Abstract
Strategies that seek to enhance musculoskeletal tissue regeneration and repair by modulating the biologic microenvironment at the site of injury have considerable therapeutic potential. Current and emerging biologic approaches include the use of growth factors, platelet-rich plasma, stem cell therapy, and scaffolds. The American Academy of Orthopaedic Surgeons hosted a research symposium in November 2015 to review the current state-of-the-art biologic treatments of articular cartilage, muscle, tendon, and bone injuries and identify knowledge gaps related to these emerging treatments. This review outlines the findings of the symposium and summarizes the consensus reached on how best to advance research on biologic treatment of orthopaedic injuries.
Biologic approaches to the treatment of orthopaedic injuries aim to optimize clinical outcomes by improving musculoskeletal tissue healing. Despite considerable research effort,1,2 biologic approaches have not yet achieved a sufficient evidence base to warrant widespread clinical application. In 2015, the American Academy of Orthopaedic Surgeons (AAOS) held a symposium to identify knowledge gaps related to biologic treatment of orthopaedic injury (BTOI) and to establish some consensus on how future research might be best directed. The experts who participated in the symposium were in universal agreement that the most effective way to achieve meaningful translation of therapies into clinical practice is to use a systematic translational approach based on sound scientific evidence and greater integration between investigators, regulators, and industry. Consensus was reached in the areas of working with regulatory bodies and industry, the use of platelet-rich plasma (PRP), stem cell therapy, augmentation of soft-tissue healing, and the use of scaffolds. The symposium participants identified fundamental changes in approach that will be necessary to expedite clinical translation of biologic therapies and recommended a framework for future research.
Systematic Approach to Research
Researchers have initiated >500 clinical trials evaluating mesenchymal stem cells (MSCs) and >180 trials evaluating PRP (see clin-icaltrials.gov) in a wide range of applications, demonstrating widespread interest in the advancement of biologic treatment approaches. However, many of these studies were initiated without sufficient understanding of the disease process being targeted or even of the attributes of the biologic therapy being evaluated. Hurried translation of new therapies into clinical use presents an expected inefficiency as a result of the use of poorly characterized and suboptimal preparations. If the results of these studies do not demonstrate a clear benefit, potentially effective treatments may be dismissed as non-effective, to the ultimate detriment of patients. This disordered approach is in direct contrast with established models of translational research that have been used successfully to deliver effective therapies across multiple medical disciplines.3,4
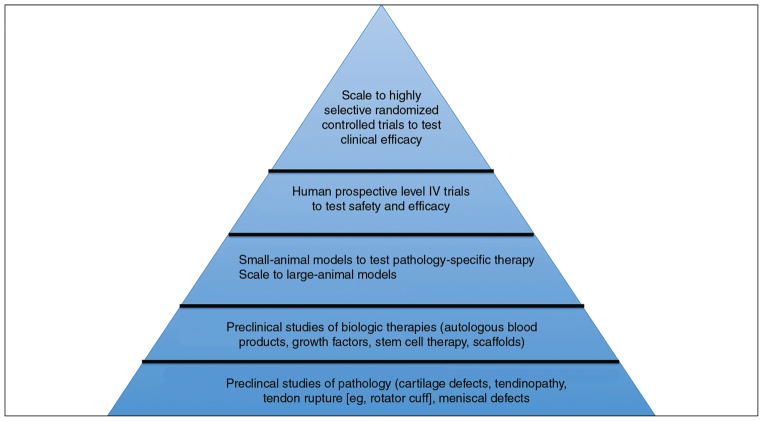
Fundamental to the translational or bench-to-bedside approach is a comprehensive understanding of the disease process. This understanding of the disease process facilitates the development of targeted therapies, which are evaluated in the laboratory setting before efficacy is studied in animal models and ultimately in patients.5 Simultaneously, clinicians provide feedback on clinical observations that might stimulate further preclinical research. This approach ensures that strategies are based on sound scientific rationale and that ineffective therapies are identified and prevented from reaching time-consuming and expensive animal and clinical studies (Figure 1). Because regulatory frameworks favor potential therapies that are extensively characterized, adoption of a systematic translational approach will facilitate progression through the regulatory process.6
Figure 1.
Schematic diagram demonstrating the pyramidal approach of translational medicine. In this bottom-up approach, preclinical understanding of the disease process facilitates identification and development of biologic therapies. Therapies optimized in vitro are studied in small-animal and large-animal models and finally in clinical studies of safety and efficacy.
In addition to being more systematic, future research into BTOI must better integrate academia, industry, and regulatory bodies. Academic researchers, industry, and regulatory bodies all strive to develop safe and effective therapies for patients. A central goal of the AAOS symposium was to foster collaboration between academia, industry, and key regulatory parties, including the FDA. The importance of establishing channels of communication between parties through regular meetings, dialogue, or online forums was identified. Strategies to foster a unified approach include improving standards of reporting, initiating new FDA pathways for the evaluation of biologic products, and the introduction of biologic treatment registries that provide real-time data on efficacy while facilitating early warning of potential adverse effects to prevent harm to patients.
The consensus statements on general approaches to future research on BTOI are therefore as follows: (1) biologics research should be based on a comprehensive understanding of the pathologic basis of disease and adequate characterization of the proposed biologic therapy, and (2) where possible, industry partnerships and dialogue with relevant regulatory bodies should be initiated early in the research process.
Working With Regulatory Bodies
A thorough discussion of BTOI requires an overview of the FDA regulatory process. It is important to differentiate regulation of biologics7 from regulation of medical devices.8 Combination products are classified as drug/device, biologic/device, drug/ biologic, or a combination of all three types.7
Human Cells, Tissues, and Cellular and Tissue-based Products
The FDA regulates human cells, tissues, and cellular and tissue-based products (HCT/Ps) as defined in Title 21, Part 1271, of the Code of Federal Regulations.7 This category encompasses a wide variety of products that are intended for implantation, transplantation, infusion, or transfer into a human recipient. Blood products and minimally manipulated bone marrow are not considered HCT/Ps. HCT/Ps are categorized on the basis of their level of risk and are further regulated under the Public Health Service Act.9 A three-tiered framework of regulation has been described by Chirba et al.10 Category 1 products are considered to be low risk and do not undergo HCT/P oversight.7
Category 2 includes lower-risk products (section 361) that meet the following criteria: minimal manipulation, homologous use only, no combination products, and lack of systemic effect.7 The primary requirement for products regulated under section 361 is manufacturing according to the Current Good Tissue Practice regulations,11 which ensures that HCT/Ps do not contain communicable disease agents, are not contaminated, and do not become contaminated.7
Category 3 includes higher-risk products (section 351) and is more heavily regulated. Products are placed into this category if they do not meet all four criteria for category 2. In addition to manufacturing according to the Current Good Tissue Practice regulations, products regulated under section 351 must undergo a process that includes communication with the FDA for completion of an investigational new drug application, development of clinical trials, and a biologics license application.1
Medical Devices
The FDA regulates medical devices as class I, class II, or class III.8 Class designation depends on the level of risk to the patient and the extent of regulatory control determined necessary by the FDA to allow marketing of the device. Since the passage of the Medical Device Amendments to the Food, Drug and Cosmetic Act in 1976, new medical devices are placed into class III regulation unless a comparable device (predicate device) is available, and reclassification may occur depending on assessment of risk.12 The Medical Device Amendments specify general controls that apply to all three device classes and pertain to adulteration, misbranding, device registration and listing, premarket notification, banned devices, notification and recall, product reporting, restricted devices, and Current Good Manufacturing Practice regulations.
Class I (eg, manual surgical instruments) represents the lowest level of risk and regulatory control.13 In addition to the general controls required for class I devices, class II devices require additional special controls, including special labeling requirements, performance standards, postmarket surveillance, and FDA guidance documents. Examples include devices such as prosthetic joints, implants for fracture fixation, and interference screws for ligament fixation. Class III devices require the general controls and must undergo a premarket approval (PMA) process before marketing.14 Sheth et al15 reviewed the process for obtaining FDA approval for marketing of new orthopaedic devices.
The 510(k) pathway to marketing is used for most newmedical devicesand requires comparison of the proposed device to a previously approved device (ie, predicate device).16,17 If the new device is found to be substantially equivalent to the predicate device, the new device is classified into the same class and is subject to the same requirements.
The FDA Safety and Innovation Act was passed in 2012 and provides an amended classification process known as de novo.18 This process provides a pathway to class I or II classification for products that have no legally marketed predicate device. Class III devices, combination products, and devices for which a classification regulation already exists are ineligible for this process.
