Abstract
Purpose
Melanocortin receptor 1 (MC1R) is overexpressed in melanoma and may be a molecular target for imaging and peptide receptor radionuclide therapy. 68Gallium (68Ga) labeling of DOTA-conjugated peptides is an established procedure in the clinic for use in positron emission tomography (PET) imaging. Aim of this study was to compare a standard labeling protocol against the 68Ga-DOTA peptide purified from the excess of unlabeled peptide.
Procedures
The MC1R ligand DOTA-NAPamide was labeled with 68Ga using a standard clinical protocol. Radioactive peptide was separated from the excess of unlabeled DOTA-NAPamide by HPLC. Immediately after the incubation of peptide and 68Ga (95°C, 15 min), the reaction was loaded on a C18 column and separated by a water/acetonitrile gradient, allowing fractionation in less than 20 minutes. Radiolabeled products were compared in biodistribution studies and PET imaging using nude mice bearing MC1R-expressing B16/F1 xenograft tumors.
Results
In biodistribution studies, non-purified 68Ga-DOTA-NAPamide did not show significant uptake in the tumor at 1 h post injection (0.78% IA/g). By the additional HPLC step, the molar activity was raised around 10,000-fold by completely removing unlabeled peptide. Application of this rapid purification strategy led to a more than 8-fold increase in tumor uptake (7.0% IA/g). The addition of various amounts of unlabeled DOTA-NAPamide to the purified product led to a blocking effect and decreased specific tumor uptake, similar to the result seen with non-purified radiopeptide. PET imaging was performed using the same tracer preparations. Purified 68Ga-DOTA-NAPamide, in comparison, showed superior tumor uptake.
Conclusions
We demonstrated that chromatographic separation of radiolabeled from excess unlabeled peptide is technically feasible and beneficial, even for short-lived isotopes such as 68Ga. Unlabeled peptide molecules compete with receptor binding sites in the target tissue. Purification of the radiopeptide therefore improved tumor uptake.
Introduction
Cutaneous malignant melanoma is one of the most lethal forms of cancer. Its incidence is increasing rapidly, making it a significant public health threat [1]. Melanocortin receptor 1 (MC1R) is overexpressed in most melanomas, making it a promising molecular target for diagnosis and peptide receptor radionuclide therapy (PRRT) [2]. Because of their low molecular weight, low immunogenicity and excellent tumor penetration, radiopeptides have attracted a steadily increasing interest in receptor-mediated tumor targeting [3, 4].
Because of its enhanced spatial resolution and high sensitivity, positron emission tomography (PET) has been developed into a valuable diagnostic tool, particularly for the detection of small metastases. Since commercial 68Germanium/68Gallium generators became widely available, 68Ga labeling of chelator-conjugated peptides turned into an established clinical procedure for use in PET imaging [5]. Due to its short half-life (67.71 min), 68Gallium (68Ga) has a higher molar activity (lower mass/activity ratio) than other nuclides in nuclear medicine, resulting in an unfavorable reaction stoichiometry [6]. In the radiochemical chelation process of 68Ga incorporation into 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), a high molar excess of DOTA-conjugated peptide over radiometal is usually used to attain high 68Ga complexation yields. The applied excess of cold peptide mass for 68Ga chelator loading typically ranges from 1,000-fold to 10,000-fold. This fraction is usually not removed before injection into the patient and it may compete for binding sites at the tumor, resulting in lower detection sensitivity. The aim of this study was to compare a standard labeling protocol against a labeling and HPLC purification protocol, which removes the excess of unlabeled peptide. We investigated the influence of peptide mass/molar activity on tumor accumulation of the MC1R ligand 68Ga-DOTA-NAPamide.
Materials and methods
Peptides
DOTA-NAPamide (Acetyl-Nle-Asp-His-D-Phe-Arg-Trp-Gly-Lys(DOTA)-NH2), an MC1R-binding peptide analogue described by Froidevaux et al. [7] was from ABX (Radeberg, Germany), [Nle4,d-Phe7]-melanocyte-stimulating hormone (NDP-MSH) from peptides&elephants (Hennigsdorf, Germany). To confirm the presence of the correct molecular mass, peptides were analyzed by a Finnigan Surveyor MSQ Plus liquid chromatography/mass spectrometry (LC-MS) system (Thermo Finnigan, Bremen, Germany). Peptides were used at a purity of greater than 95%.
Competitive binding and saturation assays with 125I-NDP-MSH
[Nle4,d-Phe7]-melanocyte-stimulating hormone (NDP-MSH) was iodinated with Na125I by the chloramine-T method and it was purified from unlabeled peptide by HPLC as described before [8]. Saturation and competitive binding studies were performed with live cells. 40.000 B16/F1 cells were seeded per well into a 96-well flat bottom cell culture plate. For competitive binding, medium was removed and 50 μL of binding buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.4, 5 mM MgCl2, 1 mM CaCl2, 0.5% BSA, protease inhibitor cocktail cOmplete [Roche Applied Science, Penzberg, Germany]) with increasing concentrations of non-radioactive peptide was added to the cells. Additionally, 50 μL binding buffer with 100,000 counts per minute (cpm) of 125I-NDP-MSH was added. After 30 minutes of incubation at 37°C, cells were washed 4 times with cold washing buffer (50 mM Tris-HCl pH 7.4, 125 mM NaCl, 0.05% BSA). 100 μL 1 N NaOH was added to lyse cells. Lysates were transferred into vials and measured using a gamma counter (Wallac 1470 Wizard, PerkinElmer, Waltham, MA, USA). The saturation assay was performed by adding 100 μL of binding buffer with increasing amounts of 125I-NDP-MSH to the cells in the presence or absence of 1 μM of unlabeled NDP-MSH.
