Abstract
Background
The fungus Paracoccidioides lutzii was recently included as a new causative species of paracoccidioidomycosis (PCM) and most cases have been reported from Brazil. According to available epidemiological information, P. lutzii is concentrated in the Middle-West region in Brazil, mainly in the state of Mato Grosso. However, clinical and laboratorial data available on patients infected with P. lutzii remain extremely limited.
Methodology/Main findings
This work describes the clinical manifestations of 34 patients suffering from PCM caused by P. lutzii, treated along 5 years (2011–2017) at a reference service center for systemic mycoses in Mato Grosso, Brazil. Adult rural workers (men), aged between 28 and 67 predominated. All patients had the chronic form of the disease, and the oral mucosa (n = 19; 55.9%), lymph nodes (n = 23; 67.7%), skin (n = 16; 47.1%) and lung (n = 28; 82.4%) were the most affected sites. Alcohol intake (n = 19; 55.9%) and smoking (n = 29; 85.3%) were frequent habits among the patients. No patient suffered from any other life-threatening disease, such as tuberculosis, cancer or other inflammatory or infectious parasitic diseases. The positivity in culture examination (97.1%) was higher than that found for the direct mycological examination (88.2%). Particularly, one patient presented fungemia at diagnosis, which lead to his death. The time elapsed between the initial symptoms and the initiation of treatment of PCM caused by P. lutzii was 19.7 (31.5) months, with most patients diagnosed 7 months after the symptoms’ onset.
Conclusions/Significance
Compared with the classical clinical-epidemiological profile of PCM caused by P. brasiliensis, the results of this descriptive study did not show significant clinical or epidemiological differences that could be attributed to the species P. lutzii. Future studies may confirm or refute the existence of clinical differences between the two fungal species.
Author summary
Paracoccidioidomycosis (PCM) is an endemic mycosis in Latin America with high incidence in Brazil. The fungi Paracoccidioides brasiliensis (including genetic groups S1, PS2, PS3 and PS4) and Paracoccidioides lutzii are the etiological agents, but little is known about the clinical manifestations of PCM caused by P. lutzii. Regarding eco-epidemiological aspects, the habitat is believed to be the soil due to the predominance of the disease among rural workers and other individuals who work in contact with the land. Paracoccidioides spp. has been isolated from aerosol samples, armadillos and dog food, but more data are needed to better understand the ecology of this fungus. The Middle-West region of Brazil presents the highest number of cases of P. lutzii infection. It is important to note that this species presents particularities regarding the serological diagnosis in patients. Thus, this study aims to verify possible clinical-epidemiological differences in 34 patients from this geographical region. Our results do not point out significant clinical or epidemiological differences between the two species causing PCM. In Brazil, the Ministry of Health has made an effort to include this disease in the list of compulsory notification diseases in order to implement a health policy aimed at an early detection, diagnosis and treatment.
Introduction
Paracoccidioidomycosis (PCM) is the most prevalent deep mycosis in Latin America, being endemic only in Brazil, Colombia and Venezuela. In Brazil, the state of Mato Grosso (Middle-West region), has a large number of cases, and the recently described new species P. lutzii [1] was recovered from the clinical isolates of patients from this geographic location. Paracoccidioides lutzii and P. brasiliensis are thermal dimorphic fungi, which grow at room temperature as mycelia and as yeasts with bipolar or multipolar buds at a temperature of 35 to 37°C (parasitic form). Estimates of annual incidence in Brazil vary from 0.71 cases to 3.70 cases per 100 thousand inhabitants [2]. According to information from the Ministry of Health, 3,181 cases of PCM deaths were recorded in Brazil between 1980 and 1995, resulting in a PCM mortality rate of 1.45 cases per million inhabitants (2.59 for the Southern region, 2.35 for the Central-West region, 1.81 for the Southeast, 1.08 for the North, and 0.20 for the Northeast) [3]. In Brazil, PCM is the 8th cause of mortality among the parasitic infectious diseases. Even so, it is still included in the group of neglected diseases, and there is no requirement for compulsory notification despite the severity of the disease and the fact that it is considered a public health problem [4]. The incidence of hospital admissions for PCM in Brazil is 7.99/1000 inhabitants, surpassing other endemic mycosis such as histoplasmosis and coccidioidomycosis [5].
The state of Mato Grosso is known as the country's granary, being the largest producer of soy, corn, cotton together with cattle breeding. This productivity is achieved due to the intense modernization of farming techniques. For this reason, most of the cases of patients affected by PCM are directly related to the agricultural activities carried out in rural properties of different territorial extensions. On the other hand, agricultural machine operators also constitute a target audience for PCM acquisition.
Recently, 65 isolates of P. brasiliensis were analyzed through nuclear and mitochondrial DNA, as well as the morphology of conidia and yeasts; in this study, the authors propose a new classification for the P. brasiliensis complex and the taxonomic recognition of the four genetic groups as P. brasiliensis (S1), P. americana (PS2) P. restrepiensis (PS3), P. venezuelensis (PS4), suggesting that they be considered as distinct species [6].
