ABSTRACT
Small GTPases play crucial roles in the maintenance of a homeostatic environment and appropriate movements of the cell. In these processes, the direct or indirect interaction between distinct small GTPases could be required for regulating mutual signaling pathways. In our recent study, ARHGEF10, known as a guanine nucleotide exchange factor (GEF) for RhoA, was indicated to interact with Rab6A and Rab8A, which are known to function in the exocytotic pathway, and colocalized with these Rabs at exocytotic vesicles. Moreover, it was suggested that ARHGEF10 is involved in the regulation of Rab6A and Rab8A localization and invasion of breast carcinoma cells, in which Rab8 also acts via regulation of membrane trafficking. These results may reveal the existence of a novel small GTPase cascade which connects the signaling of these Rabs with RhoA during membrane trafficking. In this mini-review, we consider the possible functions of ARHGEF10 and RhoA in the Rab6- and Rab8-mediated membrane trafficking pathway.
KEYWORDS: ARHGEF10, membrane trafficking, RhoGEF, RabGTPase
Introduction
Cells alter their morphology and polarity to adapt to a changing environment. Organization of the cytoskeleton and membrane trafficking plays important roles in this adaptation. These processes are mainly regulated by the small GTPases of Rab and Rho. The small GTPases act as molecular switches by cycling between active GTP-bound and inactive GDP-bound forms, and thus, they have the ability to control various effectors. The activity of these small GTPases is modulated by guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and GDP dissociation inhibitors (GDIs). GEFs catalyze the exchange of GDP for GTP to activate small GTPases; GAPs enhance the slow intrinsic GTPase activity of small GTPases, and GDIs prevent the dissociation of GDP from small GTPases.1 Evidence has accumulated recently to indicate a relationship between Rho-family proteins and the membrane trafficking pathway.1,2
The Rho family of small GTPases consists of 22 members in human cells and acts as a master regulator of the cytoskeleton, which mediates various cellular processes involved in migration, cytokinesis, and differentiation.2 Among these proteins, RhoA, Cdc42, and Rac1 are well-known inducers of stress fiber and focal adhesions (FAs), filopodia, and lamellipodia, respectively, in fibroblasts.3,4,5 Activated Rho family members interact with various effector proteins to regulate the cytoskeletal state. For example, Rho kinases (ROCKs), which are known as a serine/threonine kinase and consist of ROCK1 and ROCK2, act as downstream of the RhoA subfamily and regulate actomyosin contractility by controlling the phosphorylation state of myosin light chains (MLC).6,7,8 ROCKs phosphorylate various substrate, such as MLC itself, the MLC phosphatase and LIM kinase. Phosphorylation of MLC phosphatase downregulate its phosphatase activity and result in the activation of MLC. Phosphorylated LIM kinase phosphorylates and negatively regulate cofilin, which is known to disassemble actin filaments.9 Thus, Rho-ROCKs signals play roles in regulation of actomyosin contractility.
The GEFs for the Rho family comprise 69 members of the Dbl family, which have a Dbl homology (DH) domain as a catalytic domain, and 11 members of the dedicator of cytokinesis (DOCK) family, which have a DOCK Homology Region 2 (DHR2) domain as a catalytic domain.10 Each GEF is locally activated in response to various cellular signals to induce localized morphological changes. For example, βPIX (also known as Cool-1 and ARHGEF7), which is known to belong to the Dbl family and to activate Rac1 and Cdc42 and function in cell migration, localizes to adhesions near the leading edge through an interaction with paxillin–GIT–PAK complexes in migrating cells.10,11 It was reported that overexpression of the GIT1 (G protein-coupled receptor kinase-interacting protein 1) failed paxillin, a component of focal adhesion complexes, to localize to FA and stimulates cell motility, implicating GIT1 in FA disassembly.12 It is known that PAKs (p21-activated kinases), which contain 6 members, are downstream effectors of Rac1 and Cdc42 and phosphorylate various protein, containing paxillin.13 Phosphorylation of paxillin enhances the recruitment of the GIT1–βPIX–PAKs module to the adhesion site, and GIT1 stimulate FA disassembly and cell motility.13,14 Furthermore this module is thought to activate Rac1 at a microdomain in the leading edge.12,13 Thus, this positive-feedback loop facilitates cell migration.10,14 Moreover, the GEF for RhoA reportedly plays a role in the membrane trafficking pathway.15-17 The exocyst complex is known to be involved in the regulation of cell polarity formation by controlling the tethering of exocytotic vesicles to the plasma membrane.15,16 Pathak et al. indicated that GEF-H1, which is a member of the Dbl family of proteins, activates RhoA in response to microtubule depolymerization and is associated with various cellular functions involving migration and cytokinesis, was implicated in the regulation of exocyst assembly/localization and exocytosis through the activation of RhoA.17-19
The Rab family of small GTPases, which comprises more than 60 members, includes well-known regulators of membrane trafficking pathways.20 Each vesicle originating from the plasma membrane or various organelles must be transported along the cytoskeleton and tethered to and fused with the target membrane. Rabs specifically regulate their effectors, including sorting adaptors, tethering factors, motors, kinases, phosphatases, and modulators of small GTPases, at the donor or acceptor domain to function appropriately. For example, the Rab27 localize to melanosome and recruit melanophilin, which binds to actin-based vesicle motors of myosin Va, in a GTP-dependent manner; these melanosomes are then shuttled to the cell periphery by myosin Va.21,22 Furthermore, several studies have indicated that distinct Rabs act in coordination in the same pathway by localizing to the same or different organelles, which are occupied by different Rabs. This mechanism enables individual Rabs to function distinctively by interacting with other Rabs. An example is the Rab11–Rabin8–Rab8 cascade, wherein Rabin8, known as the GEF for Rab8, interacts with activated Rab11 to form a complex that allows activation of Rab8 at recycling endosomes. The activation of both Rab8 and Rab11 thereby facilitates an interaction between their carrier and the exocyst complex.16 Furthermore, recent findings suggest that interplay between Rabs and small GTPases other than those of the Rab family mutually influence their activity.23,24
