ABSTRACT
Arf GTPase-activating proteins (Arf GAPs) were first identified as regulators of the small GTP-binding proteins ADP-ribosylation factors (Arfs). The Arf GAPs are a large family of proteins in metazoans, outnumbering the Arfs that they regulate. The members of the Arf GAP family have complex domain structures and some have been implicated in particular cellular functions, such as cell migration, or with particular pathologies, such as tumor invasion and metastasis. The specific effects of Arfs sometimes depend on the Arf GAP involved in their regulation. These observations have led to speculation that the Arf GAPs themselves may affect cellular activities in capacities beyond the regulation of Arfs. Recently, 2 Arf GAPs, ASAP1 and AGAP1, have been found to bind directly to and influence the activity of myosins and kinesins, motor proteins associated with filamentous actin and microtubules, respectively. The Arf GAP-motor protein interaction is critical for cellular behaviors involving the actin cytoskeleton and microtubules, such as cell migration and other cell movements. Arfs, then, may function with molecular motors through Arf GAPs to regulate microtubule and actin remodeling.
Keywords: ADP-ribosylation factor GTPase-activating protein, ADP-ribosylation factors, AGAP1, Arf, Arf GAP, ASAP1, Kif2A, kinesin-13, NM2A, nonmuscle myosin 2A
Introduction
The ADP-ribosylation factors (Arfs) are guanine nucleotide binding proteins that regulate membrane traffic and the cytoskeleton.1–4 Humans have 5 isoforms, divided into 3 classes: Arf1 and 3 (class 1), Arf4 and 5 (class 2) and Arf6 (class 3). Arf function depends on switching between inactive Arf•GDP and active Arf•GTP. A cycle between the 2 forms is achieved by exchanging GTP for GDP to form Arf•GTP and then hydrolyzing GTP to switch back to Arf•GDP. Arfs, however, have low intrinsic exchange and no detectable GTPase activity. Arf function, therefore, depends on guanine nucleotide exchange factors (Arf GEFs) and Arf GTPase-activating proteins (Arf GAPs), which drive the cycle of GTP binding and hydrolysis. Studies examining the role of the GAPs in Arf-dependent cellular activities have revealed that the role of the GAPs may extend beyond inducing GTP hydrolysis by Arfs since the particular cellular activity controlled by an Arf also depends on the Arf GAP with which it associates.
The Arf GAPs are a diverse family of proteins with a common Arf GAP domain necessary for catalyzing the hydrolysis of GTP bound to Arf.2,4-6 The Arf GAPs outnumber the Arfs, with 31 human genes encoding Arf GAPs, some of which encode multiple splice variants. The Arf GAPs have complex domain structures (Fig. 1A). The function of the Arf GAPs is in part dependent on the Arf GAP activity, but GAP activity is often not sufficient to explain the cellular effect of an Arf GAP. The Arf GAPs may also function as scaffolds or adaptors, described for GITs7 and considered for ASAP18,9 and AGAP2,10–12 or as subunits of coat proteins that mediate membrane trafficking, described for Arf GAP1 and ACAP1.13–15 Arf GAPs have also been found to bind to myosins and kinesins,16,17 molecular motors associated with the actin cytoskeleton and microtubules, respectively. These molecular motors catalyze hydrolysis of ATP and use the energy released to generate force to move vesicles, slide cytoskeletal filaments or change dynamics of actin filaments or microtubules.18–20 Numerous biologic functions require force-dependent processes that are mediated by myosins and kinesins. Recent results indicate the Arf GAP-motor protein complexes may have effector function, potentially explaining how an Arf GAP could determine a specific cellular effect of an Arf. In this review, we focus our discussion on ASAP1 association with nonmuscle myosin 2A16 and AGAP1 association with kinesin-1317 and their role in the regulation of cell adhesions and migration.
Figure 1.
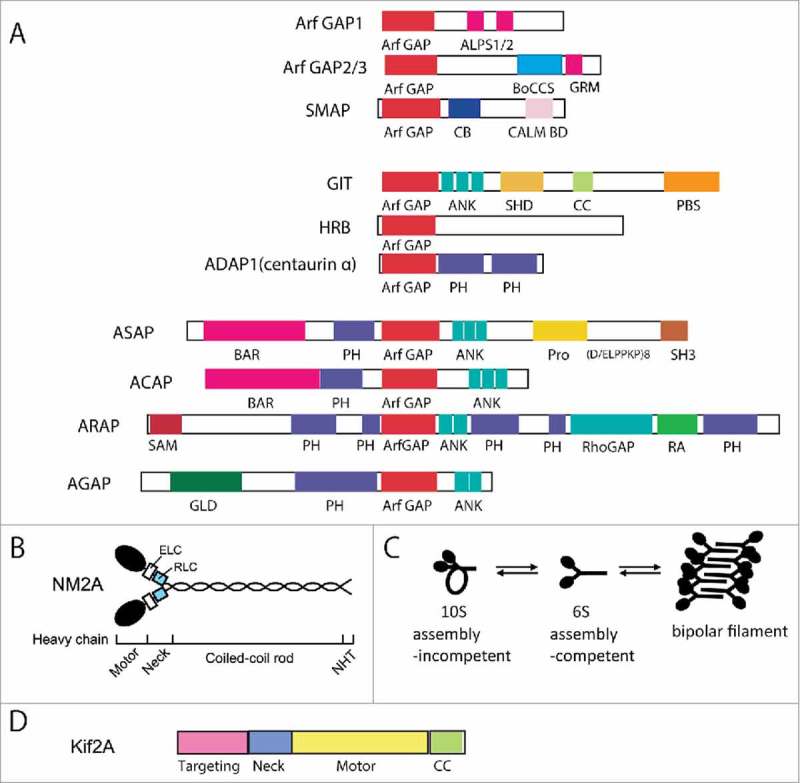
Domain structures of Arf GAPs and associated motor proteins. (A) Domain structures of the human Arf GAP subfamilies are depicted. Abbreviations are: ALPS, ArfGAP1 lipid-packing sensor; ArfGAP, ArfGAP domain; ANK, ankyrin repeat; BAR, Bin/Amphiphysin/Rvs; BoCCS, binding of coatomer, cargo and SNARE; CALM, CALM binding domain; CB, clathrin-box; CC, coiled-coil; FG repeats, multiple copies of the XXFG motif; GLD, GTP-binding protein-like domain; GRM, Glo3 regulatory motif; PBS, Paxillin binding site; PH, pleckstrin homology domain; Pro(PxxP)3, cluster of 3 Proline-rich (PxxP) motifs; Pro(D/ELPPKP)8, 8 tandem Proline-rich (D/ELPPKP) motifs; RA, Ras association motif; RhoGAP, RhoGAP domain; SAM, sterile α-motif; SH3, Src homology 3 domain; SHD, Spa-homology domain. Adapted from Kahn et al. 2009. (B) Domain structure of Nonmuscle myosin 2A. NM2A is composed of 2 heavy chains, 2 essential light chains (ELCs) and 2 regulatory light chains (RLCs). Each heavy chain contains a motor head domain, a neck region, a coiled-coil rod and a non-helical tail (NHT). (C) Conformations of NM2. D. Domain structure of Kif2A.
ASAP1 and nonmuscle myosin 2A (NM2A)
ASAP1 is composed of a BAR, PH, Arf GAP, 3 ankyrin repeat, proline rich, E/DLPPKP tandem and SH3 domains (Fig. 1A).5,21 ASAP1 prefers class 1 and 2 Arfs to class 3 Arf as substrates.21 It associates with and regulates actin based structures, including focal adhesions (FAs), invadopodia and circular dorsal ruffles (CDRs).22–24 ASAP1 binds to several oncogenes including focal adhesion kinase (FAK), Src, CrkL and cortactin.21,23,25-27 The gene for ASAP1 is amplified in several cancers, including uveal melanoma and breast, ovarian, prostate and colon cancers, and this amplification correlates with increased invasion and metastasis.8,24,28-31 ASAP1 potentiates invasion and metastasis in animal models.8 The molecular basis for the role of ASAP1 in these events is beginning to be uncovered. Part of the effect is through regulation of integrin trafficking.32 Recently, ASAP1 association with nonmuscle myosin 2A (NM2A) was discovered,16 which may directly affect actin-NM2A dynamics important for cytoskeleton remodeling, cell adhesion and the accompanying cellular changes.
