Abstract
Background:
The management of asthma has changed since the introduction of budesonide/formoterol (Symbicort®) as both maintenance and reliever therapy (SMART). SMART and its effects on bronchial hyperresponsiveness (BHR) have not been studied in primary care.
Aims:
To compare the effects of SMART and guideline-driven usual care (UC) on BHR and clinical asthma severity in primary care practice.
Methods:
Patients with mild-to-moderate stable asthma were randomised to receive SMART treatment (n=54) (budesonide/formoterol 80/4.5μg Turbuhaler®, two puffs once daily and extra inhalations as needed) or UC treatment (n=48) for 12 months. Diary data, Asthma Control Questionnaire scores, forced expiratory volume in 1 second (FEV1), and peak expiratory flow (PEF) measurements were collected during run-in and after 1, 3, 6, and 12 months of treatment. BHR, measured as the dose of histamine provoking a fall in FEV1 of 20% (PD20-histamine), was determined at randomisation and after 12 months.
Results:
One hundred and two patients with asthma participated in the study. The change in PD20-histamine during the study was not significantly different between the SMART and UC groups (p=0.26). The mean inhaled corticosteroid (ICS) dose was 326μg beclomethasone dipropionate (BDP) equivalents/day (95% CI 254 to 399) with SMART, which was significantly lower (p<0.0001) than the mean ICS dose with UC treatment (798μg BDP equivalents/day (95% CI 721 to 875). Morning and evening PEF values increased significantly with SMART treatment compared with UC; FEV1, symptoms and asthma control did not differ.
Conclusions:
Despite a 59% lower dose of ICS, BHR and other clinical outcomes remained stable during SMART treatment while PEF values improved.
Keywords: asthma, management, primary care, randomised controlled trial, SMART, bronchial hyperresponsiveness
Introduction
Asthma is a chronic inflammatory disorder of the airways associated with airway hyperresponsiveness that leads to recurrent respiratory symptoms.1 Long-term anti-inflammatory treatment with inhaled corticosteroids (ICS) is the cornerstone of therapy in persistent asthma. It reduces asthma symptoms and numbers of exacerbations, improves lung function, and is accompanied by a reduction in airway inflammation.1–3 Several studies have shown that adding a long-acting β2-agonist (LABA) to ICS may be beneficial in asthma management. This has led to the development of fixed combination inhalers of ICS and LABA.4–7
A recent development in the management of asthma is the use of the combination inhaler budesonide/formoterol (Symbicort®) both as Maintenance And as Reliever Therapy (SMART) (Symbicort® SMART®, AstraZeneca, Sweden). This approach is possible because formoterol provides rapid symptom relief, making use of a separate short acting β2-agonist redundant.8–11 When taking an extra puff for symptom relief, an additional dose of ICS and formoterol is administered simultaneously, thereby preventing a deterioration or even an exacerbation.12 Studies testing the SMART concept have shown beneficial effects on the time to first asthma exacerbation, exacerbation rate, and number of days with asthma control compared with other treatment regimens. Importantly, this approach reduces the ICS dose needed to remain in a stable state of disease.13–18
The effects of the SMART concept on bronchial hyperresponsiveness (BHR) — a hallmark of asthma — have not yet been investigated, nor has it been studied in primary care. This is of importance since primary care physicians manage the largest proportion of patients with asthma (up to 75%). These patients generally have much milder disease than patients treated in secondary care.19
This study compares the effects of budesonide/formoterol SMART on BHR with usual care (UC) treatment based on Global Initiative for Asthma (GINA) guidelines in patients with mild-to-moderate asthma in primary care.1 We anticipated that BHR with SMART would be better than with UC, or equal to UC but with a lower dose of ICS with SMART. Secondary outcomes were asthma control, exacerbations, symptoms and lung function, Asthma Control Questionnaire (ACQ-5), Satisfaction with Asthma Treatment Questionnaire (SATQ), and mean dose of ICS used.20–22
