Abstract
The potential cytotoxic effect of aggregated Aβ1–42 to neurons that express classical neurotransmitters, including acetylcholine, gamma-amino butyric acid, catecholamines and serotonin was assessed. The cholinergic system has been the central focus of the therapeutic drug strategies in amyloid-depositing pathologies such as Alzheimer’s disease. Aggregated Aβ1–42 has a multisystem cytotoxic effect causing non-specific reduction in immunoreactivity, dysfunction, or loss of retinal nerve cells. The extent of this was investigated using immunocytochemistry, TUNEL staining for apoptosis, and measurement of cell density as well as retinal surface area. There was a differential acute and/or chronic effect of Aβ on choline acetyltransferase, gamma-aminobutyric acid and 5- tryptamine hydroxylase systems, observed with the increasing time course of 6 hours to 5 months, and a bilateral/systemic effect. In contrast, the overall pattern of catecholaminergic system, as revealed by tyrosine hydroxylase immunoreactivity of the retina, appears to have remained relatively unaffected by Aβ (however this may reflect neuronal loss due to reduction in the retinal surface). This is the first in vivo evidence in a CNS model to show that not only all major neurotransmitter systems are differentially affected by Aβ aggregates but the effect may vary from one transmitter system to another under the same experimental conditions in situ and in a dose- and time-dependent manner.
Keywords: Alzheimer’s disease, neurotransmitter systems, amyloid beta, choline acetyltransferase (ChAT), gamma-aminobutyric acid (GABA), 5- tryptamine hydroxylase systems (5 TryptH)
INTRODUCTION
Alzheimer’s disease (AD) is an age-related neurodegenerative disorder that is characterised by a number of neuropathological hallmarks including the accumulation of extracellular senile plaques and loss of neurons. The amyloid hypothesis (Hardy and Allsop, 1991; Hardy and Selkoe, 2002) proposes that β-amyloid peptide (Aβ) accumulates to form senile plaques, leading to progressive and widespread degeneration of neurons in the brain of patients of AD. One of the neuronal populations that is particularly vulnerable and consistently affected in AD brain appears to be cholinergic neurons that are located primarily in the ventral basal forebrain. Importantly, the loss of cholinergic neurons, correlates well with the observation of reductions in the neurotransmitter acetylcholine (Ach) and the enzyme chloline acetyltransferase (ChAT) that is involved in the biosynthesis of Ach (Arendt et al., 1985; Procter, 1996; Palmer, 1996; Whitehouse, 1982), as well as cognitive and memory deficits and dementia of AD. These observations have led not only to the establishment of the cholinergic hypothesis (Bartus, 2007; Benzi and Moretti, 1998; Harkany et al., 1995; Hodges, 2006; Perry, et al., 1978, 1993; Schlieb and Arendt, 2006), but also the development of the commonly adopted neurotransmitter replacement strategy for treating AD. However, treatment for AD by targeting specifically the neurotransmitter acetylcholine has not been entirely successful if not controversial in view of the report by Dunnett et al implicating that dysfunction of the ventral basal forebrain, as affecting attentional states rather than the proposed memory deficits in AD (Blokland, 1996). Partly for this reason, the cholinergic hypothesis needs modification, partly also due to accumulating evidence indicating that in AD there is not only alteration in Ach and ChAT but also alterations of most other important non-cholinergic neurotransmitters, including noradrenaline, serotonin, gama-aminobutyric acid (GABA) and dopamine (Bell et al., 2006; Garcia-Alloza et al., 2006; Grudzien et al., 2007; Heneka and O’Banion, 2007; Hodges, 2006; Lanctot et al., 2004; Meltzer et al., 1998; Palmer 1996; Szot et al., 2000). It is now widely accepted that interconnections between the different neurotransmitter systems together with a number of peptides may influence the cholinergic system and thus the disruption of one system may affect others, hence producing the deficits in memory. Nonetheless, little as yet is known about the acute and chronic effects of Aβ on individual neurotransmitter systems because of a lack of suitable and well characterised and useful in vivo experimental model. The relatively novel retinal-vitreal system (Jen et al 1999, Guo et al 2007) appears to be one such model because the retina is an integral part of the central nervous system but a closed system that is highly accessible, and most important, perhaps, it contains all major neuronal populations and neurotransmitter systems that can only be found in different parts but not in the same regions of the brain.
Previous studies have already shown that the injection of Aβ aggregates to the vitreous body in the eye can affect a variety of nerve cells in the rat retina, including photoreceptors (Jen et al. 1998; Walsh et al, 2002), interneurons in the inner nuclear layer (Walsh et al. 2002; Watts et al, 2007) and retinal ganglion cells (Anderson et al 2008; Walsh et al., 2005; Guo et al., 2007). The present study was aimed at determining the cytotoxic effect of Aβ on the classical neurotransmitters with special attention to the cholinergic system.