Future Directions
The BTOI symposium consensus statement on working with regulatory bodies and industry is that partnership of academia and industry with regulators is necessary to modernize regulation of biologics and that insight should be obtained from international models when applicable. As outlined by Sheth et al15 and in an internal FDA review, critical challenges facing the FDA and its mission include funding, staffing, and necessary scientific expertise.19 Japan has recently undergone regulatory reform to support and accelerate research and development of regenerative medicine and cell therapy and to expedite access to these treatments in a safe and effective manner. The Japanese reform occurred through the passage of the Pharmaceuticals and Medical Devices Act and the Safety of Regenerative Medicine Act. Dialogue with international partners and collaboration among investigators, industry, and regulators will be necessary to advance the field while ensuring the safety of patients.
Platelet-rich Plasma
Platelets contain growth factors that stimulate the proliferation of local progenitors, direct cell differentiation, and modify local inflammatory responses.20 Knowledge of these regenerative attributes has stimulated efforts to explore the use of autologous preparations rich in platelets for the treatment of orthopaedic injuries, including tendon,21 muscle,22 cartilage,23 and bone24 defects. These preparations are made by obtaining blood from a patient and processing it to concentrate platelets above baseline levels. Some of these preparations contain leukocytes. The manipulation of blood to obtain PRP is minimal, facilitating the process of regulatory approval. Initial results achieved in introductory in vitro studies were promising25,26 but have not yet been reflected in many clinical studies to date.27,28 The AAOS symposium participants identified several obstacles to the advancement of PRP therapies and made recommendations on how future research efforts should be directed to overcome these challenges. The consensus statements on future research on PRP are summarized in Table 1.
Table 1.
Consensus Statements on Platelet-rich Plasma
| No. | Statement |
|---|---|
| 1 | An accepted nomenclature and classification system that encompasses autologous blood/plasma products and categorizes preparations in sufficient detail is required to facilitate comparison across studies. Efforts should be made to involve academics, clinicians, and industry representatives in this process to encourage widespread adoption of the system. |
| 2 | The influence of donor variance and processing and delivery factors on the composition of PRP must be established. |
| 3 | A validated assay of the efficacy of PRP should be established for each clinical application. |
| 4 | The relationship between PRP composition and efficacy should be established. |
| 5 | Minimum standards of reporting for all studies (preclinical and clinical) evaluating PRP must be established to facilitate communication and the interpretation and synthesis of scientific investigations. These standards must include measured characteristics of the PRP and factors relating to the donor, processing, and delivery of the PRP. |
| 6 | Specific formulations of PRP should be matched with specific pathologic indications. |
| 7 | Methods for establishing proof of safety and efficacy of PRP should be determined. This process may require evidence of phenotype stability or viability for each indication. |
PRP = platelet-rich plasma
Orthopaedic clinical researchers have hurried to identify clinical applications of PRP on the basis of limited scientific rationale. Symposium participants stated that PRP research should be hypothesis driven and based on an understanding of the specific pathology addressed by each treatment method. Comprehensive understanding of the disease processes may reveal the scenarios in which delivery of growth factors may be of therapeutic benefit.
Researchers have performed studies of varying levels of evidence in efforts to demonstrate the safety and beneficial effects of PRP in a broad range of applications, but the lack of standardization of PRP preparation methodology and application procedures remains a substantial barrier. Collaborative development of standardized assays or criteria to substantiate PRP safety and efficacy may accelerate regulatory pathways while minimizing risk to patients.
Nomenclature and Classification
The nomenclature of PRP products is notoriously confusing and may hinder research efforts.29 Broadly speaking, the term PRP is used to describe autologous plasma formulations containing a platelet concentration in excess of that found in peripheral blood. Other terms that have been used include platelet concentrates, autologous growth factors, plasma rich in growth factors, platelet gel, and platelet-rich fibrin matrix.
Although several authors have attempted to formally categorize PRP,30–32 no classification has achieved widespread adoption, and none describes PRP preparations in sufficient detail to enable comparison between studies. One classification system categorizes PRP formulations on the basis of the presence of leukocytes and the fibrin architecture of the formulation: pure platelet-rich plasma (P-PRP) refers to leukocyte-poor PRP; leukocyte- and platelet-rich plasma (L-PRP) refers to PRP that contains leukocytes and has a low-density fibrin network after activation; pure platelet-rich fibrin (P-PRF) designates preparations without leukocytes and with a high-density fibrin network, which are also called leukocyte-poor platelet-rich fibrin; and leukocyte-and platelet-rich fibrin (L-PRF) designates preparations that contain leukocytes in a high-density fibrin network.32 Mishra et al31 proposed a classification primarily targeting sports medicine applications. Their system also describes four types of PRP but is based on the presence of leukocytes and activation or non-activation of the PRP, with additional subgroups based on the level of platelet enrichment. The PAW classification system of PRP is based on the absolute number of platelets (P), the manner in which platelet activation (A) occurs, and the presence or absence of leukocytes (W).30
Current imprecision in terminology is confounded by emerging autologous plasma products with low numbers of platelets, termed platelet-poor plasma.33 A widely accepted, more detailed classification system should speed the process of identifying the optimal PRP preparation for each indication and allow other investigators to replicate published data or perform meta-analyses. The AAOS symposium participants stated that any new classification should incorporate all autologous blood/ plasma products.
Standardization of Preparation
PRP preparations and their growth factor profiles are highly variable. Growth factor concentrations within preparations may be influenced by donor-specific factors and by processing and delivery factors (Table 2). The blood concentrations of platelets and their contained growth factors, leukocytes, and erythrocytes have large intersubject variation,34 and patient factors that contribute to this variability have not yet been well established. Numerous PRP formulations are used in experimental or clinical research, with multiple possible combinations of blood storage, centrifuge spin protocols, and methods of activation. Currently, >17 different commercial protocols are in use,35 each yielding products with different compositions and characteristics. The effects of PRP formulation on clinical efficacy are not well understood, and protocols of application, such as the volume of PRP delivered and the timing of the injections after injury or the commencement of symptoms, have yet to be determined. These factors are not routinely included in the methods sections of research reports.36,37 The heterogeneity of PRP preparation and application precludes the establishment of consistent and successful protocols for therapeutic treatment. Future research must establish patient factors influencing PRP composition and identify the optimal delivery system, delivery timing, concentration, and proteolytic condition (which limits the half-life of many therapeutic molecules) of the PRP. The establishment of a standard reporting system for PRP is necessary to facilitate communication, interpretation, and synthesis of scientific investigations.38
Table 2.
Variables That May Influence the Growth Factor Profile of Platelet-rich Plasma
| Variable | Description |
|---|---|
| Donor | Age |
| Gender | |
| Comorbidities | |
| Concurrent medications (including anti-inflammatories) | |
| Nutritional status | |
| Processing | Blood collection and storage conditions |
| Spin protocol (speed, time) | |
| Activation protocol (agent, concentration, timing) | |
| Storage | |
| Delivery | Form of delivery (gel, solution) |
| Timing of delivery in relation to isolation | |
| Timing of delivery in relation to activation | |
| Host factors (similar to donor factors) | |
| Injury chronicity |
Indication-specific Formulations
Many of the growth factors and cytokines present in PRP act within opposing biologic pathways, with some factors having beneficial effects in certain applications and deleterious effects in others. Whereas transforming growth factor β1 (TGF-β1) may have beneficial profibrotic effects in tendon and ligament healing,39 it has been shown to be detrimental in the healing of muscle injury.40 Similarly, the pro-angiogenic effects of vascular endothelial growth factor critical to muscle regeneration negatively affect articular cartilage healing.41 Understanding the role of each growth factor in the development of specific disease processes will facilitate the identification of therapeutically relevant components of PRP for each indication and the development of customized PRP preparations most suited to the specific indication. This customization could potentially be achieved by means of manipulation of processing variables or by the removal or enrichment of certain growth factors, although the regulatory implications of this approach are unknown. Any customization strategy requires a full understanding of PRP composition. A validated assay of PRP efficacy should be established for each clinical application.
The growth factor profile of PRP is influenced by its cellular composition.42 An optimal concentration of platelets has been proposed for bone regeneration (1,000,000 platelets/ mm3),43 although no consensus has been reached for other indications. The role of leukocytes, particularly neutrophils, in PRP is controversial. Although some studies have demonstrated a positive role of leukocytes in PRP as anti-infectious and immune regulatory agents,44 other researchers have reported detrimental results of leukocyte-rich preparations in a wide range of applications.45 The optimal concentrations of leukocytes, platelets, and erythrocytes for most applications remain unclear.
