Abstract
Purpose:
To determine the rate of marginal relapse, progression-free survival (PFS), and overall survival (OS) in patients with pediatric low-grade glioma (PLGG) treated with conformal radiation therapy (CRT) with a clinical target volume (CTV) margin of 5 mm in the Children’s Oncology Group trial ACNS0221.
Methods and Materials:
Children (age 3–21 years) with unresectable progressive, recurrent, or residual PLGG were eligible for this study. Patients younger than 10 years were required to have received at least 1 chemotherapy course. Patients with neurofibromatosis type I were not eligible. All patients underwent MRI-based planning and received CRT 54 Gy in 30 fractions with a 5-mm CTV margin.
Results:
Of 85 eligible patients (median age 13.6 years) treated between March 2006 and December 2010, 14 were younger than 10 years and 36 received prior chemotherapy. Sixty-six had pilocytic astrocytoma (PA); 15 had other histologic subtypes, and 4 had unbiopsied chiasmatic lesions. Events included 23 relapses - 19 central 4 distant, and no marginal - and 7 deaths. At a median follow-up of 5.15 years, 5-year PFS was 71%±6% and OS was 93%±4%. Male gender (P=.068) and large tumor size (P=.050) trended toward significance for association with decreased PFS. Age, histology, tumor location, time between diagnosis and study entry, and MIB-1 status were not associated with PFS. OS was negatively associated with male gender (P=.064), non-PA histology (P=.010), and large tumor size (P=.0089).
Conclusions:
For patients with PLGG, CRT with a CTV margin of 5 mm yields an acceptable PFS and does not lead to a high rate of marginal relapse.
SUMMARY
We studied the rates of marginal relapse, progression-free survival, and overall survival in 85 children with pediatric low-grade glioma receiving conformal radiation therapy with a clinical target volume (CTV) margin of 0.5 cm in the Children’s Oncology Group trial ACNS0221. The progression-free and overall survival rates were acceptable and marginal relapse was not observed with this treatment strategy. Limiting the CTV may reduce the risk of treatment-related complications.
Introduction
Pediatric low-grade gliomas (PLGG) account for approximately 30% of brain tumors in children and adolescents [1,2] and can be classified as pilocytic astrocytoma, diffuse fibrillary astrocytoma, and other less common histologic subtypes [3]. Gross total resection (GTR) is often achievable and usually curative for PLGG in the cerebral or cerebellar hemisphere [4]. However, tumors arising in the hypothalamus, optic pathways, thalamus, basal ganglia, midbrain, and dorsally exophytic brain stem are not generally amenable to complete resection. Surgical intervention at these sites is usually limited to subtotal resection or biopsy. For such patients appropriate post-operative management can include observation, radiation therapy (RT), or chemotherapy [5]. Although the value of RT in improving progression-free survival (PFS) is well documented [6,7], RT is associated with numerous long-term complications including cognitive impairment, endocrinopathy, and vasculopathy, especially in children under the age of 5 years [8,9]. Chemotherapy is therefore the standard post-surgical modality for young patients with PLGG and is aimed at improving PFS and delaying the use of RT [10].
Improved imaging, along with modern treatment planning and delivery techniques such as threedimensional conformal RT (3D-CRT) [11], stereotactic RT [12,13], intensity-modulated RT (IMRT) [14], and proton therapy [15,16] allow the delivery of more conformal treatments for patients with PLGG. The use of tighter treatment volumes is expected to be associated with fewer long-term adverse effects. Merchant et al. [11] reported excellent PFS and overall survival (OS) and a low rate of marginal relapse in 78 patients treated with CRT and IMRT with a clinical target volume (CTV) margin of 1.0 cm. Although younger age at radiation exposure was significantly associated with late effects, the study suggested that the smaller CTV might reduce late effects such as cognitive impairment, endocrinopathy, and hearing loss [17].
Here we report the rate of marginal relapse, PFS, and OS in patients with PLGG receiving CRT with a CTV margin of 0.5 cm enrolled in the Children’s Oncology Group (COG) study ACNS0221.
Methods and Materials
Trial
COG trial ACNS0221 was a single-arm group-wide phase II study of CRT in patients with PLGG. The study was approved by the Pediatric Central Institutional Review Board (IRB) and IRBs of participating institutions and was opened in November 2005. Informed consent was required. Study accrual continued until December 2010, and follow-up data were collected until December 2016.
The primary objective of ACNS0221 was to determine whether the rate of early marginal failure was unacceptable in children with unresectable residual, progressive, recurrent, (symptomatic or presymptomatic) PLGG receiving a dose of 54 Gy in 30 fractions and a reduced CTV margin of 0.5 cm beyond the gross target volume (GTV). Secondary objectives were to estimate PFS, event-free survival (EFS), and OS for patients treated with reduced-field CRT and to determine whether a high MIB-1 labeling index (LI) was correlated with shortened PFS and OS in these patients.