Humans and the nine-banded armadillo (Dasypus novemcinctus) are the accidental hosts of Paracoccidioides spp. and are usually infected in rural and peri-urban environments. Despite the consensus that the fungus’ habitat is the soil, few studies were able to demonstrate the isolation from this micro niche, existing many gaps concerning the knowledge on the still unresolved eco-epidemiology of PCM. Recently, P. brasiliensis and P. lutzii were detected in soil samples from three different locations in Brazil using molecular methods [7]; nevertheless little is known about the pathogenicity, virulence of strains, and more detailed aspects relating to the eco-epidemiology of the new species P. lutzii. In 2018, Hrycyk et al. [8] confirmed that while armadillos are highly infected by P. brasiliensis, including multiple infections by distinct genotypes or species (P. brasiliensis and P. americana) in the same animal, the same does not hold true for P. lutzii, which in turn seems to present less capacity for mycelial growth and conidial production, when developing in a soil-related condition, but this deserves further investigation.
Respiratory infection occurs via inhalation of conidia present in nature, which later reach the pulmonary alveoli. Usually, the infection is controlled by the cellular immune response, but scars can remain with latency of yeast cells. Thus, there is usually asymptomatic infection or nonspecific symptoms, or even some individuals showing the progression of infection to disease [4].
When the disease develops, the classical clinical forms are known as acute or subacute ("juvenile"), prevalent in children and young adults, in which there is inadequate Th2 cell type response to control the fungal infection. The chronic form represents 80 to 95% of the cases, affects individuals in the productive age (after the third decade of life), usually affecting the lungs, upper region including lesions in the oral mucosa, nasal mucosa, skin in places adjacent to the mouth and nose, and cervical lymph nodes. The incubation period of the disease is uncertain and may develop after many years after the individual's initial contact with the fungus [2, 4].
Paracoccidioides brasiliensis is composed of a cluster of molecular siblings recognized as S1 (S1a and S1b), PS2, PS3, and PS4 [9, 10]. The phylogenetic species S1a and S1b are widespread and predominantly found in lower South America, especially in the southeast and South of Brazil, Argentina, and Paraguay [10]. PS2 has a sporadic distribution and has been less frequently reported, with human cases only being reported thus far in Venezuela and the southeast of Brazil. The PS3 and PS4 species are, to date, exclusively endemic to Colombia and Venezuela, respectively [11].
Phylogenetic analyses demonstrated that P. lutzii represents a highly divergent lineage monophyletically separated from P. brasiliensis. Paracoccidioides lutzii is often found in the Middle-West region [1] and North [12] of Brazil, and most of the genetically evaluated clinical isolates were from the state of Mato Grosso. Regarding morphology, conidia of P. lutzii are elongated (2–22 μm), while that of P. brasiliensis measure from 2 up to 5 μm [13, 14]. To date, the main difference related to P. brasiliensis and P. lutzii lies in the serological diagnosis, where there is a need to employ local antigenic preparations in serological techniques such as ELISA, immunodiffusion and latex [15, 16].
The taxonomic description of a new species has raised the curiosity of physicians due to the possible clinical implications. Furthermore, characteristics of the in vivo susceptibility of P. lutzii to drugs conventionally used in the history of PCM also raise the interest of the professionals that manage patients affected by PCM.
The objective of this work was to describe the first results concerning the epidemiological and clinical characteristics of patients affected by P. lutzii from the Middle-West (Mato Grosso) of Brazil and reflect on possible similarities or differences between these characteristics and the classical profile of the disease caused by P. brasiliensis.
Methods
Ethical approval
This study was submitted to and approved (CAAE: 17177613.6.0000.5541) by the Federal University of Mato Grosso (UFMT) and protocol number 1796–10 by the Federal University of São Paulo (UNIFESP). All adult subjects provided informed written consent and the study was approved by ethical committee under number 288.250/CEP/HUJM/UFMT.
Study scheme and fungal strains
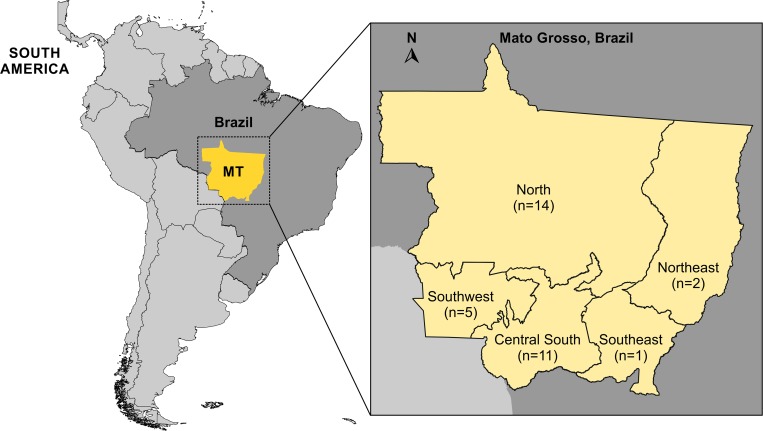
A descriptive study was carried out on 34 confirmed PCM cases caused by P. lutzii (Fig 1), that is, those with compatible clinical manifestations and positive fungal culture for Paracoccidioides spp. from different clinical materials and which were later confirmed by genotyping as P. lutzii. The patients in the study were enrolled at a reference service center of systemic mycoses of the Júlio Muller University Hospital–Federal University of Mato Grosso (UFMT / HUJM), Cuiabá, Mato Grosso—Central-West region of Brazil.