Methods
Ethical approval
The study was approved by the Medical Ethics Committee of the University Medical Center Groningen, The Netherlands (METc 2003/111). All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines
Patients
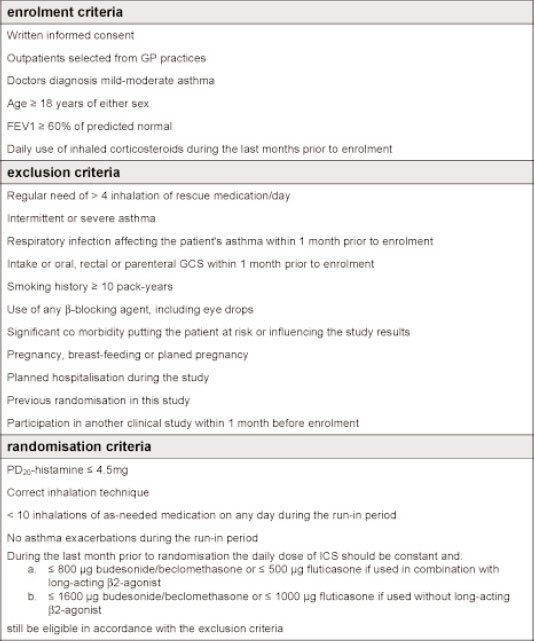
Patients with mild-to-moderate persistent asthma of either sex aged ≥18 years were selected from 32 general practices. The complete list of exclusion, enrolment and randomisation criteria are presented in Appendix 1 (available online at www.thepcrj.org). After the run-in period (Figure 1), patients were stratified according to the daily dose of ICS (<800μg vs. ≥800μg budesonide/beclomethasone dipropionate (BDP) or <500μg vs. ≥500μg fluticasone propionate) and dose of histamine provoking a fall in forced expiratory volume in 1 second (FEV1) of 20% (PD20-histamine; >1mg vs. ≤1 mg) to create balanced groups.
Figure 1. Study flow chart, V = visit, T = telephone contact.
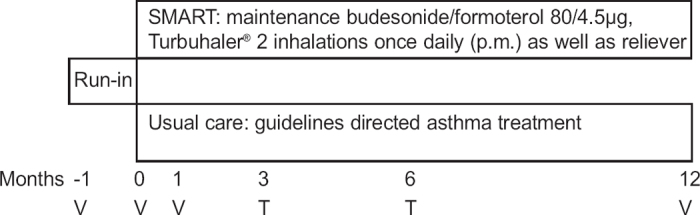
Study design and flowchart
The study was designed as an open, parallel-group, multicentre study conducted in two centres. After a 4-week run-in period, eligible patients were randomly assigned to treatment according to SMART or treatment based on GINA guidelines (UC group).1
Patients randomised to SMART treatment were instructed to take two inhalations of the lowest available budesonide/formoterol dose of 80/4.5μg once daily in the evening and additional inhalations for symptom relief as replacement for a short-acting β2-agonist. For safety reasons, patients were instructed to contact the investigator if ≥12 inhalations were needed on two consecutive days. No other asthma medication was allowed.
Patients in the UC group continued medication as before randomisation. They were treated as usual by their general practitioner (GP). Patients in both groups were instructed to contact their GP in case of a (threatening) exacerbation. Their GP treated the exacerbation but was not allowed to change the maintenance treatment in the SMART group.
Figure 1 shows the flowchart of the study. No scheduled contacts took place during the last 6 months of the study. We therefore kept contacts between investigators and patients as limited as possible as a reflection of the ‘real-life’ character of the study.
Data collection and procedures
BHR measured by PD20-histamine was the primary outcome. Histamine provocation, lung function, and reversibility tests were performed according to European Respiratory Society (ERS) guidelines by the same lung function assistant.23
PD20-histamine was assessed using the dosimeter method at randomisation and after 12 months of treatment. The second test was performed at the same time of the day (within 1 hour) as the baseline test and was postponed if the pre-challenge FEV1 was <60% of predicted normal, <1.5L, or differed by ≥15% from baseline. The PD20-histamine was calculated from a loghistamine dose versus the percentage fall in FEV1 by linear interpolation. PD20-histamine is the calculated dose in mg at which an exact fall of 20% would have been observed. In case a fall of 20% was reached at inhalation of saline, PD20 was arbitrarily set at 0.005mg. In case a fall of 20% was reached at the lowest dose (0.02mg), PD20 was arbitrarily set at 0.01mg. Since PD20 data are not normally distributed, they were transformed logarithmically with 2 as base. The differences or changes were expressed as ‘doubling dose steps’ where a difference of 1 means a doubling or halving of the PD20 value. FEV1 and forced vital capacity (FVC) measures were performed at randomisation and after 1 and 12 months of treatment. Reversibility was tested at the start of the run-in period using two inhalations of 0.5mg terbutaline (Bricanyl®, Turbuhaler®, AstraZeneca, Sweden).