Radiolabeling of DOTA-NAPamide
Radiolabeling experiments were performed on a Modular Lab PharmTracer synthesis module (Eckert & Ziegler, Berlin, Germany) which allows fully automated cassette-based labeling of gallium tracers utilizing a pharmaceutical grade 68Ge/68Ga generator (GalliaPharm, 1.85 GBq, good manufacturing practice (GMP)-certified; Eckert & Ziegler GmbH, Berlin, Germany). Cassettes were GMP-certified and sterile. They were used without pre-conditioning of the cartridges. Gallium generator at three months post calibration was eluted with aqueous HCl (0.1 M, 7 mL) and the eluate was purified on an ion-exchange cartridge followed by elution using 1 mL of 0.1 M HCl in acetone. An aliquot of DOTA-NAPamide, 50 μg (stock solution 1 mg/mL in 10% DMSO, 90% water) was mixed with 500 μL 0.1 M HEPES buffer (pH 7) and heated for 500 s at 95°C. After the reaction, the reactor was cooled with 500 μL of saline and, without post-processing, the contents of the reactor were directly used for subsequent HPLC purification.
HPLC purification
68Ga-labeled peptide was separated from non-radioactive NAPamide by reverse phase HPLC on an Agilent 1200 system (Agilent, Waldbronn, Germany). The complete mixture from the reaction chamber was loaded on an Eclipse XDB-C18 bonded silica column (Agilent, Waldbronn, Germany) and eluted with a linear gradient of acetonitrile (gradient 15–45% B in A over 20 min, flow rate of 1 mL/min, solvent A water + 0.1% trifluoroacetic acid (TFA), solvent B acetonitrile + 0.1% TFA, column at 55°C). 68Ga-DOTA-NAPamide was detected using a FlowStar LB513 detector (Berthold, Bad Wildbad, Germany) equipped with a BGO-X (5 μL) chamber. The unlabeled peptide moiety was detected via a diode array absorbance detector. 68Ga-DOTA-NAPamide was separated with the help of an automated fraction collector.
Competitive binding with 68Ga- DOTA-NAPamide
Competitive binding studies were performed according the procedure described for 125I-NDP-MSH. B16/F1 cells were incubated with 20 kBq (0.2 fmol) per well of purified 68Ga-DOTA-NAPamide alone or with 1,000-fold (0.2 pmol) or 10,000-fold (2 pmol) excess of non-radioactive DOTA-NAPamide.
Tumor inoculation and animal care
The study protocol for in vivo biodistribution and in vivo PET/MRI imaging was approved by the local committee at Landesamt für Gesundheit und Soziales for animal care according to the German law for the protection of animals. All applicable institutional and national guidelines for the care and use of animals were followed. 23 NMRI-Foxn1nu /Foxn1nu mice (Janvier Le Genest-Saint-Isle, France) were used in in vivo biodistribution experiments and 4 mice were used for PET/MRI imaging. Mean weight of the animals used was 28 gram. Mice were kept under SPF conditions with 12h/12h light/dark cycle at the animal facility of Charité –Universitätsmedizin Berlin, Campus Virchow-Klinikum. Animal health was checked at least twice weekly by visual inspection of general appearance, behavior, feces, tumor inoculation sites and by weighing the animals. A score sheet was applied to monitor health status and to take action in case a humane endpoint would be reached. During this study, humane endpoints were not reached. The maximum tumor size was below one cm3. Analgesics had not to be used for this study. Isoflurane was used as an anesthetic. Animals were sacrificed by cervical dislocation under isoflurane anesthesia. No mortality occurred outside of planned euthanasia. Data reporting was done according to ARRIVE guidelines (see S1 File).
The B16/F1 murine melanoma cell line xenograft model was chosen as it represents the standard and most frequently used tumor model for MC1R targeting in mice. This model has previously been shown to express relevant amounts of mouse MC1R. As both mouse and human MC1R bind NAPamide with similar affinity, this model is relevant to human biology. Experiments were performed throughout the day, between 9 am and 8 pm at Berlin Experimental Radionuclide Imaging Center (BERIC), Charité –Universitätsmedizin Berlin. Animals were randomly allocated to the experimental groups.
In vivo biodistribution assays
B16/F1 cells (3x106) in a volume of 150 μL 0.9% NaCl were inoculated subcutaneously on the right shoulder of NMRI-Foxn1nu /Foxn1nu mice (Janvier Labs, Saint-Berthevin, France). After 1–2 weeks of growth, tumor bearing mice were injected with approximately 5 MBq of 68Ga-DOTA-NAPamide in a volume of 100 μL 0.9% NaCl into a lateral tail vein via a catheter. Mice were sacrificed and dissected 1 h after injection. The B16F1 tumor, blood, stomach, pancreas, small intestine, colon, liver, spleen, kidney, heart, lung, muscle and femur samples were weighed and uptake of radioactivity was measured by a gamma counter (Wallac 1470 Wizard, Perkin Elmer, Waltham, MA, USA). To determine the effect of unlabeled ligand on tumor uptake, either 50 pmol or 500 pmol non-labeled DOTA-NAPamide were co-injected.