Nonmuscle myosin 2 (NM2) belongs to class II myosins, which is the largest group in the myosin superfamily.33,34 NM2 proteins are composed of 2 heavy chains, 2 essential light chains (ECL), and 2 regulatory light chains (RLC) (Fig. 1B). There are 3 different heavy chain isoforms encoded by 3 different genes, which specify the isoform of the overall NM2 to be NM2A, 2B or 2C. The heavy chains contain 2 globular head domains, which bind ATP and F-actin, followed by a neck region, a long α-helical rod domain and a non-helical tail. NM2 activity is critical for cell migration. In mesenchymal cell migration, NM2 regulates protrusion of the leading edge by generating actin retrograde flow and bundling F-actin. It also promotes adhesion maturation and is responsible for retracting the cell rear. Blebbing or the amoeboid mode of migration also depends on NM2.35 NM2 contributes to the different modes of migration due to its ability to cross-link F-actin and move along F-actin to induce contractility.
One of the most prominent contractile structure in cultured cells is the actomyosin stress fiber. Stress fibers can be divided into 3 main types: transverse arcs, dorsal or radial stress fibers, and ventral stress fibers (Fig. 2A).36 Transverse arcs are curved actomyosin structures consisting of alternate α-actinin and NM2 bundled actin filaments. Arcs orient parallel to the leading edge of the cells and move inward to the cell center as cells migrate. Dorsal stress fibers are connected to FAs at one end and elongate toward the dorsal side of the cells through VASP and Dia 1 formin-mediated actin polymerization at FAs. Dorsal stress fibers do not typically contain NM2. They transmit contractile force to FAs through the interaction with the transverse arcs, which contain NM2. Ventral stress fibers are actomyosin bundles that attach to FAs at both ends and often localize at central and posterior parts of the cells. Ventral stress fibers are thought to develop from transverse arcs captured by dorsal stress fibers. The mechanisms underlying the assembly of various contractile actomyosin structures are still being discovered.
Figure 2.
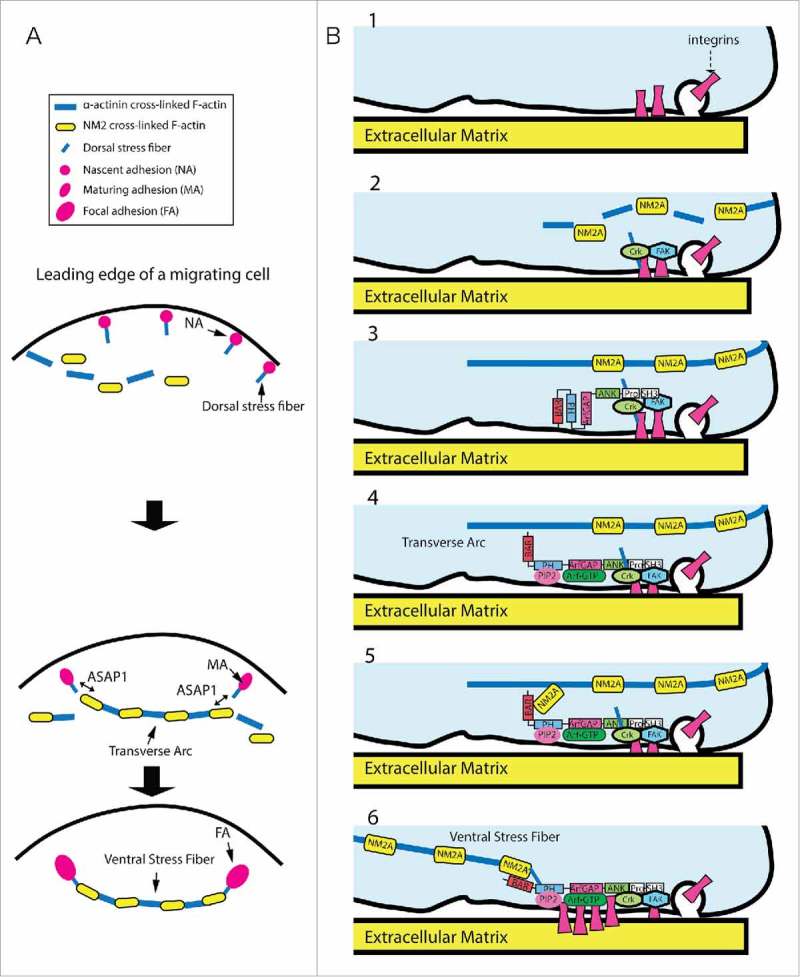
Model for ASAP1 function through NM2A to control maturation of stress fibers and associated integrin adhesions. (A) Hypothetical model for assembly of contractile stress fibers. Transverse arcs are assembled by endwise joining of NM2 and α-actinin cross-linked F-actin. The dorsal stress fibers emanating from integrin adhesions attach to arcs to form a continuous contractile stress fiber networks. The network contracts and flow toward the cell center. Ventral stress fibers are generated from transverse arcs located between 2 dorsal stress fibers. (B) ASAP1 couples assembly of actomyosin stress fibers to maturation of FAs. 1. Integrins are delivered to the cell surface and make contact with the extracellular matrix resulting in their activation. 2. Activated integrins recruit several proteins such as talin and paxillin, which recruit additional proteins including Crk and FAK. 3. ASAP1 is recruited to the nascent adhesionsvia binding of its proline rich domain to Crk and binding of its SH3 domain to FAK. 4. ASAP1 associates with PIP2 and Arf•GTP that are enriched in the forming adhesions. 5. PIP2 and Arf•GTP-bound ASAP1 interacts with NM2A (note that the roles of PIP2 and Arf in NM2A association are purely speculative, see text), stabilizing association of NM2A with actin filaments at the junction between dorsal stress fibers and transverse arcs. 6. Contractility of actomyosin stress fibers drives enlargement and maturation of the integrin adhesion.
Arfs have been implicated in control of NM2 pathways. Two Arf GEFs, BIG1 and BIG2, negatively regulate NM2 activity.37 The BIGs promote dephosphorylation of RLC by stabilizing the phosphatase (PP1/PP1cδ)-NM2A interaction and mediating a decrease in NM2-dependent contractility. Arfs may also regulate NM2 by mechanism involving Rho family members. Arf1 knockdown inhibits the EGF-stimulated activation of RhoA and RhoC, as well as the downstream activation of NM2, measured by RLC phosphorylation on Thr18/Ser19.38 Constitutively active mutants of RhoA or RhoC rescue the defect in migration in Arf1 knockdown cells. Therefore, active Arf1 promotes RhoA and RhoC activity, which in turn activate NM2 in response to EGF. Arf1 may also work downstream from or in parallel with RhoA. The constitutively active mutant Arf1[Q71L] potentiates the effect of active RhoA on assembly of stress fibers.39
Arf GAPs have also been examined as regulators of NM2 and NM2-regulated cellular structures such as the actin stress fibers and integrin adhesions. ASAP1, the most extensively studied of the Arf GAPs, controls the dynamics of actomyosin structures important for cell migration and invasion, and the effect may be caused by direct binding to and regulation of cytoskeleton proteins.16 NM2A was identified as a possible binding partner of ASAP1 in a proteomic screen. The interaction was mediated by the N-terminal BAR domain of ASAP1, which also mediates homodimerization.40 The proteins coimmunoprecipitated from cells and knockdown of NM2A and ASAP1 resulted in similar phenotypes with smaller integrin adhesions, more rapid 2-D migration, faster cell spreading and increased formation of CDRs on treatment with platelet-derived growth factor. The effect of ASAP1 knockdown could be rescued by expression of siRNA resistant ASAP1, but not by ASAP1 lacking the domain that bound to NM2A. Additionally, the effect of ASAP1 knockdown on cell spreading and CDRs could be rescued by expression of exogenous NM2A, but exogenous ASAP1 could not rescue function in NM2A knockdown. These results suggest that ASAP1 is a positive regulator of NM2A.
ASAP1 effects on integrin adhesion maturation may be related to its control of transitions between different types of actin stress fibers (Fig. 2A). ASAP1 associates with integrin adhesions, including highly dynamic nascent adhesions at the cell periphery as well as the mature, more stable FAs.16,22,25,26 Knockdown of ASAP1 disrupted actomyosin stress fibers in fibroblasts with decreased colocalization of NM2A with F-actin. Moreover, ASAP1 and NM2A appeared to colocalize at the proximal part of FAs to which dorsal and ventral stress fibers anchored. We speculate that ASAP1 could be recruited to a site of actin remodeling, such as a nascent adhesion, by binding to FAK and Crk.25–27 At the site, ASAP1 controls the association of NM2A with F-actin thereby stabilizing the connection between dorsal stress fibers and transverse arcs. Consequently, contractile force could be applied to the nascent adhesion, resulting in its maturation into FA (Fig. 2B). Understanding the possible role of the ASAP1-NM2A complex in these cellular activities will require determining the nature of the change in NM2A activity on ASAP1 binding and the mechanisms regulating the association of ASAP1 with NM2A.