METHODS
A total of 99 young female Sprague-Dawley (6–8 weeks old) rats (Harlan, UK) were used in the study. They were housed and maintained in the Comparative Biology Unit at Charing Cross Hospital. All procedures used were carried out in accordance with the UK Home Office regulations. The animals were divided into three groups. The first group (n = 18) received no treatment and hence served as naïve controls. The rest of the rats were anaesthetised with sodium pentobarbital. A hole was made at the corneo-scleral junction of the eye with a 26-gauge needle to remove some vitreal fluid before injecting 0.1M phosphate buffered saline (PBS), aggregated Aβ1–42, or reverse peptide Aβ42–1 unilaterally into the left eye with a Hamilton syringe equipped with a special needle (75SN). Both Aβ1–42 and Aβ42–1 (Bachem, UK) were incubated in PBS at 37°C over 4 days, and then injected at a concentration of 2nmol/3μl or 5nmol/3 μ l. After a survival time of 6, 24 and 48 hours or 5 months the rats were deeply anaesthetised with sodium pentobarbital (16mg/100g-body weight) and perfused initially with 0.1M PBS followed by 4% paraformaldehyde in 0.1M phosphate buffer. Both injected and un-injected eyes were dissected out, the cornea and lens removed, and then immersed in the same fixative overnight before they were processed for frozen sectioning at 20 μm thickness. Serial alternate sections collected were processed for immmunohistochemistry, as described elsewhere (Guo et al., 1990, 1991, 1992, Bresciani et al., 2002; Jen et al., 1998; Walsh et al., 2002, 2005; Yang et al., 1992). In brief, alternate sections were incubated in a solution containing monoclonal or polyclonal antibodies for a number of glial or neuronal markers, including, ChAT ((1:20), Boehringer Manheim), GABA ((1:8000), Sigma, UK), glial fibrillary acidic protein (GFAP, 1:600, Sigma), tyrosine hydroxylase (TH, 1:1000, Sigma, UK), and tryptophan hydroxylase (TryptH, 1:1000, Sigma). In addition, some sections were processed for terminal deoxynucleotidyl transferase dUTP- end-labelling (TUNEL, ApopTag kit) and Nissl (cresyl violet) staining for apoptosis and cytoarchitecture or gross morphologic changes respectively.
In order to assess the acute and chronic effects of Aβ on individual neuronal populations, cell density of ChAT-immunoreactive somata (in retinas of normal control, un-injected and injected eyes, in addition to changes in the overall patterns of immunostaining of all markers used) was determined and compared at 2 days and 5 months after injections. The total of animals in the study of dose and time dependence of the effects of Aβ was 48 (6 normal and 7 animals for the other six groups).
An Olympus microscope set at a magnification of 40X was used to observe and count the cells that showed positive immunoreactivity for ChAT in the retinal sections. For each retinal section, 5 regions/fields whose microscopic diameter = 350μm, were photographed. The most peripheral parts of the retina were counted and the midpoint taken to find the central region. Two intermediate regions located intermediately between the central and peripheral fields were additionally taken. Cell counting was performed in both injected and the contralateral un-injected retinas from the same animal to enable comparisons between the treated and untreated eye, respectively. Data were collected from 6 cases with 4 pairs of sections for each case (1 pair = injected and un-injected retina of same treatment group). As illustrated in Figure 1, each section was counted in 5 fields, producing a representative total cell count per retinal section. The average of 4 sections for each case with the same treatment but different animals was used to produce the average total cell count per retinal section of each treatment.
Figure 1.
A schematic diagram illustrating the location of the 5 fields of view chosen for cell counts of neuronal somata immunoreactive for ChAT.
Sigma Plot was used to determine whether there were significant differences in cell density between the different retinal treatment groups. Paired t-tests were used to compare both eyes of the same animal. An unpaired t-test was done for comparisons involving animals with different treatments such as, normal control cases with Aβ1–42 injected cases. The standard errors of mean were also calculated and represented on the graphs by standard error bars. To provide further information and more reliable assessment of long-term changes, the thickness and the surface area of retinas of all four groups of rats were also measured and compared at 5 months after injections. The level of significance was set for p<0.01 (Walsh, et al., 2005).
RESULTS
The effect of Aβ on different neuronal systems over a time course of 6 hours to 5 months were characterised. Qualitative changes were studied immunocytochemically in the TH, GABA neurotransmitter systems, with additional quantitative analysis in the ChAT neuronal population. The apoptotic or degenerative effect of Aβ on retinal cells, as reported in previous studies (Jen et al., 1998; Walsh et al., 2002; 2005) was confirmed by TUNEL staining and was found to primarily affect photoreceptors and neurons in the inner nuclear layer (INL) but rarely neurons in the retinal ganglion cell layer (GCL) (Fig 2). The response of retinal glia was also confirmed by immunostaining. For instance, the reaction of the predominant Müller glia was revealed by GFAP immunostaining. Although GFAP is normally expressed primarily in astrocytes on the vitreal end of the retina little if any activity was observed in the radial process of Müller glial cells of untreated retina. However, GFAP-positive immunoreactive radial processes are seen spanning the thickness of the retina, indicating that the Müller glial cells upregulate GFAP in response to injury by aggregated Aβ1–42.
Figure 2.
Apoptotic cell death as revealed by TUNEL staining (arrows) in two different regions (A and B) of a retina injected with 5 nmol Aβ1–42 at 2 days post-injection. (C) is a negative control showing no TUNEL-positive profiles in the retina. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 100 μm.
This response occurred, regardless of the dosage and survival time, with peak staining intensity by 48 hours. There was also a less intense and extensive upregulation in GFAP-immunoreactive Müller glia radial processes in eyes injected with PBS and, more interestingly, a detectable increase in GFAP immunoreactivity was observed in some Müller glial processes in retinas contralateral to the injection, reflecting a change of activity and possible bilateral stress-related response of these cells, a phenomenon that has already been described in Walsh et al., (2002).
The effects of Aβ on retinal neurons that express specific neurotransmitters was studied at different time point and doses. At 6, 24 and 48 hours survival times, none of the markers tested in this study induced changes in the pattern of immunostaining in any nerve cell populations in retinas injected with 2 nmol/3 μl of Aβ1–42. Similarly, no observable difference was found between injected and control retinas at 6 and 24 hours time points in retinas injected with 5 nmol/3 μ l. By 48 hours, however, visually detectable changes were observed in cells that are immunoreactive for ChAT, GABA and TryptH but not those reactive for TH. Interestingly, the overall pattern of immunostaining for all markers was similar to those of the normal and control despite a nearly 60% reduction in the average retinal thickness, reflecting a severe loss of cells primarily in the outer and inner nuclear layers of retinas injected with Aβ1–42, as compared with the normal or control retinas at 5 months following treatment. Furthermore, an average reduction of nearly 40% of the surface area of retinas was observed in 5-month cases injected with Aβ1–42 compared with the un-injected retinas (Table 1).