Recently, we identified ARHGEF10 as the multiple Rab-interacting GEF for RhoA.25 ARHGEF10 belongs to the Dbl family and functions as the GEF for the RhoA subfamily, consisting of RhoA, RhoB, and RhoC.26,27 Moreover, ARHGEF10 had no distinct domain structure except for DH domain and a putative PDZ-binding motif at the C terminus (Fig. 1).28 Although ARHGEF10 is proposed to be associated with various diseases, including cancer, Charcot-Marie-Tooth disease, atherothrombotic stroke, and schizophrenia,29-33 its function has not yet been elucidated. ARHGEF10 was initially identified as a GEF with a mutation in which threonine was replaced with isoleucine at codon 332 (T332I) by analyzing a family with autosomal dominant segregation of slowed nerve conduction velocities (NCV) and thin peripheral nerve myelination without the clinical phenotype (Fig. 1).34 To elucidate the cause of this phenotype, Chaya et al. performed functional analysis of the T332I and various deletion mutants of ARHGEF10 and showed that ARHGEF10 has a negative regulatory region and an essential region for GEF activity in its N-terminus containing 211–332 amino acids and C-terminus containing 1304–1342 amino acids (Fig. 1) for its GEF activity, respectively and that T332I mutant had constitutively activated GEF function and that the overexpression of this mutant but not the wild-type ARHGEF10 in HeLa or HEK293T cells induced cell contraction that was inhibited by the ROCK inhibitor Y-27632.27 Similar morphological changes were observed in the Schwann cells that overexpressed the T332I mutant.27 These results suggest that the T332I mutation-associated phenotype observed in the peripheral nerves could be due to its high GEF activity.
Figure 1.
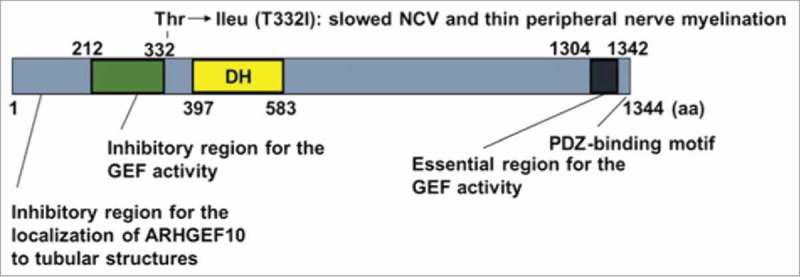
Schematic representation of ARHGEF10. The region 1–211 amino acids (aa) inhibited ARHGEF10 to localize to the Rab8-positive tubular structure. The region 212–332 aa negatively regulated the GEF activity of ARHGEF10. C-terminous 1304–1342 aa, which does not include the PDZ-binding motif (1343–1345 aa), is an essential region for the GEF activity of ARHGEF10.
To investigate the fundamental function of ARHGEF10, we performed cytochemical and functional analyses using a monoclonal antibody against ARHGEF10 as well as overexpression and knockdown techniques.25 In this study, immunofluorescence analysis using antiARHGEF10 revealed cytoplasmic vesicle-like puncta in HeLa cells. ARHGEF10 localization is affected by microtubule orientation. Since many secretory and endocytic vesicles are known to move along microtubules, we examined the colocalization of ARHGEF10 with various Rab proteins, including Rab5A, Rab6A, Rab7, Rab8A, Rab11A, and Rab27A. Among these Rabs, Rab6A and Rab8A were found to colocalize and interact with ARHGEF10 on vesicle-like puncta. Previous findings have indicated that Rab6- and Rab8-double-positive vesicles are the exocytotic vesicles containing the membrane or secreted proteins that are transported from the Golgi apparatus to the plasma membrane.35,36 Colocalization of ARHGEF10 with the exocytotic vesicle marker protein neuropeptide Y was confirmed. Further experiments suggested the involvement of ARHGEF10 in Rab6- and Rab8 localization and Rab8-mediated invasion. These results possibly show the existence of a novel small GTPase cascade, which connects the signaling of these Rabs with RhoA during membrane trafficking. In this mini-review, we discuss the possible function of ARHGEF10 and RhoA in membrane trafficking.
The localization of ARHGEF10 is altered by depolymerization of microtubules
Although ARHGEF10 is distributed as cytoplasmic vesicle-like puncta at the cell periphery, the disruption of microtubules induced the accumulation of ARHGEF10 near the centrosome in HeLa cells. This implies that ARHGEF10 localization is affected by microtubule orientation and that ARHGEF10-positive vesicles are delivered along the microtubules to the cell periphery. Furthermore, it was revealed that ARHGEF10 colocalized with Rab6A on the exocytotic vesicles. Knockdown of Rab6A or overexpression of dominant-negative Rab6A impaired ARHGEF10 localization to exocytotic vesicles. These data suggest that ARHGEF10 localizes to exocytotic vesicles in a Rab6A-dependent manner and that ARHGEF10-positive vesicles may be transported to the cell periphery together with Rab6A. It was demonstrated that Rab6A localizes to exocytotic vesicles and controls the transport of these vesicles from the Golgi apparatus to the plasma membrane via interaction with a microtubule motor, the heavy chain of kinesin-1 (KIF5B).35,37 Another study identified KIF1C as a Rab6A-interacting microtubule motor.38 Depletion of KIF1C slowed protein delivery to the cell surface and interfered with vesicle motility. These motor proteins could contribute to the localization of ARHGEF10-positive vesicles to the cell periphery. Conversely, Aoki et al. found that ARHGEF10 binds to KIF3B.39 KIF3B is known to be a component of kinesin-2 and to be associated with various cell functions, including axonal and cytoplasmic organelle transport and cilia biogenesis.40 Immunocytochemical studies show that KIF3B is localized to the centrosome and cilia.39-41 This microtubule motor could serve to deliver ARHGEF10-positive vesicles to the cell periphery or restricted sites in which KIF3B functions.
