ABSTRACT
The small GTPase Ras-related C3 botulinum toxin substrate 1 (RAC1) plays a central role in skin homeostasis, including barrier function, wound healing and inflammatory responses. Psoriasis is a common skin disease characterized by deregulation of these functions, and affected skin exhibit keratinocyte hyperproliferation, inflammation and immune cell infiltration. Although psoriasis is often triggered by environmental stimulus, there is a strong genetic association with genes expressed in both immune cells and keratinocytes, of which several are linked to Rac1 signaling. Rac1 is highly active in human psoriatic lesional skin and keratinocytes, and keratinocyte-specific overexpression of an activated mutant of Rac1, Rac1V12, in a transgenic mouse model closely mimics the presentation of human psoriasis. Both Rac1 activation in keratinocytes and immune derived stimulus are required to drive psoriasiform signaling in transgenic mouse and human xenograft models of psoriasis. Therefore, understanding how increased Rac1 activation in psoriatic epidermis is regulated is central to understanding how the abnormal crosstalk between keratinocytes and immune cells is maintained.
KEYWORDS: crosstalk, epidermis-immune, keratinocyte, psoriasis, Rac1
The small GTPase Ras-related C3 botulinum toxin substrate 1 (RAC1) plays an important role in epidermal homeostasis. Rac1 regulates epithelial junctions central for skin barrier integrity, coordinated collective migration of keratinocytes1 and stem cell maintenance.2 Further, Rac1 activity determines downstream signaling of many growth factors and cytokines,3 responsiveness to environmental extracellular stimulus4 and propagating inflammatory responses.5 These intracellular signal transduction pathways include STAT3-phosphorylation, binding and nuclear translocation,6,7 as well as NFκB signaling8,9 and promotes keratinocyte immune-cell crosstalk.10 One example of increased epidermal Rac1 activity is during wound healing, where Rac1 is essential for wound re-epithelialization.3,11,12 Another is through CD44 receptor binding on epithelial cells, which is the receptor for hyaluronic acid.13 CD44 receptor mediated Rac1 activation can also be induced by molecular mimicry of the group A Streptococcus (GAS) hyaluronic acid capsular polysaccharide. This triggers cytoskeletal re-arrangements and result in increased permeability of intercellular junctions.14
A common skin disease associated with abnormal wound healing responses (known as the Koebner phenomena), GAS infection and epidermal STAT3 activation is psoriasis,15-17 affecting roughly 1–3% of the population, and genetically associated with genes expressed in both keratinocytes and immune cells.18 Psoriasis commonly presents as erythematous scaly plaques on affected skin, and is associated with systemic co-morbidities such as psoriatic arthritis in up to 30% of cases.19 Interestingly, thioguanine, a Rac1 inhibitor20 has demonstrated efficacy in psoriasis,21 and Rac1 inhibitory peptides have shown promise in reducing antibody production and paw swelling in a murine collagen-induced arthritis model of rheumatoid arthritis.22 Therefore, we elucidated whether abnormal Rac1 activity was a feature of psoriatic lesional skin.
We found a marked activation of Rac1 in psoriatic lesional epidermis compared with normal control skin in a panel of lesional psoriasis patient skin and normal control skin. Further, increased Rac1 activation was also evident in non-lesional psoriasis skin biopsies. Isolating psoriasis patient keratinocytes and stimulating them with growth factors and cytokines such as EGF, TNFα, IL17 or IL22 induced strong Rac1 activation. This suggests that most psoriatic keratinocytes analyzed exhibited a cell intrinsic tendency toward Rac1 hyperactivation in response to various external stimuli, especially to those cytokines implicated in psoriasis pathogenesis. Overexpressing a Rac1V12 mutant under a keratin 14 promoter in a transgenic mouse model recapitulated many hallmarks of human psoriasis, including psoriasiform hyperplasia, a mixed inflammatory immune cell infiltrate, joint inflammation and a mutilating arthropathy.23 However, activated Rac1 in keratinocytes induced psoriasiform hyperplasia only in the presence of an intact immune system, as crossing Rac1V12 mice to T-cell deficient NOD/SCID mice, or treating Rac1V12 mice with immunosuppressive therapy (cyclosporine) rescued the psoriasiform phenotype. This indicated that activated Rac1 in the epidermis required immune derived stimulus, and that a skin specific defect can activate and differentiate the immune system resulting in systemic manifestations such as arthritis. Conversely, to assess whether immune derived stimulus required functional Rac1 in keratinocytes for psoriasis development, we isolated keratinocytes and fibroblasts from patient or control skin, and reconstructed organotypic 3D skin equivalents in vitro. This enabled selective modulation of Rac1 in keratinocytes before composition of skin grafts. These skin equivalents were then grafted to immunodeficient NOD/SCID mice, and after healing, grafts were intradermally injected with autologous PBMCs. Patient but not control xenografts developed profound psoriasiform hyperplasia, whereas selective inhibition of Rac1 in keratinocytes (by overexpressing a dominant negative mutant Rac1N17) rescued the phenotype. Thus, in human and murine skin, epidermal Rac1 activation is necessary to drive psoriasiform hyperplasia of the epidermis, but relies on immune derived factors.