Inclusion and exclusion criteria
A histologic diagnosis of PLGG was required for study entry, except for patients with chiasmatic tumors. The study pathologist (LRM) performed a retrospective review of tumor tissues. Patients with neurofibromatosis type I (NF-1) were ineligible because of concerns for increased risk of radiation-induced toxicities.
Patients aged 3 – 21 years with progressive, unresectable PLGG, which included pilocytic astrocytoma (PA), diffuse astrocytoma (fibrillary, gemistocytic, giant cell or pleomorphic xanthrocytoma), low-grade oligoastrocytoma, low-grade oligodendroglioma, or low-grade glioma NOS, were eligible. Patients with less than GTR were eligible post-operatively if symptomatic from their tumor or if the risk of neurologic impairment with disease progression was high enough to warrant immediate treatment. Presence of measurable disease was required and patients with leptomeningeal metastases were ineligible. Patients younger than 10 years of age were required to have received at least 1 course of chemotherapy before study entry.
Radiation therapy guidelines
MRI–based treatment planning was required. Before an institution was allowed to enroll patients on this study, it was required to demonstrate the ability to fuse diagnostic MRI and treatmentplanning CT imaging [18]. 3D-CRT, IMRT, stereotactic RT, and proton therapy were allowed. Treatment planning competence was confirmed by irradiation of the corresponding Quality Assurance Review Center (QARC) or Radiological Physics Center phantom.
All patients received a dose of 54Gy in 30 fractions of 1.8Gy each. For PA’s the GTV was taken as the entire tumor volume seen on gadolinium-enhanced T1-weighted MRI plus any additional abnormality seen on T2-weighted MRI or fluid-attenuated inversion recovery (FLAIR) imaging. For non-PA’s the GTV was based on T2 or FLAIR imaging. All tumor cysts were included in the GTV. For all tumor types, the CTV was the GTV plus a 5-mm anatomically limited margin (i.e., CTV did not extend into the calvarium). The planning target volume (PTV) was the CTV plus an institutionally determined 3- 5 mm margin. All points in the PTV were to receive at least 95% of the prescribed dose, and less than 10% of the PTV was to receive more than 107% of the prescribed dose. Mid-treatment imaging was required to monitor possible tumor enlargement during treatment. Replanning was performed if required.
All diagnostic images used for treatment planning and all treatment plans were centrally reviewed at Quality Assurance Review Center (QARC)/Imaging and Radiation Oncology Core (IROC), Rhode Island (Providence, RI) before starting treatment. Final review was performed by a pediatric neuro-radiologist (D.W.W.S.) and a radiation oncologist (J.M.C.). Also, if the treating institution suspected recurrence in a patient, all post-treatment MRIs were submitted for central review, including any imaging performed after relapse but before starting additional therapy. Relapses were classified as central, distant, or marginal [19]. A subset of relapsed patients that included all patients considered to have possible marginal relapses was independently reviewed by a second radiation oncologist (T.E.M.).
MIB-1 labeling index
Slides containing paraffin-embedded tissue from original tumor biopsy specimens of patients were retrospectively reviewed by the study pathologist (L.R.M.). Immunohistochemical analysis with the MIB-1 antibody was performed, and cells were counted using established methods [20,21]. Immunoreactive non-tumor (endothelial and hematopoietic) cells were not counted. The MIB-1 labeling index (LI) was calculated as the percentage of immunoreactive tumor cell nuclei in the most abundantly proliferative area (the so-called “hot spot”) in at least 1000 cells. Statistical analyses Efficacy measures used in this trial were PFS, EFS, and OS. PFS was defined as the time from study enrollment to the earliest of disease progression, death from any cause, or last contact for patients without events. EFS was defined the same as PFS, except that it also included second malignant neoplasms. Since no second malignant neoplasms were reported in this study, EFS was considered equivalent to PFS. OS was defined as the time from study enrollment to the earlier of death from any cause or last contact for surviving patients.
PFS and OS were estimated by the Kaplan–Meier method and standard errors were calculated by the Peto and Pike method [22]. Cox regression analysis was used to study associations between various demographic and clinical factors and survival outcomes. For PFS, no multivariable Cox regression models were identified. For OS, building a multivariable model was not feasible due to the few deaths observed (n=7).
Failure types (none, central, marginal, and distant) were also studied. For this ordinal variable, the Cochran–Armitage exact trend test, chi-squared exact test, and Spearman correlation were used as appropriate. The association of MIB-1 LI with PFS and OS was tested by univariate Cox regression analysis. A significance threshold of 0.05 was used throughout without adjusting for multiplicity. Two-sided P-values are reported.