Timing of Delivery
The optimal timing of PRP delivery remains largely unexplored. PRP can be delivered preoperatively, intra-operatively, and/or postoperatively and with repeated dosing. Limited studies to date suggest that the expression of local factors is unique to each tissue and pathology and depends on the chronicity of the injury.46,47 Each condition may have a therapeutic window in which PRP would be of most benefit. The bio-molecular environment at the site of pathology should determine the timing of PRP delivery. In the same way that growth factor profiles at a site of injury evolve over time,46 the growth factors released from platelets vary after activation.48 Growth factors are released almost immediately after activation, and most have a short life span.49 For example, the biologic half-life of vascular endothelial growth factor and platelet-derived growth factor in circulating blood is approximately 30 minutes, whereas that of fibroblast growth factor is 7.6 hours.50,51 The half-life of growth factors may be extended when heparin is administered in conjunction with the therapy.51 Future studies evaluating cytokine-release kinetics according to activation processes or methods of localization within a tissue (such as a collagen scaffold) are required for the proper application of PRP to address specific clinical indications.
Stem Cell Therapy
Stem cells are unspecialized cells that have the ability to differentiate into multiple different cell types or to replicate themselves. The ability of MSCs to differentiate into multiple musculoskeletal cell types, release regenerative growth factors, and dampen immune responses holds great promise for orthopaedic tissue engineering. Despite their widespread application in clinical trials, MSCs remain poorly understood, and their use remains controversial.52 Greater understanding of how MSCs behave in their native environment is necessary so that these characteristics can be harnessed for the benefit of orthopaedic patients.53 Key challenges to the progress of MSC-based therapies identified at the AAOS symposium include abstruse nomenclature and definitions; regulatory ambiguity; lack of understanding of the native roles and mechanisms of MSCs; the paucity of assays to define efficacy of MSCs; indefinite association between phenotypic characteristics and biologic functions; lack of consensus on the most effective source of MSCs; lack of establishment of standard preparations for specific indications; lack of data on the long-term safety of MSCs; ambiguity regarding the most effective methods of MSC delivery; and lack of consensus on appropriate clinical study design.
Stem cell therapies are the subject of regulatory ambiguity. As noted, minimal manipulation is one of the criteria used by the FDA to define the regulatory pathway that a biologic therapy must undergo. Cell preparations that are minimally manipulated, are for homologous use only, are not combined with a drug or device, and are autologous or used in a first-degree or second-degree blood relative are regulated under section 361. In contrast, products regulated under section 351 include cell and tissue products that are cultured or more than minimally manipulated, are not intended for homologous use, are combined with a drug or device, or are allogeneic. These products require an investigational new drug exemption by the FDA and a biologics license application on file with the FDA before clinical trials are initiated, whereas products regulated under section 361 bypass much of this regulation.54
A major deficiency highlighted at the AAOS symposium was the lack of strict definitions of minimal manipulation and of homologous use, although examples of preparations not meeting such criteria are available.55,56 For example, isolation of progenitor cells from adipose tissue by ex vivo enzymatic digestion has been deemed not to meet the requirement of minimal manipulation.55,56 However, some companies have been allowed to use enzymatic digestion under section 351 regulation. The response from FDA representatives taking part in the symposium was that researchers should engage with FDA officials early in the process because each case is evaluated on an individual basis. Further guidance by the FDA is required to clarify perceived ambiguity surrounding point-of-care cell therapies. Designating the use of autologous, minimally manipulated cells that are processed at the point of care, the use of which is generally accepted to be safe, as non-homologous would notably delay clinical translation while substantially increasing costs.
Nomenclature and Definition
The principal types of stem cells are embryonic stem cells (ESCs), adult stem cells, and so-called induced pluripotent stem cells (iPSCs). ESCs are pluripotent and are able to differentiate into any adult cell type (ie, all three germ layers). Their use is ethically controversial and may be associated with increased tumorogenicity because of their broad differentiation potential.57 Adult stem cells have more limited differentiation potential, usually restricted to cells of a single germ layer. They reside within each organ system and are responsible for maintaining tissue turnover by replacing cells lost through injury or aging.52 In 2006, Takahashi and Yamanaka58 described the successful dedifferentiation of mature skin fibroblasts back to cells with pluripotent potential. These iPSCs can theoretically differentiate into any cell type. The use of iPSC technology overcomes the ethical challenges associated with the use of ESCs, although concerns regarding tumorogenicity remain.59
MSCs are adult stem cells that become the cells of the musculoskeletal system, including bone, cartilage, muscle, and ligament.53 Since the publication of early descriptions of MSC-like cells obtained from bone marrow,60 cells with similar characteristics have been identified in adipose tissue,61 muscle,61 skin,62 periosteum, blood,63 and other sources. These cells have been assigned multiple names, such as multipotent adult progenitor cells,64 marrow isolated multilineage inducible cells,65 and multipotent adult stem cells.66 The relationship of these cells to MSCs is currently unclear.
Attempts have been made to standardize the nomenclature used in MSC research. However, variations in methods of isolation, culture, and assay have resulted in complicated and at times misleading nomenclature. In 2006, the International Society for Cellular Therapy produced a position statement suggesting the minimum criteria required to define MSCs.67 According to the statement, MSCs must be plastic adherent and must express the cell surface antigens CD105, CD73, and CD90. Additionally, they must not express the cell surface antigens CD45, CD34, CD14, CD11b, CD79α, CD19, or HLA-DR, and they must differentiate into osteoblasts, adipocytes, and chondroblasts in vitro. However, these criteria have several limitations. First, they are based on attributes observed in the laboratory environment and may not reflect characteristics of MSCs in their native environment.52 Furthermore, multiple distinct populations of cells have been found to fulfill these criteria.64–66 Future definitions of MSCs must reflect their identity in vivo and account for the heterogeneity of populations identified to date.
Native Roles of Stem Cells
Despite the extensive use of MSCs in clinical trials to date, the contribution of MSCs to regeneration in native tissues is not well understood. Although studies clearly demonstrate that MSCs have the capacity to perform multiple functions (differentiation, immune modulation, and release of growth factors), the timing of and reasons for the adoption of each phenotype are not clear. For MSCs to be fully exploited in therapeutic use, the mechanisms that regulate their native behavior must be understood.
A key first step is to establish the native identity of the stem cells that contribute to tissue development and healing in musculoskeletal tissues. Researchers have recently established that perivascular MSCs contribute to the regeneration of injured muscle68 and bone.69 These findings required the use of sophisticated transgenic mouse technology that labels cells with fluorescent markers and allows them to be traced as they differentiate into mature tissues. This technology should be used to identify populations of cells contributing to the regeneration of cartilage, meniscal tissue, tendon, and ligament.
Identification of progenitors contributing to tissue regeneration (and also contributing pathologically to the development of fibrotic tissue) will facilitate research into the mechanisms governing the recruitment and activation of MSCs. Key pathways that have been identified include Wnt (osteogenesis, adipogenesis, chondrogenesis),70,71 TGF-β (chondrogenesis, fibrogenesis),72,73 and Sonic Hedgehog (osteogenesis).74
Sources of Mesenchymal Stem Cells
MSCs were first isolated from bone marrow, and most studies to date have evaluated cells from this source. However, MSCs have been isolated from multiple organs and tissues, and the advantages and drawbacks of each source have not been fully evaluated. In addition to intersource variation, MSCs may differ between anatomic regions of the same tissue type (eg, MSCs isolated from iliac crest or calcaneal bone marrow, or MSCs isolated from adipose tissue in the abdomen, buttock, or infrapatellar fat pad) in terms of yield and characteristics. Understanding the specific attributes of cells from each source might reveal populations of cells most suited to a given indication.
The equivalency of MSC populations derived from distinct anatomic origins has not been robustly demonstrated. Despite fulfilling the International Society for Cellular Therapy criteria, MSC populations from different sources or states (native or cultivated) have shown differences with respect to the immunophenotype, secreted cytokine profile, and results of proteome analysis.75–77 Cloned human MSCs isolated from fat default to adipogenic potential, whereas those isolated from bone marrow default to osteogenic potential, suggesting that the tissue environment of origin influences the characteristics of MSCs.75
Studies comparing sources of MSCs have been limited by a lack of standardization in the methods of preparation of MSCs and in the outcome measures used.78 The behavior of MSCs is known to be influenced by individual differences in donors, method of isolation, duration in culture, and culture conditions; therefore, appropriate comparisons can be made only if these factors are standardized.79,80 The development of robust and reproducible assays of the efficacy of MSCs is urgently needed to allow comparison of MSC populations in these settings.