Table 1.
Retinal surface areas and cell counts (retinal thickness) of ChAT-immuno-reactive somata 5 months after injections.
| Treatmenta | Retinal surface area (mm2) | Retinal thickness (μm) | ||
|---|---|---|---|---|
| Injected | Un-injected | Injected | Un-injected | |
| Normal (6,6)b | --- | 41.6± 2.5 | --- | 230.5 ± 25.6 |
| PBS (6,6)b | 37.0 ± 2.6 | 39.5± 2.1 | 192.8 ± 29.5 | 225.0 ± 26.3 |
| Aβ42–1 (6,8)b | 34.6 ± 3.2 | 38.2±2.7 | 160.2 ± 30.2 | 202.5 ± 23.1 |
| Aβ1–42(6,7)b | 27.3 ± 3.8 * | 35.5 ± 2.9 | 88.5 ± 38.6* | 186.1 ± 33.4 |
Data as mean ±SD;
Denotes study groups that were significantly different (p < 0.01).
The numbers in the parentheses represent the number of cases)
The effects of treatments on specific individual cell populations are described separately below.
Choline acetyl transferase immunostaining
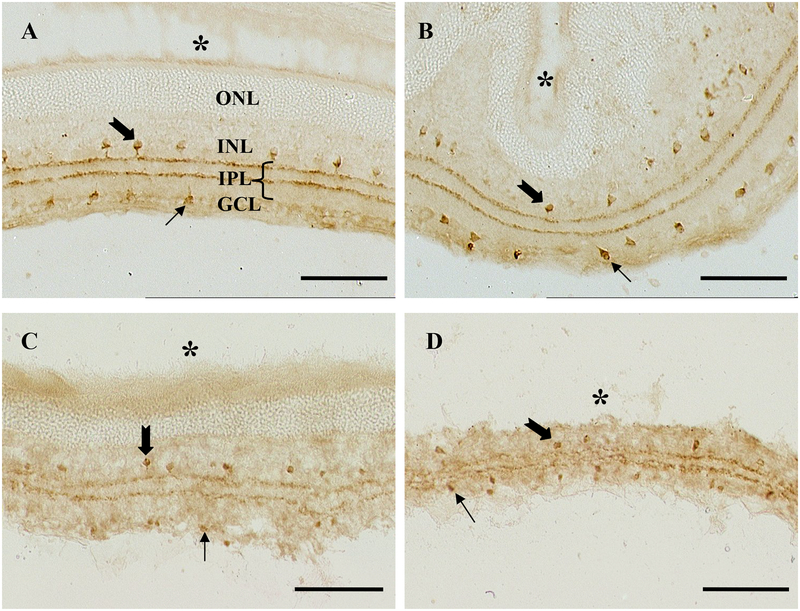
The cholinergic neurons that are immunoreactive for ChAT appeared as two populations of neurones with their somata located along the inner margins of the INL and GCL (Fig. 3A), representing amacrine and displaced amacrine cells, respectively. This is consistent with the reports of Jen et al., (1990) and Guo et al., (1991). The processes of the two sets of somata were stratified in two distinct sublaminae in the inner plexiform layer in a highly characteristic fashion. The overall pattern of ChAT-immunoreactive cells in retinas injected with Aβ1–42 was essentially the same as that of normal or un-injected side 6 to 48 hours after injection of 2 nmol. The lack of changes in the ChAT immunostaining pattern was demonstrable in retinal regions as clearly indicated by the physical distortion of the retinal tissue as a consequence of the injection (Fig. 3A, B). At 48 hours after injection of 5 nmol, however, a visually detectable reduction in immunostaining in both somata and immunoreactive sublaminae in the inner plexiform layer (IPL) was observed across the entire area of the retina (Fig. 3C). The reduction in immunostaining was confirmed by cell counting showing a reduction of ChAT-immunoreactive somata compared with the normal retinas and retinas injected with the control peptide Aβ42–1 or PBS, and the un-injected retinas from the same animals (Fig 4). The reduction of ChAT-immunoreactive somata was accompanied by the presence of less distinct immunoreactive sublaminae in the inner plexiform layer in retinas injected with 5 nmol. Interestingly, the loss of the immunostained somata and the reduction of immunoreactive sublaminae in the IPL observed in the un-injected retinas compared with the retina from normal rats and un-injected retinas from rats that were injected with the reverse peptide (Aβ42–1) (Fig 4) could indicate a bilateral or systemic effect of Aβ1–42. It was both surprising and interesting that the pattern of ChAT immunoreactivity in retinas injected with either 2 or 5 nmol returned to that of the normal and control retinas, despite a dramatic reduction in the retinal thickness (Table 1). No difference was observed between the density of ChAT somata (Fig. 4, last column) and the two highly characteristic immunoreactive sublaminae remaining, with generally slightly less or comparable staining intensity to those in the control retinas (Fig. 3D)
Figure 3.
Photomicrographs showing the characteristic pattern of cells immunoreactive to choline acetyltransferase (ChAT), in uninjected control retinas (A), and injected retinas 2 days after injection of 2 nmol (B), or 5 nmol (C) at 2 days and 5 months (D) post-injection. The thick and thin arrows indicate ChAT-immunoreactive somata of amacrine and displaced amacrine cells, respectively. The asterisk indicates the outer limit of the retina. Arrows indicate ChAT-IR cells whose somata are in the INL and the GCL. Scale bar = 100μm.
Figure 4.
