Abstract
N6-Methyladenosine (m6A) RNA methylation plays important roles during development in different species. However, knowledge of m6A RNA methylation in monocots remains limited. In this study, we reported that OsFIP and OsMTA2 are the components of m6A RNA methyltransferase complex in rice and uncovered a previously unknown function of m6A RNA methylation in regulation of plant sporogenesis. Importantly, OsFIP is essential for rice male gametogenesis. Knocking out of OsFIP results in early degeneration of microspores at the vacuolated pollen stage and simultaneously causes abnormal meiosis in prophase I. We further analyzed the profile of rice m6A modification during sporogenesis in both WT and OsFIP loss-of-function plants, and identified a rice panicle specific m6A modification motif “UGWAMH”. Interestingly, we found that OsFIP directly mediates the m6A methylation of a set of threonine protease and NTPase mRNAs and is essential for their expression and/or splicing, which in turn regulates the progress of sporogenesis. Our findings revealed for the first time that OsFIP plays an indispensable role in plant early sporogenesis. This study also provides evidence for the different functions of the m6A RNA methyltransferase complex between rice and Arabidopsis.
Author summary
N6-Methyladenosine (m6A) is the most abundant internal modification of eukaryotic mRNA, and m6A mRNA methylation affects almost every stage of mRNA metabolism. However, the components of the m6A methyltransferase complex and their functions in monocots are completely unknown. In this study, we identified the components of the m6A RNA methyltransferase complex in rice, and uncovered a hitherto unknown function of m6A RNA methylation in regulating early microspore apoptosis. We also systematically analyzed the characteristics of m6A modification during sporogenesis for the first time, and revealed the sporogenesis stage-specific distribution of m6A peaks along genes and the specific modification motif in rice, which are different from that of other species and other developmental stages. The target genes of m6A methyltransferase complex member OsFIP were also identified in this study. Given the important roles of posttranscriptional mRNA regulation in gene expression and sporogenesis in plants, the findings of this study should stimulate more studies exploring the role of plant m6A methyltransferase and other components.
Introduction
N6-methyladenosine (m6A) represents the most abundant internal modification of eukaryotic mRNA and accounts for more than 80% of all RNA base methylations in various species. m6A mRNA methylation affects almost every stage of mRNA metabolism. The deposition of m6A is achieved through a multicomponent methyltransferase complex [1]. In mammals, methyltransferase-like 3 (METTL3) is responsible for methylation activity [2]. METTL14 and Wilms’ tumor 1-associating protein (WTAP) are other components of the m6A methyltransferase complex that have also been identified [3, 4]. WTAP associates with the METTL3-METTL14 core complex and facilitates METTL3-MELLT14 complex translocation to nuclear speckles, and this activity is required for the efficient methylation of mRNA [5, 6].
Most of the progress in elucidating plant m6A methylation machineries and their functions have been achieved in Arabidopsis [7–9]. In Arabidopsis, the ortholog of METTL3 is mRNA adenosine methylase (MTA). The inactivation of MTA results in reduced m6A mRNA methylation and a failure of the developing embryo to progress past the globular stage [5, 10]. AtFIP37 is the ortholog of mammalian WTAP in Arabidopsis. AtFIP37 knockout mutants show an embryo-lethal phenotype that is caused by a strong delay in endosperm development and embryo arrest [11]. Moreover, a recent study showed that AtFIP37 plays an indispensable role in determining shoot stem cell fate in Arabidopsis [12]. All together, these studies indicate that the m6A methyltransferase components have unique functions during embryo development, shoot stem cell fate and root growth in Arabidopsis.
However, the components and functions of m6A methyltransferases in monocot species have not been reported. Here, we identified the components of the m6A methyltransferase complex in rice and uncovered a previously unknown function of m6A methylation in the regulation of pollen development. We revealed that OsFIP and OsMTA2 are the orthologues of Arabidopsis FIP37 and MTA, respectively. They interact with each other and both of them are required for mRNA methylation. The unique function of OsFIP was further revealed in this study. OsFIP is essential for early sporogenesis. Loss of function of OsFIP disrupts the m6A modifications of threonine protease and NTPase genes during sporogenesis by directly binding to them and leads to microspores being degenerate at the early microspore stage. OsFIP also affects both the chromosomes and the cytoplasmic components of microspore mother cells (MMCs) during prophase I. These findings revealed the essential roles of OsFIP in rice sporogenesis and fertility.
Results
OsFIP and OsMTA2 are the subunits of RNA N6-methyladenosine methyltransferase in rice
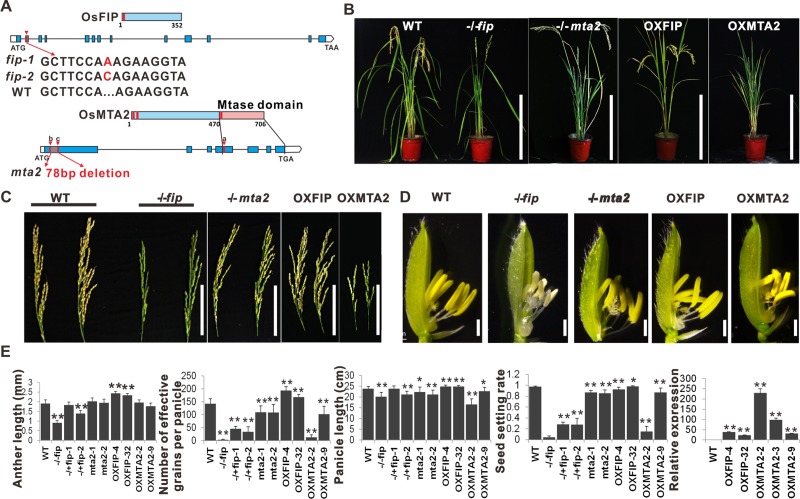
To identify the rice components of the m6A methyltransferase complex and explore their functions, we first searched for homologs of the m6A methyltransferase complex in mammals and Arabidopsis. Five rice proteins were predicted to be the m6A methyltransferase components: OsMTA2 (LOC_Os02g45110), which is 57.2% identical to AtMTA (S1A Fig); OsFIP (LOC_Os06g27970), which is 59.09% identical to AtFIP37 (S1B Fig); and OsMTA1 (LOC_Os01g16180), OsMTA3 (LOC_Os03g05420) and OsMTA4 (LOC_Os10g31030), which are 54.86%, 43.15% and 48.53% identical to AtMTB, respectively (S1C Fig). Although the functions of these five proteins are unknown, their functional regions are highly conserved (S1A and S1C Fig).
To verify whether these five proteins are the subunits of m6A RNA methyltransferase in rice, we constructed their knockout mutant lines using CRISPR-Cas9, respectively (named mta2, fip, mta1, mta3 and mta4). For the mta2 mutant, the gRNA target was first designed to target the start position of the predicted Mtase domain (the fourth exon, Fig 1A). However, no homozygous OsMTA2 knockout line with a reading frame shift mutation was obtained despite generating OsMTA2 knockout mutants twice and screening more than 300 plants. We speculated that OsMTA2 is indispensable for rice callus differentiation. We then designed two gRNAs targeting the first exon of OsMTA2, and no mutant line with a reading frame shift mutation was obtained; only two homozygous lines with a 28 amino acids deletion in the noncatalytic region or 28 amino acids substitution respectively were obtained and were used for further study (Fig 1A and S2A Fig). For the fip mutant, the gRNA was designed to target the second exon. We identified several homozygous fip mutant lines that had reading frame shifts (Fig 1A and S2B Fig). For the mta1, mta3 and mta4 mutants, the gRNA targets were designed to target the first exon (S2C Fig).
Fig 1. OsFIP and OsMTA2 are required for reproductive development.
(A) Schematic of the OsMTA2 and OsFIP knockout mutants generated by CRISPR-Cas9. The red arrowheads indicate the gRNA target sites. (B) Gross morphology of wild-type, fip, mta2, OXFIP and OXMTA2 transgenic plants. Scale bar, 40 cm. (C) Panicles of WT, fip, mta2, OXFIP and OXMTA2 plants. Scale bars, 10 cm. (D) Spikelets of WT, fip, mta2 OXFIP and OXMTA2 plants. Scale bars, 1 mm. (E) Panicle lengths, seed numbers, seed-setting rates, anther lengths and relative expression of genes of different transgenic lines. Values shown are the means ± s.d. (n > 15 plants). Significant differences were identified using Student’s t-test.