There are multiple ways that ASAP1 might affect NM2 activity. NM2 monomers exist in 2 conformations: an inactive folded 10S form and the extended 6S form (Fig. 1C).33,34 To bind F-actin and contract, NM2 must be in the 6S form that can assemble into antiparallel bipolar NM2 filaments. The transition from the 10S to 6S conformation is known to be regulated by RLC phosphorylation on Thr18/Ser19. Several kinases mediate RLC phosphorylation and are considered as activators for NM2, including Rho-associated, coiled coil-containing kinase (ROCK), myosin light chain kinase (MLCK), myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK), among others. On the other hand, dephosphorylation of RLC by myosin phosphatases like PP1, inhibits NM2 activity. In fact, ROCK activates NM2 not only by direct phosphorylation of RLC but also by phosphorylating and inhibiting PP1. ROCK, MLCK and MRCK, are regulated by Rho family members. For example, RhoA activates ROCK, leading to stress fiber formation whereas Rac activation often antagonizes RhoA function, in part by activating PAK, which inhibits MLCK.
Phosphorylation of the rod domain and non-helical tail or binding of other proteins to these regions also affect NM2 bipolar filament formation. ASAP1 may promote NM2 activity by facilitating 10S to 6S transition or antiparallel association of their rod domains through the physical interaction. ASAP1 was found to stably associate with sedimentable forms of NM2 in vitro,16 which could indicate a preference for the extended form of the protein. Alternately, the effect of ASAP1 could be through regulation of RLC phosphorylation. Since ASAP1 directly interacts with NM2A, one hypothesis is that ASAP1 stabilizes a form of NM2A that facilitates phosphorylation or acts as a scaffold to bring the kinase and NM2A together. Another possibility is that ASAP1 also binds to actin filaments (unpublished). By binding to both NM2A and F-actin, ASAP1 could control their association/dissociation kinetics. The result that colocalization of NM2A and F-actin is reduced in ASAP1 knockdown cells is consistent with this idea. These mechanisms are not mutually exclusive and are currently being investigated.
ASAP1 connects cellular signaling to actin remodeling. The C-terminal region of ASAP1 binds to several oncogenes that have effects on cell migration, invasion and metastasis including Src, FAK and Crk.8,9,21,23-25,27 Moreover, phosphorylation of ASAP1 by Src is important to ASAP1s regulation of the actin cytoskeleton.23,25,27 These interactions may regulate ASAP1-NM2A function, similar to regulation of the F-BAR domain of syndapin by its SH3 domain.41 In addition, binding partners of the ASAP1 BAR domain,42 such as FIP3, may compete with NM2A for binding and block NM2-dependent ASAP1 function. Thus, the role of the BAR domain and the C-terminal region-binding partners of ASAP1 in controlling the ASAP1-NM2A interaction represents an important area yet to be explored.
Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) and Arf•GTP may also be important for regulating ASAP1-NM2A interaction. PI(4,5)P2 has been found to be important for both ASAP1 and NM2 function. PI(4,5)P2 binding to the PH domain of ASAP1 activates Arf GAP activity of ASAP1,43–46 possibly through inducing a conformational change that increases the affinity for the substrates Arf1 and Arf5. Arf3 and Arf4 have not been examined. Several lines of evidence support the idea that, in ASAP1, the PH and Arf GAP domains contribute to the regulation of the BAR domain. First, the isolated BAR domain is not stable, but the BAR-PH tandem is, suggesting the PH domain is important to folding of the BAR domain. Second, based on modeling, the N-terminus of the BAR domain inserts an inhibitory motif into the PH-Arf GAP tandem40 and NM2A binding to the BAR domain increases PI(4,5)P2-dependent GAP activity. Third, the BAR-PH tandem binds NM2A more efficiently than a recombinant protein containing the BAR, PH and Arf GAP domains.16 Thus, ligands binding to the PH and Arf GAP domains, PI(4,5)P2 and Arf•GTP, might influence association of ASAP1 with NM2, as depicted in the hypothetical scheme presented in Fig. 3. PI(4,5)P2 is also important for NM2A function. During cellularization of Drosophila embryo, increased PI(4,5)P2 levels caused premature actomyosin contraction, indicating that PI(4,5)P2 promotes NM2 activity.47 Taken together, we propose that ASAP1 mediates effects of PI(4,5)P2 on the actomyosin networks via cycles of interactions with NM2 under the control of Arf•GTP hydrolysis (Fig. 3). In this speculative model, PI(4,5)P2 binding to ASAP1 drives a conformational change allowing Arf•GTP to bind, with changes in the BAR domain that allow binding to one form of NM2. GTP hydrolysis then facilitates transition to another form of NM2. In Fig. 3, for example, state 1 might be the 10S form, which transitions to state 2, the 6S form. Other possibilities include single 6S to 6S bipolar filaments, or unphosphorylated to phosphorylated Thr18/Ser19 of RLC. Linking the changes in NM2A to the catalytic cycle of ASAP1, which is highly regulated and rapid (kcat of 150 events/sec), may enable rapid switching between NM2A states necessary for cell movement.
Figure 3.

Model for ASAP1 interaction with NM2A regulated by PI(4,5)P2 and Arf. In this model, PI(4,5)P2 (PIP2 in figure) binding to the PH domain of ASAP1 facilitates Arf•GTP binding to ASAP1. ASAP1 undergoes a conformational change allowing interaction with NM2A. Subsequent hydrolysis of Arf•GTP induces further conformational change in ASAP1 that promotes NM2A activity and returns ASAP1 to a conformation capable for another round of NM2A binding. NM2 state 1 and state 2 represent inactive and active NM2 respectively. The transition of NM2 state 1 to state 2 could be from 10S to 6S form, or single 6S to 6S bipolar filaments, or unphosphorylated to phosphorylated Thr18/Ser19 of RLC.
ASAP1 may be representative of a new class of NM2 regulators. Five other Arf GAPs, including ASAP2, 3 and ACAP1–3, contain N-terminal BAR-PH domain tandems. Three have been studied and found to affect the actin cytoskeleton. ASAP3, for instance, has a role in stress fiber formation.48 Knockdown of ASAP3 reduced phosphorylation of RLC of NM2, and slowed migration and invasion of mammary carcinoma cells. The results are consistent with an effect of ASAP3 on NM2, which remains to be explored. Similarly, ACAP1 and ACAP2 inhibited the formation of actin-rich CDRs and membrane protrusions.49 Control of NM2 function could contribute, in part, to the effects.
AGAP1 and Kif2A
Eleven genes encode AGAP-subtype Arf GAPs.5 The first identified, AGAP1, 2 and 3, are composed of a G protein-like domain (GLD, also called a mitochondrial Rho-like protein, or miro domain), split PH, Arf GAP and Ankyrin repeat domains (Fig. 1A).50 Similar to other Arf GAPs with PH domains, the Arf GAP activity of AGAPs is stimulated by phosphoinositides. Also like other Arf GAPs, the AGAPs studied to date affect membrane trafficking and the actin cytoskeleton. AGAP1 binds directly to the clathrin adaptor protein AP-3, which together with clathrin can form a vesicular coat.10,51 AGAP1 also binds to muscarinic receptor and affects its trafficking.52 Work on the related protein AGAP2 has focused on signaling that can affect FAs, cytoskeletal dynamics and cell migration, proliferation and survival. AGAP2 destabilizes adhesions by binding to and affecting FAK phosphorylation.12,53 AGAP2 also binds to β-arrestin, which affects Erk signaling,11 and to Akt, which increases Akt activity.54 Both Erk and Akt have been reported to affect adhesion dynamics.55–57 Recently, AGAP1, 2 and 3 were found to bind to a kinesin-13 family member, Kif2A17 that depolymerizes microtubules. The interaction between AGAP1-Kif2A affected actin dependent cell movement, connecting microtubule remodeling with actin remodeling.