In vivo PET/MRI imaging
B16/F1 cells (3x106) in a volume of 150 μL 0.9% NaCl were inoculated subcutaneously on the right shoulder of NMRI-Foxn1nu /Foxn1nu mice (Janvier Labs, Saint-Berthevin, France). After 1–2 weeks of growth, tumor bearing mice were injected with approximately 15 MBq of 68Ga-DOTA-NAPamide in a volume of approximately 150 μL 0.9% NaCl into a lateral tail vein via a catheter. Positron emission tomography (PET) / magnetic resonance imaging (MRI) (1 Tesla nanoScan PET/MRI Mediso, Budapest, Hungary) was performed at Berlin Experimental Radionuclide Imaging Center (BERIC), Charité –Universitätsmedizin Berlin. Anatomic MRI scans were acquired using a T2-weighted 2D fast spin echo sequence (T2 FSE 2D) with the following parameters: coronal sequentially, matrix 256x256x20 with dimensions 0.36x0.36x1.5mm3, TR: 8695 ms, TE: 103 ms, and a flip angle of 180°. PET scans were performed for 90 min starting directly before intravenous injection of tracer. PET images were reconstructed from the raw data with the following image sequence: 6 x 10 s, 6 x 30 s, 5 x 60 s and 8 X 600 s. The tracer standardized uptake value (SUV) in the tumor tissue was determined by manual contouring of a volume of interest (VOI) of the PET images using PMOD 3.610 (PMOD Technologies, Zürich, Switzerland).
Results
DOTA-NAPamide binds to MC1R in vitro
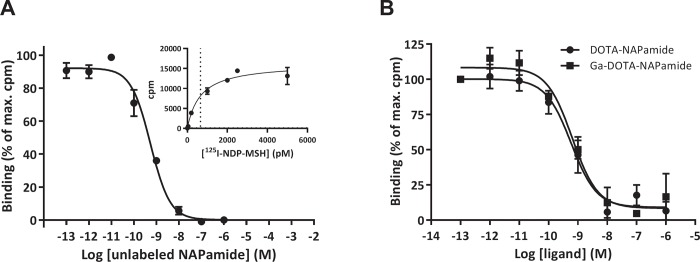
To confirm MC1R expression in the melanoma cell line B16/F1 and to assess the affinity of DOTA-NAPamide towards the receptor, competitive radioligand binding and saturation binding assays using 125I-labeled NDP-MSH were performed (Fig 1A). DOTA-NAPamide showed a high affinity for the murine MC1R expressed in the B16/F1 cells, with a calculated Ki of 0.37 nM (95% CI 0.24 nM, 0.57 nM) and a KD of 0.66 nM (95% CI 328 pM, 992 pM). To exclude a negative effect of gallium (Ga) incorporation into the DOTA chelator on binding affinity, competitive in vitro binding assays were performed using either DOTA-NAPamide or DOTA-NAPamide complexed with non-radioactive Ga. Fig 1B shows nearly identical concentration-response curves and Ki values: 0.40 nM (95% CI 0.18 nM, 0.86 nM) vs. 0.43 nM (95% CI 0.21 nM, 0.88 nM) for unlabeled and Ga-labeled DOTA-NAPamide binding to B16/F1 cells. This demonstrated that chelation with Ga did not affect the affinity of DOTA-NAPamide towards the MC1R receptor.
Fig 1. DOTA-NAPamide and Ga-DOTA-NAPamide are high affinity ligands for the melanocortin 1 receptor (MC1R): 125I-NDP-MSH ligand displacement and saturation assays performed with whole B16/F1 cells.
A Various concentrations of NAPamide were used to displace 125I-NDP-MSH. The inset shows a saturation experiment to determine the dissociation constant. B 125I-NDP-MSH ligand displacement assay to assess the impact of gallium chelation on DOTA-NAPamide. Curve fits were performed in GraphPad Prism by applying a one‐site binding equation. (n = 3; mean +/‐ SEM).
Unlabeled DOTA peptide can be removed by HPLC
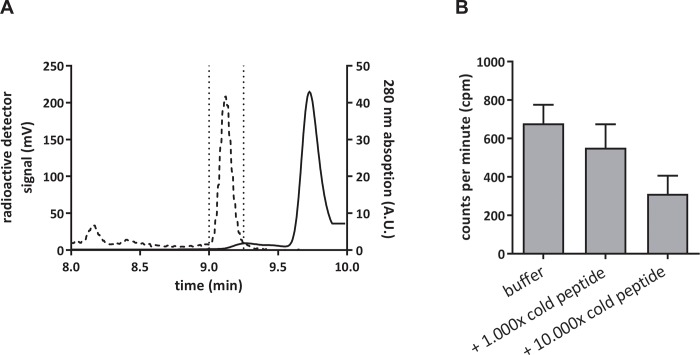
For removal of unlabeled excess of DOTA-NAPamide and of non-incorporated 68Ga after completion of the radiochemical reaction, the product was transferred from the reactor to an HPLC equipped with a C18 reverse-phase column and a fraction collector. Fig 2A shows an exemplary separation run for 68Ga-DOTA-NAPamide and unlabeled DOTA-NAPamide. Free 68Ga does not exhibit a distinct interaction with the C18 column and elutes close to the dead time of the HPLC. For the labeling reaction, the peptide was used in an excess compared to 68Ga (10,000:1 molar ratio) and it showed a slightly prolonged retention time in comparison to 68Ga-DOTA-NAPamide. This was exploited to purify 68Ga-DOTA-NAPamide (Fig 2A). To demonstrate that an excess of unlabeled peptide would displace 68Ga-DOTA-NAPamide from its receptor, the radiotracer was incubated on B16/F1 cells in vitro either alone or with a 1,000-fold or 10,000-fold excess of DOTA-NAPamide (Fig 2B). Indeed, both concentrations of unlabeled peptide were able to displace the radiopeptide as compared to incubation with buffer. In comparison to purified tracer alone, a 1,000-fold excess led to an approximately 20% decrease and a 10,000-fold excess of unlabeled peptide diminished the overall binding to less than 50% (Fig 2B).