Interestingly with increased Rac1 activity and mRNA expression in Rac1V12 skin, we found reduced levels of RhoA, and to a lesser extent CDC42. This indicates a coordinated regulation of RhoGTPases in the setting of activated Rac1 (and possibly other activated GTPases) in keratinocytes. In silico transcriptome analysis indicated that this could at least in part be due to enrichment of RhoGDI signaling, which would be in agreement with previous studies on activated Rho mutants.24 This is especially interesting as RhoA has been shown to regulate epidermal differentiation,25 and we cannot exclude that the concomitant repression of RhoA could contribute to our findings as well as to the pathogenesis of psoriasis.
A hallmark of psoriatic lesional skin is perturbed skin barrier function, and it has been established that psoriatic skin exhibit increased trans-epidermal water loss, accompanied with reduced levels of proteins important for forming the cornified envelope of the epidermis. That genome wide association studies have implicated late cornified envelope proteins with psoriasis susceptibility26 indicates that perturbation of skin barrier integrity predispose to psoriasis. This is highlighted by the fact that minor skin trauma (a phenomenon called koebnerization) is well known to induce psoriasis in those susceptible. A central transcription factor for regulating formation of the cornified cell envelope and keratinocyte differentiation is ZNF750.27,28 Our results indicate that activation of Rac1 reduces ZNF750 mRNA and protein expression,23 which would in turn lead to altered differentiation. Therefore, aberrant Rac1 activation in keratinocytes may have inhibitory effects on skin barrier integrity, through interfering with a key transcription factor underlying the terminal differentiation program in keratinocytes. Environmental stimulus such as wounding lead to Rac1 activation, and production of pro-proliferatory and pro-inflammatory cytokines such as interferons, TGFα, IL36, CCL20, TNFα and others,29,23 which in turn may lead to a perturbed epidermal-immune signaling loop. This could further accentuate Rac1-stimulating factors through immune cell recruitment.
Rac1 cycles between a GDP- bound, inactivated, and GTP- bound, activated state, regulated by a complex network of Rac1-activating factors (GEFs), Rac1 inhibiting factors (GAPs) and RhoGDP inhibitors (RhoGDIs).24 Several genetic association studies of psoriasis have implicated genes described previously to interact with Rac1, such as ZNF750,30 STAT3,6,7,31 NFκB,8 ELMO1,32 NOS2,33 IRFs34 and βdefensins.35 ZNF750, STAT3, NFκB, NOS and IRFs would likely act downstream of Rac1, indicating that a feedback activation of Rac1 could be occurring.
Innate immune responses have been genetically linked with psoriasis,18 and encompass keratinocytes as well as immune cells. These signaling pathways could be important as to how genetic susceptibility of psoriasis could be associated with a predisposition to Rac1 hyperactivation in keratinocytes (and potentially other cell types). Interactions between the epidermis and the immune system are central to maintaining skin barrier homeostasis and is an essential aspect of the skins innate immunity. Responses involve sampling and sensing of antigens as well as production of antimicrobial peptides. It is tempting to speculate that Rac1 could play a role in early antigen sensing, thereby promoting immune cell recruitment. Perturbed antigen presentation has been implicated in psoriasis pathogenesis, and Rac1 has been demonstrated to play a role in antigen-presentation, at least in part through increased endocytosis.36 This is also an enriched pathway in the Rac1V12 mouse model of psoriasis.23 Other antigen sensing pathways in keratinocytes include pathogen-associated molecular patterns (PAMPs) through pttern recognition receptors (PRR); and keratinocytes may produce antimicrobial peptides such as β-defensins and cathelicidin LL-37 in response to stimuli. These interactions are an important step in innate immunity and the immune response. β-defensins are antimicrobial peptides readily produced by keratinocytes and have been found to increase Rac1 activation in skin,35 and another host defense peptide associated with psoriasis pathogenesis, cathelicidin LL-37, has also been shown to induce Rac1 activation.37 The specific mechanism whereby these peptides would activate Rac1 in keratinocytes remains unclear, but could encompass nonselective membrane receptors for cationic peptides such as the β defensins.