ChAT-immunoreactive somata compared with the normal retinas and retinas injected with the control peptide Aβ42–1 or PBS, and the un-injected retinas from the same animals. The reduction in immunostaining was confirmed by cell counting showing a significant reduction (p< 0.01) (Table 1). The effect of Aβ1–42 at 5nmol and two days survival of the injected side was significant (*) for the dose and time dependence study.
Gamma amino butyric acid immuno-staining
The overall pattern of GABA immunoreactivity observed in the normal, un-injected and control retinas injected with PBS was similar to that described in previous studies in the normal rat (Yang et al., 1991). The numerous somata of the GABAergic neurones were amacrine cells located within the INL, with displaced amacrine cells in the GCL (Fig 5A). The number of immunoreactive sublaminae visible in the IPL varied from 2 to 5 and from region to region, a characteristic distribution pattern of GABAergic neurones in the rat retina (Fig. 5). The immunostaining pattern observed after injection of 2 nmol at all time points and after injection of 5 nmol at 6 and 24 hours was essentially unchanged. However, a very different pattern of GABA-immunoreactivity was observed in retinas at 48 hours after injection of 5 nmol Aβ1–42. (Fig 5A, B). An increase in GABA immunostaining was observed in numerous radial processes of Müller glia, a phenomenon that was never seen in any of the normal or control retinas. In contrast, a reduction in GABA-immunoreactive somata in both the INL and GCL could be observed in the same retinal regions, and the loss of immunoreactivity of these cells appeared to be more severe in the GCL abutting the vitreous. Moreover, the reduction of immunoreactive somata was always accompanied by a loss of GABA-immunoreactive sublaminae in the IPL. Most strikingly, in some regions of the same retina, hardly any immunoreactive cells or processes could be found, indicating a near total loss of GABA-immunoreactivity (Fig. 5D). A slight reduction in GABA-immunoreactive somata with occasional GABA-immunoreactive radial processes was detectable in some regions in the contralateral uninjected retinas but the effect was not consistent. Interestingly, at 5 months after the injection, the pattern of GABA-immunoreactivity in all injected retinas has returned to that comparable to the normal or control animals, indicating a complete recovery of the GABA activity in these retinas.
Figure 5.
GABA immunoreactivity in an uninjected control retina (A) and different regions in retinas injected with Aβ1–42 at 2 days (B, C & D). The thick and thin arrows indicate somata of GABA-immunoreactive amacrine and displaced amacrine cells respectively. The less intensely stained immunoreactive sublaminae (small double arrow), are found in the IPL. Note GABA-immunoreactivity is present throughout the Müller glial cells endfeet and radial processes, indicated by the arrowheads in B. Scale bar = 6100μm.
Tyrosine hydroxylase immunostaining
The distribution pattern of neurons in the catecholaminergic system is provided by the immunostaining of TH-an enzyme involved in the synthesis of dopamine/noradrenaline or adrenaline. As reported in previous studies (Guo et al., 1992; Versaiz-Botteri et al., 1986), TH-IR staining identifies two neuronal populations with large and small somata which may represent adrenergic and dopaminergic neurons, respectively. These are located at the inner margin of the INL with their extensive horizontally orientated processes ramifying the outer part of the IPL, immediately adjacent to the INL. Interestingly, these lamination processes may be observed in the outer plexiform layer (OPL) (Fig. 6). The immunoreactive pattern of TH remained unchanged in retinas injected with PBS or Aβ regardless of survival time and dosage (Fig. 6B, C, D). Both TH-immunoreactive cells with large and small somata appeared to be present with a density pattern similar to the un-injected controls (Figure 6). The preservation of the integrity of TH system is best illustrated by the highly distinctive and characteristic TH-immunoreactive band of processes located the IPL bordering the INL. However, because of the very small number of TH-immunoreactive cells observed in individual sections, cell counting was not performed to confirm whether there is a loss of TH-immunoreactive cells or not. Nonetheless, a loss of these cells can be expected in retinas injected with Aβ1–42 on the basis of a significant reduction in the retinal surface area (Table 1).
Figure 6.
TH-IR cells in uninjected control retina (A), and injected retinas at 2 days (B) and 5 months (C & D). Two sparse populations of TH-IR cells with large (thick arrows) and small (small arrows) somata located along the inner margin of the INL. These cells have extensive processes ramifying in the outer part of the IPL, immediately adjacent to the INL. The asterisk (*) marks the outer limit of the retina. Scale bar = 100μm.
Tryptophan hydroxylase immunostaining
In the present study TryptH, a serotonin/5HT rate-limiting enzyme and marker of serotonergic nerve cells, was located in association with Müller glial cells instead of retinal neurons (Fig 7A). TryptH-immunoreactivity was observed primarily in the proximal end of the radial processes of Müller glia in normal retina and retina injected with PBS. In retinas injected with 2nmol Aβ1–42 and Aβ42–1, TryptH-immunoreactivity was only slightly reduced at 2 days post-injection of 2 nmol Aβ1–42, but a near total loss was observed by 2 days after injection of 5 nmol of the peptide (Fig 7B). Interestingly, a different pattern was observed at 5 months after injection of 5 nmol Aβ1–42. Trypt H-immunoreactivity had not only returned (presumably being expressed by Müller glia) but an increase in immunostaining in radial processes was observed in some retinal regions of the injected retinas, and the increase was particularly noticeable in retinal sections that were cut obliquely or tangentially (Fig 7C). This pattern was not observed in normal or un-injected control retinas of age-matched normal retinas, or retinas injected with Aβ42–1, or PBS, indicating a long-lasting response that is specific to Aβ1–42.
Figure 7.