Fig 2.
A Reverse-phase HPLC chromatogram of a 68Ga-DOTA-NAPamide purification. The dashed line shows the radiodetector signal and the peak there represents the 68Ga-DOTA-NAPamide fraction, while the solid line shows the 280 nm absorption signal with the peak of DOTA-NAPamide. The area surrounded by dotted lines represents the purified fraction used in in vivo experiments. B In vitro displacement of 68Ga-DOTA-NAPamide from B16/F1 cells by an excess of unlabeled DOTA-NAPamide (n = 3; mean +/- SEM). Differences were not statistically significant (p = 0.12, R2 = 0.51, one-way ANOVA).
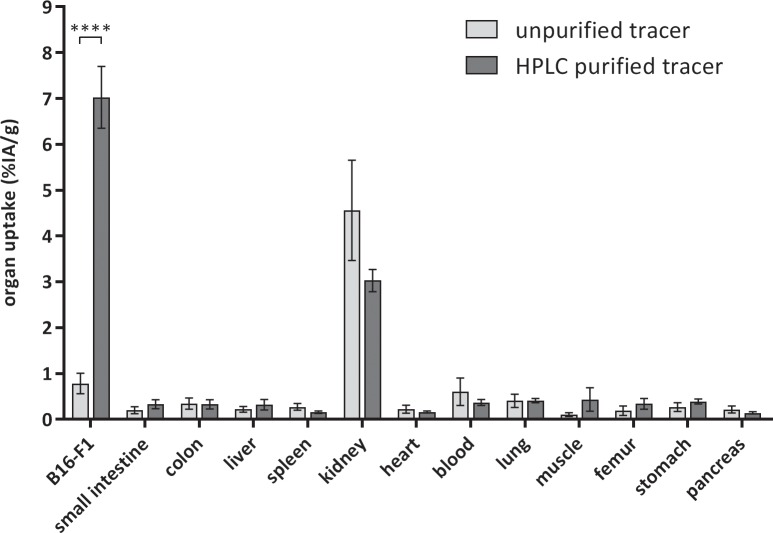
Purification of the tracer leads to an improved tumor uptake
Fig 3 shows the results of an in-vivo biodistribution experiment in B16/F1 xenograft-bearing mice 1 hour after i.v. injection of 68Ga-DOTA-NAPamide. The non-purified tracer from the standard procedure showed a very low uptake into subcutaneously grown B16/F1 tumor xenografts (0.78% ± 0.55% IA/g [95% CI]). Removal of unlabeled DOTA-NAPamide led to a more than 8-fold increase in tumor uptake, with 7.0% ± 1.7% IA/g [95% CI] in the tumor for the purified 68Ga-DOTA-NAPamide. Except for a moderate uptake in the kidneys, all other tissues showed only a small uptake of the tracer, mostly below 0.5% IA/g. In animals treated with 68Ga-DOTA-NAPamide produced by the standard procedure, the kidney uptake was slightly higher compared to the purified tracer (4.6% ± 2.7% IA/g vs. 3.0% ± 0.6% IA/g [95% CI]) (Fig 3).
Fig 3. Biodistribution at 1 h after i.v. injection of approximately 5 MBq radiotracer.
Results of 68Ga-DOTA-NAPamide produced by the standard protocol (n = 7) are shown in light gray, and results obtained with the purification protocol (n = 7) are shown in dark gray (mean +/‐ SEM; ****p = 0.0001, two-way ANOVA followed by Bonferroni post-test).