Cellular and genetic comparison may provide considerable comparative insight and guide functional assessments. Next-generation sequencing allows the entire transcriptome of populations (and even that of single cells)tobeanalyzedandcompared,and recent developments in single-cell array platforms mean that heterogeneity within populations can now be analyzed.81,82
Preparation of Mesenchymal Stem Cells
The most effective methods of preparation of MSCs in terms of purity and modification in laboratory culture have not yet been determined. MSCs represent only a small proportion of cells present within any given organ. Available MSC preparations vary in the degree to which MSCs are separated from other types of cells and in the method by which this task is accomplished. Whole bone marrow cell suspensions and the stromal vascular fraction of adipose tissue (the cellular component of lipoaspirate after removal of lipids) have been used directly with the aim of harnessing the potential of the contained stem cells. However, both represent highly heterogeneous cell populations that include inflammatory cells, hematopoietic cells, endothelial cells, nonviable cells, and other cell types.83 Studies using these heterogeneous populations have demonstrated poor and unreliable tissue formation84 or lower regenerative efficacy compared with cultured MSCs.85 Recent studies have also suggested that the presence of endothelial cells may inhibit bone differentiation and other lineages.86,87
Laboratory culture has historically been used to enrich and expand MSC populations.88 MSCs rapidly expand in laboratory conditions, overgrowing other cell types (eg, hematopoietic cells, endothelial cells) that are less suited to the conditions. A detail that is often overlooked in this process is the temporal change and convergent cell phenotype occurring through the culture process that may affect cell behavior and the therapeutic efficacy of the preparation in proportion to the number of cells it contains.76
More recently, advances in flow cytometry have enabled MSCs to be purified to homogeneity in hours without the requirement of cell culture.89 Compared with the lower yield, limited donor sites, and morbidity associated with bone marrow or periosteal harvest, adipose tissue offers a well-documented, easily accessible, abundant, and dispensable source of MSCs that can be isolated using fluorescence-activated cell sorting.90 Approximately 15 million perivascular MSCs can be purified per 100 mL of lipoaspirate, providing a sufficient number of cells to treat a broad range of musculoskeletal disorders without culture expansion.90 The high purity and potency of MSCs achieved in this process will facilitate the demonstration of product safety and efficacy required for regulatory approval.6 The trade-offs of greater MSC numbers and unaltered and heterogeneous cell preparations have not been sufficiently explored in the literature.
Further Research
Because of the documented capacity of MSCs to differentiate into musculoskeletal tissues, the authors of initial studies hypothesized that MSCs would achieve a therapeutic effect by means of tissue engraftment. However, few studies have documented substantial beneficial effects of engraftment, although some benefit is likely to be achieved through the trophic and immunomodulatory properties of MSCs. Further research is required to fully characterize the mechanism by which MSCs exert beneficial effects. If engraftment is not central to the mechanism of action, the need for actual delivery of cells could be questioned. Considerable clinical hurdles (such as rejection and tumorogenicity) and regulatory challenges could be bypassed if the responsible therapeutic cytokine released by cells were identified.
Whereas multiplerigorous assays are available to establish the function and maturation stage of distinct progenitor hematopoietic stem cells, stringent assays to demonstrate the efficacy of MSCs are lacking. Most researchers have examined potentially heterogeneous MSC populations derived from cultures with high plating density that do not accurately reflect cell behavior at the clonal level. More stringent studies using clonally derived MSC populations may enable the identification of subpopulations of cells better able to maintain their stem cell–like properties. These potency assays could help increase the efficacy and safety of cell therapies using large numbers of culture-expanded cells. Similarly, the identification of subsets of MSCs isolated through fluorescence-activated cell sorting may facilitate purification of populations most suited to specific indications. Ultimately, assays of efficacy or potency of MSCs must be indication specific.
Methods of establishing proof of the safety of stem cell therapy are needed. These methods must include identification of off-target and systemic effects of stem cells delivered locally and the ability to definitively establish the risk (currently considered minimal) of malignant transformation.
Future studies must establish the most appropriate method of delivery of MSCs. The role of concomitant delivery of growth factors and the response of MSCs to local signals should be further explored. Similarly, interactions between cells and carriers such as patches, scaffolds, and hydrogels must be established. Finally, appropriate controls for clinical studies evaluating MSC-based therapies must be clarified. Most clinical studies of articular cartilage repair have compared MSCs with microfracture. The AAOS symposium highlighted the need for consensus on appropriate control therapies for each indication to facilitate comparison between studies. The consensus statements on future stem cell therapy research are summarized in Table 3.
Table 3.
Consensus Statements on Stem Cell Therapy
| No. | Statement |
|---|---|
| 1 | The progenitors contributing to tissue development, regeneration, and healing in each specific tissue must be identified. The mechanisms regulating this contribution must be characterized. |
| 2 | The optimum preparation of stem cells for each indication must be established in a systematic fashion. Considerations should include cell number, concomitant use of growth factors, predifferentization, and vehicle. |
| 3 | Mesenchymal stem cells isolated from different tissues must be compared to identify the most appropriate cell source for each specific indication. |
| 4 | The mechanism responsible for therapeutic effects observed in applications to date must be comprehensively characterized. |
| 5 | A standardized assay of stem cell efficacy is needed. |
| 6 | Methods for establishing proof of safety of stem cell therapy should be determined in collaboration with industry and regulatory agencies. This process may require evidence of phenotype stability or viability. |
| 7 | The most appropriate control for clinical studies evaluating stem cell therapy in each indication must be identified. |
Augmentation of Soft-tissue Healing
Several strategies to augment soft-tissue healing have been attempted with mixed results. Tendon-to-bone healing was a focus of the BTOI symposium because of the known biologic limitations of rotator cuff repair. Another area of focus was the role of biologic augmentation in the treatment of tendinopathy.
Augmentation of Tendon-to-Bone Healing
Tendon-to-bone healing is critical for successful surgical treatment of soft-tissue injuries. A clinically relevant model of tendon-to-bone healing is surgical reattachment of the rotator cuff to the humerus. The rotator cuff enthesis consists of distinct zones: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and lamellar bone.91 Because surgical repair does not generate a normal enthesis, the risk of re-tear is high, especially in patients with large rotator cuff tears.92 Some studies have demonstrated that improved radiographic appearance does not necessarily result in a better subjective outcome.93 Subjective outcome measures with better sensitivity may improve correlation with imaging.
Biologic options for augmentation of healing include growth factors and cell-based therapies. The use of growth factors to augment tendon healing has shown potential in animal studies; however, growth factors are not available for clinical use in isolated form.93,94 PRP serves as a vehicle for application of a physiologic mixture of growth factors to a site of injury or surgical treatment, although the clinical efficacy is the subject of substantial debate. A recent meta-analysis of randomized controlled trials by Zhao et al95 did not support the use of PRP in arthroscopic rotator cuff repairs. In contrast, Warth et al96 reported a decreased re-tear rate in patients with large tears treated with double-row repair and PRP. As noted, variability in the formulation of PRP and between patients may contribute to the discrepancies observed among in vitro studies, preclinical studies, and human randomized controlled trials.
As noted, manipulation of HCT/Ps results in FDA regulation under section 351. However, clinicians have shown improved results without culture expansion of stem cells. A landmark case-control clinical study with 10-year follow-up by Hernigou et al97 demonstrated substantial improvement in healing outcomes in patients treated with bone marrow aspirate concentrate containing MSCs compared with the control group (87% versus 44% intact). Other strategies to augment tendon-to-bone healing, including the use of recombinant human parathyroid hormone,98 doxycycline,99 gene therapy, periosteal flaps, growth factor–coated sutures and interference screws, and osteoconductive materials,100 have been evaluated in animal studies. The BTOI consensus statements on augmentation of tendon-to-bone healing are summarized in Table 4.
Table 4.
Consensus Statements on Augmentation of Tendon-to-Bone Healing
| No. | Statement |
|---|---|
| 1 | The stem cells and growth factors involved in tissue healing after injury and repair of tendon to bone should be identified. |
| 2 | Animal models of tendon-bone injury that attempt to mimic the biology of chronic tendon pathology in humans should be developed. |
| 3 | The optimal rehabilitation protocol for patients who have undergone repair of tendon-bone injury should be established. |
| 4 | A combination of stem cells and growth factors is likely necessary to improve healing of rotator cuff tears. |
| 5 | Improved imaging methods are needed to support correlation with subjective and functional outcome measures. |
Treatment of Tendinopathy
Clinically, tendinopathy is defined as the presence of pain and dysfunction together with histologic tendon pathology. The terminology is often confusing and inconsistent, and the term tendinopathy has been recommended as an encompassing descriptor for overuse-related clinical conditions.101 A continuum of tendon pathology has been proposed, although correlation of clinical and histologic/imaging findings to facilitate treatment selection is difficult.102,103 In a study of patients with acute tendon rupture, tendinopathy was present in 97% of patients; however, it also was silently present in 34% of control subjects, suggesting that histologic findings may not correlate with clinical symptoms.104
Physical therapy is often the first-line treatment of tendinopathy, and its use has been supported in controlled studies.105–108 The authors of pre-clinical studies have evaluated the potential role of PRP in the treatment of tendinopathy by examining the effect of PRP on tendon stem cell collagen production109 and inflammation.110 As noted, different formulations of PRP are likely to have different indications and treatment effects. Clinical results of the treatment of tendinopathy are mixed. Mishra et al111 published a randomized controlled trial evaluating the efficacy of leukocyte-enriched PRP in patients with chronic tennis elbow. The researchers found no differences between the patients treated with PRP and a control group treated with saline at 12 weeks, although at 24 weeks the success rates for the PRP and saline groups were 83.9% and 68.3% (P = 0.037), respectively. Dragoo et al112 evaluated injection of leukocyte-rich PRP compared with dry needling in the treatment of refractory patellar tendinopathy; both groups were also treated with standardized eccentric exercises. The PRP group experienced significantly more clinical improvement compared with the dry needling group at 12 weeks but not at 26 weeks. In a randomized controlled trial, de Vos et al113 evaluated the treatment of chronic midportion Achilles tendinopathy with eccentric exercises and PRP or saline injection and found no difference in pain, function, satisfaction, or return to sports.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases and include the collagenases found in the extracellular matrix. MMPs may play a role in tissue remodeling through the degradation of released extracellular matrix components. Tissue inhibitors of metalloproteinases are native inhibitors and bind to MMPs in the extracellular space. An imbalance between MMPs and tissue inhibitors of metalloproteinases has been reported to lead to excessive collagen breakdown.114 Inhibition of the inflammatory phase by blockage of MMPs has been proposed.115 Orchard et al116 reported successful treatment of patellar tendinopathy with injections of the MMP inhibitor aprotinin.
