Abstract
Articular cartilage is an avascular tissue with chondrocytes in the deeper zones existing under conditions of sustained hypoxia. Using a hypoxic chamber to provide controlled hypoxia, this study was performed to determine whether sustained hypoxia enhances the production of cartilage matrix proteins. Freshly isolated primary bovine articular chondrocytes were encapsulated in three-dimensional alginate beads and maintained at 2% oxygen with media changes using media pre-equilibrated to 2% oxygen. Immunolocalization of HIF-1α was performed to verify hypoxic conditions. Sustained hypoxia resulted in an increase in proteoglycan synthesis after only 1 day, as measured by 35S-sulfate incorporation. This increase was maintained for the duration of the 17 day study. After 17 days of hypoxic culture, increases in total type II collagen and COL2A1 gene expression were probed by indirect immunofluorescence, type II collagen ELISA, and real-time qPCR; in addition, increased glycosaminoglycan deposition was observed as determined by chemical analysis. These studies show that sustained hypoxia enhances articular chondrocyte matrix synthesis and viability in three-dimensional alginate culture.
Keywords: chondrocyte, hypoxia, collagen, proteoglycan, immunofluorescence
Articular cartilage is one of the few specialized adult tissues with no direct blood supply. Local oxygen tension in cartilage ranges from 10%, near the synovial fluid, to less than 1% in the deepest layers. This indicates that the normal physiologic condition for deep cartilage is one of sustained hypoxia.1,2 Degradation of articular cartilage through conditions such as osteoarthritis (OA) has been attributed to a breakdown in the architectural structure of the extracellular matrix (ECM).3 Research aimed at restoring this matrix structure has shifted toward the physiological environment of hypoxia.
The nuclear transcription factor hypoxia-inducible factor-1α (HIF-1α) is a major regulator of cellular adaptations to hypoxia.4,5 Degradation of HIF-α-type protein is significantly decreased under oxygen tensions of <2–3%, allowing for regulation of gene expression in the adaptation to the hypoxic environment.4–9 Pfander and coworkers10 have demonstrated the role of HIF-1α in the maintenance of cartilage homeostasis and in maintaining anaerobic glycolysis in epiphyseal chondrocytes.11,12 Our lab has previously shown that HIF-1α maintains activation in the deep layers of intact articular cartilage.13 Similar to other studies that have demonstrated HIF-1α translocation to the nuclei,14,15 our lab showed translocation to the nuclei of articular chondrocytes in explants and in monolayer.13 Although there has been no direct link established between HIF-1α nuclear translocation and increased collagen type II production, both components have been shown to be stimulated in chondrocytes cultured in hypoxia.10,11,13,16
Increased production of main ECM macromolecules such as collagen type II, proteoglycans, glycosaminoglycan, and glycoproteins provides indications of chondrocyte matrix synthesis in three-dimensional culture constructs.16–19 Methods used to maintain a hypoxic environment vary throughout the literature and may explain the inconsistent results on chondrocyte matrix synthesis. An increase in mRNA for TIMP-1 and aggrecan and a decrease in COL2A1 gene expression were found in one study.14 However, in a study of chondrocyte redifferentiation in alginate culture, hypoxia caused an increase in collagen type II immunostaining.15 More recently, Koay and Athanasiou20 have shown that hypoxia (2% O2)-treated human embryonic stem cells display enhanced potential to produce collagen type II, glycosaminoglycan, and collagen type I compared to those under normoxic conditions. This finding demonstrates the differentiation potential of cells with appropriate oxygen accessibility.
In most studies of long-term hypoxic cell treatment, cells are exposed to intermittent reoxygenation upon changes of media. Cycles of hypoxia and reoxygenation have been shown to induce strong HIF-1α binding to DNA in chondrocytes leading to altered expression of TIMP-2 and MMP-9 by IL-1β.21 To distinguish the effects of hypoxia from periodic reoxygenation, a sustained hypoxic environment was maintained using a gas controlled chamber. The alginate beads used in this study have been established as a reproducible three-dimensional tissue culture model for cartilage due to their maintenance of chondrocyte phenotype, ECM synthesis, and metabolic regulation.14,15,21 Using this model, chondrocytes can be isolated from the alginate matrix and undergo quantitative analysis.