Secondary outcomes were calculated from daily diary card data during run-in and 4 weeks prior to each clinic visit or telephone contact and from two questionnaires: the Asthma Control Questionnaire (ACQ-5,20,21 all clinic visits) and the Satisfaction with Asthma Treatment Questionnaire (SATQ, after 1 and 12 months of treatment).22
Patients recorded daily the best of three morning and evening peak expiratory flow (PEF) values, asthma symptoms during night and day (3-point scale where 0=no symptoms and 3=incapacitating symptoms), nights with awakenings due to asthma symptoms, total number of budesonide/formoterol inhalations (SMART group), and intake of maintenance and as-needed medication (UC group). Diary card data were also used to calculate the number of mild asthma exacerbation days (a day with either a morning PEF <80% of the run-in average value or a night with awakenings due to asthma symptoms), the number of asthma control days (a night and day with no asthma symptoms and a night with no awakenings due to asthma symptoms), the number of severe asthma exacerbations (deterioration in asthma resulting in hospitalisation or emergency room treatment or need for oral glucocorticosteroid treatment for at least 3 days), and the time to first severe asthma exacerbation.
Safety was investigated by assessing the nature, incidence, and severity of adverse events.
Statistical analysis
Efficacy analysis was carried out on all randomised patients. All tests performed were two-sided and a p value ≤0.05 was considered statistically significant. If necessary, ‘last observation carried forward’ was used to impute missing values for ACQ and SATQ scores at 12 months of treatment if a value existed after 6 months of treatment. The change in PD20-histamine before and after 12 months of treatment was analysed by ANOVA using a multiplicative model (i.e. analysing log-transformed PD20-histamine with treatment as fixed factor and log-transformed PD20-histamine at baseline as covariate). The least-squared means resulting from this model were used to estimate the treatment difference in log PD20-histamine and to calculate two-sided 95% confidence intervals (CI), which are presented as the anti-log.
Changes in FEV1 percentage predicted, overall ACQ scores, and in both the domain and total score of the SATQ after 12 months of treatment were analysed by ANOVA. The number of asthma control days was expressed as the percentage of the total number of days recorded in the diaries. Baseline values were calculated from diary recordings of the last 10 days of the run-in period.
The daily dose of ICS used was expressed in μg/day equivalents of beclomethasone dipropionate (BDP), taking an approximate equivalence of 1000μg/day BDP=800μg/day budesonide=500μg/day fluticasone propionate.24 The mean ICS dose in the SMART group was expressed as the mean dose over all days recorded in the diaries including both maintenance and as-needed inhalations. For the UC group, the ICS dose was the prescribed dose. All different types of inhaler devices were allowed in the UC group. Data were analysed by ANOVA using an additive model with treatment as fixed factor.
The number of mild asthma exacerbation days was expressed as a percentage of the number of diary dates and compared within and between the treatment groups using a Poisson regression model. Confidence limits and p values were adjusted for over dispersion. Adverse event data were analysed by descriptive statistics.
The power calculation was based on PD20-histamine and an ANOVA of the change in log (PD20-histamine). Based on other studies, the standard deviation for 2log(PD20-histamine) was expected to be 1.7.25 Under this assumption, an ANOVA with a two-sided alternative hypothesis and a significance level of 5% can detect a difference of one with 80% power, given that the study includes 50 patients per group. A difference of 1 in log(PD20-histamine) is equal to a difference of one doubling histamine dose.
Results
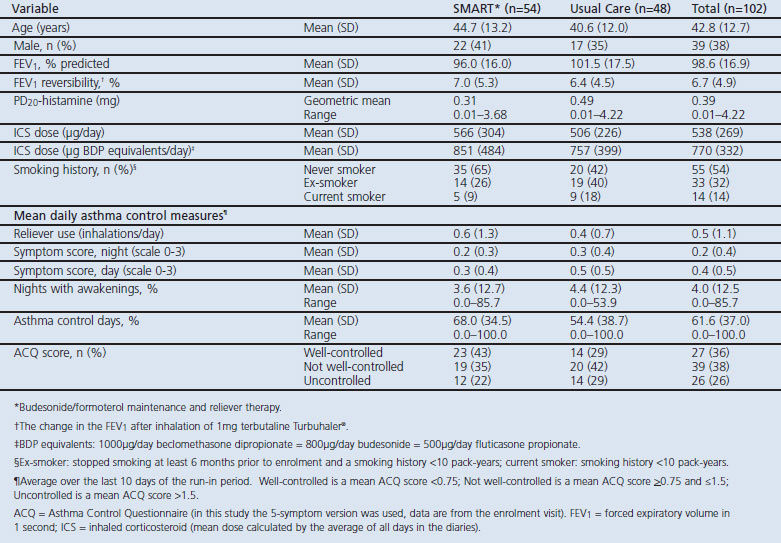
The enrolment of study subjects is summarised in Figure 2. Forty adverse events occurred in the SMART group and 42 occurred in the UC group. There were three serious adverse events, one in the SMART group (pulmonary embolism) and two in the UC group (gastritis and intervertebral disc protrusion). Four patients in the SMART group discontinued the study because of an adverse event: the abovementioned patient with a pulmonary embolism, one patient with palpitations and tremor, and two patients due to asthma deterioration. After 1 month of treatment, four patients were withdrawn because they did not fulfil eligibility criteria retrospectively. Table 1 shows the baseline characteristics of the study participants.