We next performed dot blot analyses to compare the total m6A levels in RNA from all the knockout mutant lines. As expected, knocking out of OsFIP or OsMTA2 dramatically reduced m6A levels (S3A and S3B Fig), indicating that OsFIP and OsMTA2 are required for global m6A RNA methylation in rice. However, no effects on the total m6A levels were observed in the OsMTA1, OsMTA3 and OsMTA4 knockout lines (S3C Fig). We also constructed transgenic plants overexpressing OsFIP (OXFIP) or OsMTA2 (OXMTA2) to investigate the regulatory roles of high levels of these proteins on m6A abundance and plant growth (Fig 1E). Consistently, m6A levels were slightly increased in the OXMTA2 plants and in the OXFIP lines (S3A Fig). We next examined whether the interaction between OsMTAs and OsFIP occurs in vivo using yeast two-hybrid experiments and bimolecular fluorescence complementation (BiFC). The results clearly showed that OsMTA2 interacts with OsFIP in both yeast and rice nuclei (S3D and S3E Fig). However, OsMTA1, OsMTA3 and OsMTA4 did not interact with OsMTA2 or OsFIP (S3E Fig). Together with the effects of these five proteins on the m6A levels in rice, we proposed that OsMTA2 and OsFIP are the subunits of RNA N6-methyladenosine methyltransferase in rice but that OsMTA1, OsMTA3 and OsMTA4 might not be components of the complex.
Phenotypic analysis of OsFIP and OsMTA2 mutants revealed a unique function of OsFIP in sporogenesis
Next, to investigate the functional relevance of OsFIP and OsMTA2 in rice development, we performed phenotypic analyses of the knockout mutant lines (mta2 and fip) and the overexpression lines (OXMTA2 and OXFIP) (Fig 1B). In the vegetative stage, the phenotypes of the four mutant plants appear normal and similar to that of the wild-type plants (S3F Fig), only the tiller number of homozygous fip plants (~1.4 tillers per plant) was less than that of WT plants (~4.7 tillers per plant) (S3G Fig). However, in the late stage of reproductive development, the fip plants were almost totally sterile and presented shortened panicles and anthers, and decreased effective seed number compared with that of the wild-type (WT) plants, whereas OXFIP have longer anthers, longer panicles, higher seed numbers and seed setting rates than WT plants (Fig 1C–1E). For the mta2 and OXMTA2 plants, the panicles length, fertility and effective seed number were also reduced compared with those of the WT plants but were higher than those of the fip plants (Fig 1C–1E).
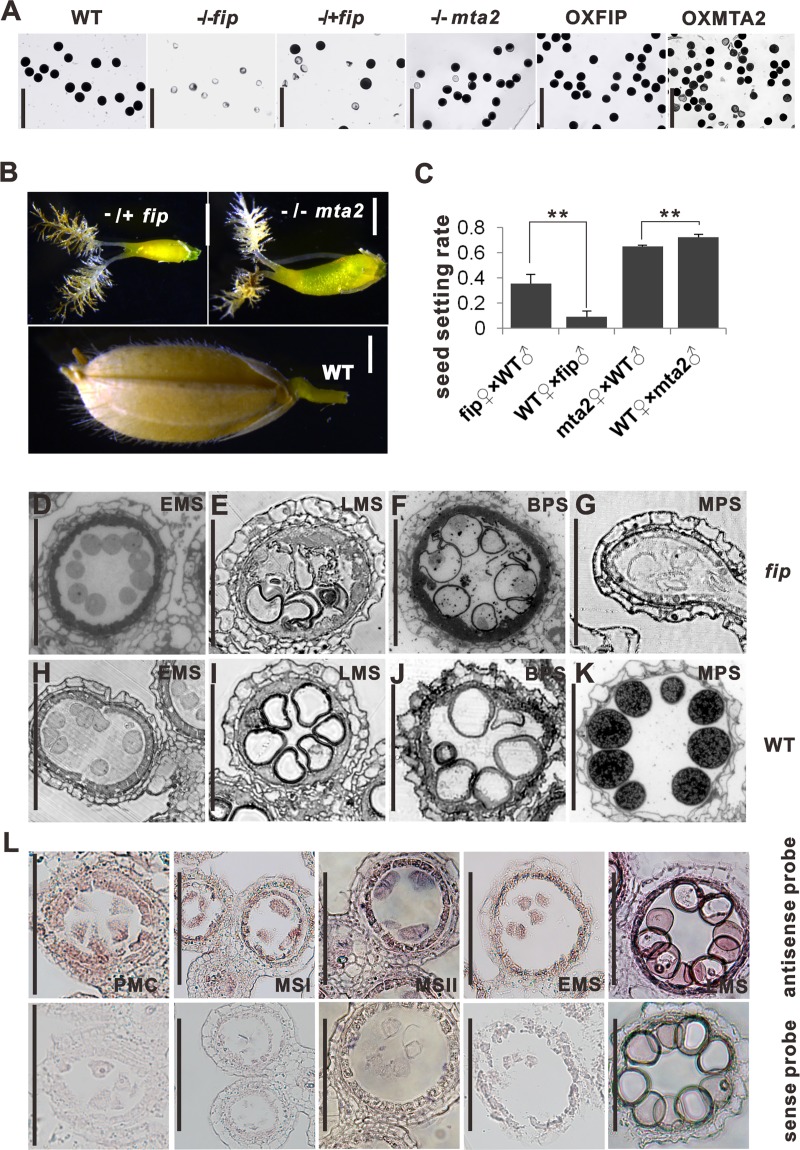
To understand what caused the sterile phenotypes of mta2 and fip, we examined the pistil and stamen structures of the four mutants mentioned above. As shown in S3D Fig, all of the mature pistils (n>60 pistils for each line) of the mutants showed normal embryo sac development (S3H Fig), suggesting that the low setting rates in mta2 and fip might not be associated with pistil development. We then examined the pollen grains of the transgenic plants, and the results showed that the fip anthers had very few pollen grains, and 84.8% of the existing pollen grains lacked starch, but mta2 and OXMTA2 only have a few abortive pollen grains (17.1% for mta2, 27.5% for OXMTA2) (Fig 2A), indicating that OsFIP plays an essential role in microspore development.
Fig 2. OsFIP and OsMTA2 regulate different stages of male reproductive development.
(A) Mature pollen grains at stage 12 stained for starch with I2-KI. Scale bars, 100 μm. (B) Seeds of WT, fip and mta2 at 21 days after flowering. Scale bars, 0.7 mm. (C) The seed setting rates of the mutants crossed with WT plants. Values shown are the means ± s.d. (n = 4 plants). Significant differences were identified using Student’s t-test. (D-K) Transverse semithin sections of homozygous fip anthers at stages 9 (D), 10 (E) 11–12 (F) and 12 (G) from left to right panels; WT anthers at stages 9 (H), 10 (I), 11–12 (J) and 12 (K) from left to right panels. Scale bars, 100 μm. (L) In situ hybridization of OsFIP mRNAs from stage 6, 7, 8, 9 and 10 anthers from left to right panels. The sense probes of OsFIP mRNAs were used as negative controls. Scale bars, 100 μm. PMC, pollen mother cell; MSI, meiosis I; MSII, meiosis II; EMS, early microspore stage; LMS, late microspore stage; BPS, binucleate pollen stage; MPS, mature pollen stage.
It is generally considered that seed development also affects plant seed-setting rates. We therefore analyzed the seeds of the mutants at 21 days after pollination to investigate the seed development process. In the abortive seeds of homozygous mta2 panicles and in OXMTA2 panicles, approximately 80% and 91.0%, respectively, of the ovaries were pollinated but did not fully develop (Fig 2B and S3H Fig). However, very different from those in the mta2 mutants, almost all of the ovaries in the homozygous fip plants at 21 days after pollination appeared unpollinated (Fig 2B and S3H Fig). To further confirm whether embryo development is normal or not in mat2 and fip mutants, we crossed fip and mta2 plants with WT plants. As expected, when we used mta2 as the female parent the seed setting rate was lower than when using mta2 as the male parent (Fig 2C). However, when we used fip as the female or male parent, the seed setting rates were also low, and much lower when using fip as male parent (Fig 2C). Together, these results indicated that OsMTA2 has a conserved role in regulating embryo development between rice and Arabidopsis, and OsFIP is required for both sporogenesis and embryo development, and the failed sporogenesis might be the dominant reason for the decreased seed setting rate in fip plants. There is no prior report about the role of RNA m6A methyltransferase in regulating sporogenesis in plants [5, 10–12], and thus, in the following experiments, we focused mainly on OsFIP.
OsFIP regulates the early degeneration of microspores at the vacuolated pollen stage
To examine how OsFIP regulates microspore development, we obtained semithin sections for observing anther development from stage 6 [13] (MMC differentiation stage) to stage 12 (late binucleate pollen stage) comparing WT and fip mutants (Fig 2D–2K, S3I and S3J Fig). The semi sections showed that no obvious abnormity in fip anthers at pollen mother cell stage, meiosis stage, and tetrad stage (S3I Fig). However, at vacuolated stage the microspores had irregular shapes and some debris in anther lobes (Fig 2E), at binucleate pollen stage the pollen degeneration became severe and had unknown particles in anther lobes (Fig 2F), and at mature stage the pollens lost their cytoplasm and were completely collapsed (Fig 2G). In the heterozygous fip plants, approximately half of the microspores could not mature after heading (S3J Fig). The results showed that the degeneration of microspores might begin from the vacuolated stage. We further examined the spatial expression pattern of OsFIP. As shown in Fig 2L, OsFIP expression was detected in MMCs, tapetal cells and microspores from stage 6 to stage 9, and the signals intensified in vacuolated microspores and tapetal cells at stage 10, indicating that OsFIP could involve in microspore developmental process.