In the current study, we have probed the effect of sustained hypoxia at a level that induces HIF-1α production and may aid in chondrocyte matrix protein formation. This study was designed to test the hypothesis that sustained hypoxia enhances chondrocyte matrix synthesis and viability in three-dimensional alginate culture.
METHODS
Alginate Bead Cultures
Full-thickness slices of cartilage were aseptically removed from 10 freshly slaughtered bovine knees and articular chondrocytes were isolated by enzymatic digestion with 0.2% pronase (EMD Chemicals, San Diego, CA) for 1.5 h and 0.25% collagenase P (Roche Applied Science, Indianapolis, IN) for 12–16 h. Cells were cultured in alginate beads (4 million per mL 1.2% alginate), as described by Masuda et al.22 The beads were stored in 10 cm tissue culture dishes in chondrocyte growth media (1:1 DMEM/Nutrient Mix F-12 [DMEM/F-12] supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin; Invitrogen, Grand Island, NY) at 37°C/5% CO2 for 3 days prior to treatment.
Experimental Groups
After the initial incubation period, 300 control beads containing chondrocytes were placed in a humidified tissue culture incubator at 37°C/5% CO2/21% O2 (normoxia) and 300 beads containing chondrocytes were placed in an incubator (for up to 17 days after adjustment) located in a hypoxic chamber after a 3 day adjustment period to the alginate (Coy Laboratory Products, Grass Lake, MI) at 37°C/5% CO2/93% N2/2% O2 (hypoxia). The hypoxic chamber is designed to rigorously maintain oxygen tension (2 ± 0.3%) during long-term culture, enabling reduction of variability and sample reoxygenation effects. Media was changed in all cultures (hypoxic and normoxic) every 2–3 days, the media level was approximately 1 mm greater than the upper bead surface, and hypoxic media was pre-equilibrated inside the chamber until O2 partial pressure was 2%. Experimental procedures were performed using a total of 10 animals with chondrocytes isolated from a minimum of three different bovine legs.
At each collection time point, chondrocytes were recovered from alginate with a 10 min incubation (at 4°C) in a solution of 50 mM sodium citrate, 150 mM NaCl, and 30 mM EDTA then centrifuged at 600 × g for 5 min to precipitate alginate and cellular debris, and washed thoroughly with HBSS (Invitrogen) to remove residual alginate. All experimental groups were handled similarly, whether cultured under hypoxic or normoxic conditions.
Histology and Immunofluorescence
Prior to analytical procedures, eight beads from each treatment group were placed in 3 mL of 10% buffered formalin acetate with 105 mM CaCl2 overnight at 4°C. The beads were then dehydrated in a histology tissue processor, embedded in paraffin, and sectioned for histological staining with Hematoxylin and Eosin (H&E), Toluidine Blue, and Safranin O (with Fast Green) using standard histology protocols. No additional counterstains were used beyond those in the standard staining protocols. Indirect immunofluorescent staining for collagen type II and type X was performed as previously described.23 We examined type II collagen and type X collagen24 as markers of proper chondrocyte phenotype for deep zone cartilage. Fluorescent images were acquired on a Nikon TE2000 microscope equipped with a Spot RT Slider digital camera. Fluorescence was quantified using Metamorph software. Total fluorescence in seven separate beads was measured per condition and the sum integrated intensity was averaged per bead.
Indirect immunofluorescent staining for HIF-1α was performed as previously described for sections of bovine cartilage13 and included appropriate controls. Nuclei were counterstained with DAPI (Molecular Probes Inc., Eugene, OR). Confocal fluorescent images were obtained at 600× magnification using an Olympus IX81 DSU. Three-dimensional reconstructions were made using ImagePro Plus software (Mediacybernetics, Silver Spring, MD). For each experimental condition, seven to nine alginate sections of three or more alginate beads were examined and 27 three-dimensional reconstructions were scored by three blinded observers to determine the percentage of nuclei containing HIF-1α versus the percentage with only extra-nuclear HIF-1α.