Results
Patients
Of the 92 patients enrolled in the study, 7 were not eligible for participation: 1 patient had a GTR; in 1 patient radiation was not started within 30 days of registration due to worsening condition; in two patients the tumor extended to the cervical spine and one of these was treated at an institution whose group membership was revoked; 2 patients received radiation at non-COG institutions; and one patient younger than 10 years had not received prior chemotherapy,. Thus, 85 patients enrolled by 44 institutions were eligible for the study.
Table 1 lists patient demographics and tumor characteristics. Of the 85 eligible patients (44 female and 41 male), 14 were younger than 10 years and 71 were 10 years or older. Median age at enrollment was 13.6 years. Median time between diagnosis and start of treatment was 7.6 months (range 0.6–183 months). Thirty-six patients had previously received chemotherapy.
Table 1.
Demographics and tumor characteristics for eligible ACNS0221 patients (n=85)
| Characteristic | N (%) | |
|---|---|---|
| Gender | Male | 41 (48) |
| Female | 44 (52) | |
| Age (y) | <5 | 3 (4) |
| 5–9 | 11 (13) | |
| 10–14 | 44 (52) | |
| 15–19 | 26 (31) | |
| ≥20 | 1 (1) | |
| Prior chemotherapy | Yes | 36 (42) |
| No | 49 (58) | |
| Time between diagnosis and start of treatment | <6 | 41 (48) |
| 6–11 | 5 (6) | |
| 12–35 | 13 (15) | |
| 36–59 | 13 (15) | |
| ≥60 | 13 (15) | |
| Histology | Pilocytic astrocytoma | 66 (78) |
| Diffuse astrocytoma | 12 (14) | |
| Unbiopsied (optic chiasm) | 4 (5) | |
| LGG NOS | 2 (2) | |
| LGG oligodendroglioma | 1 (1) | |
| Tumor location | Hypothalamic/suprasellar | 37 (44) |
| Thalamic/basal ganglia | 20 (24) | |
| Cortical | 5 (6) | |
| Midbrain | 14 (16) | |
| Brain stem | 9 (11) | |
| Tumor size (cm) | <2.5 | 25 (29) |
| 2.5–4.9 | 44 (52) | |
| ≥5 | 16 (19) | |
| MIB-1 LI | Unknown | 21 (25) |
| Low (<2) | 30 (35) | |
| High (≥2) | 34 (40) | |
Abbreviations: LGG, low-grade glioma; LI, labeling index; NOS, not otherwise specified.
Tumor characteristics
Table 1 also lists the patients’ tumor characteristics. Central pathology review performed for 72 patients revealed PA in 57 and other histologic subtypes in 15 patients. Nine patients were entered without central pathology review. All had PA per their institutional pathology report. Four patients had chiasmatic tumors and were enrolled without determining histologic diagnosis. After central review, the pathology was changed for 6 patients but all remained eligible. Almost all tumors were centrally located, with 5 tumors in the cerebral cortex and none in the cerebellar hemispheres. Infratentorial involvement was seen in 23 patients. Tumor size ranged from 1.1 cm to 10.0 cm (median 3.0 cm). MIB-1 LI was <2.0 in 30 patients, ≥2.0 in 34 patients, and unknown in 21 patients.
Radiation treatment
Of the 85 patients, 25 were treated with 3D-CRT and 60 with IMRT. None were treated with protons or stereotactic RT. Radiation treatments were not evaluable for 4 patients: appropriate benchmarks had not been filed with QARC for 2 patients, and targets were inadequately defined for 2 patients. There were 3 minor volume deviations and 1 major volume deviation.
Outcomes and relapse events
In total, the 85 patients had 23 PFS events and 7 OS events. Of the PFS events, 19 were central relapses and 4 were distant relapses; there were no marginal relapses. In the central review, 2 patients who were initially categorized as having central failures by the treating institutions were considered to have pseudo-progression [23] and were not counted as events in our analyses. Figure 1A gives Kaplan–Meier plots for OS and PFS for the 85 patients. At a median follow-up of 5.15 years, the 3- and 5-year PFS were 77%±5% and 71%±6%, respectively, and 3- and 5-year OS were 94%±3% and 93%±4%, respectively. In the univariable Cox model, male gender (Fig. 1B) and large tumor size (Fig. 1C) trended with decreased PFS, with P values (P=.068 and P=.050, respectively). Age <10 years vs. ≥10 years or more (P=.56), delivery of prior chemotherapy vs. none (P=.88), number of prior chemotherapy regimens (P=0.59), infratentorial involvement vs. none (P=.099), time from diagnosis to study entry (P=0.71), and PA vs. non-PA histology by central review (n=72) (P=.54) (Fig. 1D) were not significantly associated with PFS. OS had a significant negative association with large tumor size (HR=1.56, 95% CI 1.12–2.19, P=.0089) (Fig. 1C) and non-PA histology (hazard ratio [HR]=7.3, 95% confidence interval [CI] 1.59–33.3, P=.010) (Fig. 1D) and showed a negative trend with male gender (P=.064) (Fig. 1B). There was a significant association between failure type and gender: all 4 patients with distant failures were male (chi-squared exact test P=.042). Age, histology, time from diagnosis to study entry, and tumor size were not significantly associated with failure type. Information about first treatment after progression was collected and available for 19/23 patients. Among the 19 subjects for whom information was available, 7 were treated with surgery, 8 were treated with chemotherapy, and 4 received combined radiation and chemotherapy.