ELMO1 is a member of the engulfment and cell motility protein family, and is involved in innate immunity through TLR7- and TLR9-mediated IFN-α induction by plasmacytoid dendritic cells, ELMO1 is part of a Rac1-activating complex together with DOCK,38,39 and upon stimulation the ELMO-interacting region and the DOCK-homology region 2 guanine nucleotide exchange factor domain of DOCK2 interactions weakens their auto inhibition and induce Rac1 activation.39 This mechanism has been demonstrated in lymphocytes, although DOCK2 mediated signaling activating Rac1 has also been described in dendritic cells.40 As DOCK2 is mainly expressed in hematopoetic cells,40 the expression levels of this complex and relevance for keratinocyte mediated Rac1 activation remains to be elucidated. For instance, the ELMO-1 paralog ELMO-2 has been shown to involve epidermal growth factor mediated Rac1 activation in keratinocytes,41 and there DOCK proteins expressed in keratinocytes linked to Rac1 activation, such as DOCK1,42 indicating that this signaling axis could be relevant in increased Rac1 activation in psoriatic keratinocytes.
Besides aberrant activity Rac1 mRNA expression Rac1 is increased in psoriatic skin. Transcriptional regulation of Rac1 expression may therefore be involved in the deregulated Rac1-activation seen in psoriatic keratinocytes. It has been demonstrated in the nucleus accumbens the brain in a mouse model of stress and humans with depresson that there is a sustained inhibition of Rac1 expression and a repressive chromatin state surrounding the proximal promoter of Rac1. Further, class 1 histone deacetylaces (HDAC) rescued this repression as well as the phenotype in the mouse model.43 If the opposite mechanism with a activated chromatin state surrounding the promoter of Rac1 in susceptible cells is a feature of psoriatic patient cells, these cells could already be in an activated state when isolated from the skin. We cultured analyzed cells for several passages, and analyzed non-lesional skin keratinocytes to minimize confounding of previous activation states, but we do not have results definitely excluding that such a mechanism could persist. Thus, abnormal Rac1 activation could either occur through an intracellular signaling cascade, or indirectly through a feedback mechanism, possibly involving immune cell chemotaxis and cytokine production, promoting increase in Rac1-activating factors by both immune cells and keratinocytes.
It is also feasible that environmental stimulus or genetic susceptibility leads to an increased production of Rac1-activating factors by immune cells that once recruited into the skin potentiate and maintain a high level of Rac1 activation in the epidermis. Further, genetic factors promoting high Rac1 activity in keratinocytes could also predispose to activated Rac1 in other cell types. For instance, Rac1 has recently been demonstrated to drive interleukin 17 (IL17) production in T-cells44 by forming a complex with RORγt and activate the IL17 promoter. IL17 is a key cytokine in psoriasis pathogenesis, and stimulating keratinocytes with IL17 can lead to Rac1 activation in keratinocytes.23 This would manifest as high Rac1 activity in both keratinocytes and immune cells, and needs to be explored further. The fact the Rho-GTPase activating protein TAGAP involved in T-cell activation is genetically associated with psoriasis18 further implicates such a mechanism could be relevant. Altogether, understanding how increased Rac1 activation is induced and maintained in psoriatic cells will be central to understanding the mechanism of underlying disease-initiating factors (Fig. 1).
Figure 1.
