhoA may function in invadopodia to retract the protrusion during disassembly. Speculative pathways are labeled in red. PAK1 does not signal through PDZ-RhoGEF in invadopodia. It may function via the phosphorylation of cortactin or another substrate.
Methods and materials
Cell culture
The melanoma cell line A-375M2 was grown in Dulbecco's modified eagle's medium: nutrient F-12 ham (DMEM F-12) (containing L-glutamine), and the WM-115 cell line was grown in minimum essential medium (MEM) (containing L-glutamine). All the growth media were supplemented with 10% foetal bovine serum (FBS), penicillin and streptomycin sulfate. A-375M2 cells were transiently transfected using Lipofectamine® 2000 transfection reagent, according to the manufacturer's instructions. Where indicated ethanol washed coverslips were coated with gelatin and fixed with glutaraldehyde.
siRNA/constructs
Oligonucleotides (Dharmacon, UK) were transiently transfected at a concentration of 25nM using HiPerFect transfection reagent (Qiagen), according to the manufacturer's instructions. Control siRNA oligonucleotide (AATTCTCCGAACGTGTCACGT) PAK4 Oligo 1 siRNA oligonucleotide (GGTGAACATGTATGAGTGT) PAK4 Oligo 2 siRNA oligonucleotide (CGAGAATGTGGTGGAGATGTA). GFP-PAK4r was constructed by site-directed mutagenesis, according to the manufacturer's instructions, using the QuikChange Multisuite II kit (Stratagene). pENTR-PAK437 was used as template DNA for site-directed mutagenesis reactions. GFP Alone vector was purchased from Clontech.
Immunofluorescence
Cells were fixed with 4% (w/v) paraformaldehyde (PFA) and permeabilised using 0.2% (v/v) triton X-100 and then washed with PBS. Non-specific binding was blocked by 3% BSA. Coverslips were incubated for 2 hours with the primary antibody and then washed with PBS. Cells were incubated for 1 hour with secondary antibody and fluorophore conjugated phalloidin (Invitrogen). Coverslips were then washed with PBS and mounted using Fluorsave™ reagent. Images were acquired using an Olympus IX71 microscope.
Invadopodia lifetime Imaging
Stable control shRNA, PAK1shRNA and PAK4shRNA A-375M2 cells were infected with lentivirus to stably express LifeAct-mRFP (kind gift from Prof. Maddy Parsons). Stable control and knockdown cells expressing LifeAct-mRFP were seeded on gelatin (not fluorophore conjugated) and treated with 25uM GM6001 (VWR) overnight to inhibit invadopodia formation. The inhibitor was removed 1h before imaging by replacing media. Images were taken at 15 second intervals over 15 mins using a Nikon A1R confocal microscope. Invadopodia assembly and disassembly rates were calculated by measuring the fluorescence intensity of the actin rich invadopodia using imageJ (Intensity vs. time plot). Data values were exported and visualised in Excel and invadopodia assembly (from lowest data point to max peak) and disassembly (max peak to the following lowest data point) were calculated.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The research was funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Center based at Guyʼs and St Thomasʼ NHS Foundation Trust and Kingʼs College London (NSN and AP) and Breast Cancer Now 2014NovPR356 (MSL). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- [1].Haga RB, Ridley AJ. Rho GTPases: Regulation and roles in cancer cell biology. Small GTPases 2016; 7:207-21; PMID:27628050;http://dx.doi.org/ 10.1080/21541248.2016.1232583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev 2009; 28:137-49; PMID:19153671; http://dx.doi.org/ 10.1007/s10555-008-9176-1 [DOI] [PubMed] [Google Scholar]
- [3].Blouw B, Seals DF, Pass I, Diaz B, Courtneidge SA. A role for the podosome/invadopodia scaffold protein Tks5 in tumor growth in vivo. Eur J Cell Biol 2008; 87:555-67; PMID:18417249;http://dx.doi.org/ 10.1016/j.ejcb.2008.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gligorijevic B, Wyckoff J, Yamaguchi H, Wang Y, Roussos ET, Condeelis J. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J Cell Sci 2012; 125:724-34; PMID:22389406; http://dx.doi.org/ 10.1242/jcs.092726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Foxall E, Pipili A, Jones GE, Wells CM. Significance of kinase activity in the dynamic invadosome. Eur J Cell Biol 2016; 95:483-92; PMID:27465307; http://dx.doi.org/ 10.1016/j.ejcb.2016.07.002 [DOI] [PubMed] [Google Scholar]