Figure 2. Enrolment of patients and completion of the study.
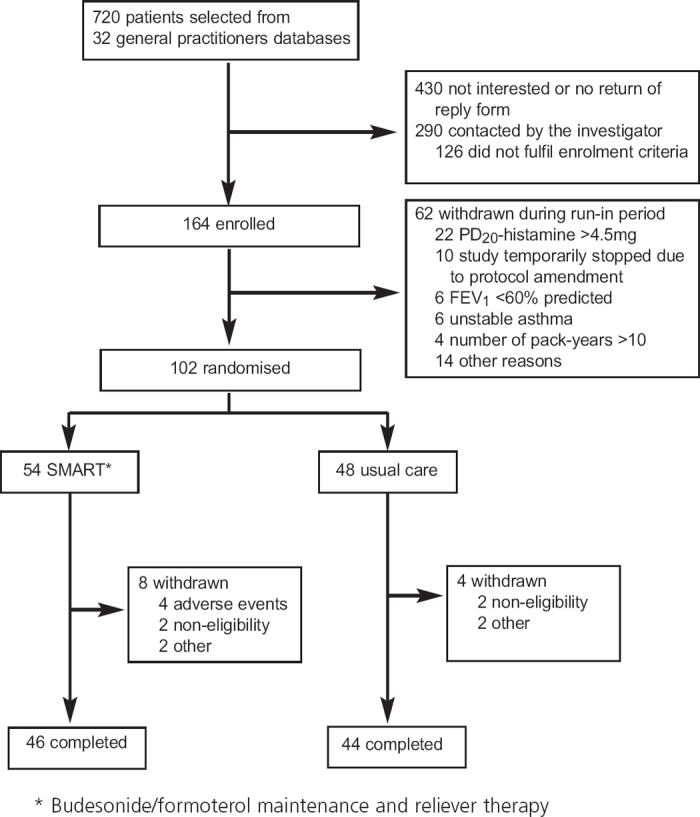
Table 1. Baseline clinical characteristics.
There was a small but clinically irrelevant improvement in PD20-histamine in both treatment groups. The ratio of PD20-histamine values at 12 months to randomisation in the SMART group was 1.05 (95% CI 0.70 to 1.56) compared with 1.46 (95% CI 0.95 to 2.22) in the UC group. The difference between the two treatment groups was not significant (SMART/UC ratio was 0.72 (95% CI 0.40 to 1.29, p=0.26)).
Table 2 summarises the outcomes of primary and secondary efficacy parameters, showing a significantly larger improvement in morning and evening PEF values and a higher rating of ease of use in the SMART group than in the UC group.
Table 2. Efficacy outcomes at the start and after 12 months of treatment in the SMART (* Budesonide/formoterol maintenance and reliever therapy) and Usual Care groups and efficacy outcomes of SMART compared with Usual Care after 12 months of treatment.
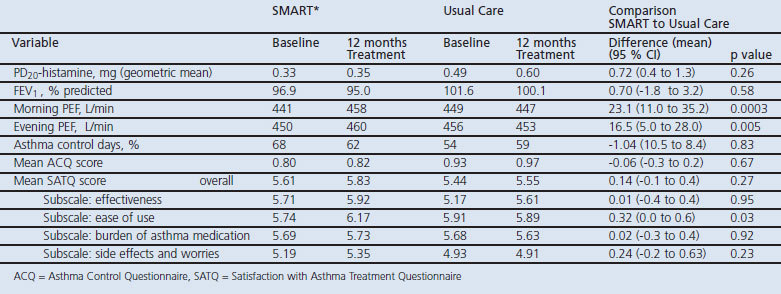
The number of days with a mild asthma exacerbation was not significantly different between SMART and UC treatment, averaging 16.4 and 16.8 mild exacerbation days/year, respectively (p=0.80). A total of 11 severe asthma exacerbations occurred in both treatment groups. Two patients in the SMART group experienced a total of four exacerbations, three occurring in one patient. Six patients in the UC group experienced a total of seven exacerbations. No asthma-related hospitalisations or emergency room treatments occurred in either group.