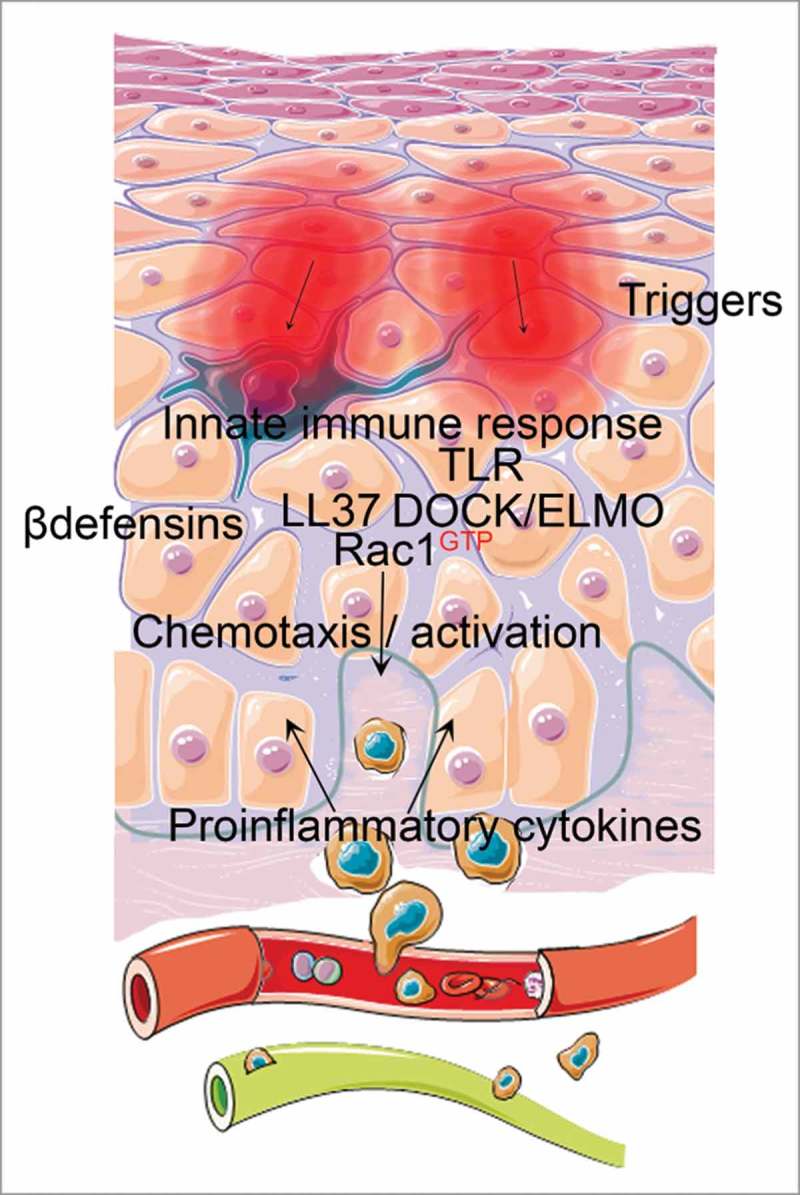
Potential upstream factors involving innate immune responses could lead to Rac1 activation in psoriatic keratinocytes and propagating a pathogenic epidermal-immune feedback loop. β-defensins, LL 37 and DOCK/ELMO signaling are examples involving innate immune signaling that both can activate Rac1 in epithelial cells and are implicated in psoriasis pathogenesis. High Rac1 activation perturbs differentiation signals central for skin barrier integrity, and leads to production of chemotactic and immunocyte-activating cytokines. Immune-derived stimulus propagates psoriasiform signaling pathways in keratinocytes involving STAT3, NFκB and interferon signaling and lead to subsequent hyperproliferation, inflammation and a perturbed skin barrier.
Several mechanisms are conceivable for the induced Rac1 activation in psoriatic lesional skin; including both increased Rac1-activating stimulus and intrinsic predisposition for Rac1 activation. This may include increased Rac1 GEF activity, diminished Rac1 GAP activity, altered binding to RhoGDI, or a spatially differential localization of Rac1 preventing its degradation or facilitating its persistent activation through differential GEF/GAP interactions. Understanding this may lead to development of targeted Rac1-inhibitory therapeutics in psoriasis.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Figures were modified from Servier Medical Art under a creative commons attribution 3.0 unported license
This work was supported by grants from the U.S. Department of Veterans Affairs Office of Research and Development; the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH grant AR47223, to M.P. Marinkovich); the National Psoriasis Foundation (to M.P. Marinkovich); the European Union's Seventh Framework Programme FP7/2007-2013 (261366, to B. Homey); and the Swedish Society of Medical Research (SSMF), the Swedish Society of Medicine (SLS), and the Fernström Founda- tion (to M.C.G. Winge).