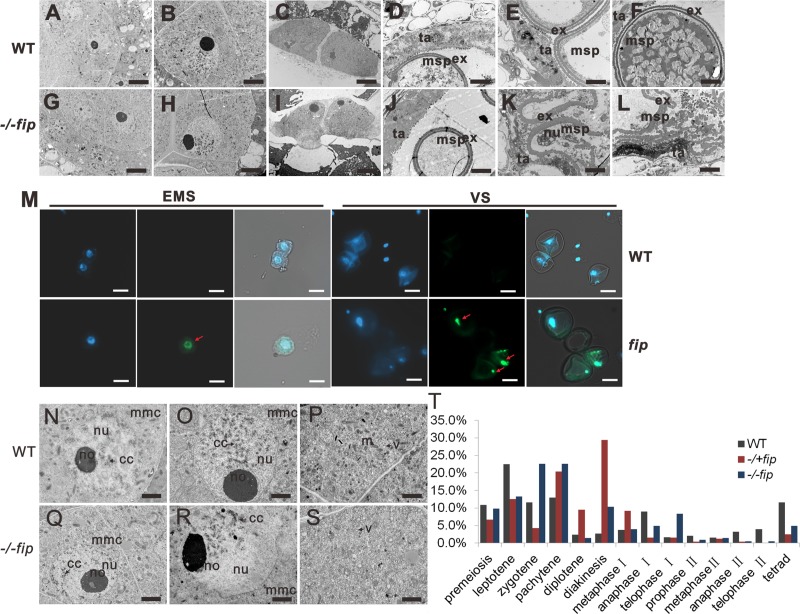
To investigate which process of microspore development OsFIP might affect, we compared the ultrastructure between WT and homozygous fip anthers from stage 6 to stage 12 (Fig 3A–3L). The most obvious abnormal phenotype of fip plants appeared at the vacuolated pollen stage. Almost all of the homozygous fip microspores degenerated at the vacuolated pollen stage (stage 10, late microspore stage) (Fig 3K). At this stage, the WT microspores have a clear single nucleus (Fig 3E); however, the homozygous fip microspores have no nucleus (Fig 3K), and 37.8% of the heterozygous fip microspores have no nucleus (S4Q Fig). S4Q and S4R Fig shows the number of nuclei of WT and fip microspores during the late micropore stage (LMS), late binucleate pollen stage (LBPS) and mature pollen stage (MPS). However, at the LMS, the nuclei and the pollen exines (ex) of the fip microspores (msp) were undergoing degeneration (Fig 3K) and degraded completely at the mature stage (Fig 3L). We also analyzed the heterozygous fip microspores. At stage 11, most of the WT microspores underwent the first mitotic division and generated a smaller generative cell and a larger vegetative cell; however, in the heterozygous fip plants, 23.2% of the fip microspores had only one nucleus, and 27.5% of the fip microspores had no nucleus. At stage 12, the WT microspores underwent a second mitosis and divided into two sperm cells, and the mature pollen grain contained three nuclei; however, 5.4% of the heterozygous fip microspores possessed one nucleus, 31.9% possessed no nucleus, and 54.0% of heterozygous fip microspores could not accumulate starch (S4Q and S4R Fig). These results showed that OsFIP affects the early apoptosis of the microspores at the vacuolated pollen stage.
Fig 3. Histological analysis of WT and homozygous fip MMCs and microspores.
(A) and (G) show the MMCs of WT (A) and fip (G) at the leptotene stage. (B) and (H) show the MMCs of WT (B) and fip (H) at the pachytene stage. (C) and (I) showed the MMCs of WT (H) and fip (K) at meiosis stage. (D) and (J) showed the microspore and tapetum of WT (D) and fip (J) at early microspore stage. (E) and (K) showed the microspore and tapetum of WT (E) and fip (K) at late microspore stage. (F) and (L) showed the microspore and tapetum of WT (F) and fip (L) at mature stage. Scale bars, 5μm. msp, microspores; ex, exine; ta, tapetum. (M) TULNEL assay of the WT and fip pollen grains at early microspore stage (EMS) and vacuolated stage (VS). Scale bars, 20 μm. (N) and (Q) show the nucleus of WT (A) and fip (D) MMCs at the leptotene stage. (O) and (P) show the nucleus (O) and cytoplasm (P) of WT MMCs at the pachytene stage. (R) and (S) show the nucleus (R) and cytoplasm (S) of fip MMCs at the pachytene stage. Scale bars, 2 μm. (T) Frequency of WT and fip MMCs at various meiotic stages in all the anthers analyzed.
To further validate whether the pollen grains degenerated from the vacuolated stage, we have performed TULNEL assay to the fip pollen grains at early microspore stage and vacuolated stage. A few signal was first detected in fip pollens at early microspore stage (23.3%), and was obvious in the nucleus of fip pollens at the vacuolated pollen stage (48.6%) which was not detected in WT samples (2.7% and 5.3%, respectively) (Fig 3M), indicating that fip pollen grains began to degenerate from early microspore stage, and the degeneration became obvious at vacuolated stage.
To identify whether the degeneration of microspores were caused by failed meiosis, we then investigated the meiosis processes of fip plants. Abnormalities were observed at early meiosis prophase. For example, at the leptotene stage (Fig 3N), WT chromosome condensation starts, and scattered chromosomes were observed, indicating DNA replication. At the pachytene stage (Fig 3O), homologous chromosome synapsis is complete, and the paired chromosomes thicken and regularly adhere to the nucleolus. However, in the fip MMCs, the chromosomes are less condensed at the leptotene stage (Fig 3Q), and the distribution of chromosomes are abnormal at the pachytene stage; the chromosomes could not adhere to the nucleolus but were always arranged in one corner (Fig 3R). In addition, we also observed clear differences between the cytoplasm of the WT and the fip MMCs. At the pachytene stage, vacuoles or autophagosome-like organelles frequently appeared in the fip MMCs but rarely appeared in the WT MMCs; other organelles were indistinct in the fip MMCs, whereas other organelles, especially an abundance of mitochondria, were visible in the cytoplasm of the WT MMCs (Fig 3P and 3S). These results showed that the loss of function of OsFIP disrupts both the chromosomes and the cytoplasmic components of MMCs. We also observed a slight arrest of both homozygous and heterozygous fip MMCs at prophase I (Fig 3T and S4A Fig) and a small portion of fip MMCs that were abnormal between WT and fip MMCs (S4B–S4P Fig). For example, in fip plants, more or less than 12 bivalents occasionally appeared, the bivalents of a small portion of MMCs could not align at the equatorial plate (S4L–S4P Fig), and sometimes chromosome bridges or chromosome fragmentation appeared in the fip MMCs (S4J and S4K Fig). It has been considered that early microspores apoptosis are frequently caused by failed meiosis [14–17]. In fip plants, the tetrad at stage 8 and the early microspores at stage 9 seem normally developed (Figs 2D, 3I–3J and S3I Fig), and fip microspores could go through meiosis stage, although the meiosis events were affected in fip MMCs (Fig 3N–3S and S4A–S4P Fig). However, we could observe degraded pollen grains from the vacuolated stage at stage 10 (Figs 2E and 3K). Thus we speculated that OsFIP is essential for microspore development from vacuolated stage to mature stage. Together, the data indicated that OsFIP is important for sporogenesis in rice, and loss of function of OsFIP mainly induces microspores degeneration at the vacuolated pollen stage and partially disturbs the meiosis events.