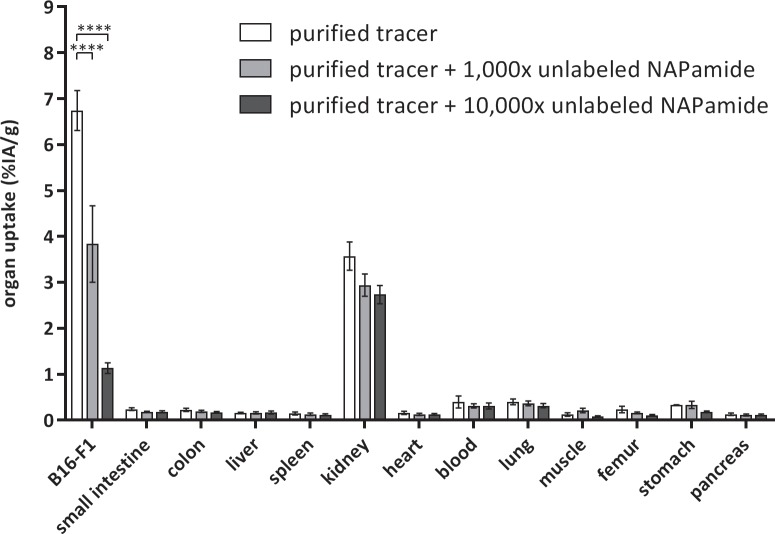
Tumor uptake enhancement is reversed by coinjection of unlabeled peptide
The effect of cold peptide mass was studied by injecting a constant amount of purified 68Ga-DOTA-NAPamide together with different amounts of unlabeled DOTA-NAPamide. Mice were injected with 5 MBq (~50 fmol) alone or with an additional 1,000-fold (50 pmol) or 10,000-fold (500 pmol) excess of unlabeled peptide. This 10,000-fold excess of unlabeled peptide corresponds to the molar ratio in the standard protocol. The coinjection of DOTA-NAPamide led to significant loss of uptake in the MC1R-expressing B16/F1 tumor xenografts (Fig 4). While purified 68Ga-DOTA-NAPamide showed an uptake of 6.7% ± 1.9% IA/g [95% CI], coinjection of 50 pmol cold DOTA-NAPamide decreased the total uptake to 3.8% ± 3.6% IA/g [95% CI]. 500 pmol coinjected peptide led to an approximately 6-fold decrease to 1.1% ± 0.5% IA/g [95% CI] (Fig 4).
Fig 4. 68Ga-DOTA-NAPamide biodistribution at 1 h after i.v. injection of approximately 5 MBq radiotracer.
Purified 68Ga-DOTA-NAPamide was injected either alone or in parallel with an excess of unlabeled DOTA-NAPamide (1,000-fold or 10,000-fold excess over radiotracer) (n = 3 per group; mean +/- SEM; ****p = 0.0001, two-way ANOVA followed by Bonferroni post-test).
PET imaging confirms the results of biodistribution studies
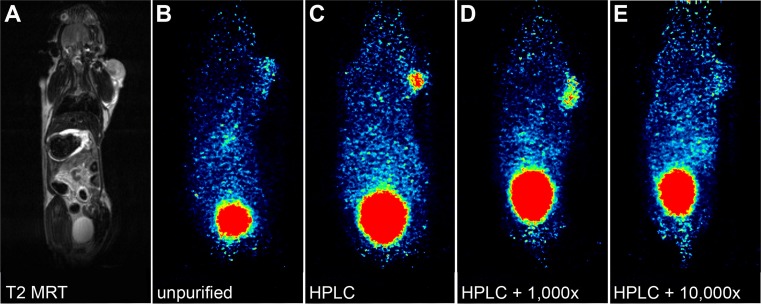
Mice bearing subcutaneous B16/F1 tumors on their right shoulder were injected with approximately 15 MBq 68Ga-DOTA-NAPamide in different formulations. Each mouse was given a different DOTA-NAPamide composition (A standard procedure, B purified, C purified + 1,000-fold excess of cold peptide, D purified + 10,000-fold excess of cold peptide). Dynamic PET images were taken from 5 to 90 minutes after injection and tracer standardized uptake value (SUV) in the tumor tissue was determined by manual contouring of a volume of interest (VOI). Fig 5 shows all four tested conditions at 1 h post injection. The tumor showed an increase in uptake of 68Ga-DOTA-NAPamide for the purified (SUV 0.420) and the purified + 1,000-fold excess of cold peptide (SUV 0.367) conditions (Fig 5B and 5C). For the injections with a 10,000-fold excess of unlabeled peptide, the tracer uptake (SUV 0.152) was on a similarly low level as for the unpurified tracer (SUV 0.119) (Fig 5A and 5D). Since the peptide is excreted through the kidneys into the bladder, a high signal was observed in both organs (kidneys not visible in Fig 5 due to their location in a different plane).
Fig 5. Coronal sections from MRI and PET scans of mice bearing B16/F1 tumors on the right shoulder at 1 h after tail vein injection of 68Ga-NAPamide with various ratios of labeled and unlabeled peptide.
A T2-weighted MRI (corresponding to PET image in B) B unpurified 68Ga-DOTA-NAPamide C HPLC-purified 68Ga-NAPamide D HPLC-purified 68Ga-NAPamide + 1,000-fold excess of DOTA-NAPamide, and E HPLC-purified 68Ga-NAPamide + 10,000-fold excess of DOTA-NAPamide.