Fig. 1.
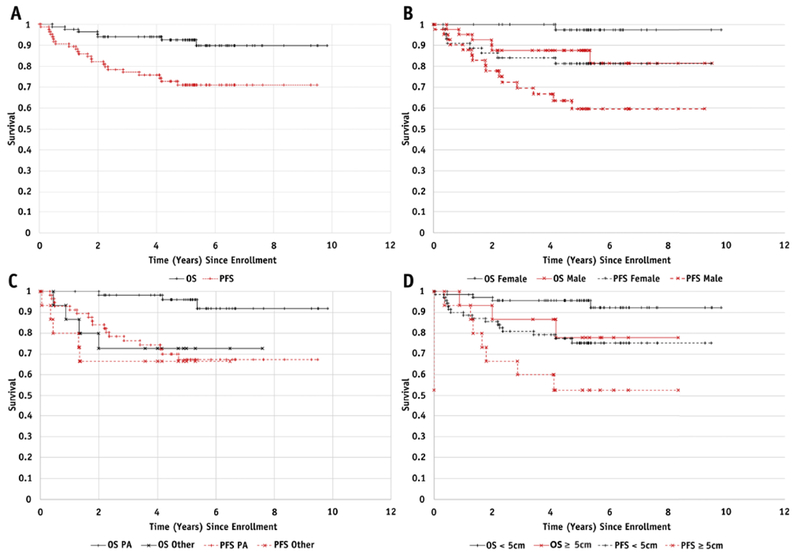
(A) Overall survival (OS) and progression-free survival (PFS) for the 85 eligible patients; (B) OS (P=.064) and PFS (P=.068) stratified by gender; (C) OS (P=.009) and PFS (P=.050) stratified by tumor size (<5 cm vs. ≥5 cm)(D) OS (P=.010) and PFS (P=.54) stratified by histology (PA vs. other) for the 72 centrally reviewed patients
Toxicity
During treatment, 2 patients developed grade IV toxicity and 2 developed grade III toxicity. One patient with a hypothalamic PA developed grade IV hypokalemia and other grade III laboratory abnormalities possibly related to therapy. A second patient with a fibrillary astrocytoma of the basal ganglia/thalamus developed grade II nausea and vomiting about 2 weeks after completing treatment. Imaging showed slight increase in tumor size and grade IV hydrocephalus. Encephalopathy increased to grade III and returned to baseline after placement of a ventriculoperitoneal shunt.
A patient with a PA of the midbrain/pons had multiple grade III neurologic toxicities related to treatment, such as hydrocephalus, tremor, multiple cranial nerve neuropathies, motor and sensory neuropathies, and depression. Imaging showed increased lesion size, but brain biopsy revealed tumor necrosis with reactive gliosis. The other grade III toxicity was an episode of back pain not related to RT.
Two patients had ocular events several months after ending RT. One with a PA of the thalamus/basal ganglia had an episode of acute visual loss arising from radiation-related edema, which was successfully treated with high-dose steroids. The other, who had a second recurrence of a midbrain PA, developed acute onset diplopia within 2 months of completing therapy. MRI revealed a new T2 abnormality, and enhancement suggesting recurrence or radiation injury. The patient was successfully treated with steroids. Biopsy after several months revealed that the new enhancement was due to radiation injury.
MIB labeling index
Tissue for MIB-1 LI analysis was available for 71 patients, but MIB-1 testing could not be performed in 7 due to insufficient/ poor quality of tissue for immunohistochemical testing. Thus, MIB-LI analysis was performed on tissue samples from 64 patients. The median MIB-1 LI was 2.2. Higher MIB-1 LI was significantly associated with shorter time from initial diagnosis to treatment (Spearman correlation −0.36, P=.003) but not associated with PFS [HR=1.04, 95% CI=0.90–1.22, P=.59] or OS [HR=1.14, 95% CI=0.91–1.43, P=.27] or failure type (Spearman correlation 0.05; P=.70). The analysis of 64 patients that had MIB-1 labeling index data available showed that MIB-1 was not associated with larger tumor size by Fisher’s exact test (P=.4022), Kruskal-Wallis test (P=0.4250) and estimated Spearman correlation coefficient of 0.02 (P=.8743).