The BTOI consensus statements on tendinopathy research are summarized in Table 5.
Table 5.
Consensus Statements on the Treatment of Tendinopathy
| No. | Statement |
|---|---|
| 1 | A uniform terminology for tendinopathy is required. Correlation of clinical and objective findings (histology and imaging) should be established to facilitate staging of tendinopathy. |
| 2 | The pathophysiology of midsubstance versus insertional tendinopathy must be further established. The timing and role of inflammation and inflammatory mediators in initiation and progression of tendinopathy should be established. |
| 3 | The role of tenocyte and tendon stem cells in tendinopathy should be considered. |
| 4 | The optimal animal model of tendinopathy should be established. This model should be supported by imaging that is sufficiently sensitive to detect differences in clinical outcome. |
| 5 | The role of mechanical stimulus in the initiation and healing of tendinopathy should be investigated. |
| 6 | Well-designed randomized controlled trials are required to evaluate treatment methods. |
Scaffold Strategies for Repair
Scaffolds can be categorized on the basis of the presence or absence of cells,117 the synthetic or biologic origin of the scaffold, mechanical properties of the scaffold, and the target location and tissue type replicated. The chemical composition and microstructure of the scaffold influence the interaction of cells with the scaffold. The use of scaffolds in the treatment of articular cartilage and osteochondral defects, meniscal pathology, and rotator cuff tears has been evaluated in preclinical studies, in vitro studies, and some early clinical studies.
Several institutions prospectively study patients undergoing novel surgical procedures such as cartilage repair.118 With industry financial support, development of a multi-center registry will allow prospective evaluation of scaffolds and other similar implants, with a goal of improving patient safety. The registry could be similar to the American Joint Arthroplasty Registry,119 a multidisciplinary effort that involves surgeons, medical device industry representatives, patient advocates, payers, and hospital administrators and has a primary mission of improving the care of patients who undergo hip and knee arthroplasty.
To advance the use of scaffolds in the treatment of orthopaedic injuries, high-quality evidence obtained through randomized controlled trials will be necessary. Microfracture has been used as a control treatment in studies of autologous chondrocyte transplantation.120 Determination of the optimal control treatment to be used with each indication in studies of scaffold technology (ie, osteochondral, meniscal, and rotator cuff defects) is necessary and is important from both scientific and regulatory standpoints. The BTOI symposium consensus statements on the use of scaffolds are summarized in Table 6.
Table 6.
Consensus Statements on the Use of Scaffolds
| No. | Statement |
|---|---|
| 1 | Because articular cartilage is a complex three-dimensional structure, the use of spatially oriented scaffolds may improve biomechanical properties and cellular organization during incorporation. |
| 2 | The ability to recruit cells into acellular scaffolds warrants further investigation. |
| 3 | The immune response to scaffolds and their breakdown products must be clarified in animal and human models. |
| 4 | Tissue-specific scaffolds should be designed to address the biochemical and biomechanical roles of native tissue. |
| 5 | Long-term in vivo behavior of polymers must be characterized in a relevant model. |
| 6 | Improvement in the ability to quantitatively evaluate scaffolds after implantation through noninvasive means (imaging) is necessary. |
| 7 | Collaboration with researchers in other disciplines (eg, dentistry, biomaterials) may be helpful in the selection of appropriate materials for scaffolds. |
| 8 | The development of sufficiently sensitive clinical outcome measures will be essential for the evaluation of advanced treatment strategies. |
| 9 | For scientific and regulatory purposes, the appropriate control group for comparison with scaffold treatment (eg, microfracture, sham surgery, hyaluronic acid injection, or rehabilitation) should be clarified. |
| 10 | Development of a registry, with industry financial support, to appropriately track scaffolds (and other bioactive implants) will be important both to ensure patient safety and to achieve regulatory approval. |
Cartilage/Osteochondral Defects
Safran et al121 outlined four primary materials used in cartilage scaffolds: protein polymers (eg, collagen, fibrin), carbohydrate polymers (eg, hyaluronic acid, polylactic acid), synthetic/artificial polymers, and composites of polymers. Safran et al121 identified the optimal tissue-engineering characteristics of cartilage scaffolds (many of which may be also applied to other target tissue types) as including biocompatibility, noncytotoxicity, biodegradability, cellular support, permeability, mechanical and chemical stability, reproducibility, availability, and versatility for use in the treatment of full-thickness or partial-thickness lesions.
In a recent systematic review of the use of scaffolds in the repair of articular cartilage lesions, Filardo et al122 compared single-stage and two-stage procedures. Limited clinical data are available for the newer single-stage procedures. More recently, Kon et al117 evaluated the use of cells in scaffold-based cartilage treatments. In situ tissue regeneration in a single-stage procedure that does not require ex vivo cell expansion is an attractive treatment option.123 Lee et al124 tested this process using a three-dimensionally printed proximal humerus articular surface in rabbits with or without TGF-β3 and found improved functional (weight-bearing) and histologic (hyaline cartilage regeneration) outcomes in the TGF-β3 group. The ability to recruit cells into acellular scaffolds warrants further investigation, including determination of the optimal chemoattractant or chemoattractants and scaffold microstructure.
Meniscal Defects
Because of the morbidity associated with meniscal defects, investigators continue to search for the optimal treatment to restore meniscal function. Methods that have been studied include allograft transplantation, cell-free scaffolds, and xenografts. Several authors have reviewed meniscal allograft transplantation and found that it provides a good solution in a difficult patient group. Noyes and Barber-Westin125 recently reviewed long-term results in patients treated with meniscal allograft transplantation and reported 88% survival at 5 years, decreasing to 63% survival at 10 years and 40% survival at 15 years.
Di Matteo et al126 recently reviewed preclinical animal studies of meniscal scaffolds, including collagen-based meniscal implants. In a recent nonrandomized level II study by Zaffagnini et al,127 the use of a xenograft collagen meniscal implant (bovine Achilles tendon) for medial meniscal defects was compared with partial medial meniscectomy. The implant demonstrated promising results in terms of clinical outcomes and imaging. A recent clinical study of 16 patients treated with a polyurethane meniscus scaffold for segmental medial meniscus defect demonstrated promising early clinical and functional improvements.128
Rotator Cuff Tears
A study by Kannus and Józsa104 revealed that most tendon ruptures are associated with preexisting degenerative lesions within the tendons. Furthermore, the number of MSCs in the greater tuberosity of patients with a rotator cuff tear decreases as a function of the time from onset to treatment of the tear, resulting in increased tear size, chronicity, and degree of fatty infiltration.129 These characteristics may be associated with difficult repairs, and the use of scaffolds may be helpful. Biologic scaffolds, typically animal or human connective tissue grafts, have a unique three-dimensional protein structure that may enhance interaction with host tissue. However, biologic scaffolds have lower biomechanical properties and less predictable degradation rates than do synthetic grafts. A recent systematic review did not demonstrate strong evidence either for or against the use of scaffolds.27
The inability of surgical repair of rotator cuff tears to produce a normal enthesis has been well documented and was highlighted by Thangarajah et al,91 who recommended further studies to optimize indications for surgical repair. Smith et al92 outlined attempts to use tissue engineering to restore the normal enthesis for tendon and ligament healing and emphasized the importance of a source of pluripotent cells and the use of a scaffold with appropriate biomechanical and biochemical properties. Derwin et al130 evaluated the biochemical and biomechanical properties of several commercially available scaffolds for rotator cuff repair. They reported similar biochemical properties of the scaffolds to tendon allograft; however, the mechanical properties of the scaffold, specifically elastic moduli, were an order of magnitude lower than those of tendon allograft.130 The ability to attract and maintain resident cells is an important characteristic of scaffolds. In an in vitro study, Beitzel et al131 found that porcine collagen scaffolds had optimal adhesion and proliferation of MSCs.