Fig 1. South America map showing sampling localities in Middle-West Brazil and total number of clinical cases of paracoccidioidomycosis caused by Paracoccidioides lutzii assessed in Mato Grosso, Brazil.
The South America map was treated using the vector graphics editor Corel Draw X8.
The Paracoccidioides spp. isolates were obtained from various clinical material (sputum, cervical lymph aspiration, blood, oral mucosa scraping, scraping of the larynx, scraping of the nasal mucosa, fragment of skin biopsy). The clinical materials were cultivated in Sabouraud Dextrose Agar (DIFCO) and incubated at a temperature of 35º C in a BOD incubator (Eletrolab) for a period of up to 20 days. Colonies with cerebriform appearance and creamy color, typical of the yeast forms, were isolated with subsequent confirmation of micromorphological characteristics of Paracoccidioides spp.
Genotyping of P. lutzii isolates
Isolates morphologically identified as Paracoccidioides spp. were subjected to molecular characterization using either HSP70 amplification [1] or via TUB1-RFLP [17]. DNA was extracted and purified from fungal colonies with the Fast DNA kit protocol (MP Biomedicals). The primer pair HSPMMT1 (5’-AAC CAA CCC CCT CTG TCT TG-3’) and PLMMT1 (5’-GAA ATG GGT GGC AGT ATG GG-3’) targeting an exclusive indel region of P. lutzii were used for PCR [1]. Isolates Pb01 and B339 were used as controls of P. lutzii (positive) and P. brasiliensis (negative) respectively. In addition, for TUB1-RFLP, the protocol described by Roberto et al. [17] was used. TUB1 fragments were amplified using the primer pair α-TubF (5′-CTG GGA GGT ATG ATA ACA CTG C-3′) and α-TubR (5′-CGT CGG GCT ATT CAG ATT TAA G-3′) [18] following a double digestion with BclI and MspI restriction endonucleases. The reaction contained 13 μL H2O, 3 μL TUB1-PCR product, 2 μL 10× fast digest buffer, and 1 μL each of the BclI (10 U/μL; Thermo Scientific) and MspI (10 U/μL; Thermo Scientific) restriction endonucleases. The digestion mixture was incubated at 37°C for 2 hours. The digested products were electrophoresed on 2.5% (w/v) agarose gels for 120 min at 100V in the presence of GelRedTM (Biotium, USA). We included a lane loaded with 50bp DNA Step Ladder (Promega, USA). Molecular identification was performed at the Medical and Molecular Mycology Laboratory (UNIFESP/EPM). The bands generated by PCR or TUB1-RFLP were visualized using the L-Pix Touch (Loccus Biotecnologia, São Paulo, Brazil) imaging system under UV illumination.
Clinical data and statistical analysis
Epidemiological and clinical data were collected from medical records of P. lutzii PCM treated patients between 2011 and 2017. The categorical variables were summarized by percentages and 95% confidence interval, and the numeric variables by mean and standard deviations. All analyses were performed by Stata Statistical Software version 12.0 (College Station, Texas, USA).
Results
Altogether 34 patients with confirmed diagnosis of PCM were evaluated, 33 men (n = 33; 97.1%) and only one woman (2.9%), with a mean (SD) age of 46.7 (9.3) years of age. Most of the patients (75.7%) resided in the north and central regions of the state of Mato Grosso (Fig 1); 73.5% in rural areas and 26.5% in urban areas. The occupations of farmer (53.6%) and rural truck driver (32.1%) were the most frequent. Smoking (85.3%) and alcohol intake (55.9%) were very frequent among patients (Table 1). None of them suffered from other life-threatening diseases.
Table 1. Demographic and behavioral characteristics of patients with paracoccidioidomycosis caused by P. lutzii.