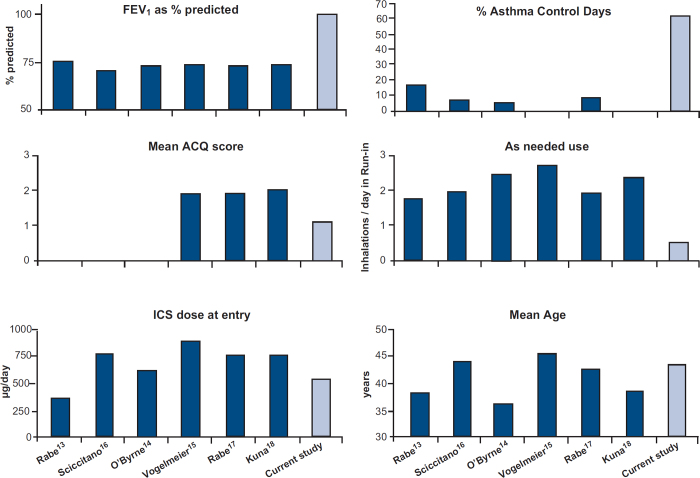
Figure 3 shows the baseline clinical characteristics of the study participants in comparison to previously published SMART studies.13–18
Figure 3. Comparison of clinical characteristics of the current study with other SMART studies at baseline.
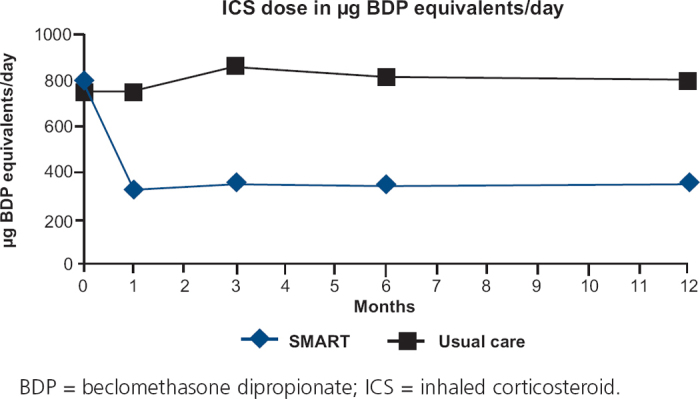
The mean ICS dose decreased to 326μg/day BDP equivalents with SMART and remained almost constant at 798μg/day BDP equivalents with UC during the 12-month treatment period (Figure 4). The difference between the treatment groups was highly significant (p<0.0001).
Figure 4. ICS dose in μg BDP equivalents at baseline and after 1, 3, 6 and 12 months of treatment.
Discussion
Main findings
This study in patients with mild-to-moderate asthma in primary care shows that BHR remains stable with SMART management despite a 59% reduction in the ICS dose compared with UC treatment. Furthermore, morning and evening PEF values improved and patients were more satisfied with the ease of use of the SMART concept. The two management approaches were comparable for all other efficacy parameters.
Baseline characteristics differ substantially between the patients in this primary care study and previous SMART studies13–18 which selected outpatients from secondary care patient record files (see Figure 3). Our patients had much better FEV1 values and ACQ scores, needed fewer inhalations of rescue medication, had more days with asthma control, and generally used a lower ICS dose at entry. Although our primary care population had milder disease in virtually every aspect of clinical characterisation, SMART treatment was beneficial in this group as well. Moreover, unlike earlier studies, there was no requirement at recruitment for symptoms/poor control so there was less potential for regression to the mean and the use of UC as a control was easier to justify. In addition to the clinical efficacy of SMART, ratings on the domain ‘ease of use’ of the questionnaire on treatment satisfaction (SATQ) were significantly higher in the SMART group. This indicates that the use of one inhaler for both maintenance treatment and symptom relief was well accepted by the patients.
Despite a lower dose of ICS, the frequency of severe asthma exacerbations in the SMART group was low. The occurrence of severe exacerbations was too infrequent in both treatment groups to analyse statistically. This is an important finding because severe exacerbations have been related to excess lung function decline in asthma, hence worse disease prognosis.26 One patient in the SMART group was responsible for three of the total number of four exacerbations. This patient did not take extra inhalations for relief, although symptom scores and PEF values in his diary clearly indicated that an exacerbation was imminent.