Functions of RhoA in membrane trafficking
ARHGEF10 colocalizes with Rab6 and Rab8 at exocytotic vesicles emanating from the Golgi apparatus.25 In this trafficking pathway, these vesicles containing newly synthesized secretory or membrane proteins must be budded and fissioned from the Golgi apparatus, transported along the cytoskeletal track, and tethered to and fused with the plasma membrane. It has been reported that Rab6 is involved in the fission and transport of these vesicles and that Rab8A localizes to these vesicles in a Rab6A-dependent manner to modulate the tethering and fusion of these vesicles with plasma membrane.35-37 In addition to localizing with these vesicles, Rab6A localizes to the Golgi apparatus and Rab8A does to the cytoplasmic tubular structures, while ARHGEF10 was only detected at exocytotic vesicles. Furthermore, the interaction of ARHGEF10 with Rab6A or Rab8A was demonstrated using immunoprecipitation assays. Conversely, overexpression of the ARHGEF10-N-terminal-deletion mutant (211∼), which lacks amino acids 1–210 (Fig. 1), altered the localization of both Rab6A and Rab8A while depletion of ARHGE10 partially inhibited the localization of Rab8A to exocytotic vesicles. These results suggest that ARHGEF10 acts as a regulator of Rab6A or Rab8A localization in the exocytotic pathway. If this is the case, how does the RhoA subfamily, downstream of ARHGEF10, play a role in this pathway?
Rab8A localizes to the tubular structure, which has been shown to originate from macropinosomes at ruffling surface membrane domains, and recycles the membrane back to the plasma membrane.42 Rab8-positive tubular structures are increased by treatment with cytochalasin D, known as an actin depolymerization drug. Huttula et al. indicated that overexpression of RhoA-G14V, the dominant active form of RhoA, altered the Rab8-positive tubular structures to the vesicular structures in HeLa cells.42 These results suggest that the control of actin filaments and RhoA-activity is implicated in the biogenesis of Rab8A-positive tubular structures (j RhoA in Fig. 2).
Figure 2.
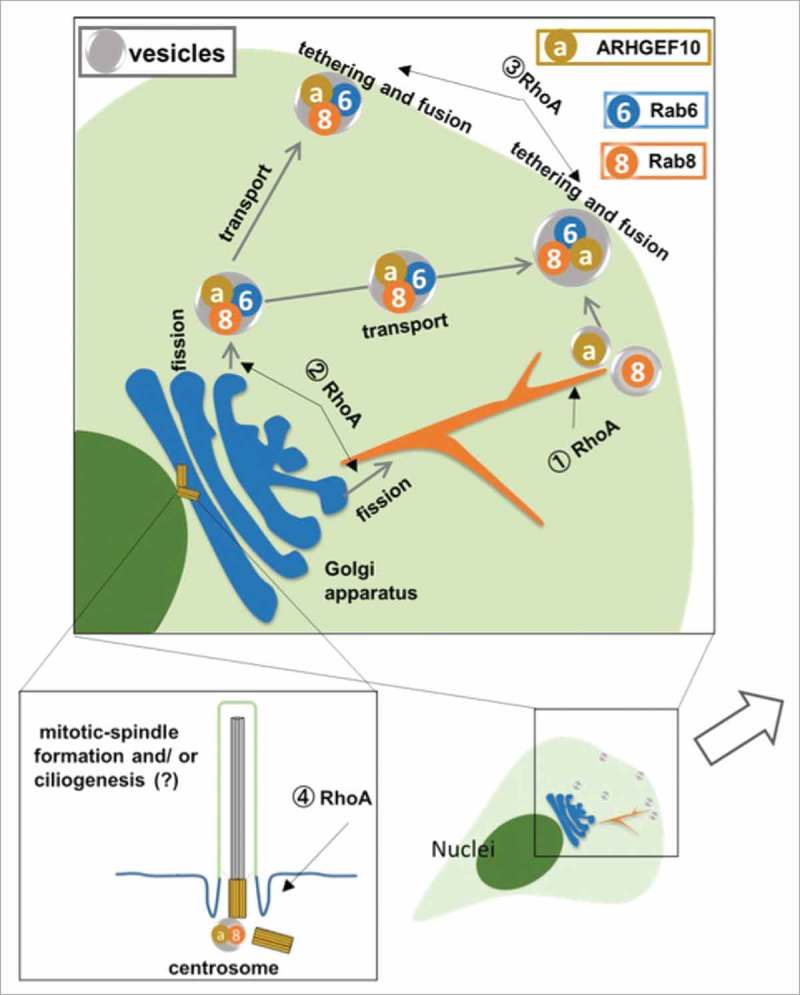
Schematic representation of exocytotic pathway, which is modulated by Rab6 and Rab8. ARHGEF10 localized to Rab6- and Rab8-positive exocytotic vesicles, but not Rab6-positive Golgi apparatus and Rab8-positive tubular structure. It is possible that RhoA might contribute to the biogenesis of Rab8-positive tubular structure (j RhoA), the fission of exocytotic vesicles from Golgi apparatus (k RhoA), tethering of exocytotic vesicles to plasma membrane (l RhoA), mitotic-spindle formation (m RhoA) and ciliogenesis (m RhoA). Rab6-positive: blue, Rab8-positive: orange, ARHGEF10-positive: brown.