Discussion
The role of RT in the management of PLGG patients has been limited by its perceived toxicity, particularly in young children. RT is currently advocated for older children with progressive or symptomatic disease after less than a GTR, and for young children only if chemotherapy is unsuccessful. Routine adjuvant radiation is not recommended as it does not offer a survival benefit over treatment at the time of progression [24].
Although the use of small treatment volumes has the intuitive appeal of reducing the risk of RT-related complications, there is a corresponding concern regarding an increased rate of marginal relapse and decreased PFS. Table 2 compares the PFS and OS of LGG patients enrolled in previous studies (2001–2013) that used modern RT techniques [11–15] with those from our study. Also, our patients did not have lower OS and PFS than those treated with conventional techniques in previous studies [25–27].
Table 2.
Comparison of progression-free and overall survival for patients enrolled in previous studies and our current study
| Author | Year | Patients (N) | Technique | CTV | Dose | Marginal Failure | PFS (%) | OS (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-y | 5-y | 6-y | 8-y | 10-y | 3-y | 5-y | 6-y | 8-y | 10-y | |||||||
| Saran et al. [13] | 2002 | 14 | SRT | GTV + 0.3–1.0 cm | 50–55 Gy | 0 | 87 | 100 | ||||||||
| Marcus et al. [12] | 2005 | 50 | SRT | GTV | 50–58 Gy | 0 | 82.5 | 68 | 97.8 | 82 | ||||||
| Merchant et al. [11] | 2009 | 78 | 3D-CRT/IMRT | GTV + 1.0 cm | 54 Gy | 1 | 87 | 74 | 98 | 96 | ||||||
| Greenberger et al. [15] | 2014 | 32 | Proton | GTV + 0.3 cm | 52.2 RBE | 90 | 83 | 100 | 100 | |||||||
| Paulino et al. [14] | 2013 | 39 | IMRT | GTV + 0.5–1.0 cm | 50.4 Gy | 0 | 78 | 94 | ||||||||
| Current study | 2017 | 85 | 3D-CRT/IMRT | GTV + 0.5 cm | 54 Gy | 0 | 77 | 71 | 94 | 93 | ||||||
Abbreviations: CTV, clinical target volume; 3D-CRT, three-dimensional conformal radiation therapy; GTV, gross target volume; IMRT, intensity-modulated radiation therapy; OS, overall survival; PFS, progression-free survival; RBE, relative biological effectiveness; SRT, stereotactic radiation therapy
Merchant et al [11] used 3D-CRT techniques similar to ours but with a CTV margin of 1.0 cm. The 5-year PFS and OS were 87%±4% and 98%±2%, respectively, for 78 patients in their study, with 1 patient having marginal relapse, versus a 5-year PFS and OS of 71%±6% and 93%±4%, respectively, and no marginal relapses for 85 patients in our study. However, 17% of their patients had NF-1, none of whom relapsed, while NF-1 patients were not included in our study. Although tumor grade was not a significant predictor of outcome in the earlier study, the PFS of our PA patients was similar to, but the OS was higher than, our non-PA patients.
Merchant et al. conducted prospective analyses of long-term toxicities in their patients [11,17,27–29]. Children irradiated at less than 5 years of age had significantly increased risks of vasculopathy [11] and cognitive decline [17]. There was no overall decrease in learning ability after CRT [28], but those given pre-irradiation chemotherapy were less able to learn new material. Endocrinopathy, common before CRT, became significantly more prevalent after 10 years of follow up [17]. Deficits in emotional and behavioral functioning were present before patients received CRT, but remained stable through 5 years of follow up [29]. Adaptive functioning was also relatively spared.[30].
Paulino et al. investigated the use of IMRT in 39 PLGG patients using 3 methods of target delineation: CTV=GTV+1 cm, CTV=GTV+0.5 cm, and dose painting (a lower dose given to a margin around the GTV) [14]. None of the 6 patients treated with CTV=GTV+0.5 cm experienced relapse. However, the 12 patients who were younger than 5 years had significantly poorer outcomes than older patients. In our series, the outcomes of the 3 patients younger than 5 years were not worse than those of older patients.
A study of neurocognitive functioning in LGG patients treated with proton irradiation found no decline in the full-scale intelligence quotient at a median of 4.5 years after treatment. However, young children and those receiving a high dose to the left temporal lobe/hippocampus experienced a significant decline in neurocognitive outcomes [15].
Post-treatment MRI changes in the absence of disease progression have been reported in irradiated PLGG patients [31,32]. A recent study [22] reported pseudo-progression [33–35] in 13 of 24 patients. Pseudo-progression began at a median of 6 months after treatment and lasted for a median of 2.1 years, with maximum tumor enlargement at a median of 8 months (range 5 months to 4.2 years). The authors suggested that pseudo-progression rather than true progression should be considered for all patients whose post-treatment images show signs of progression, and that symptomatic patients may benefit from initial treatment with steroids and/or bevacizumab [36].