Photomicrographs showing tryptophan hydroxylase immunoreactivity in control (A), and injected retinas at 2 days (B) and 5 months (C). Immunoreactivity is found within the endfeet and radial processes of the Müller glial cells (see arrows). The immunoreactive radial processes are indicated by the arrowheads. The outer margin of the retina is marked by (*). Scale bar = 100μm.
DISCUSSION
The results of this study have provided the first direct evidence in a CNS structure, in vivo, that aggregated Aβ1–42 may have an effect on a number of different neuronal populations defined by neurotansmitters expressed or produced by these cells. The positive outcome of the terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay confirmed that Aβ1–42 injection induces apoptosis in some cells, particularly in the outer and middle part of the retina, but rarely in the GCL. However, the extent of the cell density study based on immunoreactive somata reveal a different pattern of changes. A reduction in the number of neurons immunoreactive for ChAT was observed 2 days after injection of 5 nmol/3μl ofAβ1–42 but not under other experimental conditions. In addition, there was an unexpected increase in GABA immunostaining and a reduction of Trypt H-immunoreactivity in radial processes of Müller glia in these retinas, providing evidence to support a disruption in the metabolic integrity of these cells. However, there is an apparent recovery of ChAT-/GABA-immunoreactivity to near normal pattern over time as confirmed by results obtained at 5 months after injection. On the other hand, a significant reduction in the retinal surface area as well as retinal thickness suggests strongly that there is an overall and significant loss of probably all cell populations despite a lack of obvious or detectable changes in cell density 5 months after injection of 5 nmol of Aβ1–42. The significance of these findings are discussed separately below.
Effects of Aβ on the neurotransmitter systems
This study was focused on determining the potential cytotoxic effect of Aβ on the cholinergic system (a system that has been intensively studied) in relation to AD (Bartus, 2007; Benzi and Moretti, 1998; Harkany, et al., 1995; Hodges, 2006; Perry, et al., 1978; Schlieb and Arendt, 2006). This ChAT-immunoreactivity was essentially unchanged despite an initial decline in ChAT cell density observed at 2 days after injection of 5nmol/3μl. Qualitative long-time point observations at 5 months showed a relatively unaffected ChAT pattern as well as cell density per unit area, despite the dramatic decrease in retinal thickness. However, considering that there is an average of up to 40% reduction in the entire surface area of individual injected retinas, a substantial loss of cholinergic neurons is expected. In view of this, caution should be exercised about the reliability of cell counting on the basis of density which by itself may not reflect the true picture of the overall changes in the cell population concerned. It is also worth pointing out that by increasing the dose of injected Aβ1–42 from 2 to 5 nmol, the intravitreally injected peptide affected not only ChAT-immmunoreactive cells within the injected eye but also has a similar transient effect on these cells in the un-injected retinas at 2 days survival time. Thus that the transient effect could be detected at best on day 2 after 5 nmol Aβ treatment but not with the 2 nmol Aβ-treatment, suggests dose dependent effect. However, at 5 months there was a recovery suggesting time dependence regardless of the dose used. This provides additional support for a systemically mediated bilateral effect (Walsh et al., 2002).
The present study has also demonstrated for the first time in vivo that Aβ may have an effect on the GABA system. This was reflected by a temporary loss of GABA-immunoreactive somata in the INL and GCL and an increase in GABA-immunoreactivity in Müller glial cells. The implication of this observation is that Aβ aggregates may impair GABA turnover as GABA is normally released into the extracellular space at the synaptic cleft and is taken up by Müller glial cells. This, together with evidence showing an increased GFAP immunoreactivity and decrease in Bcl-2 immunostaining in Müller glia observed in the persent and previous studies (Jen et al., 1998; Walsh et al., 2002, 2005), strongly suggests a disturbance of Müller glial function or metabolic integrity after a single injection of Aβ to the vitreous. The overall pattern of GABA-immunoreactivity returned to an almost normal level, thus supporting the acute and/or chronic Aβ-induced phases. That is, initial impairment in the turnover mechanisms of this inhibitory neurotransmitter, followed again by recovery, a phenomenon similarly observed in the cholinergic system described above. These changes in the distribution of GABA-immunoreactivity have implications for the Müller glia however.
In contrast to ChAT- and GABA-immnostaining pattern, the overall pattern of TH system seems relatively intact when compared with the other neuronal populations investigated. A strikingly distinct observation is made 5 months post-Aβ1–42 injection. The pattern characteristic of TH (especially the highly distinctive immunoreactive band observed in the outer part of the IPL), was essentially unchanged despite a dramatic decrease in the thickness of the retina in some of the cases. This is likely to be a reflection of a severe overall loss of retinal cells probably in the ONL and INL. Although the present results suggest this system is relatively resistant to Aβ toxicity, further confirmation with quantitative data and increased sample sizes is needed (and this is the subject of further investigations).
The rate-limiting enzyme TrypH which involved in the biosynthesis of the neurotransmitter serotonin is present in the rat retina and its levels may vary depending on the physiological or pathological state of retinal nerve cells (Chanut et al., 2002, 2006; Liang et al., 2004). However, TryptH has been associated primarily with photoreceptors and retinal neurons such as amacrine and retinal ganglion cells but not with glia. Results from the present study, however, have demonstrated that this enzyme can be used to depict the metabolic integrity of Müller glial cells, rather than serotonergic neurons in the retina. More importantly, perhaps, we have shown for the first time to our knowledge, that two days after 5 nmol Aβ1–42 injection there is a near complete loss of TryptH-immunoreactivity in the retina, implying disturbances in Müller glial cell metabolism and function. This finding is entirely novel and potentially important considering the potential role of serotonin in modulating attentive behaviour and mood.