OsFIP is indispensable for m6A mRNA modification during early sporogenesis
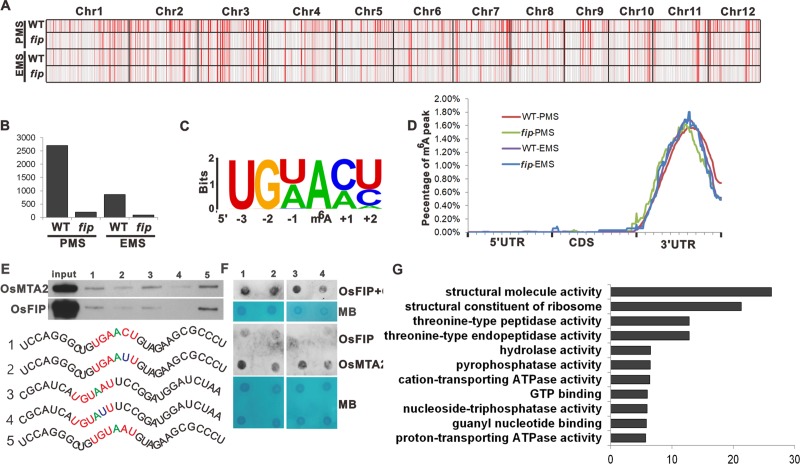
Finally, we investigated the underlying mechanism of the OsFIP regulation of sporogenesis. To understand how OsFIP contributes to the global m6A modification during pollen grains development, we performed m6A-sequencing on both WT and fip anthers at PMS and EMS and compared their transcriptome-wide m6A methylomes during meiosis (SRA: accession no. SRR8934214, SRR8934213, SRR8934212 and SRR8934211). A total of 381.9 million reads were generated from twelve libraries and uniquely aligned to the rice genome (STAR). We used exomePeak to detect the m6A peaks with an estimated p-value <0.01. Our data revealed 2699 and 863 putative high-confidence m6A peaks within 1909 and 568 genes from the WT PMS anthers and the WT EMS anthers, respectively (Fig 4A and 4B), indicating that the m6A modification was decreased after pollen mother cell meiosis. However, only 197 and 91 putative high-confidence m6A peaks within 112 and 49 genes were detected from the fip PMS anthers and the fip EMS anthers, respectively (Fig 4A and 4B), indicating that most of the m6A modifications during meiosis were OsFIP dependent.
Fig 4. Overview of Distribution of m6A modification peaks along mRNA and chromosome, and the methyltransferase activity of OsMTA2 and OsFIP through motifs.
(A) Distribution of m6A peaks along chromosomes of callus and leaf tissues. PMS, pollen mother cell stage; EMS, early microspore stage. (B) The number of m6A peaks of WT and fip panicles during PMS and EMS. (C) The UGWAMH conserved sequence motif for m6A-containing peak regions. (D) Distribution of m6A peaks in transcript segments divided into 5’UTR, CDS, and 3’UTR in wild-type and fip panicles at PMS and EMS. (E) The in vitro binding of GFP-tagged OsFIP or OsMTA2 to different RNA probes (numbered probe 1–5) with the rice panicle specific motif UGUAAU or the mammal motif GAACU or the mutated motif. (F) The in vitro RNA N6-adenosine methylation activities of GFP-tagged OsFIP or OsMTA2 as well as the combination of OsFIP and OsMTA2 were tested using different RNA probes. MB, methylene blue staining (as loading control). (G) The top enriched GO terms of the differentially m6A modified genes in fip panicles.
The conserved m6A modification motif during sporogenesis was then analyzed in both WT and fip panicles. The m6A modification motif “UGWAMH” (W = U or A; M = C or A; H = U, A or C) was significantly overrepresented (P<10−146) in WT panicles (Fig 4C), which is different from the conserved motif in Arabidopsis “RRACH” (R = G or A; H = A, C or U) [18] and is also different from that of rice callus “RAGRAG” but is similar to that of rice leaf “UGUAMM” [19], indicating that the recognition of m6A modification sites during sporogenesis might be different from other developmental stages. In the fip panicles no conserved motif was identified from the remaining m6A modifications.
Next, we analyzed the distribution of m6A peaks in genes in rice. The m6A peaks were greatly enriched at the middle of the 3’UTRs in both WT panicles and fip panicles at PMS and EMS (Fig 4D). Interestingly, the m6A modification distribution pattern during rice sporogenesis was different from that of rice leaf and callus, in which most of the m6A peaks are localized at the beginning of 3’UTRs and CDS. The difference might be related to the variant regulatory roles of m6A modification during different developmental stages. To identify the distribution of m6A peaks on mRNA abundance, we compared the expression levels of the mRNAs with fewer m6A modifications in the fip panicles between WT and fip panicles. Approximately 58% of the differentially expressed mRNAs, which also have fewer m6A modifications in the fip panicles, were upregulated in the fip panicles, and approximately 6% of them were alternatively spliced in the fip panicles (S4S–S4T Fig).
To verify the methyltransferase activity of OsMTA2 and OsFIP through this motif, we then tested N6-adenosine methylation activity. Binding efficiency for GFP-tagged OsFIP or OsMTA2 as well as the combination of OsFIP and OsMTA2 were tested using different RNA probes (numbered probe 1 to 5) with the rice panicle specific motif UGUAAU or the mammal motif GAACU or the mutated motif (Fig 4E and 4F). The methylation and binding of RNA probes was measured by immunoblotting with the m6A antibody and pulldown assays. Five probes were synthesized and applied: probe 1 has the known m6A motif in mammals “GAACU”, probe 3 and probe 5 have rice panicle specific motif “UGUAAU”, respectively, probe 2 has mutated motif “UGAAUU” and probe 4 has mutated motif “UGUAUU” (Fig 4E). As expected, the binding efficiency of OsFIP and OsMTA2 to the rice pollen specific motif or mammal motif was higher than those of mutated motifs (Fig 4E). Moreover, OsMTA2-OsFIP complexes or OsMTA2 itself in vitro exhibited m6A methyltransferase activity against rice pollen specific motif and mammal motif, but displayed less methyltransferase activity towards the mutant motif (Fig 4F). Thus, our data demonstrated that the motif identified in rice panicles was indeed a substrate of rice methyltransferase. Thus, OsFIP is essential for most of the m6A modification during sporogenesis by recognizing a rice panicle specific motif “UGWAMH”.
OsFIP directly binds to the mRNA of threonine protease and NTPase and mediates their m6A modification and expression
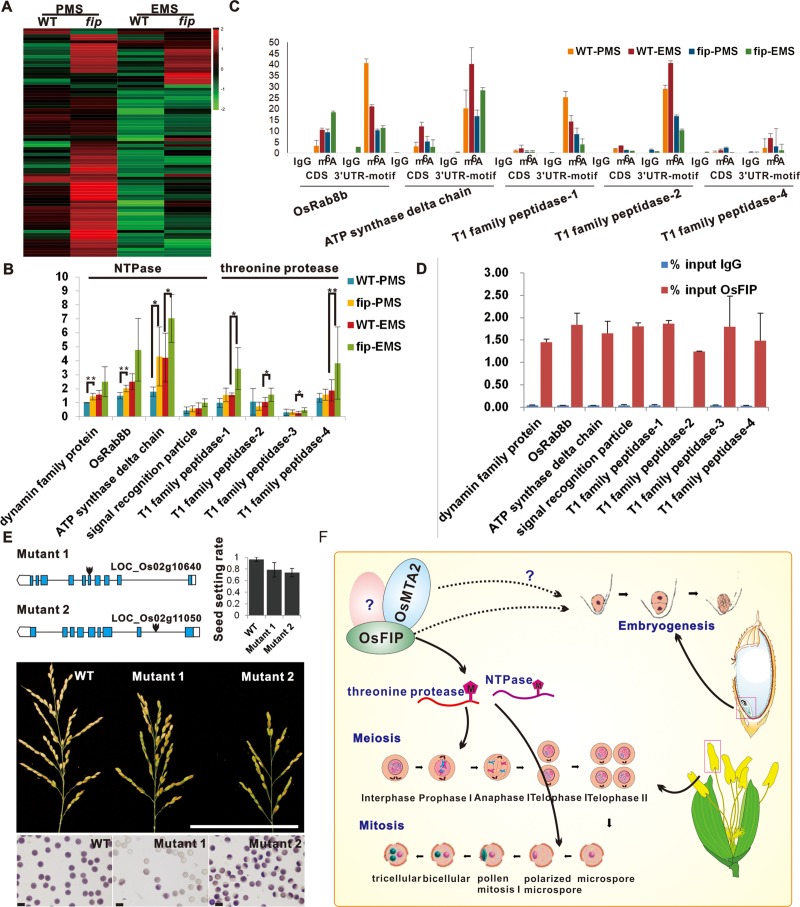
To analyze how OsFIP affects sporogenesis through m6A modification, we then performed Gene Ontology (GO) analysis of the genes that were differentially modified between fip and WT panicles during sporogenesis. Consistent with the phenotypes during meiosis, almost no GO terms related to meiosis could be enriched, indicating that OsFIP is not essential for meiosis (Fig 4G and S5 Fig). Interestingly, these genes were specifically enriched in threonine protease and NTPase GO terms (Fig 4G and S5 Fig). Threonine protease is one of the seven types of proteolytic enzymes. We then analyzed the gene expression patterns and splicing patterns of the differentially modified threonine protease and NTPase genes, and most of them are upregulated in the fip samples (Fig 5A), but their splicing patterns are only slightly affected (4%), indicating that m6A modifications mediated by OsFIP negatively regulate threonine protease and NTPase gene expression. qRT-PCR further confirmed these expression patterns (Fig 5B). However, the gene expression fold change was higher in the fip-EMS sample than in the fip-PMS sample. We speculated that the higher fold change in the EMS stage might be caused by a greater m6A modification change between WT and fip RNAs at the EMS stage. Thus, we performed m6A dot blot in WT and fip panicles in these two stages. The results showed that compared with the m6A modification level in WT, the modification level was decreased more significantly in fip at the EMS stage than that at the PMS stage (S3B Fig), which might be the reason why the fold change of gene expression was higher in the fip-EMS sample.