Kinesins are molecular motors that bind to microtubules.18,20 Kinesins bind and hydrolyze ATP, which provides energy for most kinesins to translocate along microtubules. The kinesin-13 family, comprised of Kif2A, Kif2B and Kif2C, are unconventional kinesins.58 The motor domain is located midway along the amino acid sequence of the protein with a targeting domain N-terminal to the motor and dimerization domains in both the N-terminal and C-terminal domains of the motor (Fig. 1D).59 Kif2C diffuses along the microtubule lattice independent of ATP hydrolysis. It binds the end of the microtubules where ATP hydrolysis drives the removal of tubulin dimers.60 Kinesin-13s are expressed in and have been studied in mitotic cells. Kinesin-13s have been implicated in regulating microtubule length in interphase61 and mitotic cells62 and decreasing the incidence of erroneous kinetochore attachments during mitosis.63 Kif2A is expressed in terminally differentiated cells including neurons64 and cardiomyocytes.65 In mitotic cells, Kif2A has been implicated in mitotic spindle size scaling and centrosome coalescence.66,67 In interphase cells, Kif2A has been found to affect microtubule dynamics68 and collateral axonal branch extension.64 Kif2 has been reported to affect lysosomal positioning69 and primary cilium disassembly in preparation for cell division.70 High Kif2A expression levels are an indicator of poor prognosis and higher metastatic potential, contributing to cell migration and invasion in vitro in squamous cell carcinoma and breast cancer.71,72 In transformed bronchial epithelial cells, Kif2A and Kif2C levels are regulated by K-Ras and contribute to the invasive behavior of the cells.73
Several studies have suggested a connection between the Arf and kinesin pathways. The kinesin MKLP1 was identified as a binding partner of Arf3.74 MKLP1 is part of the central spindlin complex, which organizes microtubules during cytokinesis. MKLP1 in complex with RacGAP1 directs the accumulation of the GEF Ect2 at the central spindle, an event necessary for furrow induction.75,76 MKLP1 also bundles microtubules necessary for dendrite formation in neurons.77,78 The association between Arf3 and MKLP1 depended on GTP binding to Arf3, consistent with MKLP1 being an effector of Arf3. However, a physiologic function of the Arf3/MKLP1 complex has not been identified. A proteomic screen identified Kif21 as binding partner of the Arf exchange factor BIG1.79 However, a function of the complex other than potential targeting of Kif21 to the Golgi has not been identified. The Arf GAP ADAP1 (also called centaurin α) was found to bind to Kif13b,80 a kinesin best described for function in ciliogenesis. Kif13b was found to suppress Arf6 GAP activity of ADAP1, which had consequences on ATP-stimulated human growth hormone secretion from PC12 cells. A potential role of microtubule binding was not identified.
Kinesins were identified as possible binding partners of AGAP-type Arf GAPs in proteomic and 2-hybrid screens.17 Further investigation revealed a specific association of AGAP1 with Kif2A with functional consequences for both proteins. The interaction was mediated by the GLD and PH domains of AGAP1 and the motor domain of Kif2A. The complex affected the biochemical activity of each protein in a PI(4,5)P2 dependent manner: Kif2A stimulated AGAP1 GAP activity and conversely, AGAP1 stimulated Kif2A ATPase activity.
The association of Kif2A with AGAP1 has several allosteric properties. For instance, PI(4,5)P2 is necessary for the effect of Kif2A on GAP activity, supporting the idea that PI(4,5)P2 binding to the PH domain may affect the association of Kif2A with the GLD-PH domain. PI(4,5)P2 dependent binding of β-arrestin to AGAP2 has been described previously,11 supporting the idea that the PH domain may modulate protein binding dependent on PI(4,5)P2. A second allosteric phenomenon is that PI(4,5)P2 and Kif2A, together, binding to the GLD-PH domains, affect the function of the GAP domain. One hypothesis that explains the observations, illustrated in Fig. 4, is being tested. Here, PI(4,5)P2 binding to the PH domain opens the GLD-PH domain, facilitating Kif2A binding, resulting in a second domain rearrangement allowing the substrate for the GAP, Arf•GTP, to bind to the GAP domain.
Figure 4.

Hypothesized mechanisms for control of enzymatic activity of AGAP1 by PI(4,5)P2 and Kif2A. 1. Inactive form of AGAP1. We have identified charged patches in the GLD and insert in the PH domain important for activation. The drawing shows a speculated salt bridge, but its existence has not been tested. 2. PI(4,5)P2 (PIP2 in figure) binding to PH domain. The effects of Kif2A are dependent on PI(4,5)P2 binding to the PH domain leading us to speculate that PI(4,5)P2 binding results in a domain rearrangement favoring productive interaction with Kif2A. 3. Kif2A binding to the GLD and PH domains. Kif2A binding was found to increase GAP activity, leading us to speculate that Kif2A causes further domain rearrangement that optimizes AGAP1 binding to the substrate Arf1•GTP. 4. Catalytically active PI(4,5)P2·Kif2A·AGAP1 complex. Based on work with ASAP1, another Arf GAP that contains a PH domain, we speculate that the catalytic site comprises both the Arf GAP and PH domains which are optimally oriented for binding Arf1•GTP and hydrolyzing GTP with the PI(4,5)P2- and Kif2A-induced domain rearrangements.
AGAP1 may function in complex with Kif2A to affect cellular activities. Reduced expression, by transfection with siRNA, of either Kif2A or AGAP1 inhibited wound healing and accelerated cell spreading.17 Expression of epitope-tagged AGAP1 reversed the effect of reduced expression of endogenous AGAP1 on cell spreading but expression of a point mutant or a deletion mutant of AGAP1 that bound Kif2A poorly only partly reversed the effect of reducing endogenous AGAP1. A mutant of AGAP1 containing only the Kif2A binding domains (GLD-PH) acted as a dominant negative, which accelerated spreading. These results support the idea that the AGAP1•Kif2A complex regulates cytoskeleton remodeling associated with cell movement.
Several nonexclusive mechanisms could explain the effect of the association between AGAP1 and Kif2A on cells. Preliminary data indicate that Kif2A may target AGAP1 to FAs (Luo and Randazzo, unpublished). Targeting AGAP1 with increased localized GAP activity may control Arf•GTP levels, which could have several consequences affecting FAs including control of membrane traffic or control of enzymes such as phosphoinositide kinases or phospholipase D; however, an Arf GAP dead mutant of AGAP1 partially rescued the effect of reduced endogenous AGAP1 on cell spreading,17 suggesting that Arf GAP activity is not the only function of AGAP1. AGAP1 may also function as a scaffold or adaptor protein to affect FAs and associated actin filaments. AGAP2 binds to FAK, which increases FAK activity and destabilizes FAs.12 AGAP2, in complex with the scaffolding protein RACK1, binds to and inhibits FAK, which also destabilizes FAs.53 Given the homology between AGAP1 and AGAP2, AGAP1 may also bind FAK and RACK1, affecting FAs either by activating or inactivating FAK. Because FAK activation has been correlated with both FA disassembly and assembly,12,53,81-85 cellular assays must be designed to quantify FA dynamics in response to experimental manipulations of AGAPs. Other protein partners identified in 2-hybrid screens that might be relevant include Rho-dependent kinase (ROCK), with substrates including myosin regulatory light chain, myosin light chain phosphatase and LIM kinase that affect actin dynamics.86,87
Microtubule attachment and stabilization by FAs has been suggested as a mechanism by which FA turnover can be accelerated.81,82,88 The hypothesis posits that when a microtubule contacts a FA, microtubule-dependent delivery of molecules, such as NBR1, MMP and Brag2 (in complex with MAP4K4), that promote FA disassembly will dissolve the FA and facilitate cell migration (Fig. 5).81,83,84 Counter-intuitively, Kif2A is a microtubule depolymerizer, whose loss should impair cell migration, as increased MT dynamics is correlated with increased cell migration.89 However, Kif2A could antagonize cell migration by detaching microtubules that associate with FAs, preventing their microtubule-dependent destabilization. This activity would be consistent with a coordinated role for AGAP1 in mediating endosome dependent FAK activation, FA assembly and signaling.90 In this scenario, AGAP1 would be part of the machinery antagonizing microtubule-dependent FAK inactivation and FA dissolution (Fig. 5).81 Interestingly, this model would also predict that AGAP1 and AGAP2 are antagonistic with respect to control of FA assembly and disassembly. Unfortunately, the precise mechanism describing the effect of microtubules on FAs is still under investigation, but preliminary data suggest an intriguing connection to membrane traffic and protein transport. AGAP1 regulation of Arf•GTP levels could be coordinated with the regulation of Kif2A to control microtubule dynamics with the membrane remodeling mediated by changes in Arf activity. This possibility is currently being explored.
Figure 5.