Several reports have proposed that the contraction of actomyosin and the polymerization of actin filaments, known to be regulated by RhoA signals, play roles in the fission of exocytotic vesicles from the Golgi apparatus.37,43 Miserey-Lenkei et al. demonstrated that Rab6 bound to myosin II and that the cells treated with Y-27632 (a ROCK inhibitor) and ML-7 (a myosin light chain kinase inhibitor) exhibited the formation of tubular structures, which extended from the Golgi apparatus to the cytoplasm and contained secretory proteins, due to the failure of scission of exocytotic vesicles.37 Furthermore, it was reported that knockdown of mammalian Diaphanous-related formin 1 (mDia1), which acts as a direct target for RhoA44 and activates the polymerization of actin filaments,45 resulted in an increase in Rab6-positive tubular structures.46 Serine/threonine kinase LIM-kinase1, another RhoA downstream protein, is also proposed to be involved in the formation of vesicles originating from the Golgi apparatus destined for the apical plasma membrane of epithelial cells.47 It is known that LIM-kinase1 activated by RhoA suppresses the activity of cofilin, which acts as an actin-depolymerizing protein.9 The overexpression of kinase-dead LIM-kinase1, of LIM-kinase1 small interfering RNA, or of an activated cofilin mutant interfered with exit from the Golgi apparatus of these vesicles.47 From these data, it appears that RhoA could function to bring about fission of exocytotic vesicles from the Golgi apparatus (k RhoA in Fig. 2).
Several studies also support a role for RhoA in the tethering and fusion between exocytotic vesicles and the plasma membrane. In yeast, exocyst, an evolutionarily conserved octameric protein complex, is shown to be involved in tethering and fusion processes. In these processes, Rho is crucial in regulating the formation and localization of the exocyst.15 A similar mechanism is conserved in mammals. In invading breast carcinoma MDA-MB231 cells, it was reported that IQGAP, which functions as a scaffold protein in the formation of cell polarity through regulation of the tethering point of exocytotic vesicles, colocalized and interacted with Sec 3 and Sec 8, which are components of the exocyst, in the invadopodia. In this pathway, activation of RhoA is required to stimulate this interaction.48 These results indicate a role for RhoA in the regulation of exocytotic vesicles tethering at the restricted or overall plasma membrane (l RhoA in Fig. 2). Therefore, ARHGEF10 functioning in relation to these processes may be mediated via activation of RhoA.
Conversely, inhibition of Rab8A localization to the exocytotic vesicles observed in the ARHGEF10-depleted cells was rescued by the expression of the ARHGEF10 mutant, which has suppressed GEF activity. This result indicates that the GEF activity of ARHGEF10 is not essential for localization of Rab8A to the exocytotic vesicles. It is known that 2 distinct Rabs simultaneously interact with the same effector protein, thereby enabling association between 2 membrane compartments. One example is rabenosyn 5, which has separate binding sites for Rab4 and Rab5.49 Overexpression of rabenosyn 5 promotes the generation of Rab4- and Rab5-overlaped membrane domains. Thus, it has been suggested that rabenosyn 5 connects a Rab4-positive compartment with a Rab5-positive compartment. In our study, some ARHGEF10-positive vesicles were observed at or near the Rab8A-positive tubular structures. Moreover, the deletion mutant of ARHGEF10 (211∼) inappropriately localizes to the Rab8A-positive tubular structures. These results suggest that the region containing 1–211 amino acids inhibits ARHGEF10 to localize to Rab8A-positive tubular structures (Fig. 1) and full-length ARHGEF10 localizes temporally and spatially to this tubular structure through certain modifications or interactions with unknown factors in response to a transient stimulation. Conversely, overexpression of 211∼ led to the generation of large vesicle-like structures containing both Rab6 and Rab8. The large vesicle-like structures generated by the overexpression of 211∼ could result from fusion of Rab6A-positive vesicles with Rab8A-positive tubular structures. Unfortunately, we could not find the characteristical domain structure in the amino acids 1–210. From these results, it appears that ARHGEF10 could fuse Rab6A-positive vesicles with the Rab8A-positive membrane, as exemplified by the relationship between rabenosyn 5 and Rab4 and Rab5.
The function of ARHGEF10 in cell invasion
Various Rabs and Rho family proteins have been implicated in the migration and invasion of cells.10 In migrating or invading cells, these small GTPases contribute to the establishment of cell polarity by regulating membrane trafficking and orientation of the cytoskeleton. It has been proposed that Rab8 plays a crucial role in the invasion of MDA-MB231 cells via polarized exocytosis.50 In this study, Rab8 was detected at the vesicles that originate from the Golgi apparatus and contain membrane type 1-matrix metalloproteinase (MT1-MMP), known as an essential protease in matrix degradation and cell invasion. These vesicles are focally delivered to the invasive structures in the invading cells. Depletion of Rab8 reduced invasive activity due to the failure of polarized exocytosis of MT1-MMP. It is interesting to note that the exocyst complexes were implicated in the tethering of the vesicles containing MT1-MMP to the invadopodia through an interaction with IQGAP and that this interaction was enhanced by the activation of RhoA.48 Since a similar reduction in invasive activity was observed by knockdown of ARHGEF10 in MDA-MB231 cells, it might appear that ARHGEF10 functions to effect polarized exocytosis through an interaction with Rab8.