| Characteristic | n | % | |
|---|---|---|---|
| Sex | Male | 33 | 97.1 |
| Female | 1 | 2.9 | |
| Age (years) | 27–50 | 21 | 61.8 |
| > 50 | 13 | 38.2 | |
| Mean (SD): 46.7 (9.3) | |||
| Region of origin in MT | North | 14 | 42.4 |
| (n = 33) | Central South | 11 | 33.3 |
| Southwest | 5 | 15.2 | |
| Northeast | 2 | 6.1 | |
| Southeast | 1 | 3.0 | |
| Residence Area | Rural | 25 | 73.5 |
| Urban | 9 | 26.5 | |
|
Occupation (n = 28) |
Farming | 15 | 53.6 |
| Rural Transport | 9 | 32.1 | |
| Other | 4 | 14.3 | |
| Smoking | Yes | 29 | 85.3 |
| No | 5 | 14.7 | |
| Alcohol intake | Yes | 19 | 55.9 |
| No | 5 | 44.1 | |
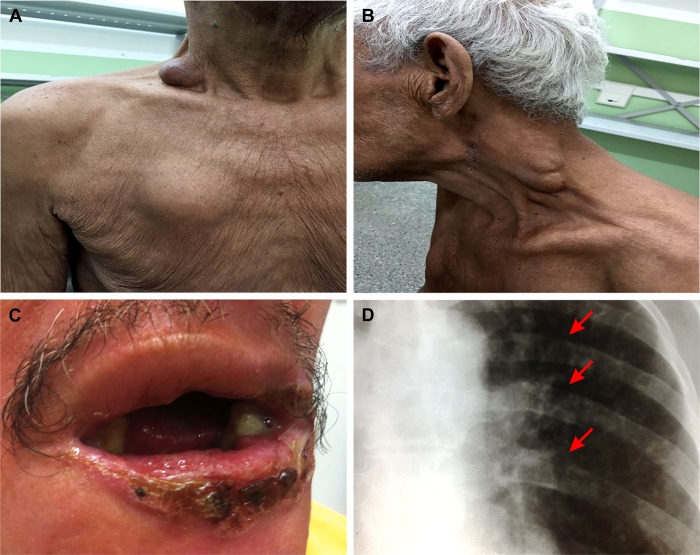
The species P. lutzii was identified by TUB1-RFLP in all 34 patients described, 30 (88.2%) being new cases and 4 (11.8%) relapsed cases of the disease. The PCM in this series of cases was multifocal in 88.2% (n = 30) and unifocal in 11.8% (n = 4). All patients had the chronic clinical form of the disease, with pulmonary involvement in 82.4%, lymph nodes (Fig 2A and 2B) in 67.7%, oral (Fig 2C) in 55.9%, cutaneous in 47.1%, laryngeal in 32.8%, nasal in 11.8%, bone (Fig 2D) in 11.8% and 2.9% in adrenal glands. One of these patients had symptoms of fungemia by P. lutzii. No patient presented central nervous system or genital involvement. The average time (SD) elapsed between the initial symptoms and the initiation of treatment of PCM by P. lutzii was 19.7 (31.5) months, with most patients diagnosed 7 months after the symptoms’ onset (Table 2).
Fig 2. Clinical features of paracoccidioidomycosis due to Paracoccidioides lutzii.
(A) Ganglion in the second right intercostal space on the anterior wall of the thorax in a patient with PCM caused by Paracoccidioides lutzii; patient 22. (B) Left cervical ganglion in a patient with paracoccidioidomycosis caused by P. lutzii; patient 09. (C) Oral lesion by P. lutzii in a patient with paracoccidioidomycosis; patient 06. (D) Bone fracture (arrow) caused by P. lutzii; patient 09.
Table 2. Clinical and laboratory characteristics of patients with paracoccidioidomycosis caused by P. lutzii at the time of diagnosis.
| Characteristic | n | % | |
|---|---|---|---|
| Admission | New Case | 30 | 88.2 |
| Recurrence | 4 | 11.8 | |
| Clinical form | Chronic | 34 | 100.0 |
| Other | 0 | - | |
| Classification | Multifocal | 30 | 88.2 |
| Unifocal | 4 | 11.8 | |
| Affected Sites | Lung | 28 | 82.4 |
| Lymph nodes | 23 | 67.7 | |
| Mouth | 19 | 55.9 | |
| Skin | 16 | 47.1 | |
| Larynx | 11 | 32.8 | |
| Nose | 4 | 11.8 | |
| Bone | 4 | 11.8 | |
| Adrenal glands | 1 | 2.9 | |
| Duration of symptoms | 1–6 | 13 | 39.4 |
| (months) | 7–12 | 8 | 24.2 |
| > 12 | 12 | 36.4 | |
| Diagnostic method | Culture | 33 | 97.1 |
| Direct mycological examination | 30 | 88.2 | |
| Histopathological examination | 12 | 35.3 | |
| Clinical Specimen | Lymph node aspiration | 14 | 41.2 |
| Oral lesion scraping | 13 | 38.2 | |
| Sputum | 3 | 8.8 | |
| Skin fragment | 3 | 8.8 | |
| Blood | 1 | 3.0 | |
| Drugs used for the treatment | SMX+TMP | 30 | 88.2 |
| SMX+TMP and itraconazole | 7 | 20.5 | |
| Itraconazole | 2 | 5.9 | |
| Amphotericin B | 2 | 5.9 | |
| Mean (SD) | |||
| Hematology/Biochemistry | Hemoglobin (g/dL) | 11.7 (2.6) | |
| Hematocrit (%) | 36.6 (7.1) | ||
| Leukocytes (/mm3) | 10,284 (3,947) | ||
| Eosinophils (/mm3) | 544 (500) | ||
| Albumin (g/dL) | 3.2 (0.6) | ||
| Glucose (g/dL) | 90.9 (21.1) | ||
SD: standard deviation
SMX+TMP: sulfamethoxazole + trimethoprim
The diagnosis of PCM was initially confirmed by culture in 97.1% (n = 33) of cases, direct mycological examination (DME) in 88.2% (n = 30), histopathological examination in 35.3% (n = 12). Clinical specimens used for the mycological exams were ganglionic secretion (n = 14), scraped oral mucosa lesion (n = 13), sputum samples (n = 3), skin biopsy (n = 3) and blood (n = 1) (Table 1).