Tracer kinetics
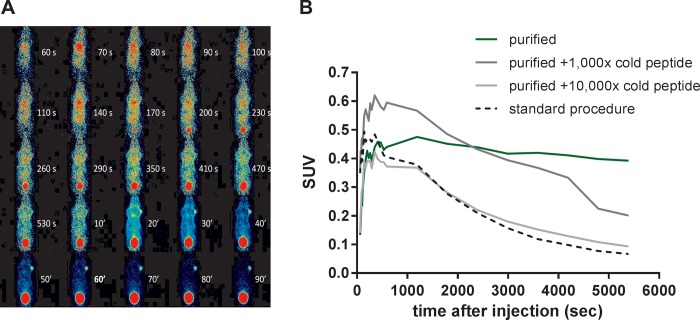
To obtain kinetic profiles, mice were imaged for up to 90 minutes after injection of any of the four different tracer formulations. Images were reconstructed over short intervals: 10/30/60 seconds until 10 minutes p.i. and over 10 minutes thereafter. Fig 6A shows the kinetics for the purified 68Ga-DOTA-NAPamide. After defining VOIs for the tumor in each mouse, the resulting SUVs were plotted over time from dynamic PET data. Early tracer kinetics in the tumor confirm the advantage of tracer purification: in the first 5–10 minutes after injection, the tracer reaches a maximum level in the tumors for all tested conditions (Fig 6B). Thereafter, uptake was slowly decreasing over the next 90 minutes. In the animal treated with a 1,000-fold excess of cold peptide mass, the initial amount of radioactivity in the tumor is slightly higher (SUV 0.63) than in the other tumors (mean SUV 0.43). Of note, the slope of tracer decrease in B16/F1 tumors was less steep for the purified 68Ga-DOTA-NAPamide.
Fig 6.
A Short-interval reconstruction from dynamic PET data of a mouse bearing a B16/F1 tumor on the right shoulder after tail vein injection of 5 MBq purified 68Ga-NAPamide. Injected peptide amounts: 500 pmol (unpurified and 10,000-fold excess), 50 pmol (1,000-fold excess) and 0.05 pmol (purified). B Time-activity relationships of 68Ga-DOTA-NAPamide B16/F1 tumor accumulation derived from dynamic PET data obtained after injection of four different tracer formulations (n = 1).
Discussion
The feasibility of targeting metastatic melanoma with MC1R ligands for nuclear medicine has been studied as early as 1990 and has since resulted in a multitude of ligand-chelator conjugates applied for biodistribution, SPECT and PET experiments [9, 10]. Froidevaux et al. developed the 8mer metabolically stable high-affinity MC1R ligand DOTA-NAPamide that showed favorable biodistribution and tumor uptake values ranging from 7.56% to 9.43% IA/g at 4 h p.i. [7]. The radionuclides used were of moderate half-life (2.81 d for 111In, 3.26 for 67Ga). Another study with 64Cu-DOTA-NAPamide reported ~4% IA/g at 4 h p.i. [11]. Only recently, one study used the short-lived 68Ga with this tracer demonstrating tumor demarcation in PET imaging yet not reporting biodistribution data for the B16/F10 tumors used [12]. However, no clinical study has been reported for the use of MC1R analogs in melanoma imaging so far.
In experiments preceding this study, we performed biodistribution studies using the MC1R ligands 68Ga-DOTA-NDP-MSH and 68Ga-DOTA-NAPamide in the B16/F1 melanoma model, yet failed to achieve the high tumor uptake previously reported for the longer-lived 111In and 67Ga complexes. We hypothesized that the unfavorable stoichiometry of the chelation reaction of the peptide conjugate with 68Ga and the resulting high excess of unlabeled ligand prevented higher tumor uptake due to competition for MC1R binding sites. Therefore, in the current study we aimed to compare a standard 68Ga labeling protocol against a labeling and HPLC-purification protocol, which removes the excess of unlabeled peptide. We investigated the influence of peptide mass/molar activity on tumor accumulation of the MC1R ligand 68Ga-DOTA-NAPamide using biodistribution and PET imaging.
For the standard labeling protocol used in this study, the product of 350 MBq 68Ga-DOTA-NAPamide (3.5 pmol 68Ga) is accompanied by 35 nmol (50 μg) of unlabeled peptide, resulting in a 10,000-fold excess over the radioligand. Due to this stoichiometry, the theoretical molar activity of 103,000 GBq/μmol for pure 68Ga-DOTA-NAPamide is diminished to an effective molar activity of just 10 Gbq/μmol. Lowering the peptide amount in the chelation reaction was no remedy as it dramatically reduces the radiochemical yield.
HPLC purification of the radiopeptide, however, improved tumor uptake in biodistribution studies by a factor of more than 8-fold, resulting in a tumor-to-kidney ratio of 2.33 and tumor-to-tissue ratios of better than 15 for all other organs investigated (Fig 3). While others have argued HPLC purification was not convenient in clinical practice and developed solid phase extraction of 68Ga-exendin as an alternative method [13], we found using an HPLC to fractionate tracer was feasible and efficient. In an in vitro binding assay (Fig 2B), however, the differences between purified tracer and combinations with either a 1,000-fold or 10,000-fold excess of unlabeled peptide were less pronounced than expected (only 50% reduction). Adding a 10,000-fold higher amount of competitor should lead to a higher reduction in binding (e.g. to about 10%). The lack of a more drastic reduction may be explained by non-specific binding to the cells e.g. hydrophobic interaction with unrelated cell surface receptors. The impact of this, however, seemed to be restricted to the in vitro situation, as the in vivo competition (Fig 4) led to a considerably higher displacement (more than 8-fold difference).
As most radiochemical laboratories have this instrumentation in place for routine quality control, cost may not be limiting. The procedure can also be performed rapidly—within 15 to 20 min. Similar successful approaches to purify 11C, 18F and 67Ga tracers have been reported [14–16]. However, with production of 68Ga tracers shifting towards kit preparation, HPLC purification may not have much clinical future. Whether the HPLC purification strategy is applicable with other tracers needs to be established on a case-to-case basis. We have attempted to apply this method with a CMKLR1-targeting peptide but failed to improve tumor uptake above the level of unpurified tracer (about 6%, unpublished results). In addition, the enhancement of molar activity may not be of critical importance for the imaging of humans as the applied amount of activity (and thus, peptide) is several orders of magnitude lower than for rodents. A human of 70 kg mass (2,500 times that of a 28-gram mouse) and 7 liters of blood volume (4,400 times that of the mouse) would typically receive about 150 MBq instead of 15 MBq for mice in this study (only 10-fold more). Consequently, the resulting concentration of the competing peptide would be much lower in the clinical setting.