References
- [1].Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol 2015; 17(3):276-87; PMID:25706233; http://dx.doi.org/ 10.1038/ncb3115 [DOI] [PubMed] [Google Scholar]
- [2].Benitah SA, Frye M, Glogauer M, Watt FM. Stem cell depletion through epidermal deletion of Rac1. Science 2005; 309(5736):933-5; PMID:16081735; http://dx.doi.org/ 10.1126/science.1113579 [DOI] [PubMed] [Google Scholar]
- [3].DiPersio CM. Double duty for Rac1 in epidermal wound healing. Sci STKE 2007; 2007(391):pe33; PMID:17579242; http://dx.doi.org/ 10.1126/stke.3912007pe33 [DOI] [PubMed] [Google Scholar]
- [4].Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci 2003; 116(Pt 17):3543-56; PMID:12865436; http://dx.doi.org/ 10.1242/jcs.00663 [DOI] [PubMed] [Google Scholar]
- [5].Keestra AM, Winter MG, Auburger JJ, Frässle SP, Xavier MN, Winter SE, Kim A, Poon V, Ravesloot MM, Waldenmaier JF, et al.. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 2013; 496(7444):233-7; PMID:23542589; http://dx.doi.org/ 10.1038/nature12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science 2000; 290(5489):144-7; PMID:11021801; http://dx.doi.org/ 10.1126/science.290.5489.144 [DOI] [PubMed] [Google Scholar]
- [7].Kawashima T, Bao YC, Nomura Y, Moon Y, Tonozuka Y, Minoshima Y, Hatori T, Tsuchiya A, Kiyono M, Nosaka T, et al.. Rac1 and a GTPase-activating protein, MgcRacGAP, are required for nuclear translocation of STAT transcription factors. J Cell Biol 2006; 175(6):937-46; PMID:17178910; http://dx.doi.org/ 10.1083/jcb.200604073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frost JA, Swantek JL, Stippec S, Yin MJ, Gaynor R, Cobb MH. Stimulation of NFkappa B activity by multiple signaling pathways requires PAK1. J Biol Chem 2000; 275(26):19693-9; PMID:10779525; http://dx.doi.org/ 10.1074/jbc.M909860199 [DOI] [PubMed] [Google Scholar]
- [9].Jefferies CA, O'Neill LA. Rac1 regulates interleukin 1-induced nuclear factor kappaB activation in an inhibitory protein kappaBalpha-independent manner by enhancing the ability of the p65 subunit to transactivate gene expression. J Biol Chem 2000; 275(5):3114-20; PMID:10652294; http://dx.doi.org/ 10.1074/jbc.275.5.3114 [DOI] [PubMed] [Google Scholar]
- [10].Pedersen E, Wang Z, Stanley A, Peyrollier K, Rösner LM, Werfel T, Quondamatteo F, Brakebusch C. RAC1 in keratinocytes regulates crosstalk to immune cells by Arp2/3-dependent control of STAT1. J Cell Sci 2012; 125(Pt 22):5379-90; PMID:22956547; http://dx.doi.org/ 10.1242/jcs.107011 [DOI] [PubMed] [Google Scholar]
- [11].Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, Hartwig B, Herzog V, Klein HW, Krieg T, et al.. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci 2007; 120(Pt 8):1480-90; PMID:17389689; http://dx.doi.org/ 10.1242/jcs.03426 [DOI] [PubMed] [Google Scholar]
- [12].Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. The Rac activator Tiam1 is required for (alpha)3(beta)1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol 2005; 171(5):871-81; PMID:16330714; http://dx.doi.org/ 10.1083/jcb.200509172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Oliferenko S, Kaverina I, Small JV, Huber LA. Hyaluronic acid (HA) binding to CD44 activates Rac1 and induces lamellipodia outgrowth. J Cell Biol 2000; 148(6):1159-64; PMID:10725329; http://dx.doi.org/ 10.1083/jcb.148.6.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cywes C, Wessels MR. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature 2001; 414(6864):648-52; PMID:11740562; http://dx.doi.org/ 10.1038/414648a [DOI] [PubMed] [Google Scholar]
- [15].Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol 1992; 128(1):39-42; PMID:1739285; http://dx.doi.org/ 10.1001/archderm.1992.01680110049004 [DOI] [PubMed] [Google Scholar]