In an update of the St. Jude series including 221 patients [37], 62 developed pseudoprogression with a 10-year cumulative incidence of 29%. The median time to pseudoprogression was 6.1 months with 32% requiring some level of intervention. Patients with PA had 5.4-fold greater odds of developing pseudoprogression relative to other PLGG histologic subtypes. Pseudoprogression was associated with significantly better PFS and OS. In our cooperative group trial, all available imaging studies performed before and after an institutional call of recurrence were centrally reviewed. Two patients had pseudo-progression and were not categorized as failures in the analysis but the numbers of patients and events in our trial were too few to compare to other series.
These findings suggest that the PFS reported for irradiated PLGG patients may be lower than the true value because of mislabeling of pseudo-progression as true progression. Pseudo-progression may also overstate the benefit of salvage chemotherapy, as resolution of pseudo-progression could be misinterpreted as response to chemotherapy.
In a study of 141 children with newly-diagnosed PA, most of whom were treated with aggressive surgical resection followed by observation, MIB-1 LI was of possible prognostic significance for PFS [21]. In our study, an elevated MIB-1 LI was associated with a shorter interval between initial diagnosis and enrollment, but did not correlate with PFS or OS.
Two studies found higher risk of death in PLGG patients receiving RT than those not receiving RT [38,39]. A population-based analysis [39] showed that for patients who did not undergo GTR and survived more than 5 years after diagnosis, RT within 1 year of diagnosis was associated with increased risk of tumor-related and overall late deaths.
The association between male gender and progression-free survival was a trend and not statistically significant. The association between large tumor size and overall survival was statistically significant. There was no leading explanation for this finding. One might consider the potential for sampling error in the histologic assessment of large tumors, larger tumors at presentation may be a surrogate for more aggressive tumor subtypes, and larger tumors might harbor more radiation resistant features. That larger tumors were associated with a lower rate of overall survival could be a spurious finding. In pediatric low-grade glioma chemotherapy trials larger tumors have been consistently associated with worse progression-free survival [10].
To evaluate the possibility that larger tumors might be associated with more aggressive tumor subtypes, we assessed the association between MIB-1 and tumor size and found that MIB-1 was not associated with larger tumor size.
The results and conclusions drawn from this cooperative group trial are more likely to be generalizable than data from a single institution trial. However, we are aware of some limitations of the current study. Despite instructions in the protocol, institutions followed many different policies about starting salvage chemotherapy after apparent tumor progression. Thus, we may have misclassified instances of pseudoprogression as true radiation failures. Also, we do not have any neuro-cognitive data to support our assumption that the reduction in CTV margin from the 1.0 cm used by Merchant et al. [11] will lead to further reduction in deficits.
Conclusions
We show that treatment of PLGG patients with CRT using a CTV margin of 0.5 cm does not lead to high rates of marginal relapse and is associated with an acceptable 5-year EFS and OS. It is important to distinguish local failure from pseudo-progression. CRT is associated with lesser toxicity than is traditional wide-field radiation and has an important role in the multi-disciplinary management of progressive or recurrent PLGG. However, its use in young children should be limited due to considerable toxicity.
Acknowledgments
Funding: This clinical trial was supported by COG Group Operations U10 CA098543, COG Statistics and Data Center U10 CA098413, COG Group Operations U10 CA180886 and COG Statistics and Data Center U10 CA180899.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: JC, JH, and LMreport grants from NCI to COG, CureSearch, QARC, and/or IROC, during the conduct of the study. KM reports medical direction for cancer pathways for Lymphoma and ownership of SINK cancer, a medical physics and dosimetry staffing company outside the submitted work. DB, PC, MF, AG, SK, TM, AOT, IP, DS, JW, and TZ reports no conflicts of interest concerning the materials or methods used in this study of the findings reported in this paper.
Statistical Analysis: Jie Huang, MS, and Tianni Zou, PhD were responsible for statistical analysis.