When vascular endothelial cells are artificially subjected to uniaxial cyclic stretch, they become elongated and align perpendicular to the stretch axis. Moreover, as perpendicular alignment of the cells proceeds, actin stress fibers themselves align perpendicularly to the stretch axis. Since these changes to the cell morphology and cytoskeletal alignment occur as a result of sensing mechanical force through the cell–substrate and cell–cell adhesion sites, integrin and cadherin play important roles in these process.51,52 Abiko et al. indicated that reorientation of the cell and stress fibers induced by cyclic-stretch stress were suppressed by knockdown of ARHGEF10 in human umbilical vein endothelial cells (HUVECs).53 This result supports the idea that ARHGEF10 could act to modulate reorientation of the cell and stress fibers. However, several studies have also proposed the involvement of Rab8 in the regulation of cell morphology.42,54 A recent report indicated that the overexpression of dominant active Rab8 suppressed the activity of RhoA, resulting in the loss of the stress fibers.55 These results suggest that Rab8 also modulates the formation of stress fibers through regulation of RhoA activity. Thus, ARHGEF10 and Rab8 could contribute to the change in cell morphology, which is involved in the establishment of cell polarity, through regulation of stress fiber reorientation. It is noteworthy that overexpression of T332I mutants induced cell contraction, which could also be attributed to the force generated by formation of stress fibers.27 Two recent studies support a correlation between the functions of Rab6 and Rab8 and polarized cell migration. Directional migration of the cells requires the formation of front–rear cell polarity, such as the leading edge and tail. In the leading edge of migrating cells, endocytosed or newly synthesized integrins have to be delivered to the leading edge to generate a new focal adhesion, and the mature adhesion should be turned over. Hence, coordinated focal adhesion dynamics facilitate polarized cell migration. It has been reported that Rab8 is involved in the turnover of focal adhesion via the polarized transport of these exocytotic vesicles containing MT1-MMP near the focal adhesion.55 Rab6 was also suggested to play a role in the formation of cell polarity through regulation of polarized β1 integrin distribution.56 Based on these data, ARHGEF10 possibly functions to establish cell polarity in invading or migrating cells through association with these small GTPases.
The function of centrosomal ARHGEF10
Aoki et al. showed that ARHGEF10 and RhoA localized to the centrosome, and knockdown of these proteins or treatment with ROCK inhibitor resulted in multipolar-spindle formation. These results showed the involvement of ARHGEF10 in the regulation of mitotic-spindle formation in HeLa cells (m RhoA in Fig. 2).39 Our study could not confirm the centrosomal localization of ARHGEF10 in HeLa cells at the endogenous or exogenous level. However, this discrepancy could be attributable to differences in antibodies or cell culture conditions (for example, the serum used in the culture medium). Primary cilia project from proximal mother centrioles, known as basal bodies, and play roles in sensing extracellular signals.40,57 It was reported that Rab8 localized to the basal body to control the trafficking of vesicles in primary ciliogenesis.58-60 In addition to the regulation of mitotic-spindle formation, the localization of ARHGEF10 to the centrosome could also play a role in ciliogenesis. Several studies indicated the linkage between modulation of Rho GTPase activity and the actin cytoskeleton and ciliogenesis.61-67 The inhibition of either Rho kinase (ROCK) or F-actin polymerization promoted ciliogenesis in retinal pigmented epithelial (RPE1) cells.66 Moreover, loss of p190A Rho GAP, which have GAP activity for RhoA and Rac1, induced the ciliogenesis defects and this defect was rescued by the inhibition of either Rho kinase (ROCK) or F-actin polymerization.67 These results suggested that RhoA might negatively regulate the ciliogenesis. On the other hand, it was reported that docking of basal bodies at the apical membrane were dependent on RhoA activation in the mouse primary culture airway epithelial cells.61 Thus, ARHGEF10 localizing to the centrosome might function in the ciliogenesis by modulating RhoA activity. Aoki et al. identified KIF3B as an ARHGEF10-binding partner.39 It was noteworthy that this motor protein was proposed to be required for ciliogenesis.57
Conclusion
Although our study revealed that ARHGEF10 localized to Rab6- and Rab8 positive exocytotic vesicles, interacted with these Rabs, and was involved in cell invasion, its fundamental role in cell functions, such as invasion, migration, and cytokinesis, as well as in its contribution to Rabs signaling has not yet been completely elucidated. Taking into consideration the aforementioned example, ARHGEF10 could act to provide a connection between Rabs signaling and RhoA signaling to mediate diverse biologic processes. Further studies should unravel the precise and basal functions of ARHGEF10 in the Rab- and Rho- associated biologic systems.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgment
We are very grateful to our staffs for their assistance.
Funding
This work has been funded by a Grant-in Aid for Scientific Research from Japan Society for the Promotion of Sciences (#24700379 to SS, #16K08442 to SI).