Fig 5. OsFIP regulates the m6A modification and expression by binding to the rice panicle motif.
(A) Expression pattern of NTPase or threonine protease genes in WT and fip panicles during PMS and EMS. (B) qRT-PCR validation of eight differentially m6A modified NTPase or threonine protease genes in WT and fip panicles during PMS and EMS. Values shown are the means ± s.d. (n = 3 biological replications). Significant differences were identified using Student’s t-test. (C) m6A-IP-qPCR of five NTPase or threonine protease genes at the CDS regions without a motif and at the 3’ UTR regions with a motif in WT and fip panicles during PMS and EMS. (D) OsFIP-RIP-qPCR of eight NTPase or threonine protease genes using Flag antibody in the OXFIP-Flag plants. (E) The panicle and pollen grain phenotypes and seed setting rates of two T-DNA insertion mutants of two threonine protease genes. The red arrows indicate the T-DNA insertion site. Scale bars for panicles, 10cm; Scale bars for pollen grains, 100μm. (F) Proposed model describing the roles of OsFIP and OsMTA2 in regulating sporogenesis and embryo development.
To confirm the m6A peak position in these threonine protease and NTPase genes as OsFIP targets at PMS and EMS, we further performed m6A-RIP-qPCR in WT and fip plants (Fig 5C). Except for two of them that were undetected, five of the other six genes were shown to have m6A modifications at the predicted 3’UTR rice panicle specific motif, which were diminished in fip plants but not in the gene body region (Fig 5C). We also identified the direct binding of OsFIP to these genes by performing OsFIP-RIP assays in the OXFIP plants using anti-FLAG antibody (Fig 5D). The results showed that all of these genes were bound by OsFIP (Fig 5D). These results indicate that OsFIP directly binds to threonine protease and NTPase genes and mediates their m6A modification at the rice panicle specific motif.
Proteases have been reported to be important for sporogenesis through inducing the apoptosis-like programmed cell death (PCD) of microspores in plants, although the role of threonine protease has not been reported in plants. NTPase has also been reported to affect cytoplasmic male sterility in rice. To verify the roles of these differentially m6A modified and expressed NTPases and threonine proteases on sporogenesis, we chose five NTPase genes (LOC_Os02g11050, LOC_Os02g10640, LOC_Os09g32800, LOC_Os03g50520 and LOC_Os12g44150) that are m6A modified in an OsFIP dependent way. We obtained their mutant plants from the RMD rice mutant database. Insertion mutant of the two genes LOC_Os02g11050 and LOC_Os02g10640 have abnormal pollen grains, showing the probable role of NTPase genes on sporogenesis (S4U and S4V Fig). Thus, we concluded that OsFIP is essential for threonine protease and NTPase gene expression and/or splicing, which then prevents microspores from early PCD at the early microspores stage.
In summary, OsFIP and OsMTA2 were revealed as the major components of the m6A methyltransferase complex in rice, and both proteins interact with each other and they are required for rice reproductive development. OsFIP is essential for early sporogenesis by mediating m6A modification of a set of threonine protease and NTPase genes. We also reported the characteristics of rice m6A modification during sporogenesis in both WT and fip plants, and identified a rice sporogenesis stage specific m6A modification motif “UGWAMH”. The proposed functions of rice OsFIP are shown in Fig 5E. This is the first study to report that the m6A RNA methyltransferase complex plays an essential role in plant sporogenesis.
Discussion
Identification of the methyltransferase complex for catalyzing m6A formation in RNA is essential for understanding the functions of m6A modification. In mammals, the core components of the complex are METTL3, METTL14 and WTAP. In Arabidopsis, the orthologs of METTL3, METTL14 and WTAP have been identified as AtMTA, AtMTB and AtFIP37. In this study, we characterized the core components of the rice m6A methyltransferase complex and demonstrated that OsFIP and OsMTA2 are orthologs of METTL3 and WTAP. Knocking out of OsFIP or OsMTA2 decreased m6A mRNA methylation and resulted in sterile phenotypes. Moreover, OsFIP and OsMTA2 interact with each other in the nucleus. We predicted the homologous genes of Arabidopsis AtMTB and mammalian METTL14 and found that OsMTA1, OsMTA3 and OsMTA4 are highly homologous with AtMTB and METTL14. However, OsMTA1, 3 and 4 do not affect m6A methylation levels; moreover, they do not interact with OsMTA2 or OsFIP. These results suggested that these proteins might not be the subunits of the rice m6A methyltransferase complex. Additional studies are needed to demonstrate whether plant m6A methyltransferases have other components.
Most of the molecular functional studies of the plant m6A methyltransferase complex have been performed in Arabidopsis. AtMTA and AtFIP37 regulate embryo development, and AtFIP37 determines shoot stem cell fate in Arabidopsis. The ortholog of METTL14, MTB, which is involved in root development, was identified as a component required for m6A in Arabidopsis [9]. In this study, we uncovered novel functions of OsFIP in rice. OsFIP regulates pollen development by affecting the m6A modification of threonine protease and NTPase genes, which has not been reported for WTAP in mammals or AtFIP37 in Arabidopsis. OsFIP is important for normal sporogenesis progress in rice, and the complete deletion of OsFIP causes early degeneration of pollen grains during the vacuolated pollen stage. A small portion of the fip MMCs also has abnormal chromosome distribution and the vacuolated cytoplasm at meiosis prophase I.
The m6A-Seq of WT and fip panicles showed that OsFIP is indispensable for m6A modification of threonine protease and NTPase genes during early sporogenesis. Threonine protease was first described in 1995 in animals, but no study has been performed on plant threonine protease. Interestingly, other kinds of proteases are closely related to sporogenesis. For example, OsCP1 is a rice cysteine protease that is essential for early microspores development [20]. A36 and A39 are two aspartic proteases in Arabidopsis that affect pollen apoptosis-like PCD [21]. It is intriguing to speculate about the specific roles of threonine protease during early sporogenesis under the regulation of m6A modification. Many NTPases, especially ATPases, were also regulated by m6A modification mediated by OsFIP during sporogenesis. ATPases are well known to be involved in energy metabolism and cytoplasmic male sterility [22–25]. It is possible that m6A modification is also important for cytoplasmic male sterility by affecting ATPases during sporogenesis. We also showed that two NTPase genes might function during sporogenesis. This report is the first to describe that the homolog of WTAP regulates the early sporogenesis process.
It is worth mentioning that the roles of OsMTA2 in rice reproductive development were different from those of OsFIP. We observed that the mutation or overexpression of OsMTA2 leads mainly to aborted seeds, which is conserved in Arabidopsis and that the loss of function of AtMTA leads to embryo-lethal phenotypes. These differences between the functions of OsFIP and of OsMTA2 imply that there might be other methyltransferases that interact with OsFIP to modify the mRNAs of gametogenesis-related genes or imply that OsFIP might play an independent role in gametogenesis. Whether WTAP or AtFIP37 have similar roles needs to be further studied.
Materials and methods
Plant growth conditions and generation of transgenic rice plants
The growth conditions and generation of transgenic plants were conducted according to Zhang et al. [26]. Briefly, the Zhonghua 11 (Oryza sativa japonica) rice cultivar was used in these experiments. Rice plants were grown in the field in Guangzhou, China (23°08′ N, 113°18′ E), where the growing season extends from late April to late September. The average low temperature range is 22.9–25.5°C, and the average high temperature range is 29.7–32.9°C. The day length ranged from 12 to 13.5 h. The plants were maintained with routine management practices. As ZhongHua 11, a japonica variety usually cultivated in Northern part of China, when grown in Gruangdong Province, it flowers earlier and has less tiller numbers and grain numbers. OsFIP and OsMTA1/2/3,4 were overexpressed under the control of the CaMV35S promoter. Three overexpression lines with higher expression level than WT plants of these genes respectively were used for the next phenotype analysis. The OsFIP and OsMTA2 knockout mutants were generated using CRISPR-Cas9-based genome editing technology as previously described [27]. T1, T2 and T3 generations of fip plants and T3 generation of mta2 plants were used to analyze the phenotypes. The heterozygous fip plants were harvested and used to screen homozygous fip plants in the next generation, as homozygous fip plants are almost completely sterility. The phenotypes of T1, T2 and T3 generations of fip and mta2 plants are stable. See Supplementary Methods for details.The following primers were used: OsFIP Target site: 5’-GTTGGACGTTTTCGCTTCCAAGA-3 and 5’- AAACTCTTGGAAGCGAAAACGTC -3’; OsMTA2 Target site 1: 5’- GCCGCGGATTCTGGCAGCTCCTTG -3’ and 5’- AAACCAAGGAGCTGCCAGAATCCG -3’; OsMTA2 Target site 2: 5’- GTTGCCCCCCTCTGAGACCGATGC -3’ and 5’- AAACGCATCGGTCTCAGAGGGGGG -3’; OsMTA1 Target site: 5’ GCCGTACGGGAGATAACTCAAGGG-3’ and 5’-AAACCCCTTGAGTTATCTCCCGTA-3’; OsMTA3 Target site:5’-GCCGAAAGGTGATAGACCCTCCAG-3’ and 5’AAACCTGGAGGGTCTATCACCTTT-3’; OsMTA4 Target site:5’-GCCGAGATTGTCCGACGGGTACA-3’ and 5’-AAACTGTACCCGTCGGACAATCT-3’.