The decision on the treatment of patients was based on the II Brazilian Consensus of Paracoccidioidomycosis [4], using sulfamethoxazole + trimethoprim in 88.2% of the patients. Out of these, 7 (23.3%) also used itraconazole and another 2 (6.9%) amphotericin B deoxycholate. The initial hematological and biochemical evaluation of the patients did not present any relevant changes (Table 2).
Discussion
In the present study on 34 patients with confirmed infection by P. lutzii, there were no clinical or epidemiological differences that could be attributed to the P. lutzii species.
The epidemiological profile of PCM has been revealing remarkable changes in frequency, demographic characteristics and geographical distribution. More than a decade ago studies published by our research group showed differences between isolates of Paracoccidioides spp. Initial investigations were conducted looking for correlations between clinical forms of the disease, geographical origin of same, susceptibility to antifungal drugs and epidemiological findings [19, 20]. In 2009, Batista et al. [21], showed significant differences in serological test results using double radial immunodiffusion for diagnosis of PCM when sera from patients from the Middle-West and Southeast regions of Brazil were evaluated. The exoantigens obtained from isolates from patients from these geographical regions affected by PCM presented strong evidence of antigenic variation among the isolates [15, 22]. It was also observed through the RAPD technique that clinical isolates from different anatomical sites (arm and face) of a same patient presented genetic differences [23]. All evidence collected related to possible antigenic differences whenever exoantigens from different geographic locations [21] were used by different researchers who obtained results from the use of different molecular techniques seeking correlation with virulence of isolates [24] and clinical forms of the disease [20], was important for the proposal of a new species: P. lutzii [1].
However, the vast literature related to clinical, demographic and epidemiological aspects of P. brasiliensis as a single etiologic agent of the disease until 2009, highlights classical presentations fairly known by medical professionals. For the physician, it is important to assess the epidemiological, clinical, diagnostic and therapeutic impact on the disease of different species of Paracoccidioides, i.e., whether there are indeed differences regarding clinical manifestations between the two species: P. lutzii and P. brasiliensis, possibly attributed to the antigenic differences of clinical isolates [15], or even to the virulence of the strains [24, 25].
Taking into account the acute/subacute forms according to Ferreira [26], a multisystemic involvement of the disease is observed; the presence of lymphadenomegaly, cutaneous lesions, hepatosplenomegaly or abdominal masses. Jaundice, ascites, and peripheral edema may also be present. The latter justify the investigation of hypoalbuminemia. Signs of adrenal involvement, as well as neurological involvement, are rare in this clinical form. Digestive complaints, such as abdominal pain, chronic malabsorptive diarrhea and vomiting, are also quite frequent. Fever and weight loss complete the clinical picture, presence of growth or pain in the bone region requires the identification of bone lesions. According to Mendes [27] and Valle et al. [28], the chronic form is assessed through signs and symptoms related to the pulmonary, tegumentary and laryngeal involvement (cough, dyspnea, mucopurulent expectoration, ulcerated skin lesions and nasopharyngeal mucosa, odynophagia, dysphagia and dysphonia); lymphatic (adenomegaly); adrenal [29, 30] (asthenia, weight loss, hypotension, darkening of skin, abdominal pain). Relating to the central nervous system, according to Pereira et al. [31] and Almeida et al. [32] the following may be observed: headache, motor deficit, convulsive syndrome, changes in behavior and/or level of consciousness. Regarding the digestive impairment, diarrhea and sometimes malabsorption syndrome are reported [33]. In this study, all patients evaluated presented the chronic form of the disease, where pathognomonic signs and symptoms of this form were recognized, mainly showing pulmonary, lymphatic, oral and cutaneous impairment. There was no clinical evidence in this sample of patients evaluated (n = 34) that could be highlighted, considering the etiology of PCM caused by P. lutzii. One case of fungemia was observed [34], but it is not possible to infer that P. lutzii is more virulent than P. brasiliensis because of this finding. Moreover, out of the 34 cases evaluated with etiology of PCM by P. lutzii, only two were classified as severe chronic form, the majority (n = 32) being classified as moderate clinical form.
Considering the proposed species (P. brasiliensis S1a, S1b, PS2, PS3, PS4 and P. lutzii), Macedo et al. [35] described an autochthonous clinical case in the southeast of Brazil (Rio de Janeiro), classified as P. brasiliensis PS2. These authors reported that few cases with this molecular taxonomy have been recorded in the literature when compared with S1 and PS3, and that among the cases registered pointing PS2 as the etiologic agent a higher frequency of the chronic form of the disease was observed. This finding was also observed in 34 patients affected by PCM caused by P. lutzii assessed in this study. Associated habits (smoking and drinking) were also frequent, as well as the frequency in male individuals in the productive age. These characteristics coincide with those described in the literature for classical PCM caused by P. brasiliensis (smoking (>20 cigarettes/day for >20 years) and alcohol intake (>50g/day). They are also often associated with the mycosis) [36]. Regarding the duration of symptoms in months for patients affected by P. lutzii in this series of cases, two groups were the most frequent: 13 patients allocated in the range of 1 to 6 months, and 12 patients ranged higher than 12 months, corroborating the classical data already published for P. brasiliensis.