The appropriate amount of cold peptide mass or the best molar activity for optimal tumor imaging has been a matter of investigation for more than two decades now. Using the somatostatin receptor ligand 111In-pentetreotide, an early study by Breeman et al. found moderate molar activities (0.6, 6.0 MBq/μg) to yield the highest uptake in rat pancreas, while higher and lower values showed reduced uptake.[17] However, the majority of subsequent studies demonstrated that for efficient receptor targeting, low peptide doses should be administered. De Jong et al e.g. systematically investigated the effects of varying peptide mass at constant or varying molar activity on organ activity using 111In- [DOTA0,Tyr3]octreotide in tumor-bearing rats [18]. This study identified a principal detrimental effect of increasing peptide doses in both experimental settings with pancreas and tumor only showing limited benefit of an increased peptide dose of 0.2 (pancreas) or 0.5 μg (CA20948 tumors). Increasing amounts of radiolabeled peptide at constant molar activity were also used investigating the 67Ga-labeled GRPR ligand BZH3 [19]. A peptide amount of 15 pmol per mouse showed highest tumor uptake (10.9% IA/g), with 5, 45 and 135 pmol yielding somewhat lower results (7.98, 8.0, 6.25% IA, respectively). Of note, intestines showed a continuously decreasing % IA/g uptake with increasing amounts of peptide, suggested to be due to increasing saturation of receptors. With limited molar activity of the 68Ga tracer, significant peptide dose reduction was only feasible at the expense of very small activities, insufficient for high-quality imaging in mice.[19]
Brom et al. compared the effect of co-injected unlabeled peptide over 5 orders of magnitude in in vivo biodistribution studies [13]. Low doses of unlabeled exendin-3, in the molar range of co-injected radioactive [Lys40(111In)]exendin-3 did not show a clear effect on tumor uptake. Eventually, the addition of 0.3 μg (~60 pmol) cold peptide led to a decrease in tumor uptake of the radioactive tracer. This dose was approximately 300-fold higher than the administered amount of 111Indium-labeled exendin. The same group compared the effect of co-injected unlabeled DOTA-minigastrin over four orders of magnitude (0.1–100 μg) in in vivo biodistribution studies. They observed an increasing drop in tumor uptake of their labeled DOTA-minigastrin for all tested amounts of cold co-injections (50 pmol—50 nmol) [20]. Using the standard labeling procedure in the current study, 500 pmol of unlabeled peptide were injected with an activity of 5 MBq 68Ga-DOTA-NAPamide. A detrimental effect of higher peptide dose had also been described in the first study describing DOTA-NAPamide as an MC1R tracer: tumor uptake at 170 pmol or 420 pmol peptide was much lower than with just 20 pmol [7].
Chelators with higher labeling efficiency than DOTA may represent an alternative approach to obtaining high molar activities. Recently, the effect of varying molar activities (0.5–1,000 GBq/μmol) was studied for the two integrin-targeting 68Ga-TRAP peptides 68Ga-aquibeprin and 68Ga-avebetrin in biodistribution and PET imaging [12]. With this chelator (TRAP), very high molar activities (up to 5,000 GBq/μmol) could be produced during radiochemical labeling without additional HPLC purification [21]. Again, highest tumor uptake was found with highest molar activities with similar tumor-to-kidney ratios for all tested conditions. However, tumor-to-muscle ratios reached a maximum at 6 nmol (~3.5 GBq/μmol), indicating a benefit for moderate molar activities when muscle signal is limiting [12]. A variety of factors may lead to this, including target expression levels in muscle and other organs. Further studies will have to elucidate the relationship between expression, biodistribution and optimal molar activity for tumor imaging.
Conclusions
Choosing the right molar activity and finding a way to obtain it in an efficient way remains to be a challenge during the introduction of new tracers. We showed that separation of radiolabeled and cold peptide via HPLC is technically feasible and beneficial, even for short-lived isotopes such as 68Ga. Unlabeled peptide molecules can strongly compete with receptor binding sites in the target tissue. Purification of the radiopeptide improved tumor uptake in in biodistribution studies and PET/MRI scans. Production of higher molar activity radiotracers may not only be important for imaging, but may also improve uptake and thus efficacy in therapeutic procedures such as PRRT.
Supporting information
(PDF)
Data Availability
The data for this manuscript are available from the public repository zenodo (http://doi.org/10.5281/zenodo.2554341).