- [6].King H, Nicholas NS, Wells CM. Role of p-21-Activated Kinases in Cancer Progression. Int Rev Cell Mol Biol 2014; 309:347-387; PMID:24529727 [DOI] [PubMed] [Google Scholar]
- [7].Moshfegh Y, Bravo-Cordero JJ, Miskolci V, Condeelis J, Hodgson L. A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat Cell Biol 2014; 16:574-86; PMID:24859002; http://dx.doi.org/ 10.1038/ncb2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ayala I, Baldassarre M, Giacchetti G, Caldieri G, Tetè S, Luini A, Buccione R. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci 2008; 121:369-78; PMID:18198194; http://dx.doi.org/ 10.1242/jcs.008037 [DOI] [PubMed] [Google Scholar]
- [9].Furmaniak-Kazmierczak E, Crawley SW, Carter RL, Maurice DH, Cote GP. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res 2007; 100:1328-36; PMID:17446433; http://dx.doi.org/ 10.1161/CIRCRESAHA.106.147744 [DOI] [PubMed] [Google Scholar]
- [10].Webb BA, Eves R, Crawley SW, Zhou S, Côté GP, Mak AS. PAK1 induces podosome formation in A7r5 vascular smooth muscle cells in a PAK-interacting exchange factor-dependent manner. Am J Physiol Cell Physiol 2005; 289:C898-907; PMID:15944209; http://dx.doi.org/ 10.1152/ajpcell.00095.2005 [DOI] [PubMed] [Google Scholar]
- [11].Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, Linder S. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol 2006; 209:568-79; PMID:16897755; http://dx.doi.org/ 10.1002/jcp.20777 [DOI] [PubMed] [Google Scholar]
- [12].Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell 2008; 100:97-108; PMID:18199048; http://dx.doi.org/ 10.1042/BC20070109 [DOI] [PubMed] [Google Scholar]
- [13].Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, Dagostino E, Knighton D, Loi CM, Zager M, et al.. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A 2010; 107:9446-51; PMID:20439741; http://dx.doi.org/ 10.1073/pnas.0911863107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther 2009; 8:2559-65; PMID:19723886; http://dx.doi.org/ 10.1158/1535-7163.MCT-09-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, Cheng M, Zhao D, Li F. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 2012; 317:24-32; PMID:22085492; http://dx.doi.org/ 10.1016/j.canlet.2011.11.007 [DOI] [PubMed] [Google Scholar]
- [16].Nicholas NS, Pipili A, Lesjak MS, Ameer-Beg SM, Geh JL, Healy C, MacKenzie Ross AD, Parsons M, Nestle FO, Lacy KE, et al.. PAK4 suppresses PDZ-RhoGEF activity to drive invadopodia maturation in melanoma cells. Oncotarget 2016; 7(43):70881-97; PMID:27765920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Barac A, Basile J, Vázquez-Prado J, Gao Y, Zheng Y, Gutkind JS. Direct interaction of p21-activated kinase 4 with PDZ-RhoGEF, a G protein-linked Rho guanine exchange factor. J Biol Chem 2004; 279:6182-9; PMID:14625312; http://dx.doi.org/ 10.1074/jbc.M309579200 [DOI] [PubMed] [Google Scholar]
- [18].Driessens MH, Olivo C, Nagata K, Inagaki M, Collard JG. B plexins activate Rho through PDZ-RhoGEF. FEBS Lett 2002; 529:168-72; PMID:12372594; http://dx.doi.org/ 10.1016/S0014-5793(02)03323-9 [DOI] [PubMed] [Google Scholar]
- [19].Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, Arnheiter H. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 2004; 9:49-58; PMID:14723707; http://dx.doi.org/ 10.1111/j.1356-9597.2004.00701.x [DOI] [PubMed] [Google Scholar]