In terms of distribution by regions in the state of Mato Grosso, the North (n = 14) and Central South (n = 11) regions were responsible for the largest number of cases. The concentration of the highest number of cases in the Northern region can be explained by environmental factors due to the opening of new agricultural frontiers with forest felling, especially in the Amazon—Mato Grosso region [37]. In addition, the occurrence of different species of Paracoccidioides may also be contributing to the change in the epidemiological pattern [37].
The suspected diagnosis of PCM occurs through clinical and epidemiological data, but the confirmation is done primarily by the identification of the etiologic agent in fresh tissue examinations, cultures and histopathologic preparations, which are considered the gold standard in the definition of the disease, being known as direct techniques in the diagnosis of PCM. Indirect techniques are represented by the presence of antibodies and circulating antigens in the serum of patients with PCM. A very interesting result was found in this study, with higher positivity for the culture identification (97.1%) when compared with that found by direct mycological examination (88.2%).
Generally speaking, it is not possible, so far, to establish important clinical differences that can be attributed to P. lutzii or P. brasiliensis complex. In 2017, our research group evaluated, in another study, a total of 554 patients who were treated at the same hospital during the study period (1998 to 2014), 527 had confirmed PCM diagnosis. Out of 527 patients, 244 (46.3%) patients (mean age, 48.4 [10.9] years; range, 14–83 years), classified as the chronic form of PCM. All patients were living in rural areas, and most performed activities related to agriculture [38]. These data show that the acute form of PCM is less frequent in the state of Mato Grosso, central region of Brazil, a geographical region where a higher frequency of P. lutzii has been observed so far.
This is the first study that presents a series of cases of P. lutzii, identified by molecular methods and correlating them with the clinical and epidemiological profile of affected patients. The actual incidence of each phylogenetic species and its involvement in clinical practice should include other studies in different regions of Brazil and Latin America to compare the forms of PCM and clinical manifestations with the genetic profile of these entities.
Only a few studies are found to date in the literature offering the molecular identification of clinical isolates and their association with clinical characteristics of patients affected by PCM. For comparison purposes, considering clinical findings and molecular characterization, Macedo et al. [39] recently carried out phylogenetic analysis of 54 Paracoccidioides spp. clinical strains from Rio de Janeiro, Brazil where P. brasiliensis (n = 48) and P. americana (n = 6) were identified as the causative agents of PCM. Considering the clinical classification, the authors reported that 41 strains were identified as P. brasiliensis, 23 corresponded to the chronic form, and 16 were acute. In Mato Grosso, all 34 clinical cases infected by P. lutzii corresponded to the chronic form of PCM. In relation to the affected organs, for both P. lutzii (Table 2) and P. brasiliensis [39], lungs and lymph nodes were the most affected. Regarding the severity of the disease, 7 were classified as mild, 18 (moderate), and 16 (severe) in the case of P. brasiliensis [39], in contrast to 32 cases (moderate form) PCM caused by P. lutzii, and only 2 cases were classified as severe.
The number of clinical cases evaluated by Macedo et al [39] concerning P. americana is very small. Thus, it is difficult to make any inference or comparison considering clinical manifestations. This has proven to be a limitation for the study [39].
Based on the clinical findings regarding P. lutzii and P. brasiliensis complex, there is no evidence that allows us to point out significant clinical differences between species. For this reason, we believe that studies with a greater number of isolates should be conducted to confirm or refute the hypothesis that there are clinical differences related to the different species. However, the genetic susceptibility of the host should always be an important parameter to be considered, as well as the virulence of the strain, regardless of the species that causes PCM.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported, in part, by grants from São Paulo Research Foundation (FAPESP), the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES). RCH acknowledges the financial support of Mato Grosso Research Foundation (FAPEMAT). ZPdC acknowledges the financial support of FAPESP (2009/54024-2) and CNPq (CNPq 429594/2018-6). AMR acknowledges the financial support of FAPESP (2017/27265-5) and CAPES (88887.177846/2018-00). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Teixeira MM, Theodoro RC, de Carvalho MJA, Fernandes L, Paes HC, Hahn RC, et al. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol. 2009;52(2):273–83. 10.1016/j.ympev.2009.04.005 [DOI] [PubMed] [Google Scholar]