The loss of TryptH in Müller glia is in stark contrast to an increase in GFAP and GABA immunoreacitivties observed in their radial processes, thus signifying that any of the changes seen were not a consequence of cellular loss. Moreover, TryptH had reappeared 5months later in the Müller cell endfeet and even their radial processes suggesting not only recovery from the initial insult but also a possible continuous disturbance of metabolic activity in these cells. This, together with the pattern revealed by using other markers, as reported in the present and previous studies (Jen et al., 1998; Walsh et al., 2002; 2005) indicate clearly that dysfunction of Müller glia, in addition to activation of microglia, is another key feature of retinal changes or dysfunction after intravitreal injection of Aβ aggregates. It remains unequivocal that the acute as well chronic effects of Aβ on the various cell populations and neurotransmitter systems in the retina have provided new insight into the potentially cytotoxic effect of amyloid peptides. This paper demonstrates that Aβ’s toxic effects are not exclusive to the cholinergic system, a point supported by previous reports showing similar acute and chronic effects on photoreceptors, neurons that are rich in the calcium-binding protein parvalbumin as well as retinal ganglion cells (Jen et al., 1998; Walsh et al., 2002; 2005). These findings are of special importance as far as future development of potential neuroprotective and therapeutic strategies.
The role Müller glia in Aβ toxicity
Müller cells are the predominant glial cells of the retina, with radial processes extending throughout its depth. They are intimately related to all the major neuronal populations in the retina, and their normal function is critical for the maintenance and survival of neurons by removing excess ions and neurotransmitters from the synaptic cleft after neuronal depolarisation has occurred (Reichenbach et al., 1993; Brew and Attwell, 1987). This function is mirrored in astroglial-neuronal interactions within the brain (Kimelberg and Norenberg, 1989). The existences of macroglial-neuronal communications and lactate or glycogen for energy-dependent processes have been widely documented (Magistretti, et al., 1999; Poitry-Yamate, et al., 1995; Barres 1991; Tsacopoulos and Magistretti, 1996). Apparent dysfunction of the Müller glial cells is again evident with GABA’s impaired uptake/removal or synthesis pathway ((Kimelberg and Norenberg, 1989).
The accumulation of GABA seen within the Müller glial cells infers a disturbance in the metabolism of glial. Hence, release of potentially toxic ammonia and glutamate may further exacerbate damage, manifesting in a number of ways such as, excitotoxicity and/or an impaired release of neurotrophic factors required for the maintenance and growth of neurons. Müller glia cells are known to produce glutamine synthetase (Riepe and Norenburg, 1977; Reichenbach, 1989), and its level can be reduced after intravitreal injection of Aβ. This may provide a mechanism for Aβ neurotoxicity via Müller cell dysfunction in the retina. The compromised metabolic glial functions are additionally represented by the depletion of TryptH and upregulation of GFAP. However, this appears to be an acute phase response as restoration of Müller glial cell function also restores TryptH and GABA at the long term point of 5 months. The continuous upregulation of GFAP and TryptH suggests progression of Aβ1–42 toxicity into a chronic phase.
The disruption of the efficacy normally associated with the glial system presents in a myriad of destructive cascades triggering eventual irreversible damage and death. Indeed astrogliosis remain a profound hall mark of the brain (Little and O’Callaghan, 2001). It has been postulated by Arispe et al., (1993), that Aβ may form Ca2+ channels in lipid bilayers. Changes in Ca2+ influx may thus affect or cause injury to Müller glial cells. There is also evidence that G-protein activation via Aβ-Müller cell membrane disturbances may inhibit protein kinase C production, believed to be involved in the synthesis of certain proteins including the secreted form of amyloid precursor protein (Cowburn, et al., 1996). Alternatively, the possible amphiphilic nature of Aβ, may combine to mediate its toxicity via a direct perturbation of the glial cell membranes. Indeed Schubert et al., (1995) reported that ApoE4 may potentiate this interaction via its binding region for β-sheet or amphiphilic structures. The imbalances of these interactions may induce oxidative processes which could act as catalysts for neuronal damage or to initiate destruction of systems via lipid peroxidation, carbonyl protein modifications and mitochondrial DNA and/or RNA oxidation in vulnerable neurons (Yankner, 1996; Palmer, 1996; Smith, et al., 1997; Nunomura, et al., 1999). Interestingly, antioxidants have been shown to produce a substantial slowing and prevention of the cell death associated with Aβ (Jang et al 2004; Jen, et al., 1998; Nunomura, et al., 1999, Aruoma et al., 2003), which raises an important issue concerning the possible protective effects conferred by natural dietary antioxidants in relation to toxicity and AD. In this vein, Rezai-Zadeh et al., (2008) have shown that tea catechins (epigallocatechin gallate was protective of cognitive impairment in Alzheimer transgenic mice. Further it would be of interest to corroborate the results reported here by evaluating the expression of choline acetyltransferase and 5-tryptamine hydroxylase and this work is ongoing.
In conclusion, this study has provided novel information concerning potential cytotoxic effects which could be acute, chronic or long-lasting cytotoxic after one single intravitreal injection of 5 nmol/3ul of Aβ. Importantly, while the cell density and the overall distribution pattern of the various cell populations as defined by their neurotransmitters remain largely unchanged at 5 months after injection of Aβ, there is overall loss of retinal nerve cells over a time course of 6 hours −5 months, a conclusion inferred by reductions in the area or volume of the injected retinas. Evidence of dysfunction of Müller glia cell supports further the notion that these cells may play a central role in mediating what appears to be an indirect toxic mechanism(s) induced by Aβ.
Acknowledgements:
This study was supported by grants from American Alzheimer’s Association, National Institutes of Health (USA), and Wellcome Trust (UK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest Statement:
“The authors declare that there are no conflicts of interest.”