BiFC and yeast two-hybrid assays
Two-week-old rice shoots were used to isolate protoplasts. A bundle of rice plants (approximately 30 seedlings) were cut together into approximately 0.5-mm strips with propulsive force using sharp razors. The strips were incubated in an enzyme solution (1.5% cellulose RS, 0.75% macerozyme R-10, 0.6 M mannitol, 10 mM MES, pH 5.7, 10 mM CaCl2 and 0.1% BSA) for 4–5 h in the dark with gentle shaking (40–50 rpm). After the enzymatic digestion, an equal volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl and 2 mM MES, pH 5.7) was added, followed by shaking (60–80 rpm) for 30 min. Protoplasts were released by filtering through 40-μm nylon mesh into round bottom tubes, followed by washing 3–5 times with W5 solution. The pellets were collected by centrifugation at 800 rpm for 3 min in a swinging bucket. After washing once with W5 solution, the pellets were then resuspended in MMG solution (0.4 M mannitol, 15 mM MgCl2 and 4 mM MES, pH 5.7) at a concentration of 2×106 cells mL-1. BIFC PEG-mediated transfections were performed as previously described [28]. Protoplasts were observed using a confocal laser-scanning microscope (Zeiss 7 DUO NLO) at 488 and 561 nm excitation. All manipulations described above were performed at room temperature.
m6A dot blot assay
m6A dot blot assay was performed as previously described[29] with some modifications. Briefly, total RNA was isolated from panicles of different transgenic lines with RNAiso plus (TAKARA) according to the manufacturer's instructions. The RNA samples were loaded to the nylon membrane and UV crosslinked to the membrane. Then the membrane was stained with 0.02% methylene blue (sigma) (in 0.3M NaAc, PH 5.5). After the staining, the membrane was washed by 0.5% SDS and TBST, and then blocked with 5% nonfat dry milk (in 1X TBST) for 1 hours and incubated with a specific anti-m6A antibody (1:5000 dilution, Abcam) overnight at 4°C. Then the HRP-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology) was added to the blots for 1 hour at room temperature and the membrane was developed with Amersham ECL Prime Western Blotting Detection Reagent (Milipore).
DAPI staining
The 4',6-diamidino-2-phenylindole (DAPI) staining was performed as previously described, with minor modifications [30]. The fixed tissue was washed twice with water and twice with 10 mM citrate buffer, pH 4.5. Four to six anthers were placed in a small drop of 60% acetic acid on a slide and pressed with another slide to release microspore mother cells. The slides were then separated, and the samples were dried at room temperature for 5 min. A total of 5 μL DAPI solution (1 μg/mL DAPI in a buffer with 50% glycerol and 10 mM citrate, pH 4.5) was placed onto the slide, covered with a cover glass and sealed with clear nail polish. The slides were examined under a fluorescence microscope (Leica DM5000B).
Eosin B staining
Eosin B staining was performed previously described [31]. The ovaries were dissected in 70% ethanol under a binocular dissecting microscope, and sequentially hydrated in 50% ethanol, 30% ethanol and distilled water. After that, the ovaries were pretreated in 2% aluminium potassium sulphate for 20 min. The ovaries were then stained with 10 mg/l of eosin B solution for 10–12 h at room temperature. The samples were post-treated in 2% aluminium potassium sulphate for 20 min and rinsed three times with distilled water, followed by dehydration with a series of ethanol solutions (30%, 50%, 70%, 90% and 100%). Subsequently, the dehydrated samples were transferred to a mixture of absolute ethanol and methyl salicylate (1:1) for 1 h and then cleared in pure methyl salicylate solution for at least 1 h. The slides were examined under a confocal laser scanning microscope (Zeiss 7 DUO NLO).
Semi-thin sections for light microscopy of anthers
The samples were fixed in 2.5% paraformaldehyde—3.0% glutaraldehyde in 0.1 mol/L PBS (pH 7.2) for 4 h at 4°C and then washed 3 times in the same buffer, which was followed by post-fixation in 1% osmium tetroxide for 2 h at room temperature and 3 rinses using the same buffer. Specimens were dehydrated in a graded ethanol series and embedded in Epon812 (SPI Supplies Division of Structure Probe Inc., West Chester, PA, USA). Polymerization took place for 24 h at 40°C, which was followed by 24 h at 60°C. Specimens were cut to a thickness of 1 μm on a Leica RM2155 and were stained with 0.5% toluidine blue. Sections were observed and photographed with a Leica DMLB microscope.
Examination of gene expression by qRT-PCR analysis
Total RNAs from rice seedlings at 14 d after germination or panicles before heading were reverse transcribed using the PrimeScript RT reagent kit (Takara, Japan). Real-time PCR was performed using SYBR Premix Ex Taq (Takara, Japan) to detect the PCR products. Actin2 was used as the reference gene. Real-time PCR was performed according to the manufacturer’s instructions (Takara, Japan), and the resulting melting curves were visually inspected to ensure the specificity of the product detection. Gene expression was quantified using the comparative Ct method. The experiments were performed in triplicate, and the results are represented as the mean ± s.d. For Actin2, the primers were Actin2-F (5’-GTGCTTTCCCTCTATGCT-3’) and Actin2-R (5’-CTCGGCAGAGGT GGTGAA-3’); and for OsFIP, the primers were OsFIP-F (5-GGAAGAAAGTGCGCCAGGTG-3) and OsFIP-R (5- GATTTGGCAGCCTCCCGTTC -3); for OsMTA2, the primers were OsMTA2-F (5’-AGGTGGTTCCCAGCTGAAGG-3’) and OsMTA2-R (5’-GCAGGTCTTTGTGTGACGGC-3’); for MEL1, the primers were MEL1-F (5’-GCTATACCTATGCGCGATG-3’) and MEL1-R (5’-ATCCGAACTCTCTCCTTCCA-3’); for MEL2, the primers were MEL2-F (5’-TGTGATGCAGCTTGTCCCAT-3’) and MEL2-R (5’-CGCTCCATGACTCCCACATA-3’); for SPO11-4, the primers were SPO11-4-F (5’-CAATGCGAATCAGCGGGAAG-3’) and SPO11-4-R (5’-TCAATCCAGCCCCAAGTGTC-3’); for PAIR1, the primers were PAIR1-F (5’-AAAGGTGGAGCAGGGAAAGG-3’) and PAIR1-R (5’-TGCTGACTGGTGCCTTCTTT-3’); for RPA2C, the primers were RPA2C-F (5’-CAGCACCGGGAAGATCCCAC-3’) and RPA2C-R (5’-TGCAGGGGGTAGTCCTTGGT-3’); for CRC1, the primers were CRC1-F (5’-AGGGTGGCAATTCTCTCTGG-3’) and CRC1-R (5’-ATCAAAGCGTGCAGGAAAGC-3’); for ZIP4, the primers were ZIP4-F (5’-ACTCTCTTCACCGAAGCACT-3’) and ZIP4-R (5’-CTTGAGCCCCTCTAGATTTG-3’); for Rec8, the primers were Rec8-F (5’-TCCGGAAGGTCCAAGAGGCA -3’) and Rec8-R (5’-TGAGTTGCTAAAACGCATGCTTGA-3’).
In situ hybridization
RNA in situ hybridization was performed as previously described, with minor modifications [32]. Briefly, the plant materials were fixed in FAA fixative for 8 h at 4°C after vacuum infiltration and dehydrated using a graded ethanol series, followed by a xylene series, and embedded in Paraplast Plus (Sigma-Aldrich). Microtome sections (9 μm) were mounted on Probe-On Plus microscope slides (Fisher). The 141-bp regions of OsFIP was amplified using the primers 5’- GGAAGAAAGTGCGCCAGGTG -3’ and 5’- GATTTGGCAGCCTCCCGTTC -3’ and then subcloned into the pEASY-T3 (TransGen Biotech) vector and used as the template to generate sense and antisense RNA probes. The OsMTA2 probe was amplified using the primers 5’- AGGTGGTTCCCAGCTGAAGG -3’ and 5’- GCAGGTCTTTGTGTGACGGC -3’. The antisense probe was transcribed using T7 RNA polymerase, and the sense probe was synthesized using SP6 RNA polymerase. Digoxigenin-labeled RNA probes were prepared using a DIG RNA Labeling Kit (SP6/T7) (Roche) according to the manufacturer's instructions. Photomicrographs were obtained using a bright-field microscope (Leica DM5000B).
m6A - Sequencing
The panicles at the pollen mother cell meiosis stage (2–5 mm spikelet) and the early microspore stage (7–8 mm spikelet) from the wild-type and fip plants (n > 20 plants for each sample) were collected to extract the total RNA. Two biological replicates of m6A RIP sequencing were performed for the two WT samples, but only one replicate were performed for fip samples because of the insufficient samples of fip plants. m6A sequencing was performed as previously described with modifications using ant- m6A antibody (Synaptic Systems, cat. No. 202003) [33]. The RNA-seq was performed on the Illumina Hiseq 2500 platform.