- 2.Martinez R. New trends in paracoccidioidomycosis epidemiology. J Fungi. 2017;3(1):1 10.3390/jof3010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutinho ZF, Silva D, Lazera M, Petri V, Oliveira RM, Sabroza PC, et al. Paracoccidioidomycosis mortality in Brazil (1980–1995). Cad Saude Publica. 2002;18(5):1441–54. . [DOI] [PubMed] [Google Scholar]
- 4.Shikanai-Yasuda MA, Mendes RP, Colombo AL, Queiroz-Telles F, Kono ASG, Paniago AMM, et al. Brazilian guidelines for the clinical management of paracoccidioidomycosis. Rev Soc Bras Med Trop. 2017;50(5):715–40. 10.1590/0037-8682-0230-2017 . [DOI] [PubMed] [Google Scholar]
- 5.Giacomazzi J, Baethgen L, Carneiro LC, Millington MA, Denning DW, Colombo AL, et al. The burden of serious human fungal infections in Brazil. Mycoses. 2016;59(3):145–50. 10.1111/myc.12427 . [DOI] [PubMed] [Google Scholar]
- 6.Turissini DA, Gomez OM, Teixeira MM, McEwen JG, Matute DR. Species boundaries in the human pathogen Paracoccidioides. Fungal Genet Biol. 2017;106(Supplement C):9–25. 10.1016/j.fgb.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arantes TD, Theodoro RC, Teixeira MdM, Bosco SdMG, Bagagli E. Environmental mapping of Paracoccidioides spp. in Brazil reveals new clues into genetic diversity, biogeography and wild host association. PLoS Negl Trop Dis. 2016;10(4):e0004606 10.1371/journal.pntd.0004606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hrycyk MF, Garcia Garces H, Bosco SdMG, de Oliveira SL, Marques SA, Bagagli E. Ecology of Paracoccidioides brasiliensis, P. lutzii and related species: infection in armadillos, soil occurrence and mycological aspects. Med Mycol. 2018:myx142-myx. 10.1093/mmy/myx142 [DOI] [PubMed] [Google Scholar]
- 9.Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, et al. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol. 2006;23(1):65–73. 10.1093/molbev/msj008 [DOI] [PubMed] [Google Scholar]
- 10.Munoz JF, Farrer RA, Desjardins CA, Gallo JE, Sykes S, Sakthikumar S, et al. Genome diversity, recombination, and virulence across the major lineages of Paracoccidioides. mSphere. 2016;1(5). 10.1128/mSphere.00213-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teixeira MM, Theodoro RC, Nino-Vega G, Bagagli E, Felipe MSS. Paracoccidioides species complex: Ecology, phylogeny, sexual reproduction, and virulence. PLoS Pathog. 2014;10(10):e1004397 10.1371/journal.ppat.1004397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques-da-Silva SH, Rodrigues AM, de Hoog GS, Silveira-Gomes F, de Camargo ZP. Occurrence of Paracoccidioides lutzii in the Amazon region: Description of two cases. Am J Trop Med Hyg. 2012;87(4):710–4. 10.4269/ajtmh.2012.12-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theodoro RC, Teixeira MdM, Felipe MSS, Paduan KdS, Ribolla PM, San-Blas G, et al. Genus Paracoccidioides: Species recognition and biogeographic aspects. PLoS ONE. 2012;7(5):e37694 10.1371/journal.pone.0037694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira MM, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, et al. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol. 2014;52(1):19–28. 10.3109/13693786.2013.794311 . [DOI] [PubMed] [Google Scholar]
- 15.Gegembauer G, Araujo LM, Pereira EF, Rodrigues AM, Paniago AM, Hahn RC, et al. Serology of paracoccidioidomycosis due to Paracoccidioides lutzii. PLoS Negl Trop Dis. 2014;8(7):e2986 10.1371/journal.pntd.0002986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dos Santos PO, Rodrigues AM, Fernandes GF, da Silva SH, Burger E, de Camargo ZP. Immunodiagnosis of paracoccidioidomycosis due to Paracoccidioides brasiliensis using a latex test: detection of specific antibody anti-gp43 and specific antigen gp43. PLoS Negl Trop Dis. 2015;9(2):e0003516 10.1371/journal.pntd.0003516 ; PubMed Central PMCID: PMCPmc4334539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberto TN, Rodrigues AM, Hahn RC, de Camargo ZP. Identifying Paracoccidioides phylogenetic species by PCR-RFLP of the alpha-tubulin gene. Med Mycol. 2016;54(3):240–7. 10.1093/mmy/myv083 . [DOI] [PubMed] [Google Scholar]
- 18.Kasuga T, White TJ, Taylor JW. Estimation of nucleotide substitution rates in Eurotiomycete fungi. Mol Biol Evol. 2002;19(12):2318–24. 10.1093/oxfordjournals.molbev.a004056 [DOI] [PubMed] [Google Scholar]
- 19.Hahn RC, Hamdan JS. In vitro susceptibilities of Paracoccidioides brasiliensis yeast form to antifungal drugs. Mycoses. 2000;43(11–12):403–7. . [PubMed] [Google Scholar]