Funding Statement
This study was supported by grant 03IPT614A in the InnoProfile program of the German Federal Ministry of Education and Research (BMBF; https://www.bmbf.de/) to CG, by grant INST 335/454-1FUGG from Deutsche Forschungsgemeinschaft (DFG; https://www.dfg.de/) to WB and by grant 20161123 from Sonnenfeld-Stiftung to Samantha Exner. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gladfelter P, Darwish NHE, Mousa SA. Current status and future direction in the management of malignant melanoma. Melanoma Res. 2017;27(5):403–10. 10.1097/CMR.0000000000000379 . [DOI] [PubMed] [Google Scholar]
- 2.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49(22):6352–8. . [PubMed] [Google Scholar]
- 3.Fani M, Maecke HR. Radiopharmaceutical development of radiolabelled peptides. Eur J Nucl Med Mol Imaging. 2012;39 Suppl 1:S11–30. 10.1007/s00259-011-2001-z . [DOI] [PubMed] [Google Scholar]
- 4.Reubi JC, Maecke HR. Peptide-based probes for cancer imaging. J Nucl Med. 2008;49(11):1735–8. 10.2967/jnumed.108.053041 . [DOI] [PubMed] [Google Scholar]
- 5.Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007;34(7):982–93. 10.1007/s00259-006-0317-x . [DOI] [PubMed] [Google Scholar]
- 6.Velikyan I, Beyer GJ, Bergstrom-Pettermann E, Johansen P, Bergstrom M, Langstrom B. The importance of high specific radioactivity in the performance of 68Ga-labeled peptide. Nucl Med Biol. 2008;35(5):529–36. 10.1016/j.nucmedbio.2008.03.002 . [DOI] [PubMed] [Google Scholar]
- 7.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, et al. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45(1):116–23. . [PubMed] [Google Scholar]
- 8.Exner S, Prasad V, Wiedenmann B, Grotzinger C. Octreotide Does Not Inhibit Proliferation in Five Neuroendocrine Tumor Cell Lines. Front Endocrinol (Lausanne). 2018;9:146 10.3389/fendo.2018.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bard DR, Knight CG, Page-Thomas DP. A chelating derivative of alpha-melanocyte stimulating hormone as a potential imaging agent for malignant melanoma. Br J Cancer. 1990;62(6):919–22. 10.1038/bjc.1990.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raposinho PD, Correia JD, Oliveira MC, Santos I. Melanocortin-1 receptor-targeting with radiolabeled cyclic alpha-melanocyte-stimulating hormone analogs for melanoma imaging. Biopolymers. 2010;94(6):820–9. 10.1002/bip.21490 . [DOI] [PubMed] [Google Scholar]
- 11.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjug Chem. 2007;18(3):765–72. 10.1021/bc060306g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notni J, Steiger K, Hoffmann F, Reich D, Schwaiger M, Kessler H, et al. Variation of Specific Activities of 68Ga-Aquibeprin and 68Ga-Avebetrin Enables Selective PET Imaging of Different Expression Levels of Integrins alpha5beta1 and alphavbeta3. J Nucl Med. 2016;57(10):1618–24. 10.2967/jnumed.116.173948 . [DOI] [PubMed] [Google Scholar]
- 13.Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. Eur J Nucl Med Mol Imaging. 2010;37(7):1345–55. 10.1007/s00259-009-1363-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsifis A, Papazian V, Jackson T, Loc'h C. A rapid and efficient preparation of [123I]radiopharmaceuticals using a small HPLC (Rocket) column. Appl Radiat Isot. 2006;64(1):27–31. 10.1016/j.apradiso.2005.06.006 . [DOI] [PubMed] [Google Scholar]
- 15.Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, et al. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50(10):1638–45. 10.2967/jnumed.109.065870 . [DOI] [PubMed] [Google Scholar]
- 16.Van Laeken N, Kersemans K, De Meestere D, Goethals I, De Vos F. Improved HPLC purification strategy for [11C]raclopride and [11C]DASB leading to high radiochemical yields and more practical high quality radiopharmaceutical formulations. Appl Radiat Isot. 2013;78:62–7. 10.1016/j.apradiso.2013.04.009 . [DOI] [PubMed] [Google Scholar]
- 17.Breeman WA, Kwekkeboom DJ, Kooij PP, Bakker WH, Hofland LJ, Visser TJ, et al. Effect of dose and specific activity on tissue distribution of indium-111-pentetreotide in rats. J Nucl Med. 1995;36(4):623–7. . [PubMed] [Google Scholar]
- 18.de Jong M, Breeman WA, Bernard BF, van Gameren A, de Bruin E, Bakker WH, et al. Tumour uptake of the radiolabelled somatostatin analogue [DOTA0, TYR3]octreotide is dependent on the peptide amount. Eur J Nucl Med. 1999;26(7):693–8. . [DOI] [PubMed] [Google Scholar]
- 19.Schuhmacher J, Zhang H, Doll J, Macke HR, Matys R, Hauser H, et al. GRP receptor-targeted PET of a rat pancreas carcinoma xenograft in nude mice with a 68Ga-labeled bombesin(6–14) analog. J Nucl Med. 2005;46(4):691–9. . [PubMed] [Google Scholar]
- 20.Brom M, Joosten L, Laverman P, Oyen WJ, Behe M, Gotthardt M, et al. Preclinical evaluation of 68Ga-DOTA-minigastrin for the detection of cholecystokinin-2/gastrin receptor-positive tumors. Mol Imaging. 2011;10(2):144–52. [PMC free article] [PubMed] [Google Scholar]
- 21.Notni J, Simecek J, Hermann P, Wester HJ. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chemistry. 2011;17(52):14718–22. 10.1002/chem.201103503 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The data for this manuscript are available from the public repository zenodo (http://doi.org/10.5281/zenodo.2554341).