Limitations of this study
There are some limitations to our study. It was based on BHR resulting in relatively low numbers of patients. The effects on BHR were observed during 1-year follow-up and might have been larger after a longer duration of follow-up. Indeed, earlier studies investigating the effects of ICS on BHR for more than 1 year showed larger differences in PC20 values. However, the differences were already significant with 1 year of follow-up.27,28 Nevertheless, we cannot fully exclude the possibility that longer treatment with SMART would have led to further differences. Patients starting with SMART had somewhat more severe asthma based on reliever use and hyperresponsiveness, although the difference was not significant. If they indeed did have somewhat more severe asthma, this might have affected the study outcome, particularly in this group. However, our results showed similar clinical outcomes with a lower cumulative ICS dose in the SMART group after 12 months of treatment. Finally, this was an open study which might affect the results, but this was necessary in order to compare the two arms of the study in a real-life situation of care in general practice.
Interpretation of findings in relation to previously published work
BHR is a risk factor for the development of asthma and impaired lung function later in life.29–31 More severe BHR predicts asthma progression, a feature generally but not invariably associated with airway wall inflammation and remodelling.32 ICS have a beneficial effect on BHR given their anti-inflammatory characteristics, with most studies showing an improvement of up to twofold.33,34 It is therefore surprising that the PD20-histamine remained stable with SMART treatment while the dose of ICS was reduced by 59%. Doctors may have concerns about ICS reduction during SMART treatment and question whether this would lead to lower suppression of airway inflammation. Our data show that BHR is not worse with SMART treatment. Additionally, a Canadian study in patients with mild-to-severe asthma showed that SMART treatment had similar effects on the control of eosinophilic airway inflammation with a 26% lower overall ICS dose compared with best clinical practice treatment.35 These findings may result from enhancement of the anti-inflammatory effects of ICS by simultaneously administering LABA via the same inhaler.12,36 Alternatively, since small flare-ups of inflammation may be immediately suppressed with SMART treatment but not with UC, SMART may have prevented deterioration of BHR.
Both cumulative exposure to ICS and total daily ICS dose correlate significantly with the risk of experiencing local and systemic side effects of ICS.37 Doctors should therefore strive to treat asthma patients with the lowest ICS dose possible without losing effectiveness. Our study shows that SMART treatment in a primary care setting is indeed able to do this. Papi et al.38 showed in their study in patients with mild asthma that a strictly symptom-driven use of the combination inhaler beclomethasone/salbutamol was as effective as maintenance therapy with beclomethasone alone with respect to improvements in lung function and exacerbation rates, despite a lower cumulative dose of ICS in the former group.
Conclusions
In summary, we conclude that budesonide/formoterol maintenance and reliever therapy appears to be a well-tolerated and beneficial concept for the management of patients with mild-to-moderate asthma in primary care. It reduces the dose of inhaled corticosteroids needed and can be considered as a good alternative for guideline-based treatment. Our data thus provides supportive evidence to apply SMART strategies in primary care as suggested in the recently revised GINA guidelines.39
Acknowledgments
Handling editor Mike Thomas
Statistical review Gopal Netuveli
Funding This study was supported by an unrestricted educational grant by AstraZeneca BV, The Netherlands. The sponsors were involved in the study design and interpretation of the data, always in conjunction with the study investigators.
Trial registration This trial is registered at: http://www.clinicaltrials.gov, identifier: NCT00235911.
Appendix 1: Enrolment, exclusion and randomisation criteria
Footnotes
RAR has received a research grant from AstraZeneca (AZ); and speaking fees, consulting fees, and reimbursement for attending conferences from AZ, GlaxoSmithKline (GSK), Boehringer, and MSD. DP has received a research grant from AZ; speaking fees, consulting fees, and reimbursement for attending conferences from AZ, GSK, MSD, Novartis, and Nycomed; funding for research from AZ, GSK, and Nycomed; travel to the ERS or ATS partially funded by AZ, GSK, Chiesi, and Nycomed; and she has been consultant to AZ, Boehringer Ingelheim, Chiesi, GSK, Nycomed, and TEVA. TvdM has received research grants from AZ, Nicomed, and MSD; speaking fees, consulting fees, and reimbursement for attending conferences from AZ and GSK; and has been consultant to AZ, MSD, Nicomed, and Novartis.