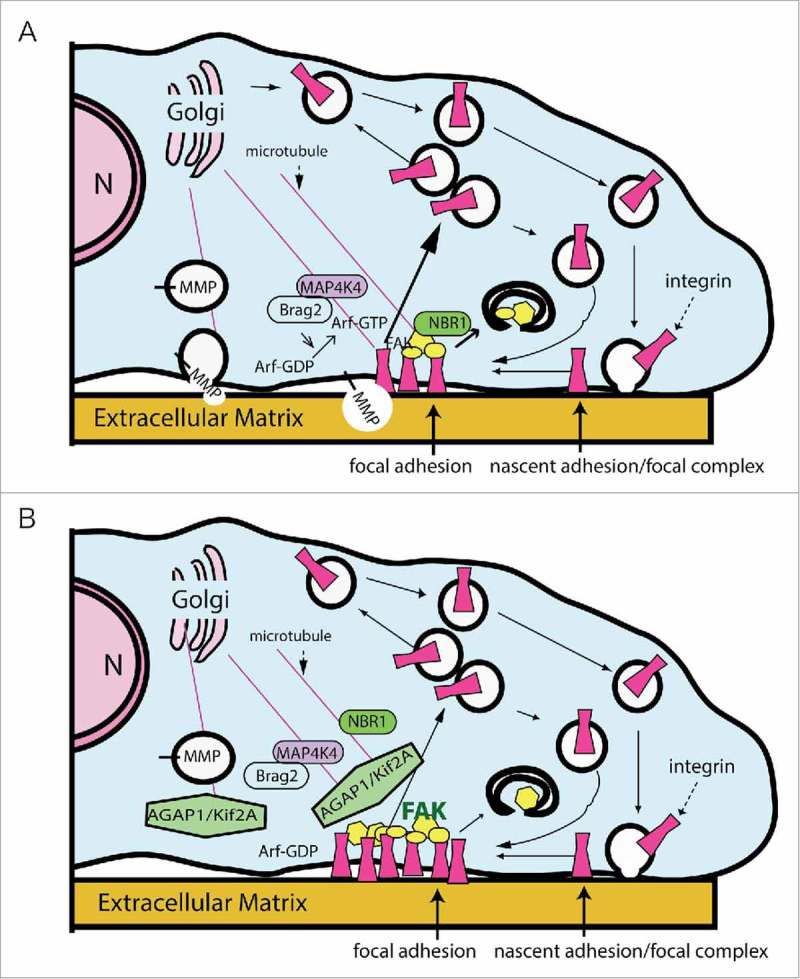
AGAP1 association with Kif2A may affect microtubule dependent FA dynamics. (A) Docking of microtubules with FAs results in disassembly of FAs through multiple mechanisms, including delivery of an Arf exchange factor, Brag2 (transported when associated with MAP4K4), which controls endocytosis of integrins, delivery of NBR1, which controls autophagy of FA plaque proteins (illustrated as yellow geometric shapes) and delivery of metalloproteinase (MMP), which hydrolyzes the extracellular part of the FA. Microtubule contact limits FAK activity. (B) AGAP1-Kif2A may increase the depolymerization of microtubules, preventing their association with FAs and consequent delivery of molecules that drive disassembly. The reduced microtubule contact results in increased FAK activity, indicated by the bold green “FAK,” by mechanisms that may involve AGAP1 directly as described in the text. As a result, the FA would be stabilized. N, nucleus.
Conclusions
In addition to the 2 examples of Arf GAP-motor protein complexes described above, AGAP2 and AGAP3 also were reported to bind Kif2A, although the functions of the complexes have not been examined.17 In unpublished work, Kif2A, Kif2B, Kif2C and Kif15 were identified as possible binding partners of ASAP3, Kif5A as a binding partner of ASAP1 and myo18 and Kif26A as binding partners of ARAP1 (Randazzo, unpublished). If binding motor proteins is a common property of Arf GAPs, are the proteins in the complexes similarly affected or are there diverse consequences? Evidence already indicates that the effects may be diverse. ADAP1 was inhibited by Kif13b in contrast to AGAP1, for which Kif2A stimulated Arf GAP activity and ASAP1, where NM2A stimulated GAP activity. Furthermore, there is no evidence for an effect of ADAP1 on Kif13b, whereas AGAP1 positively affects Kif2A ATPase activity and ASAP1 may positively affect NM2A activity (Heissler, Chen, Randazzo and Sellers, unpublished).
A network of interactions could coordinate Arf GAP and motor activities to bring about a specific cellular behavior. For instance, ASAP1/NM2A and AGAP1/Kif2A function could be simultaneously controlled by Arf1•GTP and phosphoinositides. Actin remodeling and membrane traffic would, consequently, be coordinately regulated to control adhesion dynamics (Fig. 6). In this model, FAs are stabilized by concurrently increasing NM2 activity and decreasing microtubule-induced endocytosis of integrins, owing to the ASAP1/NM2A and AGAP1/Kif2A interactions. The network of Arf GAPs and molecular motors could potentially exert more complex control, with one Arf GAP associating with more than one motor, one motor associating with more than one Arf GAP, imparting diverse consequences to the biochemical functions of the proteins.
Figure 6.
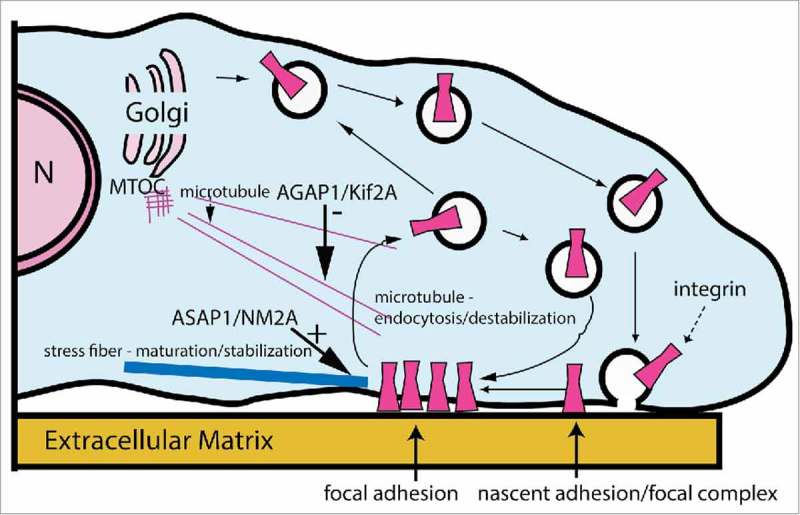
ASAP1 and AGAP1 cooperate to stabilize FAs. In this speculative model, ASAP1 association with NM2A stabilizes NM2A association with actin filaments at the contact sites between FAs and actomyosin stress fibers, which stabilizes FAs. At the same time, AGAP1 functions through Kif2A to destabilize microtubules, preventing the contact of microtubules with FAs, contributing to stabilization of the FAs by preventing endocytosis of integrins that accompanies FA turnover. N, nucleus.
Regardless of the specifics of the mechanisms, the association of Arf GAPs with either or both actin and microtubule motors could help explain the cellular activity of the Arf GAPs to control specific membrane and actin remodeling events and is the basis for hypotheses related to connections between the actin and microtubule based cytoskeletons. The possible connections of the ASAP1/NM2A and the AGAP1/Kif2A interactions in controlling FAs are described above. Other intriguing possibilities include ASAP family members that may bind to both kinesins and NM2A through their BAR domains. Possible mechanisms by which cell behavior could be affected will depend on the specific motors and whether the motors can bind simultaneously or mutually exclusively. Also important to learn will be the role of Arf and phospholipid binding to the Arf GAP for controlling binding of the motors. One would anticipate such an effect given that motors affect GAP activity – by the principle of microreversibility – and could be a means by which Arf could coordinately affect the actin and microtubule cytoskeletons.
Abbreviations
- ADAP1
ArfGAP With Dual PH Domains 1
- Arf
ADP-ribosylation factor
- Arf GAP
Arf GTPase-activating protein
- Arf GEF
Arf guanine nucleotide exchange factor
- Ank
Ankyrin repeat
- BAR
Bin/Amphiphysin/Rvs
- BIG
Brefeldin A‐Inhibited Guanine Nucleotide‐Exchange Factor
- BRAG2
Brefeldin A resistant Arf GEF
- Crk
CT10 regulator of a tyrosine kinase
- FA
focal adhesion
- FAK
focal adhesion kinase
- CDR
circular dorsal ruffle
- EGF
epidermal growth factor
- ELC
essential light chain
- FIP3
RAB11 Family Interacting Protein 3
- GIT
G-protein-coupled receptor kinase interactor-1
- MAP4K4
Mitogen-Activated Protein Kinase Kinase Kinase Kinase 4
- MKLP1
Mitotic kinesin-Like protein 1
- MMP
matrix metalloproteinase
- MT
microtubule
- NBR1
neighbor of BRCA1 gene 1
- NM2
non-muscle myosin 2
- PH
pleckstrin-homology domain
- PI(4,5)P2
Phosphatidylinositol 4,5-bisphosphate
- PP1,Src p56lck
p59fynT inhibitor
- Rack1
Receptor for activated C kinase1
- RLC
regulatory light chain
- Rock1
Rho associated coiled-coil containing protein kinase 1
- SH3
SRC Homology 3 Domain
- Src
Rous Sarcoma virus family Non-Receptor Tyrosine Kinase
Acknowledgments
We thank Marielle E. Yohe for insightful discussions and critical review of the manuscript and James Sellers and Sarah Heissler for insightful discussions. The work was supported by the intramural program of the National Institutes of Health (Project BC 007365) and by GM069429 to LW.