References
- 1.Ostrom QT, de Blank PM, Kruchko C, Petersen CM, Liao P, Finlay JL, Stearns DS, Wolff JE, Wolinsky Y, Letterio JJ, Barnholtz-Sloan JS (2015) Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol 16 Suppl 10:x1–x36. doi: 10.1093/neuonc/nou327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol 18 Suppl 1 :i1–i50. doi: 10.1093/neuonc/nov297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131 (6):803–820. doi: 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 4. Wisoff JH, Sanford RA, Heier LA, Sposto R, Burger PC, Yates AJ, Holmes EJ, Kun LE (2011) Primary neurosurgery for pediatric low-grade gliomas: a prospective multi-institutional study from the Children’s Oncology Group. Neurosurgery 68 (6):1548–1554; discussion 1554-1545. doi: 10.1227/NEU.0b013e318214a66e [DOI] [PubMed] [Google Scholar]
- 5.Freeman CR, Farmer JP, Montes J (1998) Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys 41 (5):979–987 [DOI] [PubMed] [Google Scholar]
- 6.Grabenbauer GG, Schuchardt U, Buchfelder M, Rodel CM, Gusek G, Marx M, Doerr HG, Fahlbusch R, Huk WJ, Wenzel D, Sauer R (2000) Radiation therapy of optico-hypothalamic gliomas (OHG)--radiographic response, vision and late toxicity. Radiother Oncol 54 (3):239–245 [DOI] [PubMed] [Google Scholar]
- 7.Wallner KE, Gonzales MF, Edwards MS, Wara WM, Sheline GE (1988) Treatment results of juvenile pilocytic astrocytoma. J Neurosurg 69 (2):171–176. doi: 10.3171/jns.1988.69.2.0171 [DOI] [PubMed] [Google Scholar]
- 8.Chadderton RD, West CG, Schuller S, Quirke DC, Gattamaneni R, Taylor R (1995) Radiotherapy in the treatment of low-grade astrocytomas. II. The physical and cognitive sequelae. Childs Nerv Syst 11 (8):443–448 [DOI] [PubMed] [Google Scholar]
- 9.Kortmann RD, Timmermann B, Taylor RE, Scarzello G, Plasswilm L, Paulsen F, Jeremic B, Gnekow AK, Dieckmann K, Kay S, Bamberg M (2003) Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part II: Treatment-related late toxicity. Strahlenther Onkol 179 (9):585–597. doi: 10.1007/s00066-003-8104-0 [DOI] [PubMed] [Google Scholar]
- 10.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30 (21):2641–2647. doi: 10.1200/JCO.2011.36.6054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA (2009) Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 27 (22):3598–3604. doi: 10.1200/JCO.2008.20.9494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus KJ, Goumnerova L, Billett AL, Lavally B, Scott RM, Bishop K, Xu R, Young Poussaint T, Kieran M, Kooy H, Pomeroy SL, Tarbell NJ (2005) Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys 61 (2):374–379. doi: 10.1016/j.ijrobp.2004.06.012 [DOI] [PubMed] [Google Scholar]
- 13.Saran FH, Baumert BG, Khoo VS, Adams EJ, Garre ML, Warrington AP, Brada M (2002) Stereotactically guided conformal radiotherapy for progressive low-grade gliomas of childhood. Int J Radiat Oncol Biol Phys 53 (1):43–51 [DOI] [PubMed] [Google Scholar]
- 14.Paulino AC, Mazloom A, Terashima K, Su J, Adesina AM, Okcu MF, Teh BS, Chintagumpala M (2013) Intensity-modulated radiotherapy (IMRT) in pediatric low-grade glioma. Cancer 119 (14):2654–2659. doi: 10.1002/cncr.28118 [DOI] [PubMed] [Google Scholar]
- 15.Greenberger BA, Pulsifer MB, Ebb DH, MacDonald SM, Jones RM, Butler WE, Huang MS, Marcus KJ, Oberg JA, Tarbell NJ, Yock TI (2014) Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys 89 (5):1060–1068. doi: 10.1016/j.ijrobp.2014.04.053 [DOI] [PubMed] [Google Scholar]
- 16.Hug EB, Muenter MW, Archambeau JO, DeVries A, Liwnicz B, Loredo LN, Grove RI, Slater JD (2002) Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol 178 (1): 10–17 [DOI] [PubMed] [Google Scholar]
- 17.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X (2009) Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol 27 (22):3691–3697. doi: 10.1200/JCO.2008.21.2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulin K, Urie MM, Cherlow JM (2010) Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys 77 (5): 1584–1589. doi: 10.1016/j.ijrobp.2009.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SW, Fraass BA, Marsh LH, Herbort K, Gebarski SS, Martel MK, Radany EH, Lichter AS, Sandler HM (1999) Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys 43 (1):79–88 [DOI] [PubMed] [Google Scholar]
- 20.Going JJ (1994) Efficiently estimated histologic cell counts. Hum Pathol 25 (4):333–336 [DOI] [PubMed] [Google Scholar]
- 21.Bowers DC, Gargan L, Kapur P, Reisch JS, Mulne AF, Shapiro KN, Elterman RD, Winick NJ, Margraf LR (2003) Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol 21 (15):2968–2973. doi: 10.1200/JCO.2003.01.017 [DOI] [PubMed] [Google Scholar]