References
- [1].Curtis de I, Meldolesi J. Cell surface dynamics – how Rho GTPases orchestrate the interplay between the plasma membrane and the cortical cytoskeleton. J Cell Sci 2012; 125:4435-44; PMID:23093576; http://dx.doi.org/ 10.1242/jcs.101501 [DOI] [PubMed] [Google Scholar]
- [2].Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol 2006; 16(10):522-9; PMID:16949823; http://dx.doi.org/ 10.1016/j.tcb.2006.08.006 [DOI] [PubMed] [Google Scholar]
- [3].Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70(3):389-99; PMID:1643657; http://dx.doi.org/ 10.1016/0092-8674(92)90163-7 [DOI] [PubMed] [Google Scholar]
- [4].Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70(3):401-10; PMID:1643658; http://dx.doi.org/ 10.1016/0092-8674(92)90164-8 [DOI] [PubMed] [Google Scholar]
- [5].Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81(1):53-62; PMID:7536630; http://dx.doi.org/ 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- [6].Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Kaibuchi K. et al.. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273(5272):245-8; PMID:8662509; http://dx.doi.org/ 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- [7].Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271(34):20246-9; PMID:8702756;http://dx.doi.org/ 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- [8].Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4(6):446-56; PMID:12778124; http://dx.doi.org/ 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- [9].Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999; 285(5429):895-8; PMID:10436159; http://dx.doi.org/ 10.1126/science.285.5429.895 [DOI] [PubMed] [Google Scholar]
- [10].Goicoechea SM, Awadia S, Garcia-Mata R. I'm coming to GEF you: Regulation of RhoGEFs during cell migration. Cell Adh Migr 2014; 8(6):535-49; PMID:25482524; http://dx.doi.org/ 10.4161/cam.28721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1998; 1(2):183-92; PMID:9659915; http://dx.doi.org/ 10.1016/S1097-2765(00)80019-2 [DOI] [PubMed] [Google Scholar]
- [12].Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol 2000; 20(17):6354-63; PMID:10938112; http://dx.doi.org/ 10.1128/MCB.20.17.6354-6363.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rane CK, Minden A. P21 activated kinases: structure, regulation, and functions. Small GTPases 2014; 5:e28003; PMID:24658305; http://dx.doi.org/ 10.4161/sgtp.28003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 2006; 173(4):587-9; PMID:16717130; http://dx.doi.org/ 10.1083/jcb.200509075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 2009; 21(4):537-42; PMID:19473826; http://dx.doi.org/ 10.1016/j.ceb.2009.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Das A, Guo W. Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol 2011; 21(7):383-6; PMID:21550243; http://dx.doi.org/ 10.1016/j.tcb.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pathak R, Delorme-Walker VD, Howell MC, Anselmo AN, White MA, Bokoch GM, Dermardirossian C. The microtubule-associated Rho activating factor GEF-H1 interacts with exocyst complex to regulate vesicle traffic. Dev Cell 2012; 23(2):397-411; PMID:22898781; http://dx.doi.org/ 10.1016/j.devcel.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell 2007; 12(5):699-712; PMID:17488622; http://dx.doi.org/ 10.1016/j.devcel.2007.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nalbant P, Chang YC, Birkenfeld J, Chang ZF, Bokoch GM. Guanine nucleotide exchange factor-H1 regulates cell migration via localized activation of RhoA at the leading edge. Mol Biol Cell 2009; 20(18):4070-82; PMID:19625450; http://dx.doi.org/ 10.1091/mbc.E09-01-0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91(1):119-49; PMID:21248164; http://dx.doi.org/ 10.1152/physrev.00059.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wu XS, Rao K, Zhang H, Wang F, Sellers JR, Matesic LE, Copeland NG, Jenkins NA, Hammer JA 3rd. Identification of an organelle receptor for myosin-Va. Nat Cell Biol 2002; 4(4):271-8; PMID:11887186; http://dx.doi.org/ 10.1038/ncb760 [DOI] [PubMed] [Google Scholar]
- [22].Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem 2002; 277(14):12432-6; PMID:11856727; http://dx.doi.org/ 10.1074/jbc.C200005200 [DOI] [PubMed] [Google Scholar]
- [23].Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J Cell Sci 2012; 125(Pt 9):2235-43; PMID:22344257; http://dx.doi.org/ 10.1242/jcs.098657 [DOI] [PubMed] [Google Scholar]
- [24].Baschieri F, Farhan H. Crosstalk of small GTPases at the Golgi apparatus. Small GTPases 2012; 3(2):80-90; PMID:22790194; http://dx.doi.org/ 10.4161/sgtp.19842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shibata S, Kawanai T, Hara T, Yamamoto A, Chaya T, Tokuhara Y, Tsuji C, Sakai M, Tachibana T, Inagaki S. ARHGEF10 directs the localization of Rab8 to Rab6-positive executive vesicles. J Cell Sci 2016; 129(19):3620-3634; PMID:27550519; http://dx.doi.org/ 10.1242/jcs.186817 [DOI] [PubMed] [Google Scholar]
- [26].Mohl M, Winkler S, Wieland T, Lutz S. Gef10-the third member of a Rho-specific guanine nucleotide exchange factor subfamily with unusual protein architecture. Naunyn Schmiedebergs Arch Pharmacol 2006; 373(5):333-41; PMID:16896804; http://dx.doi.org/ 10.1007/s00210-006-0083-0 [DOI] [PubMed] [Google Scholar]