References
- [1].Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 2006; 172:645–50; PMID:16505163; http://dx.doi.org/ 10.1083/jcb.200512057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gillingham AK, Munro S. The small G proteins of the arf family and their regulators. Ann Rev Cell Dev Biol 2007; 23:579–611; http://dx.doi.org/ 10.1146/annurev.cellbio.23.090506.123209 [DOI] [PubMed] [Google Scholar]
- [3].D'Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 2006; 7:347–58; PMID:16633337; http://dx.doi.org/ 10.1038/nrm1910 [DOI] [PubMed] [Google Scholar]
- [4].Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 2011; 12:362–75; PMID:21587297; http://dx.doi.org/ 10.1038/nrm3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kahn RA, Bruford E, Inoue H, Logsdon JM, Nie ZZ, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, et al. . Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol 2008; 182:1039–44; PMID:18809720; http://dx.doi.org/ 10.1083/jcb.200806041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic 2007; 8:1465–75; PMID:17666108; http://dx.doi.org/ 10.1111/j.1600-0854.2007.00624.x [DOI] [PubMed] [Google Scholar]
- [7].Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling and the cytoskeleton. Curr Opin Cell Biol 2001; 13:593–9; PMID:11544028; http://dx.doi.org/ 10.1016/S0955-0674(00)00256-8 [DOI] [PubMed] [Google Scholar]
- [8].Sabe H, Hashimoto S, Morishige M, Ogawa E, Hashimoto A, Nam JM, Miura K, Yano H, Onodera Y. The EGFR-GEP100-Arf6-AMAP1 Signaling Pathway Specific to Breast Cancer Invasion and Metastasis(dagger). Traffic 2009; 10:982–93; PMID:19416474; http://dx.doi.org/ 10.1111/j.1600-0854.2009.00917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sabe H, Onodera Y, Mazaki Y, Hashimoto S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr Opin Cell Biol 2006; 18:558–64; PMID:16904307; http://dx.doi.org/ 10.1016/ceb.2006.08.002 [DOI] [PubMed] [Google Scholar]
- [10].Nie ZZ, Fei JJ, Premont RT, Randazzo PA. The Arf GAPs AGAP1 and AGAP2 distinguish between the adaptor protein complexes AP-1 and AP-3. J Cell Sci 2005; 118:3555–66; PMID:16079295; http://dx.doi.org/ 10.1242/jcs.02486 [DOI] [PubMed] [Google Scholar]
- [11].Wu YJ, Zhao Y, Ma XJ, Zhu YJ, Patel J, Nie ZZ. The Arf GAP AGAP2 interacts with beta-arrestin2 and regulates beta(2)-adrenergic receptor recycling and ERK activation. Biochem J 2013; 452:411–21; PMID:23527545; http://dx.doi.org/ 10.1042/BJ20121004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhu YJ, Wu YJ, Kim JI, Wang ZM, Daaka Y, Nie ZZ. Arf GTPase-activating Protein AGAP2 Regulates Focal Adhesion Kinase Activity and Focal Adhesion Remodeling. J Biol Chem 2009; 284:13489–96; PMID:19318351; http://dx.doi.org/ 10.1074/jbc.M900469200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lee SY, Yang JS, Hong WJ, Premont RT, Hsu VW. ARFGAP1 plays a central role in coupling COPI cargo sorting with vesicle formation. J Cell Biol 2005; 168:281–90; PMID:15657398; http://dx.doi.org/ 10.1083/jcb.200404008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang JS, Lee SY, Gao MG, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol 2002; 159:69–78; PMID:12379802 http://dx.doi.org/ 10.1083/jcb.200206015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J, Peters PJ, Bai M, Dai J, Bos E, Kirchhausen T, Kandror KV, Hsu VW. An ACAP1-containing clathrin coat complex for endocytic recycling. J Cell Biol 2007; 178:453–64; PMID:17664335; http://dx.doi.org/ 10.1083/jcb.200608033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen PW, Jian X, Heissler SM, Le K, Luo R, Jenkins LM, Nagy A, Moss J, Sellers JR, Randazzo PA. The Arf GTPase-activating Protein, ASAP1, Binds Nonmuscle Myosin 2A to Control Remodeling of the Actomyosin Network. J Biol Chem 2016; 291:7517–26; PMID:26893376;http://dx.doi.org/ 10.1074/jbc.M115.701292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Luo R, Chen PW, Wagenbach M, Jian X, Jenkins L, Wordeman L, Randazzo PA. Direct Functional Interaction of the Kinesin-13 family membrane Kinesin Like Protein 2A (Kif2A) and Arf GAP with GTP-Binding Protein-Like, Ankyrin Repeats and PH domains1 (AGAP1). J Biol Chem 2016; 291:21350–62; http://dx.doi.org/ 10.1074/jbc.M116.732479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 2009; 10:682–96; PMID:19773780; http://dx.doi.org/ 10.1038/nrm2774 [DOI] [PubMed] [Google Scholar]
- [19].Sellers JR, Goodson HV, Wang F. A myosin family reunion. J Mus Res Cell Mot 1996; 17:7–22; http://dx.doi.org/ 10.1007/BF00140320 [DOI] [PubMed] [Google Scholar]
- [20].Hirokawa N, Takemura R. Kinesin superfamily proteins and their various functions and dynamics. Exp Cell Res 2004; 301:50–9; PMID:15501445; http://dx.doi.org/ 10.1016/j.yexcr.2004.08.010 [DOI] [PubMed] [Google Scholar]
- [21].Brown MT, Andrade J, Radhakrishna H, Donaldson JG, Cooper JA, Randazzo PA. ASAP1, a phospholipid-dependent Arf GTPase-activating protein that associates with and is phosphorylated by Src. Mol Cell Biol 1998; 18:7038–51; PMID:9819391; http://dx.doi.org/ 10.1128/MCB.18.12.7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton. Proc Natl Acad Sci USA 2000; 97:4011–6; PMID:10725410; http://dx.doi.org/ 10.1073/pnas.070552297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bharti S, Inoue H, Bharti K, Hirsch DS, Nie Z, Yoon HY, Artym V, Yamada KA, Mueller SC, Barr VA, et al. . Src-dependent phosphorylation of ASAP1 regulates podosomes. Mol Cell Biol 2007; 27:8271–83; PMID:17893324; http://dx.doi.org/ 10.1128/MCB.01781-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Onodera Y, Hashimoto S, Hashimoto A, Morishige M, Mazaki Y, Yamada A, Ogawa E, Adachi M, Sakurai T, Manabe T, et al. . Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J 2005; 24:963–73; PMID:15719014; http://dx.doi.org/ 10.1038/sj.emboj.7600588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu YH, Loijens JC, Martin KH, Karginov AV, Parsons JT. The association of ASAP1, an ADP ribosylation factor-GTPase activating protein, with focal adhesion kinase contributes to the process of focal adhesion assembly. Mol Biol Cell 2002; 13:2147–56; PMID:12058076; http://dx.doi.org/ 10.1091/mbc.E02-01-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Liu YH, Yerushalmi GM, Grigera PR, Parsons JT. Mislocalization or reduced expression of arf GTPase-activating protein ASAP1 inhibits cell spreading and migration by influencing Arf1 GTPase cycling. J Biol Chem 2005; 280:8884–92; PMID:15632162; http://dx.doi.org/ 10.1074/jbc.M412200200 [DOI] [PubMed] [Google Scholar]
- [27].Oda A, Wada I, Miura K, Okawa K, Kadoya T, Kato T, Nishihara H, Maeda M, Tanaka S, Nagashima K, et al. . CrkL directs ASAP1 to peripheral focal adhesions. J Biol Chem 2003; 278:6456–60; PMID:12522101; http://dx.doi.org/ 10.1074/jbc.M210817200 [DOI] [PubMed] [Google Scholar]
- [28].Ehlers JP, Worley L, Onken MD, Harbour JW. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin Cancer Res 2005; 11:3609–13; PMID:15897555; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-1941 [DOI] [PubMed] [Google Scholar]
- [29].Lin D, Watahiki A, Bayani J, Zhang F, Liu L, Ling V, Sadar MD, English J, Fazli L, So A, et al. . ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res 2008; 68:4352–9; PMID:18519696; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5237 [DOI] [PubMed] [Google Scholar]
- [30].Buffart TE, Coffa J, Hermsen M, Carvalho B, van der Sijp JRM, Ylstra B, Pals G, Schouten JP, Meijer GA. DNA copy number changes at 8q11-24 in metastasized colorectal cancer. Cell Oncol 2005; 27:57–65; PMID:15750208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hou T, Yang C, Tong C, Zhang H, Xiao J, Li J. Overexpression of ASAP1 is associated with poor prognosis in epithelial ovarian cancer. Int J Clin Exp Path 2014; 7:280–7. [PMC free article] [PubMed] [Google Scholar]