References
- Global Initiative for Asthma (GINA). Global strategy for Asthma Management and Prevention (updated 2004). NIH Publication No. 02–3659. Bethesda, MD: National Institutes of Health, 2004. [Google Scholar]
- Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) Study. Eur Respir J 2000;16:802–7. http://dx.doi.org/10.1183/09031936.00.16580200 [DOI] [PubMed] [Google Scholar]
- Partridge MR, van der Molen T, Myrseth SE, Busse WW. Attitudes and actions of patients with asthma on regular maintenance therapy: the INSPIRE study. BMC Pulm Med 2006;6:13. http://dx.doi.org/10.1186/1471-2466-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels RA, Lofdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. N Engl J Med 1997;337:1412–18. http://dx.doi.org/10.1056/NEJM199711133372001 [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Barnes PJ, Rodriguez Roisin R, et al. Low dose inhaled budesonide and formoterol in mild persistent asthma: the OPTIMA randomized trial. Am J Respir Crit Care Med 2001;164:1392–7. [DOI] [PubMed] [Google Scholar]
- Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of inhaled steroid or addition of salmeterol in symptomatic asthma (MIASMA). BMJ 2000;320:1368–73. http://dx.doi.org/10.1136/bmj.320.7246.1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanranta H, Lahdensuo A, Moilanen E, Barnes PJ. Add-on therapy options in asthma not adequately controlled by inhaled corticosteroids: a comprehensive review. Respir Res 2004;5:17–42. http://dx.doi.org/10.1186/1465-9921-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seberová E, Andersson A. Oxis (formoterol given by Turbuhaler) showed as rapid an onset of action as salbutamol given by a pMDI. Respir Med 2000;94:607–11. http://dx.doi.org/10.1053/rmed.2000.0788 [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Sears MR, Campbell M, et al. for the RELIEF Study Investigators. Formoterol as relief medication in asthma: a worldwide safety an effectiveness trial. Eur Respir J 2003;22:787–94. http://dx.doi.org/10.1183/09031936.03.00055803 [DOI] [PubMed] [Google Scholar]
- Tattersfield AE, Löfdahl CG, Postma DS, et al. Comparison of formoterol and terbutaline for as-needed treatment of asthma: a randomised trial. Lancet 2001;357:257–61. http://dx.doi.org/10.1016/S0140-6736(00)03611-4 [DOI] [PubMed] [Google Scholar]
- Molen van der T, Postma DS, Turner MO, et al. Effects of the long acting p2-agonist formoterol on asthma control in asthmatic patients using inhaled corticosteroids. Thorax 1997;6:535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Scientific rationale for using a single inhaler for asthma control. Eur Respir J 2007;29:1–9. http://dx.doi.org/10.1183/09031936.00080306 [DOI] [PubMed] [Google Scholar]
- Rabe KF, Pizzichini E, Ställberg B, et al. Budesonide/formoterol in a single inhaler for maintenance and relief in mild-to-moderate asthma. Chest 2006;129:246–56. http://dx.doi.org/10.1378/chest.129.2.246 [DOI] [PubMed] [Google Scholar]
- O'Byrne PM, Bisgaard H, Godard P, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med 2005;171:129–36. http://dx.doi.org/10.1164/rccm.200407-884OC [DOI] [PubMed] [Google Scholar]
- Vogelmeier C, D'Urzo A, Pauwels R, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J 2005;26:819–28. http://dx.doi.org/10.1183/09031936.05.00028305 [DOI] [PubMed] [Google Scholar]
- Scicchitano R, Aalbers R, Ukena D, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin 2004;20:1403–18. http://dx.doi.org/10.1185/030079904X2051 [DOI] [PubMed] [Google Scholar]
- Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lallo G. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet 2006;368:744–53. http://dx.doi.org/10.1016/S0140-6736(06)69284-2 [DOI] [PubMed] [Google Scholar]
- Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 2007;61:725–36. http://dx.doi.org/10.1111/j.1742-1241.2007.01338.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy 2007;62:591–604. http://dx.doi.org/10.1111/j.1398-9995.2007.01394.x [DOI] [PubMed] [Google Scholar]
- Juniper EF, O'Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway calibre and rescue p2-agonist use? Respir Med 2001;95:319–23. http://dx.doi.org/10.1053/rmed.2001.1034 [DOI] [PubMed] [Google Scholar]