- [27].Chaya T, Shibata S, Tokuhara Y, Yamaguchi W, Matsumoto H, Kawahara I, Kogo M, Ohoka Y, Inagaki S. Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10). J Biol Chem 2011; 286(34):29511-20; PMID:21719701; http://dx.doi.org/ 10.1074/jbc.M111.236810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].García-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol 2007; 17(1):36-43; PMID:17126549; http://dx.doi.org/ 10.1016/j.tcb.2006.11.004 [DOI] [PubMed] [Google Scholar]
- [29].Cooke SL, Pole JC, Chin SF, Ellis IO, Caldas C, Edwards PA. High-resolution array CGH clarifies events occurring on 8p in carcinogenesis. BMC Cancer 2008; 8:288; PMID:18840272; http://dx.doi.org/ 10.1186/1471-2407-8-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Matsushita T, Ashikawa K, Yonemoto K, Hirakawa Y, Hata J, Amitani H, Doi Y, Ninomiya T, Kitazono T, Kubo M, et al.. Functional SNP of ARHGEF10 confers risk of atherothrombotic stroke. Hum Mol Genet 2010; 19(6):1137-46; PMID:20042462; http://dx.doi.org/ 10.1093/hmg/ddp582 [DOI] [PubMed] [Google Scholar]
- [31].Ekenstedt KJ, Becker D, Minor KM, Shelton GD, Patterson EE, Bley T, Oevermann A, Bilzer T, Leeb T, Mickelson JR, et al.. An ARHGEF10 deletion is highly associated with a juvenile-onset inherited polyneuropathy in Leonberger and Saint Bernard dogs. PLoS Genet 2014; 10(10):e1004635; PMID:25275565; http://dx.doi.org/ 10.1371/journal.pgen.1004635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boora GK, Kulkarni AA, Kanwar R, Beyerlein P, Qin R, Banck MS, Ruddy KJ, Pleticha J, Lynch CA, Beutler AS, et al.. Association of the Charcot-Marie-Tooth disease gene ARHGEF10 with paclitaxel induced peripheral neuropathy in NCCTG N08CA (Alliance). J Neurol Sci 2015; 357(1-2):35-40; PMID:26143528; http://dx.doi.org/ 10.1016/j.jns.2015.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jungerius BJ, Hoogendoorn ML, Bakker SC, Van't Slot R, Bardoel AF, Ophoff RA, Wijmenga C, Kahn RS, Sinke RJ. An association screen of myelin-related genes implicates the chromosome 22q11 PIK4CA gene in schizophrenia. Mol Psychiatry 2008; 13(11):1060-8; http://dx.doi.org/ 10.1038/sj.mp.4002080 [DOI] [PubMed] [Google Scholar]
- [34].Verhoeven K, De Jonghe P, Van de Putte T, Nelis E, Zwijsen A, Verpoorten N, De Vriendt E, Jacobs A, Van Gerwen V, Timmerman V, et al.. Slowed conduction and thin myelination of peripheral nerves associated with mutant rho Guanine-nucleotide exchange factor 10. Am J Hum Genet 2003; 73(4):926-32; PMID:14508709; http://dx.doi.org/ 10.1086/378159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Akhmanova A, et al.. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell 2007; 13(2):305-14; PMID:17681140;http://dx.doi.org/ 10.1016/j.devcel.2007.06.010 [DOI] [PubMed] [Google Scholar]
- [36].Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peränen J, Pasterkamp RJ, Akhmanova A, et al.. Rab6, Rab8, and MICAL3 cooperate in controlling docking and fusion of exocytotic carriers. Curr Biol 2011; 21(11):967-74; PMID:21596566; http://dx.doi.org/ 10.1016/j.cub.2011.04.030 [DOI] [PubMed] [Google Scholar]
- [37].Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol 2010; 12(7):645-54; PMID:20562865; http://dx.doi.org/ 10.1038/ncb2067 [DOI] [PubMed] [Google Scholar]
- [38].Lee PL, Ohlson MB, Pfeffer SR. Rab6 regulation of the kinesin family KIF1C motor domain contributes to Golgi tethering. Elife 2015; 4; http://dx.doi.org/ 10.7554/eLife.06029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aoki T, Ueda S, Kataoka T, Satoh T. Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10. BMC Cell Biol 2009; 10:56; PMID:19635168; http://dx.doi.org/ 10.1186/1471-2121-10-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].He M, Agbu S, Anderson KV. Microtubule Motors Drive Hedgehog Signaling in Primary Cilia. Trends Cell Biol 2016; PMID:27765513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Malicki J, Besharse JC. Kinesin-2 family motors in the unusual photoreceptor cilium. Vision Res 2012; 75:33-6; PMID:23123805; http://dx.doi.org/ 10.1016/j.visres.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hattula K, Furuhjelm J, Tikkanen J, Tanhuanpää K, Laakkonen P, Peränen J. Characterization of the Rab8-specific membrane traffic route linked to protrusion formation. J Cell Sci 2006; 119(Pt 23):4866-77; PMID:17105768; http://dx.doi.org/ 10.1242/jcs.03275 [DOI] [PubMed] [Google Scholar]
- [43].Müsch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the TGN. J Cell Biol 1997; 138(2):291-306; PMID:9230072; http://dx.doi.org/ 10.1083/jcb.138.2.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J 1997; 16(11):3044-56; PMID:9214622;http://dx.doi.org/ 10.1093/emboj/16.11.3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol 2003; 13(15):1335-40; PMID:12906795; http://dx.doi.org/ 10.1016/S0960-9822(03)00540-2 [DOI] [PubMed] [Google Scholar]