- [16].Weiss G, Shemer A, Trau H. The Koebner phenomenon: review of the literature. J Eur Acad Dermatol Venereol 2002; 16(3):241-8; PMID:12195563; http://dx.doi.org/ 10.1046/j.1473-2165.2002.00406.x [DOI] [PubMed] [Google Scholar]
- [17].Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 2005; 11(1):43-9; PMID:15592573; http://dx.doi.org/ 10.1038/nm1162 [DOI] [PubMed] [Google Scholar]
- [18].Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, Ding J, Li Y, Tejasvi T, Gudjonsson JE, et al.. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat Genet 2012; 44(12):1341-8; PMID:23143594; http://dx.doi.org/ 10.1038/ng.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boehncke WH, Schon MP. Psoriasis. Lancet 2015; 386(9997):983-94; PMID:26025581; http://dx.doi.org/ 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- [20].Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, et al.. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003; 111(8):1133-45; PMID:12697733; http://dx.doi.org/ 10.1172/JCI16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mason C, Krueger GG. Thioguanine for refractory psoriasis: a 4-year experience. J Am Acad Dermatol 2001; 44(1):67-72; PMID:11148479; http://dx.doi.org/ 10.1067/mjd.2001.109296 [DOI] [PubMed] [Google Scholar]
- [22].Abreu JR, Dontje W, Krausz S, de Launay D, van Hennik PB, van Stalborch AM, Ten Klooster JP, Sanders ME, Reedquist KA, Vervoordeldonk MJ, et al.. A Rac1 inhibitory peptide suppresses antibody production and paw swelling in the murine collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther 2010; 12(1):R2; PMID:20053277; http://dx.doi.org/ 10.1186/ar2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Winge MC, Ohyama B, Dey CN, Boxer LM, Li W, Ehsani-Chimeh N, Truong AK, Wu D, Armstrong AW, Makino T, et al.. RAC1 activation drives pathologic interactions between the epidermis and immune cells. J Clin Invest 2016; 126(7):2661-77; PMID:27294528; http://dx.doi.org/ 10.1172/JCI85738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol 2011; 12(8):493-504; PMID:21779026; http://dx.doi.org/ 10.1038/nrm3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dubash AD, Koetsier JL, Amargo EV, Najor NA, Harmon RM, Green KJ. The GEF Bcr activates RhoA/MAL signaling to promote keratinocyte differentiation via desmoglein-1. J Cell Biol 2013; 202(4):653-66; PMID:23940119; http://dx.doi.org/ 10.1083/jcb.201304133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].de Cid R, Riveira-Munoz E, Zeeuwen PL, Robarge J, Liao W, Dannhauser EN, Giardina E, Stuart PE, Nair R, Helms C, et al.. Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat Genet 2009; 41(2):211-5; PMID:19169253; http://dx.doi.org/ 10.1038/ng.313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boxer LD, Barajas B, Tao S, Zhang J, Khavari PA. ZNF750 interacts with KLF4 and RCOR1, KDM1A, and CTBP1/2 chromatin regulators to repress epidermal progenitor genes and induce differentiation genes. Genes Dev 2014; 28(18):2013-26; PMID:25228645; http://dx.doi.org/ 10.1101/gad.246579.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, Johnston D, Siprashvili Z, Khavari PA. ZNF750 Is a p63 Target Gene that Induces KLF4 to Drive Terminal Epidermal Differentiation. Dev Cell 2012; 22(3):669-77; PMID:22364861; http://dx.doi.org/ 10.1016/j.devcel.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garber K. Genetics: Deep exploration. Nature 2012; 492(7429):S56-7; PMID:23254973; http://dx.doi.org/ 10.1038/492S56a [DOI] [PubMed] [Google Scholar]
- [30].Birnbaum RY, Zvulunov A, Hallel-Halevy D, Cagnano E, Finer G, Ofir R, Geiger D, Silberstein E, Feferman Y, Birk OS. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nat Genet 2006; 38(7):749-51; PMID:16751772; http://dx.doi.org/ 10.1038/ng1813 [DOI] [PubMed] [Google Scholar]