References
- Anderson PJB, Watts HR, Jen SSM, Gentleman SM, Moncaster JA, Walsh DT, Jen LS, 2008. Differential effects of interleukin-1β and S100B on amyloid precursor protein in rat retinal neurons. Clin Ophthalmol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Tennstedt A, Arendt A, 1985. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neuroscience 14, 1–14. [DOI] [PubMed] [Google Scholar]
- Arispe N, Rojas E, Pollard HB, 1993. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: Blockade by tromethamine and aluminu. Proc. Natl. Acad. Sci. (USA) 90, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, 2000. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol 163: 495–529. [DOI] [PubMed] [Google Scholar]
- Barres BA, 1991. New role for glia. J. Neurosci 11, 3685–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, Cucatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Casudio CA, 2006. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging 27, 1644–1657. [DOI] [PubMed] [Google Scholar]
- Benzi G, Moretti A, 1998. Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s Disease? Eur. J. Pharmacol 346, 1–13. [DOI] [PubMed] [Google Scholar]
- Blokland A, 1996. Acetylcholine: a neurotransmitter for learning and memory? Brain Res. Rev 21, 285–300. [DOI] [PubMed] [Google Scholar]
- Bresciani LG, Walsh DT, Gentleman SM, Jen LS, 2002. Developmental regulation and possible alternative cleavage of presenilin 1 in the rat retina. Mol. Cell Neurosci 21, 239–249. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D, 1987. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature 327, 707–709. [DOI] [PubMed] [Google Scholar]
- Chanut E, Nguyen-Legros J, Labarthe B, Trouvin JH, Versaux-Botteri C, 2002. Serotonin synthesis and its light-dark variation in the rat retina. J. Neurochem 83, 863–869. [DOI] [PubMed] [Google Scholar]
- Chanut E, Labarthe B, Lacroix B, Noda A, Gasdeblay S, Bondie J-R, Versaux-botteri C, 2006. Variations of dopamine, serotonin, and amino acid concentrations in Noda epileptic rat (NER) retina. Brain Res. 1070, 56–64. [DOI] [PubMed] [Google Scholar]
- Cowburn RF, Fowler CJ, O’Neill C, 1996. Neurotransmitter receptor/G-protein mediated signal transduction in Alzheimer’s Disease brain. Neurodegeneration 5, 483–488. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Everitt BJ, Robbins TW., 1991. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trend. Neurosci 14, 494–501. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Tsang SW, Gil-Bea FJ, Francis PT, Lai MK, Marcos B, Chen CP, Ramierez MJ, 2006. Involvement of the GABAergic system in depressive symptoms of Alzheimer’s disease. Neurobiol Aging 27, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Gasbarri A, Introini-Collison IN, Packard MG, Pacitti C, McGaugh JL, 1993. Interaction of cholinergic-dopaminergic systems in the regulation of memory storage in aversively motivated learning tasks. Brain Res. 627, 72–78. [DOI] [PubMed] [Google Scholar]
- Grudzien A, Sahw P, Weintraub S, Bigio E, Mash DC, Mesulam MM, 2007. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging 28, 327–335. [DOI] [PubMed] [Google Scholar]
- Guo QX, Chau RMW, Yang SZ, Jen LS, 1991. Development of choline-acetyltransferase- immunoreactive neurons in normal and intracranially transplanted retinas in rats. Dev. Brain Res 62, 177–187. [DOI] [PubMed] [Google Scholar]
- Guo QX, Yang SZ, Liang CL, Tsang DT, Jen LS, 1992. Tyrosine-hydroxylase- immunoreactive neurons in retinal transplants in the rat. Biol. Sig 1, 46–56. [DOI] [PubMed] [Google Scholar]
- Guo QX, Yu MC, Garey LJ, Jen LS, 1992. Development of parvalbumin immunoreactive neurons in normal and intracranially transplanted retinas in the rat. Exp. Brain Res 90, 359–368. [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF, 2007. Targeting amyloid-beta in glaucoma treatment. Proc. Natl. Acad. Sci. (USA) 104, 13444–13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Allsop D, 1991. Amyloid deposition as the central event in the aetiology of Alzheimer’s Disease. Trend Pharmacol. Sci 12, 383–388. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ, 2002. The amyloid hypothesis of Alzheimer’s disease: progress and problems of the road to therapeutics. Science 297, 353–336. [DOI] [PubMed] [Google Scholar]
- Harkany T, Lengyel Z, Soós K, Penke B, Luiten PGM, Gulya K, 1995. Cholinotoxic effects of β-amyloid (1–42) peptide on cortical projections of the rat nucleus basalis magnocellularis. Brain Res. 695, 71–75. [DOI] [PubMed] [Google Scholar]
- Henneka MT, O’Banion MK, 2007. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol 184, 69–91. [DOI] [PubMed] [Google Scholar]
- Hodges JR, 2006. Alzheimer’s centennial legacy: origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain 129, 2811–2822 [DOI] [PubMed] [Google Scholar]
- Jang J-H, Aruoma OI, Jen L-S, Chung HY, Surh Y-J, 2004. Ergothioneine rescues PC12 cells from β-amyloid-induced apoptotic death. Free Rad. Biol. Med 36, 288–299. [DOI] [PubMed] [Google Scholar]
- Jen LS, Hart AJ, Jen A, Relvas JB, Gentleman SM, Garey LJ, Patel AJ, 1998. Alzheimer’s peptide kills cells of retina in vivo. Nature 392, 140–141. [DOI] [PubMed] [Google Scholar]
- Jen LS, Tsang D, Chau RMW, Shen WZ, 1990. The cholinergic system in retinal transplants in rats. Brain Res. 523, 156–160. [DOI] [PubMed] [Google Scholar]
- Kimelburg HK, Norenburg MD, 1989. Astrocytes. Scientific Am. Apr, 44–52. [Google Scholar]