Advanced Imaging for Monitoring of Scaffolds
Previously, surgeons used second-look arthroscopy to assess intra-articular surgical procedures. However, the clinical trend toward the use of non-invasive advanced imaging affords investigators the ability to qualitatively evaluate the status of implants and surrounding tissue. This literature has largely focused on the integrity of cartilage repair, including microfracture and autologous chondrocyte implantation. More recently, advanced imaging has been used to evaluate bony incorporation with scaffolds.
In one study, CT was used to evaluate the use of a synthetic multiphase implant to fill donor sites in patients undergoing osteochondral autograft transplantation.132 The authors of the study reported incorporation of bone in the autograft transplants, whereas the donor sites with synthetic plugs had evidence of fibrous tissue alone. On the basis of these findings, the authors of the study recommended against the use of this implant to fill osteochondral defects. In a different study evaluating the characteristics of this synthetic implant, cartilage-sensitive MRI and quantitative T2-mapping MRI were used assess the morphologic characteristics and incorporation of the implant.133 The authors of the study reported that the appearance of the plug on imaging improved with increasing postoperative duration, and T2 relaxation times approached those of normal articular cartilage. To protect patient safety and evaluate efficacy of scaffolds, the continued use of noninvasive imaging to monitor scaffolds is recommended. Validation of imaging findings with appropriately sensitive outcome measures is necessary. As imaging technology improves and the use of scaffolds expands, the establishment of consistent imaging protocols will be necessary.
Biocompatibility
Some authors have raised concerns regarding the biocompatibility of scaffolds. Natural products such as collagen derivatives, hyaluronic acid, alginate, agarose, fibrin glue, and chitosan have been reported to have better biocompatibility.117 Algul et al134 evaluated a three-layered scaffold consisting predominantly of chitosan, alginate, and tricalcium phosphate for the treatment of osteochondral defects and reported noncytotoxicity according to the International Organization for Standardization standard.
Previous attempts to use synthetic materials for treatment of knee pathology, such as in anterior cruciate ligament reconstruction, have been plagued by long-term complications resulting from the breakdown of graft material.135 Therefore, characterization of the biomechanical properties and biocompatibility of materials at the time of implantation is not sufficient. The immune response to scaffolds and their breakdown products, as well as the long-term behavior of such polymers in vivo, must be clarified in appropriate animal models before these products are approved for human use.
Summary
The use of biologic treatments that contribute to a regenerative micro-environment has great potential to improve healing rates and function in patients with musculoskeletal injury. In efforts to speed clinical translation, investigators have moved forward with clinical trials evaluating biologic therapies despite limited understanding of the underlying pathologic basis of disease and without full characterization of the therapeutic agents. To reach the full potential of biologic therapies, future research must return to a pyramid approach whereby clinical trials are inspired by comprehensive scientific understanding and supported with appropriate animal studies. The common aim of academia, industry, and regulatory bodies is to translate scientific discoveries into safe and effective therapies for patients. Greater communication and collaboration between these parties will accelerate this translation.
Acknowledgments
Dr. LaPrade or an immediate family member has received royalties from Arthrex and Smith & Nephew; serves as a paid consultant to Arthrex, Össur, and Smith & Nephew; has received research or institutional support from Arthrex, ConMed Linvatec, Össur, and Smith & Nephew; and serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, the European Society for Sports Traumatology, Knee Surgery and Arthroscopy, and the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine. Dr. Dragoo or an immediate family member serves as a paid consultant to or is an employee of Becton Dickinson, ConMed Linvatec, DePuy Synthes, Genzyme, Moximed, Össur, and RNL Bio; has received research or institutional support from ConMed Linvatec and Össur; has received nonincome support (such as equipment or services), commercially derived honoraria, or other non-research–related funding (such as paid travel) from EmCyte; and serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine. Dr. Koh or an immediate family member serves as a paid consultant to or is an employee of Aesculap/B. Braun Medical, Aperion Biologics, and Arthrex; has stock or stock options held in Aperion Biologics; and serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine, the Arthroscopy Association of North America, the Illinois Association of Orthopaedic Surgeons, and the Patellofemoral Foundation. Dr. Chu or an immediate family member serves as a board member, owner, officer, or committee member of the American Orthopaedic Society for Sports Medicine. Neither of the following authors nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Murray and Dr. Geeslin.
References
Evidence-based Medicine: Levels of evidence are described in the table of contents. In this article, references 22, 36, 37, 105, 107, 108, and 111–113 are level I studies. References 23, 95, 96, 127, and 129 are level II studies. References 87, 97, 114, and 118 are level III studies. References 28, 91, 116, 117, 120, 122, 125, 128, 132, 133, and 135 are level IV studies.
References printed in bold type are those published within the past 5 years.
- 1.Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68–79. doi: 10.5435/JAAOS-22-02-68. [DOI] [PubMed] [Google Scholar]
- 2.Gobbi A, Bathan L. Biological approaches for cartilage repair. J Knee Surg. 2009;22(1):36–44. doi: 10.1055/s-0030-1247726. [DOI] [PubMed] [Google Scholar]
- 3.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: A translational success story. Gut. 2015;64(5):830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabel GJ. Designing tomorrow’s vaccines. N Engl J Med. 2013;368(6):551–560. doi: 10.1056/NEJMra1204186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keramaris NC, Kanakaris NK, Tzioupis C, Kontakis G, Giannoudis PV. Translational research: From benchside to bedside. Injury. 2008;39(6):643–650. doi: 10.1016/j.injury.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 6.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Department of Health and Human Services. [Accessed December 21, 2015];Human cells, tissues, and cellular and tissue-based products. 21 CFR §1271. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271&showFR=1.
- 8.US Department of Health and Human Services. [Accessed December 21, 2015];Medical device classification procedures. 21 CFR §860. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=860.
- 9.US Department of Health and Human Services. [Accessed December 21, 2015];Regulation of biological products. 42 USC §262. http://www.fda.gov/RegulatoryInformation/Legislation/ucm149278.htm.
- 10.Chirba MA, Sweetapple B, Hannon CP, Anderson JA. FDA regulation of adult stem cell therapies as used in sports medicine. J Knee Surg. 2015;28(1):55–62. doi: 10.1055/s-0034-1398470. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. [Accessed April 8, 2016];Guidance for industry: Current Good Tissue Practice (CGTP) and additional requirements for manufacturers of human cells, tissues, and cellular and tissue-based products (HCT/Ps) http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/tissue/ucm091408.pdf.
- 12.Jarow JP, Ahmed HU, Choyke PL, Taneja SS, Scardino PT. Partial gland ablation for prostate cancer: Report of a Food and Drug Administration, American Urological Association, and Society of Urologic Oncology public workshop. Urology. 2016;88:8–13. doi: 10.1016/j.urology.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services. [Accessed December 21, 2015];General controls for medical devices. http://www.fda.gov/medicaldevices/deviceregulationandguidance/overview/generalandspecialcontrols/ucm055910.htm.
- 14.US Department of Health and Human Services. [Accessed December 21, 2015];Premarket approval of medical devices. 21 CFR §814. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?cfrpart=814.
- 15.Sheth U, Nguyen NA, Gaines S, Bhandari M, Mehlman CT, Klein G. New orthopedic devices and the FDA. J Long Term Eff Med Implants. 2009;19(3):173–184. doi: 10.1615/jlongtermeffmedimplants.v19.i3.20. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services. [Accessed December 21, 2015];Establishment registration and device listing for manufacturers and initial importers of devices. 21 CFR §807. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=807.
- 17.US Department of Health and Human Services. [Accessed December 21, 2015];Premarket approval (PMA) http://www.fda.gov/Medicaldevices/Deviceregulationandguidance/Howtomarketyourdevice/Premarketsubmissions/Premarketapprovalpma/Default.Htm.
- 18.US Department of Health and Human Services. [Accessed December 21, 2015];Food and Drug Administration Safety and Innovation Act (FDASIA) http://www.fda.gov/regulatoryinformation/legislation/significantamendmentstothefdcact/fdasia/default.htm.
- 19.FDA Science Board. [Accessed April 4, 2016];FDA science and mission at risk: Report of the Subcommittee on Science and Technology. 2007 Nov; http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007-4329b_02_01_FDA%20Report%20on%20Science%20and%20Technology.pdf.