- [31].Pelletier S, Duhamel F, Coulombe P, Popoff MR, Meloche S. Rho family GTPases are required for activation of Jak/STAT signaling by G protein-coupled receptors. Mol Cell Biol 2003; 23(4):1316-33; PMID:12556491; http://dx.doi.org/ 10.1128/MCB.23.4.1316-1333.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Li H, Yang L, Fu H, Yan J, Wang Y, Guo H, Hao X, Xu X, Jin T, Zhang N. Association between Galphai2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat Commun 2013; 4:1706; PMID:23591873; http://dx.doi.org/ 10.1038/ncomms2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE, Li Y, Weidinger S, Eberlein B, Gieger C, et al.. Genome-wide association analysis identifies 3 psoriasis susceptibility loci. Nat Genet 2010; 42(11):1000-4; PMID:20953189; http://dx.doi.org/ 10.1038/ng.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ehrhardt C, Kardinal C, Wurzer WJ, Wolff T, von Eichel-Streiber C, Pleschka S, Planz O, Ludwig S. Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett 2004; 567(2–3):230-8; PMID:15178328; http://dx.doi.org/ 10.1016/j.febslet.2004.04.069 [DOI] [PubMed] [Google Scholar]
- [35].Kiatsurayanon C, Niyonsaba F, Smithrithee R, Akiyama T, Ushio H, Hara M, Okumura K, Ikeda S, Ogawa H. Host defense (Antimicrobial) peptide, human beta-defensin-3, improves the function of the epithelial tight-junction barrier in human keratinocytes. J Invest Dermatol 2014; 134(8):2163-73; PMID:24633129; http://dx.doi.org/ 10.1038/jid.2014.143 [DOI] [PubMed] [Google Scholar]
- [36].Shurin GV, Tourkova IL, Chatta GS, Schmidt G, Wei S, Djeu JY, Shurin MR. Small rho GTPases regulate antigen presentation in dendritic cells. J Immunol 2005; 174(6):3394-400; PMID:15749872; http://dx.doi.org/ 10.4049/jimmunol.174.6.3394 [DOI] [PubMed] [Google Scholar]
- [37].Akiyama T, Niyonsaba F, Kiatsurayanon C, Nguyen TT, Ushio H, Fujimura T, Ueno T, Okumura K, Ogawa H, Ikeda S. The human cathelicidin LL-37 host defense peptide upregulates tight junction-related proteins and increases human epidermal keratinocyte barrier function. J Innate Immun 2014; 6(6):739-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Y, Xu X1, Pan M, Jin T. ELMO1 Directly Interacts with Gbetagamma Subunit to Transduce GPCR Signaling to Rac1 Activation in Chemotaxis. J Cancer 2016; 7(8):973-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hanawa-Suetsugu K, Kukimoto-Niino M, Mishima-Tsumagari C, Akasaka R, Ohsawa N, Sekine S, Ito T, Tochio N, Koshiba S, Kigawa T, et al.. Structural basis for mutual relief of the Rac guanine nucleotide exchange factor DOCK2 and its partner ELMO1 from their autoinhibited forms. Proc Natl Acad Sci U S A 2012; 109(9):3305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gotoh K, Tanaka Y, Nishikimi A, Nakamura R, Yamada H, Maeda N, Ishikawa T, Hoshino K, Uruno T, Cao Q, et al.. Selective control of type I IFN induction by the Rac activator DOCK2 during TLR-mediated plasmacytoid dendritic cell activation. J Exp Med 2010; 207(4):721-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ho E, Dagnino L. Epidermal growth factor induction of front-rear polarity and migration in keratinocytes is mediated by integrin-linked kinase and ELMO2. Mol Biol Cell 2012; 23(3):492-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Erasmus JC, Welsh NJ, Braga VM. Cooperation of distinct Rac-dependent pathways to stabilise E-cadherin adhesion. Cell Signal 2015; 27(9):1905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, Cahill ME, Dias C, Ribeiro E, Ables JL, et al.. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med 2013; 19(3):337-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kurdi AT, Bassil R, Olah M, Wu C, Xiao S, Taga M, Frangieh M, Buttrick T, Orent W, Bradshaw EM. Tiam1/Rac1 complex controls Il17a transcription and autoimmunity. Nat Commun 2016; 7:13048.. [DOI] [PMC free article] [PubMed] [Google Scholar]