- Lanctot KL, Herrmann N, Mazzotta P, Khan LR, Ingber N, 2004. GABAergic function in Alzheimer’s disease: evidence for dysfunction and potential s a therapeutic target for the treatment of behavioural and psychological symptoms of dementia. Can. J. Psychiatry 49, 439–453. [DOI] [PubMed] [Google Scholar]
- Liang J, Wessel JH, Iuvone PM, Tosini G, Fukuhara C, 2004. Diurnal rhythms of tryptophan hycroxylase 1 and 2 mRNA expression in the rat retina. Neuroreport 15, 1497–1500. [DOI] [PubMed] [Google Scholar]
- Little AR, O’Callaghan JP, 2001. Astrogliosis in the Adult and Developing CNS: Is There a Role for Proinflammatory Cytokines? NeuroToxicology 22, 607–618 [DOI] [PubMed] [Google Scholar]
- Lowe SL, Francis PT, Proctor AW, Palmer AM, Davidson AN, Bowen DM, 1988. Gamma aminobutyric acid concentration in brain tissue at two stages of Alzheimer’s disease. Brain 111, 785–799. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG, 1999. Energy on demand. Science 283, 496–497. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Pardo CA, Cork LC, Price DL, 1994. Synaptic pathology and glial responses to neuronal injury precede the formation of senile plaques and amyloid deposits in the aging cerebral cortex. Am. J. Pathol 145, 1358–1381. [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF III., 1998. Serotonin in aging, late-life depression, and Alzheimer’s disease: the emerging role of functional imaging. Neuropsychopharmacology 18: 407–430. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA, 1999. RNA Oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci 19, 1959–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AM, 1996. Neurochemical studies of Alzheimer’s disease. Neurodegeneration 5, 381–391. [DOI] [PubMed] [Google Scholar]
- Perry EK, Piggott MA, Court JA, Johnson M, Perry RH, Transmitters in the developing and senescent human brain. Ann. NY Acad. Sci 1993, 695, 69–72. [DOI] [PubMed] [Google Scholar]
- Poitry-Yamate CL, Poitry S, Tsacopoulos M, 1995. Lactate released by Müller glial cells is metabolised by photoreceptors from mammalian retina. J. Neurosci 15, 5179–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procter AW, 1996. Neurochemical correlates of dementia. Neurodegeneration 5, 403–407. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Stolzenberg JU, Eberhardt W, Chao TI, Dettmer D, Hertz L, 1993. What do retinal Müller (glial) cells do for their neuronal ‘small siblings’? J. Chem. Neuroanat 6, 201–213. [DOI] [PubMed] [Google Scholar]
- Riepe RE, Norenburg M, 1997. Müller cell localisation of glutamine synthetase in rat retina. Nature 268, 654–655. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T, 2006. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural. Transm 113, 1625–1644. [DOI] [PubMed] [Google Scholar]
- Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H, 1995. Amyloid peptides are toxic via a common oxidative mechanism. Proc. Natl. Acad. Sci. (USA) 92, 1989–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Harris PLR, Sayre LM, Beckman JS, Perry G, 1997. Widespread peroxynitrite- mediated damage in Alzheimer’s disease. J. Neurosci 17, 2653–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, Leverenz JB, Peskind ER, Kiyasu E, Rohde K, Miller MA, Raskind MA, 2000. Tyrosine hydroxylase and norepinephrine transporter mRNA expression in the locus coeruleus in Alzheimer’s disease. Mol. Brain Res 84, 135–140. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ, 1996. Metabolic coupling between glia and neurons. J. Neurosci 16: 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn JE, Femglietti EV Jr., Baber RP, Saitao K, Roberts E, Ribak CE, 1981. GABAergic amacrine cells in rat retina: immunocytchemical identification and synaptic connectivity. J. Compar. Neurol 197, 113–127. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Matin-Martinelli E, Nguyen-Legros J, Geffard M, Vigny A, Denoroy L, 1996. Regional specialization of the rat retina: catecholaminergic-containing amacrine cell characterization and distribution. J. Compar. Neurol 243, 422–433. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Pochet R, Nguyen-Legros J, 1989. Immunohistochemical localization of GABA-containing neurons during postnatal development of the rat retina. Invest Ophthalmol Vis. Sci 30, 652–659. [PubMed] [Google Scholar]
- Walsh DT, Bresciani L, Saunders D, Manca MF, Jen A, Gentleman SM, Jen LS, 2005. Amyloid beta peptide causes chronic glial cell activation and neuro-degeneration after intravitreal injection. Neuropathol. Appl. Neurobiol 31, 491–502. [DOI] [PubMed] [Google Scholar]
- Walsh DT, Montero RM, Bresciani LG, Jen AYT, Leclercq PD, Saunders D, El-Amir AN, Gbadamoshi L, Gentleman SM, Jen LS, 2002. Amyloid-beta peptide is toxic to neurons in vivo via indirect mechanisms. Neurobiol. Dis 10, 20–27. [DOI] [PubMed] [Google Scholar]
- Watts HR, Vince V, Walsh DT, Bresciani LG, Gentleman SM, Jen LS, Anderson PJB, 2007. Alterations in presenilin 1 processing by amyloid-beta peptide in the rat retina. Exp. Brain Res 181, 69–77. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, 1982. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239. [DOI] [PubMed] [Google Scholar]
- Yang SZ, Guo QX, Tsang D, Jen LS, 1992. Development of gamma-aminobutyric acid-immunoreactive neurons in normal and intracranially transplanted retinas in rats. Brain Res. Bull 28, 543–550. [DOI] [PubMed] [Google Scholar]
- Yanker BA, 1996. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16, 921–932. [DOI] [PubMed] [Google Scholar]