- 20.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: Current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602–608. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Andia I, Sanchez M, Maffulli N. Tendon healing and platelet-rich plasma therapies. Expert Opin Biol Ther. 2010;10(10):1415–1426. doi: 10.1517/14712598.2010.514603. [DOI] [PubMed] [Google Scholar]
- 22.A Hamid MS, Mohamed Ali MR, Yusof A, George J, Lee LP. Platelet-rich plasma injections for the treatment of hamstring injuries: A randomized controlled trial. Am J Sports Med. 2014;42(10):2410–2418. doi: 10.1177/0363546514541540. [DOI] [PubMed] [Google Scholar]
- 23.Kon E, Mandelbaum B, Buda R, et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: From early degeneration to osteoarthritis. Arthroscopy. 2011;27(11):1490–1501. doi: 10.1016/j.arthro.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742–1749. doi: 10.1177/0363546512452713. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaky SH, Ottonello A, Strada P, Cancedda R, Mastrogiacomo M. Platelet lysate favours in vitro expansion of human bone marrow stromal cells for bone and cartilage engineering. J Tissue Eng Regen Med. 2008;2(8):472–481. doi: 10.1002/term.119. [DOI] [PubMed] [Google Scholar]
- 27.Longo UG, Lamberti A, Maffulli N, Denaro V. Tissue engineered biological augmentation for tendon healing: A systematic review. Br Med Bull. 2011;98:31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 28.Andia I, Sánchez M, Maffulli N. Platelet rich plasma therapies for sports muscle injuries: Any evidence behind clinical practice? Expert Opin Biol Ther. 2011;11(4):509–518. doi: 10.1517/14712598.2011.554813. [DOI] [PubMed] [Google Scholar]
- 29.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, Bielecki T. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: Current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 30.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: The PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 31.Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. Curr Pharm Biotechnol. 2012;13(7):1185–1195. doi: 10.2174/138920112800624283. [DOI] [PubMed] [Google Scholar]
- 32.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Floryan KM, Berghoff WJ. Intraoperative use of autologous platelet-rich and platelet-poor plasma for orthopedic surgery patients. AORN J. 2004;80(4):668–674. doi: 10.1016/s0001-2092(06)61320-3. quiz 675–678. [DOI] [PubMed] [Google Scholar]
- 34.Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308–316. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 35.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 36.Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: A randomized, single-blind, parallel-group trial. Am J Sports Med. 2013;41(10):2240–2248. doi: 10.1177/0363546513497925. [DOI] [PubMed] [Google Scholar]
- 37.Wang A, McCann P, Colliver J, et al. Do postoperative platelet-rich plasma injections accelerate early tendon healing and functional recovery after arthroscopic supraspinatus repair? A randomized controlled trial. Am J Sports Med. 2015;43(6):1430–1437. doi: 10.1177/0363546515572602. [DOI] [PubMed] [Google Scholar]
- 38.Mautner K, Malanga GA, Smith J, et al. A call for a standard classification system for future biologic research: The rationale for new PRP nomenclature. PM R. 2015;7(4 suppl):S53–S59. doi: 10.1016/j.pmrj.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Beye JA, Hart DA, Bray RC, McDougall JJ, Salo PT. Injury-induced changes in mRNA levels differ widely between anterior cruciate ligament and medial collateral ligament. Am J Sports Med. 2008;36(7):1337–1346. doi: 10.1177/0363546508316283. [DOI] [PubMed] [Google Scholar]
- 40.Huard J, Li Y, Fu FH. Muscle injuries and repair: Current trends in research. J Bone Joint Surg Am. 2002;84(5):822–832. [PubMed] [Google Scholar]
- 41.Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: How the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthritis Cartilage. 2008;16(3):279–286. doi: 10.1016/j.joca.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 43.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 44.Moojen DJ, Everts PA, Schure RM, et al. Antimicrobial activity of platelet-leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26(3):404–410. doi: 10.1002/jor.20519. [DOI] [PubMed] [Google Scholar]
- 45.Dragoo JL, Braun HJ, Durham JL, et al. Comparison of the acute inflammatory response of two commercial platelet-rich plasma systems in healthy rabbit tendons. Am J Sports Med. 2012;40(6):1274–1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi M, Itoi E, Minagawa H, et al. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15(3):371–377. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Otis JS, Niccoli S, Hawdon N, et al. Pro-inflammatory mediation of myoblast proliferation. PLoS One. 2014;9(3):e92363. doi: 10.1371/journal.pone.0092363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh JH, Kim W, Park KU, Roh YH. Comparison of the cellular composition and cytokine-release kinetics of various platelet-rich plasma preparations. Am J Sports Med. 2015;43(12):3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- 49.Mooren RE, Hendriks EJ, van den Beucken JJ, et al. The effect of platelet-rich plasma in vitro on primary cells: Rat osteoblast-like cells and human endothelial cells. Tissue Eng Part A. 2010;16(10):3159–3172. doi: 10.1089/ten.tea.2009.0832. [DOI] [PubMed] [Google Scholar]
- 50.Tengood JE, Kovach KM, Vescovi PE, Russell AJ, Little SR. Sequential delivery of vascular endothelial growth factor and sphingosine 1-phosphate for angiogenesis. Biomaterials. 2010;31(30):7805–7812. doi: 10.1016/j.biomaterials.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beenken A, Mohammadi M. The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray IR, West CC, Hardy WR, et al. Natural history of mesenchymal stem cells, from vessel walls to culture vessels. Cell Mol Life Sci. 2014;71(8):1353–1374. doi: 10.1007/s00018-013-1462-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray IR, Péault B. Q&A: Mesenchymal stem cells. Where do they come from and is it important? BMC Biol. 2015;13(1):99. doi: 10.1186/s12915-015-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parson A. The long journey from stem cells to medical product. Cell. 2006;125(1):9–11. doi: 10.1016/j.cell.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Malarkey MA. [Accessed April 4, 2016];Letter to Regenerative Sciences, Inc. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/ComplianceActivities/Enforcement/UntitledLetters/ucm091991.htm.
- 56.Malarkey MA. [Accessed April 4, 2016];Letter to CellTex Therapeutics Corporation. http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2012/ucm323853.htm.
- 57.Blum B, Benvenisty N. The tumorogenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Knoepfler PS. Deconstructing stem cell tumorogenicity: A roadmap to safe regenerative medicine. Stem Cells. 2009;27(5):1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20(3):263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 61.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 62.Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 63.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36(5):642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 65.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 66.Beltrami AP, Cesselli D, Bergamin N, et al. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110(9):3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 67.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 68.Dellavalle A, Maroli G, Covarello D, et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 69.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci U S A. 2011;108(16):6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Cawthorn WP, Bree AJ, Yao Y, et al. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone. 2012;50(2):477–489. doi: 10.1016/j.bone.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am J Pathol. 2004;164(3):1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Serra R, Johnson M, Filvaroff EH, et al. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139(2):541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21(4):393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 75.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23(3):412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell JB, McIntosh K, Zvonic S, et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24(2):376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 77.Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212(3):702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 78.Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications: A systematic review of the literature. Open Orthop J. 2011;5(suppl 2):242–248. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adamzyk C, Emonds T, Falkenstein J, et al. Different culture media affect proliferation, surface epitope expression, and differentiation of ovine MSC. Stem Cells Int. 2013;2013:387324. doi: 10.1155/2013/387324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wegmeyer H, Bröske AM, Leddin M, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013;22(19):2606–2618. doi: 10.1089/scd.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freeman BT, Jung JP, Ogle BM. Single-cell RNA-Seq of bone marrow-derived mesenchymal stem cells reveals unique profiles of lineage priming. PLoS One. 2015;10(9):e0136199. doi: 10.1371/journal.pone.0136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wechsler ME, Hermann BP, Bizios R. Adult human mesenchymal stem cell differentiation at the cell population and single-cell levels under alternating electric current. Tissue Eng Part C Methods. 2016;22(2):155–164. doi: 10.1089/ten.tec.2015.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paredes B, Santana A, Arribas MI, et al. Phenotypic differences during the osteogenic differentiation of single cell-derived clones isolated from human lipoaspirates. J Tissue Eng Regen Med. 2011;5(8):589–599. doi: 10.1002/term.351. [DOI] [PubMed] [Google Scholar]