- [32].Onodera Y, Nam JM, Hashimoto A, Norman JC, Shirato H, Hashimoto S, Sabe H. Rab5c promotes AMAP1-PRKD2 complex formation to enhance beta 1 integrin recycling in EGF-induced cancer invasion. J Cell Biol 2012; 197:983–96; PMID:22734003; http://dx.doi.org/ 10.1083/jcb.201201065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vicente-Manzanares M, Ma XF, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 2009; 10:778–90; PMID:19851336; http://dx.doi.org/ 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heissler SM, Manstein DJ. Nonmuscle myosin-2: mix and match. Cell Mol Life Sci 2013; 70:1–21; PMID:22565821; http://dx.doi.org/ 10.1007/s00018-012-1002-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Aguilar-Cuenca R, Juanes-Garcia A, Vicente-Manzanares M. Myosin II in mechanotransduction: master and commander of cell migration, morphogenesis, and cancer. Cell Mol Life Sci 2014; 71:479–92; PMID:23934154; http://dx.doi.org/ 10.1007/s00018-013-1439-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Tojkander S, Gateva G, Schevzov G, Hotulainen P, Naumanen P, Martin C, Gunning PW, Lappalainen P. A molecular pathway for myosin II recruitment to stress fibers. Curr Biol 2011; 21:539–50; PMID:21458264; http://dx.doi.org/ 10.1016/j.cub.2011.03.007 [DOI] [PubMed] [Google Scholar]
- [37].Le K, Li CC, Ye G, Moss J, Vaughan M. Arf guanine nucleotide-exchange factors BIG1 and BIG2 regulate nonmuscle myosin IIA activity by anchoring myosin phosphatase complex. Proc Natl Acad Sci USA 2013; 110:E3162–E70; PMID:23918382; http://dx.doi.org/ 10.1073/pnas.1312531110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schlienger S, Campbell S, Claing A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol Biol Cell 2014; 25:17–29; PMID:24196838; http://dx.doi.org/ 10.1091/mbc.E13-06-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol 1998; 143:1981–95; PMID:9864369; http://dx.doi.org/ 10.1083/jcb.143.7.1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jian XY, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA. Autoinhibition of Arf GTPase-activating Protein Activity by the BAR Domain in ASAP1. J Biol Chem 2009; 284:1652–63; PMID:19017632; http://dx.doi.org/ 10.1074/jbc.M804218200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rao Y, Ma Q, Vahedi-Faridi A, Sundborger A, Pechstein A, Puchkov D, Luo L, Shupliakov O, Saenger W, Haucke V. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc Natl Acad Sci U S A 2010; 107:8213–8; PMID:20404169; http://dx.doi.org/ 10.1073/pnas.1003478107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating Protein ASAP1 Interacts with Rab11 Effector FIP3 and Regulates Pericentrosomal Localization of Transferrin Receptor-positive Recycling Endosome. Mol Biol Cell 2008; 19:4224–37; PMID:18685082; http://dx.doi.org/ 10.1091/mbc.E08-03-0290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kam JL, Miura K, Jackson TR, Gruschus J, Roller P, Stauffer S, Clark J, Aneja R, Randazzo PA. Phosphoinositide-dependent activation of the ADP-ribosylation factor GTPase-activating protein ASAP1 - Evidence for the pleckstrin homology domain functioning as an allosteric site. J Biol Chem 2000; 275:9653–63; PMID:10734117; http://dx.doi.org/ 10.1074/jbc.275.13.9653 [DOI] [PubMed] [Google Scholar]
- [44].Che MM, Boja ES, Yoon HY, Gruschus J, Jaffe H, Stauffer S, Schuck P, Fales HM, Randazzo PA. Regulation of ASAP1 by phospholipids is dependent on the interface between the PH and Arf GAP domains. Cell Signal 2005; 17:1276–88; PMID:16038802; http://dx.doi.org/ 10.1016/j.cellsig.2005.01.007 [DOI] [PubMed] [Google Scholar]
- [45].Jian X, Tang WK, Zhai P, Roy NS, Luo R, Gruschus JM, Yohe ME, Chen PW, Li Y, Byrd RA, et al. . Molecular Basis for Cooperative binding of Anionic Phospholipids to the PH Domain of the Arf GAP ASAP1. Structure 2015; 23:1977–88; PMID:26365802; http://dx.doi.org/ 10.1016/j.str.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Randazzo PA, Kahn RA. GTP Hydrolysis by ADP-Ribosylation Factor Is Dependent On Both an ADP-Ribosylation Factor GTPase-Activating Protein and Acid Phospholipids. J Biol Chem 1994; 269:10758–63; PMID:8144664 [PubMed] [Google Scholar]
- [47].Reversi A, Loeser E, Subramanian D, Schultz C, De Renzis S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J Cell Biol 2014; 205:395–408; PMID:24798734; http://dx.doi.org/ 10.1083/jcb.201309079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ha VL, Bharti S, Inoue H, Vass WC, Campa F, Nie ZZ, de Gramont A, Ward Y, Randazzo PA. ASAP3 is a focal adhesion-associated Arf GAP that functions in cell migration and invasion. J Biol Chem 2008; 283:14915–26; PMID:18400762; http://dx.doi.org/ 10.1074/jbc.M709717200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jackson TR, Brown FD, Nie ZZ, Miura K, Foroni L, Sun JL, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are Arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol 2000; 151:627–38; PMID:11062263; http://dx.doi.org/ 10.1083/jcb.151.3.627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nie ZZ, Stanley KT, Stauffer S, Jacques KM, Hirsch DS, Takei J, Randazzo PA. AGAP1, an endosome-associated, phosphoinositide-dependent ADP-ribosylation factor GTPase-activating protein that affects actin cytoskeleton. J Biol Chem 2002; 277:48965–75; PMID:12388557; http://dx.doi.org/ 10.1074/jbc.M202969200 [DOI] [PubMed] [Google Scholar]
- [51].Nie ZZ, Boehm M, Boja ES, Vass WC, Bonifacino JS, Fales HM, Randazzo PA. Specific regulation of the adaptor protein complex AP-3 by the Arf GAP AGAP1. Dev Cell 2003; 5:513–21; PMID:12967569; http://dx.doi.org/ 10.1016/S1534-5807(03)00234-X [DOI] [PubMed] [Google Scholar]
- [52].Bendor J, Lizardi-Ortiz JE, Westphalen RI, Brandstetter M, Hemmings HC, Sulzer D, Flajolet M, Greengard P. AGAP1/AP-3-dependent endocytic recycling of M-5 muscarinic receptors promotes dopamine release. EMBO J 2010; 29:2813–26; PMID:20664521; http://dx.doi.org/ 10.1038/emboj.2010.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dwane S, Durack E, O'Connor R, Kiely PA. RACK1 promotes neurite outgrowth by scaffolding AGAP2 to FAK. Cell Signal 2014; 26:9–18; PMID:24056044; http://dx.doi.org/ 10.1016/j.cellsig.2013.08.036 [DOI] [PubMed] [Google Scholar]
- [54].Ahn JY, Hu YX, Kroll TG, Allard P, Ye KQ. PIKE-A is amplified in human cancers and prevents apoptosis by up-regulating Akt. Proc Natl Acad Sci USA 2004; 101:6993–8; PMID:15118108; http://dx.doi.org/ 10.1073/pnas.0400921101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Devreotes P, Horwitz AR. Signaling networks that regulate cell migration. Cold Spring Harbor Perspectives Biol 2015; 7:a005959; PMID:26238352; http://dx.doi.org/ 10.1101/cshperspect.a005959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Broussard JA, Lin WH, Majumdar D, Anderson B, Eason B, Brown CM, Webb DJ. The endosomal adaptor protein APPL1 impairs the turnover of leading edge adhesions to regulate cell migration. Mol Biol Cell 2012; 23:1486–99; PMID:22379109; http://dx.doi.org/ 10.1091/mbc.E11-02-0124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol 2004; 6:154–61; PMID:14743221; http://dx.doi.org/ 10.1038/ncb1094 [DOI] [PubMed] [Google Scholar]
- [58].Walczak CE, Gayek S, Ohi R. Microtubule-Depolymerizing Kinesins. In: Schekman R, ed. Ann Rev Cell Dev Biol 2013; 29:417–41; http://dx.doi.org/ 10.1146/annurev-cellbio-101512-122345 [DOI] [PubMed] [Google Scholar]
- [59].Maney T, Wagenbach M, Wordeman L. Molecular dissection of the microtubule depolymerizing activity of mitotic centromere-associated kinesin. J Biol Chem 2001; 276:34753–8; PMID:11466324; http://dx.doi.org/ 10.1074/jbc.M106626200 [DOI] [PubMed] [Google Scholar]