- [20].Jaiswal M, Gremer L, Dvorsky R, Haeusler LC, Cirstea IC, Uhlenbrock K, Ahmadian MR. Mechanistic insights into specificity, activity, and regulatory elements of the regulator of G-protein signaling (RGS)-containing Rho-specific guanine nucleotide exchange factors (GEFs) p115, PDZ-RhoGEF (PRG), and leukemia-associated RhoGEF (LARG). J Biol Chem 2011; 286:18202-12; PMID:21454492;http://dx.doi.org/ 10.1074/jbc.M111.226431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A novel spatiotemporal RhoC activation pathway locally regulates cofilin activity at invadopodia. Curr Biol 2011; 21:635-44; PMID:21474314; http://dx.doi.org/ 10.1016/j.cub.2011.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes Cells 2003; 8:1019-27; PMID:14750956; http://dx.doi.org/ 10.1111/j.1365-2443.2003.00695.x [DOI] [PubMed] [Google Scholar]
- [23].Sedgwick AE, Clancy JW, Olivia Balmert M, D'Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Sci Rep 2015; 5:14748; PMID:26458510; http://dx.doi.org/ 10.1038/srep14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yu CH, Rafiq NB, Krishnasamy A, Hartman KL, Jones GE, Bershadsky AD, Sheetz MP. Integrin-matrix clusters form podosome-like adhesions in the absence of traction forces. Cell Rep 2013; 5:1456-68; PMID:24290759; http://dx.doi.org/ 10.1016/j.celrep.2013.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schramp M, Ying O, Kim TY, Martin GS. ERK5 promotes Src-induced podosome formation by limiting Rho activation. J Cell Biol 2008; 181:1195-210; PMID:18573916; http://dx.doi.org/ 10.1083/jcb.200801078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berdeaux RL, Diaz B, Kim L, Martin GS. Active Rho is localized to podosomes induced by oncogenic Src and is required for their assembly and function. J Cell Biol 2004; 166:317-23; PMID:15289494; http://dx.doi.org/ 10.1083/jcb.200312168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol 2010; 189:541-56; PMID:20421424; http://dx.doi.org/ 10.1083/jcb.200909113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wells CM, Whale AD, Parsons M, Masters JR, Jones GE. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. J Cell Sci 2010; 123:1663-73; PMID:20406887; http://dx.doi.org/ 10.1242/jcs.055707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 2007; 12:699-712; PMID:17488622; http://dx.doi.org/ 10.1016/j.devcel.2007.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 2005; 118:1861-72; PMID:15827085; http://dx.doi.org/ 10.1242/jcs.02313 [DOI] [PubMed] [Google Scholar]
- [31].Tian X, Tian Y, Gawlak G, Sarich N, Wu T, Birukova AA. Control of vascular permeability by atrial natriuretic peptide via a GEF-H1-dependent mechanism. J Biol Chem 2014; 289:5168-83; PMID:24352660; http://dx.doi.org/ 10.1074/jbc.M113.493924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Meiri D, Marshall CB, Mokady D, LaRose J, Mullin M, Gingras AC, Ikura M, Rottapel R. Mechanistic insight into GPCR-mediated activation of the microtubule-associated RhoA exchange factor GEF-H1. Nature communications 2014; 5:4857; PMID:25209408; http://dx.doi.org/ 10.1038/ncomms5857 [DOI] [PubMed] [Google Scholar]
- [33].Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14-3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 2004; 279:18392-400; PMID:14970201; http://dx.doi.org/ 10.1074/jbc.M400084200 [DOI] [PubMed] [Google Scholar]
- [34].Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol 2008; 28:4162-72; PMID:18411304;http://dx.doi.org/ 10.1128/MCB.01532-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kosoff R, Chow HY, Radu M, Chernoff J. Pak2 kinase restrains mast cell FcepsilonRI receptor signaling through modulation of Rho protein guanine nucleotide exchange factor (GEF) activity. J Biol Chem 2013; 288:974-83; PMID:23204526;http://dx.doi.org/ 10.1074/jbc.M112.422295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Meiri D, Marshall CB, Greeve MA, Kim B, Balan M, Suarez F, Bakal C, Wu C, Larose J, Fine N, et al.. Mechanistic insight into the microtubule and actin cytoskeleton coupling through dynein-dependent RhoGEF inhibition. Mol Cell 2012; 45:642-55; PMID:22405273; http://dx.doi.org/ 10.1016/j.molcel.2012.01.027 [DOI] [PubMed] [Google Scholar]
- [37].hmed T, Shea K, Masters JR, Jones GE, Wells CM. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal 2008; 20:1320-8; PMID:18424072;http://dx.doi.org/ 10.1016/j.cellsig.2008.02.021 [DOI] [PubMed] [Google Scholar]