- 84.Müller AM, Mehrkens A, Schäfer DJ, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cell Mater. 2010;19:127–135. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 85.Cheung WK, Working DM, Galuppo LD, Leach JK. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12(4):554–562. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 86.Meury T, Verrier S, Alini M. Human endothelial cells inhibit BMSC differentiation into mature osteoblasts in vitro by interfering with osterix expression. J Cell Biochem. 2006;98(4):992–1006. doi: 10.1002/jcb.20818. [DOI] [PubMed] [Google Scholar]
- 87.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 88.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corselli M, Crisan M, Murray IR, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytometry A. 2013;83(8):714–720. doi: 10.1002/cyto.a.22313. [DOI] [PubMed] [Google Scholar]
- 90.James AW, Zara JN, Corselli M, et al. An abundant perivascular source of stem cells for bone tissue engineering. Stem Cells Transl Med. 2012;1(9):673–684. doi: 10.5966/sctm.2012-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thangarajah T, Pendegrass CJ, Shahbazi S, Lambert S, Alexander S, Blunn GW. Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med. 2015;3(6):2325967115587495. doi: 10.1177/2325967115587495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith L, Xia Y, Galatz LM, Genin GM, Thomopoulos S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect Tissue Res. 2012;53(2):95–105. doi: 10.3109/03008207.2011.650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodeo SA, Potter HG, Kawamura S, Turner AS, Kim HJ, Atkinson BL. Biologic augmentation of rotator cuff tendon-healing with use of a mixture of osteoinductive growth factors. J Bone Joint Surg Am. 2007;89(11):2485–2497. doi: 10.2106/JBJS.C.01627. [DOI] [PubMed] [Google Scholar]
- 94.Kovacevic D, Gulotta LV, Ying L, Ehteshami JR, Deng XH, Rodeo SA. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res. 2015;473(5):1644–1654. doi: 10.1007/s11999-014-4020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhao JG, Zhao L, Jiang YX, Wang ZL, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: A meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125–135. doi: 10.1016/j.arthro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: A meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 97.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int Orthop. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 98.Hettrich CM, Beamer BS, Bedi A, et al. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res. 2012;30(5):769–774. doi: 10.1002/jor.22006. [DOI] [PubMed] [Google Scholar]
- 99.Bedi A, Fox AJ, Kovacevic D, Deng XH, Warren RF, Rodeo SA. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38(2):308–317. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 100.Kovacevic D, Fox AJ, Bedi A, et al. Calcium-phosphate matrix with or without TGF-β3 improves tendon-bone healing after rotator cuff repair. Am J Sports Med. 2011;39(4):811–819. doi: 10.1177/0363546511399378. [DOI] [PubMed] [Google Scholar]
- 101.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: Time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 102.McCreesh K, Lewis J. Continuum model of tendon pathology: Where are we now? Int J Exp Pathol. 2013;94(4):242–247. doi: 10.1111/iep.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cook JL, Purdam CR. Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy. Br J Sports Med. 2009;43(6):409–416. doi: 10.1136/bjsm.2008.051193. [DOI] [PubMed] [Google Scholar]
- 104.Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon: A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 105.Kongsgaard M, Kovanen V, Aagaard P, et al. Corticosteroid injections, eccentric decline squat training and heavy slow resistance training in patellar tendinopathy. Scand J Med Sci Sports. 2009;19(6):790–802. doi: 10.1111/j.1600-0838.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 106.Scott A, Huisman E, Khan K. Conservative treatment of chronic Achilles tendinopathy. CMAJ. 2011;183(10):1159–1165. doi: 10.1503/cmaj.101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bisset L, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med. 2005;39(7):411–422. doi: 10.1136/bjsm.2004.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holmgren T, Oberg B, Sjöberg I, Johansson K. Supervised strengthening exercises versus home-based movement exercises after arthroscopic acromioplasty: A randomized clinical trial. J Rehabil Med. 2012;44(1):12–18. doi: 10.2340/16501977-0889. [DOI] [PubMed] [Google Scholar]
- 109.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 110.Zhang J, Middleton KK, Fu FH, Im HJ, Wang JH. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS One. 2013;8(6):e67303. doi: 10.1371/journal.pone.0067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: A double-blind, prospective, multicenter, randomized controlled trial of 230 patients. Am J Sports Med. 2014;42(2):463–471. doi: 10.1177/0363546513494359. [DOI] [PubMed] [Google Scholar]
- 112.Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: A double-blind, randomized controlled trial. Am J Sports Med. 2014;42(3):610–618. doi: 10.1177/0363546513518416. [DOI] [PubMed] [Google Scholar]
- 113.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: A randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 114.Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum. 2006;54(3):832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- 115.Garofalo R, Cesari E, Vinci E, Castagna A. Role of metalloproteinases in rotator cuff tear. Sports Med Arthrosc. 2011;19(3):207–212. doi: 10.1097/JSA.0b013e318227b07b. [DOI] [PubMed] [Google Scholar]
- 116.Orchard J, Massey A, Brown R, Cardon-Dunbar A, Hofmann J. Successful management of tendinopathy with injections of the MMP-inhibitor aprotinin. Clin Orthop Relat Res. 2008;466(7):1625–1632. doi: 10.1007/s11999-008-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kon E, Roffi A, Filardo G, Tesei G, Marcacci M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy. 2015;31(4):767–775. doi: 10.1016/j.arthro.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 118.Krych AJ, Nawabi DH, Farshad-Amacker NA, et al. Bone marrow concentrate improves early cartilage phase maturation of a scaffold plug in the knee: A comparative magnetic resonance imaging analysis to platelet-rich plasma and control. Am J Sports Med. 2016;44(1):91–98. doi: 10.1177/0363546515609597. [DOI] [PubMed] [Google Scholar]
- 119. [Accessed April 4, 2016];American Joint Replacement Registry (AJRR) http://www.ajrr.net/
- 120.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 121.Safran MR, Kim H, Zaffagnini S. The use of scaffolds in the management of articular cartilage injury. J Am Acad Orthop Surg. 2008;16(6):306–311. doi: 10.5435/00124635-200806000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: A systematic review and technical note. Arthroscopy. 2013;29(1):174–186. doi: 10.1016/j.arthro.2012.05.891. [DOI] [PubMed] [Google Scholar]
- 123.Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013;45:e57. doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet. 2010;376(9739):440–448. doi: 10.1016/S0140-6736(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Noyes FR, Barber-Westin SD. Meniscal transplantation in symptomatic patients under fifty years of age: Survivorship analysis. J Bone Joint Surg Am. 2015;97(15):1209–1219. doi: 10.2106/JBJS.N.01340. [DOI] [PubMed] [Google Scholar]
- 126.Di Matteo B, Perdisa F, Gostynska N, Kon E, Filardo G, Marcacci M. Meniscal scaffolds: Preclinical evidence to support their use. A systematic review. Open Orthop J. 2015;9:143–156. doi: 10.2174/1874325001509010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zaffagnini S, Marcheggiani Muccioli GM, Lopomo N, et al. Prospective long-term outcomes of the medial collagen meniscus implant versus partial medial meniscectomy: A minimum 10-year follow-up study. Am J Sports Med. 2011;39(5):977–985. doi: 10.1177/0363546510391179. [DOI] [PubMed] [Google Scholar]
- 128.Schüttler KF, Haberhauer F, Gesslein M, et al. Midterm follow-up after implantation of a polyurethane meniscal scaffold for segmental medial meniscus loss: Maintenance of good clinical and MRI outcome. Knee Surg Sports Traumatol Arthrosc. 2015 Aug 23; doi: 10.1007/s00167-015-3759-5. Epub ahead of print. [DOI] [PubMed]
- 129.Hernigou P, Merouse G, Duffiet P, Chevalier N, Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop. 2015;39(6):1219–1225. doi: 10.1007/s00264-015-2724-8. [DOI] [PubMed] [Google Scholar]
- 130.Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair: Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88(12):2665–2672. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 131.Beitzel K, McCarthy MB, Cote MP, et al. Properties of biologic scaffolds and their response to mesenchymal stem cells. Arthroscopy. 2014;30(3):289–298. doi: 10.1016/j.arthro.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 132.Barber FA, Dockery WD. A computed tomography scan assessment of synthetic multiphase polymer scaffolds used for osteochondral defect repair. Arthroscopy. 2011;27(1):60–64. doi: 10.1016/j.arthro.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 133.Bedi A, Foo LF, Williams RJ, III, Potter HG Cartilage Study Group. The maturation of synthetic scaffolds for osteochondral donor sites of the knee: An MRI and T2-mapping analysis. Cartilage. 2010;1(1):20–28. doi: 10.1177/1947603509355970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Algul D, Sipahi H, Aydin A, Kelleci F, Ozdatli S, Yener FG. Biocompatibility of biomimetic multilayered alginate-chitosan/β-TCP scaffold for osteochondral tissue. Int J Biol Macromol. 2015;79:363–369. doi: 10.1016/j.ijbiomac.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 135.Rushton N, Dandy DJ, Naylor CP. The clinical, arthroscopic and histological findings after replacement of the anterior cruciate ligament with carbon-fibre. J Bone Joint Surg Br. 1983;65(3):308–309. doi: 10.1302/0301-620X.65B3.6841400. [DOI] [PubMed] [Google Scholar]