- [60].Helenius J, Brouhard G, Kalaidzidis Y, Diez S, Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature 2006; 441:115–9; PMID:16672973; http://dx.doi.org/ 10.1038/nature04736 [DOI] [PubMed] [Google Scholar]
- [61].Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, Gordon-Weeks P, Galjart N, Bornens M, Wordeman L, et al. . Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol 2006; 174:839–49; PMID:16954346; http://dx.doi.org/ 10.1083/jcb.200512058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Domnitz SB, Wagenbach M, Decarreau J, Wordeman L. MCAK activity at microtubule tips regulates spindle microtubule length to promote robust kinetochore attachment. J Cell Biol 2012; 197:231–7; PMID:22492725; http://dx.doi.org/ 10.1083/jcb.201108147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell 2004; 15:1146–59; PMID:14699064; http://dx.doi.org/ 10.1091/mbc.E03-08-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Homma N, Takei Y, Tanaka Y, Nakata T, Terada S, Kikkawa M, Noda Y, Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell 2003; 114:229–39; PMID:12887924; http://dx.doi.org/ 10.1016/S0092-8674(03)00522-1 [DOI] [PubMed] [Google Scholar]
- [65].Drum BM, Yuan C, Li L, Liu Q, Wordeman L, Santana LF. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J Mol Cell Cardiol 2016; 93:32–43; PMID:26902968; http://dx.doi.org/ 10.1016/j.yjmcc.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wilbur JD, Heald R. Mitotic spindle scaling during Xenopus development by kif2a and importin alpha. Elife 2013; 2:e00290; PMID:23425906; http://dx.doi.org/ 10.7554/eLife.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Eagleson G, Pfister K, Knowlton AL, Skoglund P, Keller R, Stukenberg PT. Kif2a depletion generates chromosome segregation and pole coalescence defects in animal caps and inhibits gastrulation of the Xenopus embryo. Mol Biol Cell 2015; 26:924–37; PMID:25568341; http://dx.doi.org/ 10.1091/mbc.E13-12-0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mennella V, Rogers GC, Rogers SL, Buster DW, Vale RD, Sharp DJ. Functionally distinct kinesin-13 family members cooperate to regulate microtubule dynamics during interphase. Nat Cell Biol 2005; 7:235–45; PMID:15723056; http://dx.doi.org/ 10.1038/ncb1222 [DOI] [PubMed] [Google Scholar]
- [69].Santama N, Krijnse-Locker J, Griffiths G, Noda Y, Hirokawa N, Dotti CG. KIF2 beta, a new kinesin superfamiIy protein in non-neuronal cells, is associated with lysosomes and may be implicated in their centrifugal translocation. EMBO J 1998; 17:5855–67; PMID:9774330; http://dx.doi.org/ 10.1093/emboj/17.20.5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Miyamoto T, Hosoba K, Ochiai H, Royba E, Izumi H, Sakuma T, Yamamoto T, Dynlacht BD, Matsuura S. The Microtubule-Depolymerizing activity of a Mitotic Kinesin protein KIF2A drives primary Cilia Disassembly coupled with cell proliferation. Cell Rep 2015; 10:664–73; http://dx.doi.org/ 10.1016/j.celrep.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang CQ, Qu X, Zhang XY, Zhou CJ, Liu GX, Dong ZQ, Wei FC, Sun SZ. Overexpression of Kif2a promotes the progression and metastasis of squamous cell carcinoma of the oral tongue. Oral Oncology 2010; 46:65–9; PMID:20005768; http://dx.doi.org/ 10.1016/j.oraloncology.2009.11.003 [DOI] [PubMed] [Google Scholar]
- [72].Wang J, Ma S, Ma R, Qu X, Liu W, Lv C, Zhao S, Gong Y. KIF2A silencing inhibits the proliferation and migration of breast cancer cells and correlates with unfavorable prognosis in breast cancer. Bmc Cancer 2014; 14:461; PMID:24950762; http://dx.doi.org/ 10.1186/1471-2407-14-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zaganjor E, Osborne JK, Weil LM, Diaz-Martinez LA, Gonzales JX, Singel SM, Larsen JE, Girard L, Minna JD, Cobb MH. Ras regulates kinesin 13 family members to control cell migration pathways in transformed human bronchial epithelial cells. Oncogene 2014; 33:5457–66; PMID:24240690; http://dx.doi.org/ 10.1038/onc.2013.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Boman AL, Kuai J, Zhu XJ, Chen J, Kuriyama R, Kahn RA. Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Mot Cyto 1999; 44:119–32; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- [75].Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol 2009; 7:e1000110; PMID:19468300; http://dx.doi.org/ 10.1371/journal.pbio.1000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, Zhang C, Shokat KM, Carr SA, Yaffe MB, et al. . Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol 2009; 7:e1000111; PMID:19468302; http://dx.doi.org/ 10.1371/journal.pbio.1000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sharp DJ, Kuriyama R, Baas PW. Expression of a kinesin-related motor protein induces Sf9 cells to form dendrite-like processes with nonuniform microtubule polarity orientation. J Neurosci 1996; 16:4370–5; PMID:8699247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a microtubule-associated motor protein essential for dendritic differentiation. J Cell Biol 1997; 138:833–43; PMID:9265650; http://dx.doi.org/ 10.1083/jcb.138.4.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Shen XY, Meza-Carmen V, Puxeddu E, Wang GH, Moss J, Vaughan M. Interaction of brefeldin A-inhibited guanine nucleotide-exchange protein (BIG) 1 and kinesin motor protein KIF21A. Proc Natl Acad Sci USA 2008; 105:18788–93; PMID:19020088; http://dx.doi.org/ 10.1073/pnas.0810104105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Venkateswarlu K, Hanada T, Chishti AH. Centaurin-alpha(1) interacts directly with kinesin motor protein KIF13B. J Cell Sci 2005; 118:2471–84; PMID:15923660; http://dx.doi.org/ 10.1242/jcs.02369 [DOI] [PubMed] [Google Scholar]
- [81].Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol 2005; 7:581–90; PMID:15895076; http://dx.doi.org/ 10.1038/ncb1262 [DOI] [PubMed] [Google Scholar]
- [82].Nader GPF, Ezratty EJ, Gundersen GG. FAK, talin and PIPKI[gamma] regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol 2016; 18:491–503; PMID:27043085; http://dx.doi.org/ 10.1038/ncb3333 [DOI] [PubMed] [Google Scholar]
- [83].Wu X, Kodama A, Fuchs E. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell 2008; 135:137–48; PMID:18854161; http://dx.doi.org/ 10.1016/j.cell.2008.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Yue J, Xie M, Gou X, Lee P, Schneider MD, Wu X. Microtubules regulate focal adhesion dynamics through MAP4K4. Dev Cell 2014; 31:572–85; PMID:25490267; http://dx.doi.org/ 10.1016/j.devcel.2014.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Rondé P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci 2005; 118:4415; PMID:16159962; http://dx.doi.org/ 10.1242/jcs.02565 [DOI] [PubMed] [Google Scholar]
- [86].Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res 2000; 261:44–51; PMID:11082274; http://dx.doi.org/ 10.1006/excr.2000.5046 [DOI] [PubMed] [Google Scholar]
- [87].Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003; 4:446–56; PMID:12778124; http://dx.doi.org/ 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- [88].Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 2004; 303:836–9; PMID:14764879; http://dx.doi.org/ 10.1126/science.1091325 [DOI] [PubMed] [Google Scholar]
- [89].Kaverina I, Straube A. Regulation of cell migration by dynamic microtubules. Seminars Cell Dev Biol 2011; 22:968–74; http://dx.doi.org/ 10.1016/j.semcdb.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Alanko J, Mai A, Jacquemet G, Schauer K, Kaukonen R, Saari M, Goud B, Ivaska J. Integrin endosomal signalling suppresses anoikis. Nat Cell Biol 2015; 17:1412–21; PMID:26436690; http://dx.doi.org/ 10.1038/ncb3250 [DOI] [PMC free article] [PubMed] [Google Scholar]