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–07. http://dx.doi.org/10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- Campbell JL, Kiebert GM, Partidge MR. Development of the Satisfaction with inhaled Asthma Treatment Questionnaire. Eur Respir J 2003;22:127–34. http://dx.doi.org/10.1183/09031936.03.00097503 [DOI] [PubMed] [Google Scholar]
- Sterk PF, Fabbri LM, Quanjer PH, et al. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party, Standardization of Lung Function Tests, European Community for Steel and Coal. Official statement of the European Respiratory Society. Eur Respir J 1993;6(Suppl):53–83. [PubMed] [Google Scholar]
- Mollmann H, Wagner M, Krishnaswami S, et al. Single-dose and steady-state pharmacokinetic and pharmacodynamic evaluation of therapeutically clinically equivalent doses of inhaled fluticasone propionate and budesonide, given as Diskus or Turbohaler dry-powder inhalers to healthy subjects. J Clin Pharmacol 2001;41:1329–38. http://dx.doi.org/10.1177/00912700122012913 [DOI] [PubMed] [Google Scholar]
- Osterman K, Carlholm M, Ekelund J, et al. Effect of 1 year daily treatment with 400 lg budesonide (Pulmicort® Turbuhaler®) in newly diagnosed asthmatics. Eur Respir J 1997;10:2210–15. http://dx.doi.org/10.1183/09031936.97.10102210 [DOI] [PubMed] [Google Scholar]
- Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J 2007;30:452–6. http://dx.doi.org/10.1183/09031936.00165106 [DOI] [PubMed] [Google Scholar]
- Kerstjens HA, Brand PL, Hughes MD, et al. A comparison of bronchodilator therapy with or without inhaled corticosteroid therapy for obstructive airways disease. Dutch Chronic Non-Specific Lung Disease Study Group. N Eng J Med 1992;327:1413–19. http://dx.doi.org/10.1056/NEJM199211123272003 [DOI] [PubMed] [Google Scholar]
- Haahtela T, Jarvinen M, Kava T, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. N Engl J Med 1994;331:700–5. http://dx.doi.org/10.1056/NEJM199409153311103 [DOI] [PubMed] [Google Scholar]
- Hargrave FE, Ryan G, Thomson NC, et al. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol 1981;68:347–55. http://dx.doi.org/10.1016/0091-6749(81)90132-9 [DOI] [PubMed] [Google Scholar]
- Jansen DF, Rijcken B, Schouten JP, et al. The relationship of skin test positivity, high serum total IgE levels, and peripheral blood eosinophilia to symptomatic and asymptomatic airway hyperresponsiveness. Am J Respir Crit Care Med 1999;159:924–31. [DOI] [PubMed] [Google Scholar]
- Xu X, Rijcken B, Schouten JP, Weiss ST. Airways responsiveness and development and remission of chronic respiratory symptoms in adults. Lancet 1997;350:1431–4. http://dx.doi.org/10.1016/S0140-6736(97)10041-1 [DOI] [PubMed] [Google Scholar]
- James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134–55. http://dx.doi.org/10.1183/09031936.00146905 [DOI] [PubMed] [Google Scholar]
- Kraan J, Koëter GH, Mark van der ThW, Sluiter HJ, Vries de K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. J Allergy Clin Immunol 1985;76:628–36. http://dx.doi.org/10.1016/0091-6749(85)90786-9 [DOI] [PubMed] [Google Scholar]
- Kraan J, Koëter GH, Mark van der TW, et al. Dosage and time effects of inhaled budesonide on bronchial hyperreactivity. Am Rev Respir Dis 1988;137:44–8. http://dx.doi.org/10.1164/ajrccm/137.L44 [DOI] [PubMed] [Google Scholar]
- Sears MR, Boulet LP, Laviolette M, et al. Budesonide/formoterol maintenance and reliever therapy: impact on airway inflammation in asthma. Eur Respir J 2008;31:982–9. http://dx.doi.org/10.1183/09031936.00104007 [DOI] [PubMed] [Google Scholar]
- Kauer M, Chivers JE, Giembycz MA, Newton R. Long-acting p2-adrenoceptor agonists synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Mol Pharmacol 2008;73:203–14. http://dx.doi.org/10.1124/mol.107.040121 [DOI] [PubMed] [Google Scholar]
- Foster JM, Aucott L, van der Werf RH, et al. Higher patient perceived side effects related to higher daily doses of inhaled corticosteroids in the community: a cross-sectional analysis. Respir Med 2006;100:1318–36. http://dx.doi.org/10.1016/j.rmed.2005.1 L029 [DOI] [PubMed] [Google Scholar]
- Papi A, Canonica G, Mestrelli P, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med 2007;356:2040–52. http://dx.doi.org/10.1056/NEJMoa063861 [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention, revised 2006. www.ginasthma.com