The m6A modification peaks were called with the exomePeak program with strict criteria (false discovery rate (FDR) <0.05, P-value <0.01and fold change (FC)>2). The de novo motif identification of the m6A peak data was performed by susing the HOMER software to obtain their position weight matrices and accurate motif regions. We assigned all modification sites to gene regions covering CDS, 3’UTR, 5’ UTR, intron and exon region. The gene expression level was calculated using RPKM method (Read Per kb per Million reads). The differentially expressed genes were then screened. Gene Ontology (GO) enrichment analysis was performed to decipher the biological processes involving the differentially modified genes.
m6A -IP-qPCR
m6A-IP-qPCR was performed using the magna RIP kit (Millipore, 2982054). 50μg total RNA was used, after treated with DNaseⅠNthermo, EN0525), the RNA was fragmented by 0.1 M ZnCl2 at 94℃ for 100 s then immunoprecipitated with 3 μg anti-m6A antibody (Synaptic Systems, 202003). RNAs isolated were analyzed by RT-PCR.
Biochemistry assay for m6A methyltransferase activity in vitro
The in vitro methyltransferase activity assay was performed in a standard 50 μL of reaction mixture containing the following components: 0.15 nmol RNA probe, fresh purified FIP or MTA proteins, 0.8 mM d3-SAM, 80 mM KCl, 1.5 mM MgCl2, 0.2 U μL−1 RNasin, 10 mM DTT, 4% glycerol and 15 mM HEPES (pH 7.9). The reaction was incubated at 16°C for 12 h. The methylation of RNA-probe was measured by immunoblotting with the m6A antibody.
Biotinylated RNA probes pulldown assay
The pulldown assay was performed using the Pierce Magnetic RNA-Protein Pull-Down Kit (thermo scientific, 20164) according to its instruction. Cells were lysed in 200 μl of lysis buffer (150 mM KCl, 25 mM Tris pH 7.4, 0.5 mM DTT, 0.5% NP40, with 1 nM PMSF) in 4°C for 1h. Add 50 pmol of RNA probe to 50 μL of streptavidin magnetic beads, then incubated the tube for 30 minutes at room temperature with rotation. After washed with 50μL 20mM Tris (pH 7.5) twice, the RNA-bound beads were incubated with the lysate for 1 h at 4°C with rotation. Beads were washed three times with 500 μl wash buffer (20mM Tris (pH 7.5), 10mM NaCl, 0.1% Tween-20 Detergent). Finally, beads were boiled for 10 min in SDS sample buffer, and followed by Western blotting analysis.
Supporting information
The functional regions are indicated by black boxes. (A) Conservation analysis of OsMTA2. (B) Conservation analysis of OsFIP. (C) Conservation analysis of OsMTA1/3/4.
(JPG)
(A) Editing types of the heterozygous OsMTA2 knockout plants. (B) Editing types of the homozygous and heterozygous OsFIP knockout plants. (C) Editing types of the heterozygous OsMTA1, 3, and 4 knockout plants.
(JPG)
(A) Dot blot analysis of RNA m6A levels in wild-type, fip, mta2, OXFIP and OXMTA2 panicles. MB, methylene blue staining (as loading control). (B) Dot blot analysis of RNA m6A levels in wild-type and fip panicles at PMS and EMS stage. MB, methylene blue staining (as loading control). (C) Dot blot analysis of RNA m6A levels in wild-type, mta1, mta3 and mta4 seedlings. (D-E) OsMTA2 interacts with OsFIP in both rice nuclei (D) and yeast (E), Scal bar, 2μm. (E) Yeast two-hybrid between OsMTA1, 3, 4 and OsFIP and OsMTA2. (F) The morphology of WT and fip plants during vegetable stage. (G) Tiller number per plants of WT and the transgenic plants. Values shown are the means ± s.d. (n > 20 plants). Significant differences were identified using Student’s t-test. (H) embry sacs of WT, fip and mta2 plants before flowering or 21 Days after flowering. (I) Transverse semithin sections of homozygous fip anthers at stages 5, 6 7 and 8 from left to right panels. (J) Transverse semithin sections of heterozygous fip anthers at stages 12. SDS, sporogenous cells differentiation stage; PMC, pollen mother cell; MSI, meiosis I; MSII, meiosis II; MPS, mature pollen stage.
(JPG)
(A) Frequency of MMCs at various meiotic stages in anthers ranging from 0.3–0.8 mm in length. Gray and red bars indicate the frequency of WT and heterozygous fip MMCs at various stages. (B-P) The meiosis processes of WT (B-H), heterozygous fip (I-K) and homozygous fip (L-P) MMCs. The arrows indicate the chromosome bridge and chromosome fragments. Scale bars, 4 μm. (R) The number of nucleus of WT and fip microspores during late micropore stage (LMS), late binucleate pollen stage (LBPS) and mature pollen stage (MPS). mmc, microspore mother cells; nu, nucleus; no, nucleolus; cc, condensed chromosome; m, mitochondria; v, vacuoles. (Q) The microspores of WT and fip plants during late microspore stage (LMS), late binucleate pollen stage (LBPS) and mature pollen stage (MPS). Red scircles indicate the nucleus. Scale bars, 20 μm. (S) Expression pattens of genes which are m6A modified in a OsFIP dependent way. (T) Splicing patterns of genes which are m6A modified in a OsFIP dependent way.
(JPG)
(PNG)
Acknowledgments
We thank Prof Yungui Yang at Beijing Institute of Genomics and Prof Zhukuan Cheng at Institute of Genetic and Developmental Biology for helpful comments when we designed and carried out the study.