- [46].Zilberman Y, Alieva NO, Miserey-Lenkei S, Lichtenstein A, Kam Z, Sabanay H, Bershadsky A. Involvement of the Rho-mDia1 pathway in the regulation of Golgi complex architecture and dynamics. Mol Biol Cell 2011; 22(16):2900-11; PMID:21680709; http://dx.doi.org/ 10.1091/mbc.E11-01-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Salvarezza SB, Deborde S, Schreiner R, Campagne F, Kessels MM, Qualmann B, Caceres A, Kreitzer G, Rodriguez-Boulan E. LIM kinase 1 and cofilin regulate actin filament population required for dynamin-dependent apical carrier fission from the trans-Golgi network. Mol Biol Cell 2009; 20(1):438-51; PMID:18987335; http://dx.doi.org/ 10.1091/mbc.E08-08-0891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. J Cell Biol 2008; 181(6):985-98; PMID:18541705; http://dx.doi.org/ 10.1083/jcb.200709076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].de Renzis S, Sönnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol 2002; 4(2):124-33; PMID:11788822; http://dx.doi.org/ 10.1038/ncb744 [DOI] [PubMed] [Google Scholar]
- [50].Bravo-Cordero JJ, Marrero-Diaz R, Megías D, Genís L, García-Grande A, García MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J 2007; 26(6):1499-510; PMID:17332756; http://dx.doi.org/ 10.1038/sj.emboj.7601606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 2009; 10(1):21-33; PMID:19197329; http://dx.doi.org/ 10.1038/nrm2593 [DOI] [PubMed] [Google Scholar]
- [52].Huveneers S, de Rooij J. Mechanosensitive systems at the cadherin-F-actin interface. J Cell Sci 2013; 126(Pt 2):403-13; PMID: 23524998; http://dx.doi.org/ 10.1242/jcs.109447 [DOI] [PubMed] [Google Scholar]
- [53].Abiko H, Fujiwara S, Ohashi K, Hiatari R, Mashiko T, Sakamoto N, Sato M, Mizuno K. Rho guanine nucleotide exchange factors involved in cyclic-stretch-induced reorientation of vascular endothelial cells. J Cell Sci 2015; 128(9):1683-95; PMID:25795300; http://dx.doi.org/ 10.1242/jcs.157503 [DOI] [PubMed] [Google Scholar]
- [54].Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 2002; 13(9):3268-80; PMID:12221131; http://dx.doi.org/ 10.1091/mbc.E02-03-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Bravo-Cordero JJ, Cordani M, Soriano SF, Díez B, Muñoz-Agudo C, Casanova-Acebes M, Boullosa C, Guadamillas MC, Montoya MC, et al.. A novel high-content analysis tool reveals Rab8-driven cytoskeletal reorganization through Rho GTPases, calpain and MT1-MMP. J Cell Sci 2016; 129(8):1734-49; PMID:26940916; http://dx.doi.org/ 10.1242/jcs.174920 [DOI] [PubMed] [Google Scholar]
- [56].Shafaq-Zadah M, Gomes-Santos CS, Bardin S, Maiuri P, Maurin M, Iranzo J, Gautreau A, Lamaze C, Caswell P, Johannes L, et al.. Persistent cell migration and adhesion rely on retrograde transport of β(1) integrin. Nat Cell Biol 2016; 18(1):54-64; PMID:26641717; http://dx.doi.org/ 10.1038/ncb3287 [DOI] [PubMed] [Google Scholar]
- [57].Hsiao YC, Tuz K, Ferland RJ. Trafficking in and to the primary cilium. Cilia 2012; 1(1):4; PMID:23351793; http://dx.doi.org/ 10.1186/2046-2530-1-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Jackson PK, et al.. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007; 129(6):1201-13; PMID:17574030; http://dx.doi.org/ 10.1016/j.cell.2007.03.053 [DOI] [PubMed] [Google Scholar]
- [59].Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 2007; 178(3):363-9; PMID:17646400; http://dx.doi.org/ 10.1083/jcb.200703047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Jackson PK. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci U S A 2011; 108(7):2759-64; PMID:21273506;http://dx.doi.org/ 10.1073/pnas.1018823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 2007; 120(Pt 11):1868-76; PMID:17488776; http://dx.doi.org/ 10.1242/jcs.005306 [DOI] [PubMed] [Google Scholar]
- [62].Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 2008; 40(7):871-9; PMID:18552847; http://dx.doi.org/ 10.1038/ng.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 2010; 464(7291):1048-51; PMID:20393563; http://dx.doi.org/ 10.1038/nature08895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Cao J, Shen Y, Zhu L, Xu Y, Zhou Y, Wu Z, Li Y, Yan X, Zhu X. miR-129-3p controls cilia assembly by regulating CP110 and actin dynamics. Nat Cell Biol 2012; 14(7):697-706; PMID:22684256; http://dx.doi.org/ 10.1038/ncb2512 [DOI] [PubMed] [Google Scholar]
- [65].Hernandez-Hernandez V, Pravincumar P, Diaz-Font A, May-Simera H, Jenkins D, Knight M, Beales PL. Bardet-Biedl syndrome proteins control the cilia length through regulation of actin polymerization. Hum Mol Genet 2013; 22(19):3858-68; PMID:23716571; http://dx.doi.org/ 10.1093/hmg/ddt241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee JK, Heo WD, Kim J. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun 2015; 6:6781; PMID:25849865; http://dx.doi.org/ 10.1038/ncomms7781 [DOI] [PubMed] [Google Scholar]
- [67].Stewart K, Gaitan Y, Shafer ME, Aoudjit L, Hu D, Sharma R, Tremblay M, Ishii H, Marcotte M, Bouchard M, et al.. A point mutation in p190A RhoGAP affects Ciliogenesis and leads to Glomerulocystic kidney defects. PLoS Genet 2016; 12(2):e1005785; PMID:26859289; http://dx.doi.org/ 10.1371/journal.pgen.1005785 [DOI] [PMC free article] [PubMed] [Google Scholar]