- 22.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG (1977) Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer 35 (1): 1–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naftel RP, Pollack IF, Zuccoli G, Deutsch M, Jakacki RI (2015) Pseudoprogression of low-grade gliomas after radiotherapy. Pediatr Blood Cancer 62 (1):35–39. doi: 10.1002/pbc.25179 [DOI] [PubMed] [Google Scholar]
- 24.Mishra KK, Puri DR, Missett BT, Lamborn KR, Prados MD, Berger MS, Banerjee A, Gupta N, Wara WM, Haas-Kogan DA (2006) The role of up-front radiation therapy for incompletely resected pediatric WHO grade II low-grade gliomas. Neuro Oncol 8 (2):166–174. doi: 10.1215/15228517-2005-011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huynh-Le MP, Walker AJ, Burger PC, Jallo GI, Cohen KJ, Wharam MD, Terezakis SA (2016) Management of pediatric intracranial low-grade gliomas: long-term follow-up after radiation therapy. Childs Nerv Syst 32 (8):1425–1430. doi: 10.1007/s00381-016-3100-8 [DOI] [PubMed] [Google Scholar]
- 26.Mansur DB, Rubin JB, Kidd EA, King AA, Hollander AS, Smyth MD, Limbrick DD, Park TS, Leonard JR (2011) Radiation therapy for pilocytic astrocytomas of childhood. Int J Radiat Oncol Biol Phys 79 (3):829–834. doi: 10.1016/j.ijrobp.2009.11.015 [DOI] [PubMed] [Google Scholar]
- 27.Raikar SS, Halloran DR, Elliot M, McHugh M, Patel S, Gauvain KM (2014) Outcomes of pediatric low-grade gliomas treated with radiation therapy: a single-institution study. J Pediatr Hematol Oncol 36 (6):e366–370. doi: 10.1097/MPH.0000000000000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Pinto M, Conklin HM, Li C, Merchant TE (2012) Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys 84 (3):e363–369. doi: 10.1016/j.ijrobp.2012.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willard VW, Conklin HM, Wu S, Merchant TE (2015) Prospective longitudinal evaluation of emotional and behavioral functioning in pediatric patients with low-grade glioma treated with conformal radiation therapy. J Neurooncol 122 (1): 161–168. doi: 10.1007/s11060-014-1696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netson KL, Conklin HM, Wu S, Xiong X, Merchant TE (2013) Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int J Radiat Oncol Biol Phys 85 (5):1301–1306. doi: 10.1016/j.ijrobp.2012.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakardjiev AI, Barnes PD, Goumnerova LC, Black PM, Scott RM, Pomeroy SL, Billett A, Loeffler JS, Tarbell NJ (1996) Magnetic resonance imaging changes after stereotactic radiation therapy for childhood low grade astrocytoma. Cancer 78 (4):864–873. doi: [DOI] [PubMed] [Google Scholar]
- 32.Chawla S, Korones DN, Milano MT, Hussain A, Hussien AR, Muhs AG, Mangla M, Silberstein H, Ekholm S, Constine LS (2012) Spurious progression in pediatric brain tumors. J Neurooncol 107 (3):651–657. doi: 10.1007/s11060-011-0794-z [DOI] [PubMed] [Google Scholar]
- 33.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9 (5):453–461. doi: 10.1016/S1470-2045(08)70125-6 [DOI] [PubMed] [Google Scholar]
- 34.Hoffman WF, Levin VA, Wilson CB (1979) Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg 50 (5):624–628. doi: 10.3171/jns.1979.50.5.0624 [DOI] [PubMed] [Google Scholar]
- 35.Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13 (4):389–403. doi: 10.1586/ern.13.7 [DOI] [PubMed] [Google Scholar]
- 36.Foster KA, Ares WJ, Pollack IF, Jakacki RI (2015) Bevacizumab for symptomatic radiation-induced tumor enlargement in pediatric low grade gliomas. Pediatr Blood Cancer 62 (2):240–245. doi: 10.1002/pbc.25277 [DOI] [PubMed] [Google Scholar]
- 37.Tsang DS, Murphy ES, Lucas JT Jr., Lagiou P, Acharya S, Merchant TE (2017) Pseudoprogression in pediatric low-grade glioma after irradiation. J Neurooncol 135 (2):371–379. doi: 10.1007/s11060-017-2583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales La Madrid A, Marcus KJ, Guo D, Ullrich NJ, Robison NJ, Chi SN, Beroukhim R, Kieran MW, Manley PE (2014) Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61 (7):1173–1179. doi: 10.1002/pbc.24958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnatry R, Zhukova N, Guerreiro Stucklin AS, Pole JD, Mistry M, Fried I, Ramaswamy V, Bartels U, Huang A, Laperriere N, Dirks P, Nathan PC, Greenberg M, Malkin D, Hawkins C, Bandopadhayay P, Kieran MW, Manley PE, Bouffet E, Tabori U (2016) Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 122 (8):1261–1269. doi: 10.1002/cncr.29907 [DOI] [PubMed] [Google Scholar]