Data Availability
Large-scale sequencing data are available from the NCBI SRA database (SRR8934214, SRR8934213, SRR8934212 and SRR8934211). All other relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by the National Natural Science Foundation of China (No. 91640202, 31770883 and 91335104), National Key R&D Program of China (No. 2017YFA0504400), and the grants from Guangdong Province (No. 2014T70833, 2016A030308015 and 2017TQ04N779) and Guangzhou (201707020018 and 201710010029). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. The Journal of biological chemistry. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. 1994. July 01;269(26):17697–704. [PubMed] [Google Scholar]
- 2.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.]. 1997. November;3(11):1233–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nature chemical biology. [Research Support, N.I.H., Extramural]. 2014. February;10(2):93–5. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nature cell biology. [Research Support, Non-U.S. Gov't]. 2014. February;16(2):191–8. 10.1038/ncb2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong S, Li H, Bodi Z, Button J, Vespa L, Herzog M, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. The Plant cell. [Research Support, Non-U.S. Gov't]. 2008. May;20(5):1278–88. 10.1105/tpc.108.058883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell research. [Research Support, Non-U.S. Gov't]. 2014. February;24(2):177–89. 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei LH, Song P, Wang Y, Lu Z, Tang Q, Yu Q, et al. The m6A Reader ECT2 Controls Trichome Morphology by Affecting mRNA Stability in Arabidopsis. Plant Cell. 2018. April 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arribas-Hernandez L, Bressendorff S, Hansen MH, Poulsen C, Erdmann S, Brodersen P. An m6A-YTH Module Controls Developmental Timing and Morphogenesis in Arabidopsis. Plant Cell. 2018. April 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruzicka K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, et al. Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol. 2017. July;215(1):157–72. 10.1111/nph.14586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodi Z, Zhong S, Mehra S, Song J, Graham N, Li H, et al. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3' End and Reduced Levels Cause Developmental Defects. Frontiers in plant science. 2012;3:48 10.3389/fpls.2012.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vespa L, Vachon G, Berger F, Perazza D, Faure JD, Herzog M. The immunophilin-interacting protein AtFIP37 from Arabidopsis is essential for plant development and is involved in trichome endoreduplication. Plant physiology. [Research Support, Non-U.S. Gov't]. 2004. April;134(4):1283–92. 10.1104/pp.103.028050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen L, Liang Z, Gu X, Chen Y, Teo ZW, Hou X, et al. N(6)-Methyladenosine RNA Modification Regulates Shoot Stem Cell Fate in Arabidopsis. Developmental cell. 2016. July 25;38(2):186–200. 10.1016/j.devcel.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang D, Luo X, Zhu L. Cytological analysis and genetic control of rice anther development. J Genet Genomics. 2011. September 20;38(9):379–90. 10.1016/j.jgg.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 14.Nonomura K, Morohoshi A, Nakano M, Eiguchi M, Miyao A, Hirochika H, et al. A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell. 2007. August;19(8):2583–94. 10.1105/tpc.107.053199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, Wang C, Higgins JD, Yu J, Zong J, Lu P, et al. MEIOTIC F-BOX Is Essential for Male Meiotic DNA Double-Strand Break Repair in Rice. Plant Cell. 2016. August;28(8):1879–93. 10.1105/tpc.16.00108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Che L, Tang D, Wang K, Wang M, Zhu K, Yu H, et al. OsAM1 is required for leptotene-zygotene transition in rice. Cell Res. 2011. April;21(4):654–65. 10.1038/cr.2011.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong L, Tang D, Zhu K, Wang K, Li M, Cheng Z. Somatic and reproductive cell development in rice anther is regulated by a putative glutaredoxin. Plant Cell. 2012. February;24(2):577–88. 10.1105/tpc.111.093740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wan Y, Tang K, Zhang D, Xie S, Zhu X, Wang Z, et al. Transcriptome-wide high-throughput deep m(6)A-seq reveals unique differential m(6)A methylation patterns between three organs in Arabidopsis thaliana. Genome Biol. 2015. December 14;16:272 10.1186/s13059-015-0839-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Wang X, Li C, Hu S, Yu J, Song S. Transcriptome-wide N(6)-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014;11(9):1180–8. 10.4161/rna.36281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, Jung KH, An G, Chung YY. Isolation and characterization of a rice cysteine protease gene, OsCP1, using T-DNA gene-trap system. Plant Mol Biol. 2004. March;54(5):755–65. 10.1023/B:PLAN.0000040904.15329.29 [DOI] [PubMed] [Google Scholar]
- 21.Gao H, Zhang Y, Wang W, Zhao K, Liu C, Bai L, et al. Two Membrane-Anchored Aspartic Proteases Contribute to Pollen and Ovule Development. Plant Physiol. 2017. January;173(1):219–39. 10.1104/pp.16.01719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Paepe R, Forchioni A, Chetrit P, Vedel F. Specific mitochondrial proteins in pollen: presence of an additional ATP synthase beta subunit. Proc Natl Acad Sci U S A. 1993. July 1;90(13):5934–8. 10.1073/pnas.90.13.5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li WQ, Zhang XQ, Xia C, Deng Y, Ye D. MALE GAMETOPHYTE DEFECTIVE 1, encoding the FAd subunit of mitochondrial F1F0-ATP synthase, is essential for pollen formation in Arabidopsis thaliana. Plant Cell Physiol. 2010. June;51(6):923–35. 10.1093/pcp/pcq066 [DOI] [PubMed] [Google Scholar]
- 24.Li J, Pandeya D, Jo YD, Liu WY, Kang BC. Reduced activity of ATP synthase in mitochondria causes cytoplasmic male sterility in chili pepper. Planta. 2013. April;237(4):1097–109. 10.1007/s00425-012-1824-6 [DOI] [PubMed] [Google Scholar]
- 25.Wen L, Ruesch KL, Ortega VM, Kamps TL, Gabay-Laughnan S, Chase CD. A nuclear restorer-of-fertility mutation disrupts accumulation of mitochondrial ATP synthase subunit alpha in developing pollen of S male-sterile maize. Genetics. 2003. October;165(2):771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YC, Yu Y, Wang CY, Li ZY, Liu Q, Xu J, et al. Overexpression of microRNA OsmiR397 improves rice yield by increasing grain size and promoting panicle branching. Nature biotechnology. [Research Support, Non-U.S. Gov't]. 2013. September;31(9):848–52. 10.1038/nbt.2646 [DOI] [PubMed] [Google Scholar]
- 27.Ma X, Zhang Q, Zhu Q, Liu W, Chen Y, Qiu R, et al. A Robust CRISPR/Cas9 System for Convenient, High-Efficiency Multiplex Genome Editing in Monocot and Dicot Plants. Molecular plant. [Research Support, Non-U.S. Gov't]. 2015. August;8(8):1274–84. 10.1016/j.molp.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant methods. 2011. September 30;7(1):30 10.1186/1746-4811-7-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N6- Methyladenosine RNA Demethylase. Cancer Cell. 2017. January 09;31(1):127–41. 10.1016/j.ccell.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross KJ, Fransz P, Jones GH. A light microscopic atlas of meiosis in Arabidopsis thaliana. Chromosome research: an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. [Research Support, Non-U.S. Gov't]. 1996. November;4(7):507–16. [DOI] [PubMed] [Google Scholar]
- 31.Zeng YX, Hu CY, Lu YG, Li JQ, Liu XD. Abnormalities occurring during female gametophyte development result in the diversity of abnormal embryo sacs and leads to abnormal fertilization in indica/japonica hybrids in rice. Journal of integrative plant biology. [Research Support, Non-U.S. Gov't]. 2009. January;51(1):3–12. 10.1111/j.1744-7909.2008.00733.x [DOI] [PubMed] [Google Scholar]
- 32.Kouchi H, Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Molecular & general genetics: MGG. [Comparative Study Research Support, Non-U.S. Gov't]. 1993. April;238(1–2):106–19. [DOI] [PubMed] [Google Scholar]
- 33.Dominissini D, Moshitch-Moshkovitz S, Salmon-Divon M, Amariglio N, Rechavi G. Transcriptome-wide mapping of N(6)-methyladenosine by m(6)A-seq based on immunocapturing and massively parallel sequencing. Nat Protoc. 2013. January;8(1):176–89. 10.1038/nprot.2012.148 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The functional regions are indicated by black boxes. (A) Conservation analysis of OsMTA2. (B) Conservation analysis of OsFIP. (C) Conservation analysis of OsMTA1/3/4.
(JPG)
(A) Editing types of the heterozygous OsMTA2 knockout plants. (B) Editing types of the homozygous and heterozygous OsFIP knockout plants. (C) Editing types of the heterozygous OsMTA1, 3, and 4 knockout plants.
(JPG)
(A) Dot blot analysis of RNA m6A levels in wild-type, fip, mta2, OXFIP and OXMTA2 panicles. MB, methylene blue staining (as loading control). (B) Dot blot analysis of RNA m6A levels in wild-type and fip panicles at PMS and EMS stage. MB, methylene blue staining (as loading control). (C) Dot blot analysis of RNA m6A levels in wild-type, mta1, mta3 and mta4 seedlings. (D-E) OsMTA2 interacts with OsFIP in both rice nuclei (D) and yeast (E), Scal bar, 2μm. (E) Yeast two-hybrid between OsMTA1, 3, 4 and OsFIP and OsMTA2. (F) The morphology of WT and fip plants during vegetable stage. (G) Tiller number per plants of WT and the transgenic plants. Values shown are the means ± s.d. (n > 20 plants). Significant differences were identified using Student’s t-test. (H) embry sacs of WT, fip and mta2 plants before flowering or 21 Days after flowering. (I) Transverse semithin sections of homozygous fip anthers at stages 5, 6 7 and 8 from left to right panels. (J) Transverse semithin sections of heterozygous fip anthers at stages 12. SDS, sporogenous cells differentiation stage; PMC, pollen mother cell; MSI, meiosis I; MSII, meiosis II; MPS, mature pollen stage.
(JPG)
(A) Frequency of MMCs at various meiotic stages in anthers ranging from 0.3–0.8 mm in length. Gray and red bars indicate the frequency of WT and heterozygous fip MMCs at various stages. (B-P) The meiosis processes of WT (B-H), heterozygous fip (I-K) and homozygous fip (L-P) MMCs. The arrows indicate the chromosome bridge and chromosome fragments. Scale bars, 4 μm. (R) The number of nucleus of WT and fip microspores during late micropore stage (LMS), late binucleate pollen stage (LBPS) and mature pollen stage (MPS). mmc, microspore mother cells; nu, nucleus; no, nucleolus; cc, condensed chromosome; m, mitochondria; v, vacuoles. (Q) The microspores of WT and fip plants during late microspore stage (LMS), late binucleate pollen stage (LBPS) and mature pollen stage (MPS). Red scircles indicate the nucleus. Scale bars, 20 μm. (S) Expression pattens of genes which are m6A modified in a OsFIP dependent way. (T) Splicing patterns of genes which are m6A modified in a OsFIP dependent way.
(JPG)
(PNG)
Data Availability Statement
Large-scale sequencing data are available from the NCBI SRA database (SRR8934214, SRR8934213, SRR8934212 and SRR8934211). All other relevant data are within the manuscript and its Supporting Information files.