- 20.Hahn RC, Macedo AM, Fontes CJ, Batista RD, Santos NL, Hamdan JS. Randomly amplified polymorphic DNA as a valuable tool for epidemiological studies of Paracoccidioides brasiliensis. J Clin Microbiol. 2003;41(7):2849–54. 10.1128/JCM.41.7.2849-2854.2003 ; PubMed Central PMCID: PMCPmc165335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batista J Jr, de Camargo ZP, Fernandes GF, Vicentini AP, Fontes CJF, Hahn RC. Is the geographical origin of a Paracoccidioides brasiliensis isolate important for antigen production for regional diagnosis of paracoccidioidomycosis? Mycoses. 2010;53(2):176–80. 10.1111/j.1439-0507.2008.01687.x [DOI] [PubMed] [Google Scholar]
- 22.Queiroz Junior Lde P, de Camargo ZP, Tadano T, Rodrigues AM, Takarara DT, Gegembauer G, et al. Serological and antigenic profiles of clinical isolates of Paracoccidioides spp. from Central Western Brazil. Mycoses. 2014;57(8):466–72. 10.1111/myc.12183 . [DOI] [PubMed] [Google Scholar]
- 23.Batista Junior J, Berzaghi R, Arnaud AD, Fontes CJ, de Camargo ZP, Hahn RC. Simultaneous infection of human host with genetically distinct isolates of Paracoccidioides brasiliensis. Mem Inst Oswaldo Cruz. 2010;105(1):62–5. . [DOI] [PubMed] [Google Scholar]
- 24.de Oliveira HC, da Silva Jde F, Scorzoni L, Marcos CM, Rossi SA, de Paula ESAC, et al. Importance of adhesins in virulence of Paracoccidioides spp. Front Microbiol. 2015;6:303 10.3389/fmicb.2015.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camacho E, Nino-Vega GA. Paracoccidioides spp.: Virulence factors and immune-evasion strategies. Mediators Inflamm. 2017;2017:5313691 10.1155/2017/5313691 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferreira MS. Paracoccidioidomycosis. Paediatr Respir Rev. 2009;10(4):161–5. 10.1016/j.prrv.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Mendes RP. The gamut of clinical manifestations In: Franco M, Lacaz CS, Restrepo-Moreno A, G DN, editors. Paracoccidioidomycosis. 2 Boca Raton: CRC Press; 1994. p. 233–58. [Google Scholar]
- 28.do Valle AC, Aprigliano Filho F, Moreira JS, Wanke B. Clinical and endoscopic findings in the mucosae of the upper respiratory and digestive tracts in post-treatment follow-up of paracoccidioidomycosis patients. Rev Inst Med Trop Sao Paulo. 1995;37(5):407–13. . [DOI] [PubMed] [Google Scholar]
- 29.Del Negro G, Melo EH, Rodbard D, Melo MR, Layton J, Wachslicht-Rodbard H. Limited adrenal reserve in paracoccidioidomycosis: cortisol and aldosterone responses to 1–24 ACTH. Clin Endocrinol. 1980;13(6):553–9. . [DOI] [PubMed] [Google Scholar]
- 30.Colombo AL, Faical S, Kater CE. Systematic evaluation of the adrenocortical function in patients with paracoccidioidomycosis. Mycopathologia. 1994;127(2):89–93. . [DOI] [PubMed] [Google Scholar]
- 31.Pereira WC, Tenuto RA, Raphael A, Sallum J. [Brain localization of South American blastomycosis. Considerations apropos of 9 cases]. Arq Neuropsiquiatr. 1965;23(2):113–26. . [DOI] [PubMed] [Google Scholar]
- 32.de Almeida SM, Queiroz-Telles F, Teive HA, Ribeiro CE, Werneck LC. Central nervous system paracoccidioidomycosis: clinical features and laboratorial findings. J Infect. 2004;48(2):193–8. . [DOI] [PubMed] [Google Scholar]
- 33.Laudanna A, Bettarello A, Van Bellen B, Kieffer J. South American blastomycosis as a cause of malabsorption and protein-losing enteropathy. Arch Gastroenterol. 1975;12(3):195–8. [Google Scholar]
- 34.Hahn RC, Rodrigues AM, Fontes CJ, Nery AF, Tadano T, de Padua Queiroz Junior L, et al. Fatal Fungemia due to Paracoccidioides lutzii. Am J Trop Med Hyg. 2014;91(2):394–8. 10.4269/ajtmh.13-0482 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Macedo PM, Almeida-Paes R, de Medeiros Muniz M, Oliveira MM, Zancope-Oliveira RM, Costa RL, et al. Paracoccidioides brasiliensis PS2: First autochthonous paracoccidioidomycosis case report in Rio de Janeiro, Brazil, and literature review. Mycopathologia. 2016;181(9–10):701–8. 10.1007/s11046-016-0015-6 . [DOI] [PubMed] [Google Scholar]
- 36.dos Santos WA, da Silva BM, Passos ED, Zandonade E, Falqueto A. [Association between smoking and paracoccidioidomycosis: a case-control study in the State of Espirito Santo, Brazil]. Cad Saude Publica. 2003;19(1):245–53. . [DOI] [PubMed] [Google Scholar]
- 37.Millington MA, Nishioka SA, Martins ST, Santos Z, Lima Junior FEF, Alves RV. [Paracoccidioidomycosis: historical approach and perspectives for implementation of surveillance and control]. Epidemiol Serv Saude. 2018;27(spe):e0500002 10.5123/S1679-49742018000500002 . [DOI] [PubMed] [Google Scholar]
- 38.Nery AF, Crepaldi NP, Rossi S, Tadano T, Leal-Santos FA, Hahn RC, et al. Therapeutic response in adult patients with nonsevere chronic paracoccidioidomycosis treated with sulfamethoxazole-trimethoprim: A retrospective study. Am J Trop Med Hyg. 2017;97(2):556–62. 10.4269/ajtmh.16-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Macedo PM, Teixeira MdM, Barker BM, Zancopé-Oliveira RM, Almeida-Paes R, Francesconi do Valle AC. Clinical features and genetic background of the sympatric species Paracoccidioides brasiliensis and Paracoccidioides americana. PLoS Negl Trop Dis. 2019;13(4):e0007309 10.1371/journal.pntd.0007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.