Proteoglycan Synthesis
Proteoglycan synthesis was measured by addition of 40 μCi/mL 35S-sulfate (Amersham) into the chondrocyte growth media for 6 hours at 37°C. Conditioned media was then removed and 35S-sulfate labeled macromolecules were extracted by placing the beads in 0.5 mL of 4 M guanidine HCl extraction buffer on a shaker at 4°C for 48 h.25 Cell extracts were centrifuged at 2,000 × g for 5 min to precipitate cellular debris. Aliquots of the cell extract supernatent and conditioned media were eluted on Sephadex G-25M in PD10 columns (Boeringer Ingleheim) with 4 M guanidine HCl extraction buffer to separate 35S-sulfate-incorporated macromolecules from unincorporated label.25 The proteoglycan fractions were counted in a scintillation counter (Packard Instruments).
Collagen Type II ELISA
After 17 days of hypoxic and normoxic culture, chondrocytes were recovered from 50 alginate beads per experimental group. Isolated cell pellets were resuspended in 0.05 M acetic acid (pH 2.8–3.0) and 1/10 starting volume of 10 mg/mL pepsin (dissolved in 0.05 M acetic acid) and incubated for 48 h at 4°C, followed by 48 h of digestion at 4°C in pancreatic elastase solution (1 mg/mL dissolved in 1X TBS, pH adjusted to 7.8–8.0). Following digestion, the pH was adjusted to 8.0 with 1 N NaOH, according to the manufacturer’s protocol for the collagen type II ELISA (M.D. Bioscience, St. Paul, MN), and a Centriplus Centrifugal Filter Device was used to concentrate the macromolecular solutions (Millipore, Billerica, MA). Concentrated samples were normalized to the lowest total protein concentration for each sample group and diluted 1:100 with assay buffer. The remainder of the protocol was completed per the manufacturer’s instructions.
Quantitative PCR
After 17 day culture in hypoxic and normoxic environments chondrocytes were recovered from alginate (25 beads per group). mRNA was isolated from the cell pellet using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and cDNA was constructed with the RT2 first strand kit (Supperarray, Frederick, MD). qPCR was performed with the RT2 SYBR Green qPCR Master Mix (Supperarray) to detect collagen type II a1 and β-actin gene expression between hypoxic and normoxic culture conditions. The following primer sequences were used: COL2A1, forward 5′-TGTCCCTCCAGAAATGTGGCTTCT-3′ and reverse 5′-TCCATGTTCAAGACAGGCTGTGGA-3′, and β-actin, forward 5′-TGGATCG-CAAGCAGGAGTACGAT-3′ and reverse 5′-AAGGGTGTAACGCAGCTAACAGTC-3′. Each cDNA sample was combined with 12.5 μL RT2 SYBR Green/ROX qPCR Master Mix, 10.5 μL ddH2O, 1.0 μL template cDNA, and 1.0 μL gene-specific 10 μM forward and reverse PCR primers for a final volume of 25 μL per well. The mixture was cycled and quantified with a real-time thermal cycler (Mx3000P; Stratagene, Cedar Creek, TX) as follows: one cycle of 95°C (10 min), followed by 40 cycles of 95°C (15 s) and 65°C (45 s).
Statistical Analysis
Statistical significance was evaluated by one-way analysis of variance (ANOVA) and Bonferroni t post-test or t-test. A level of p < 0.05 was considered significant. Values are reported as mean ± SEM unless otherwise noted.
RESULTS
Translocation of HIF-1α
Indirect immunofluorescence of HIF-1α in thin sections of alginate beads revealed that HIF-1α was either excluded from the nuclei with normoxia (perinuclear location; Fig. 1A) or found within the nucleus of chondrocytes with hypoxia (Fig. 1B). Scoring of three-dimensional confocal reconstructions of HIF-1α stained alginate cultures showed that in normoxic culture, intranuclear HIF-1α was observed in 37 ± 5% of chondrocytes. After incubation in 2% oxygen for 6 h, intranuclear HIF-1α was observed in 79 9% of chondrocytes (Fig. 1C).
Figure 1.
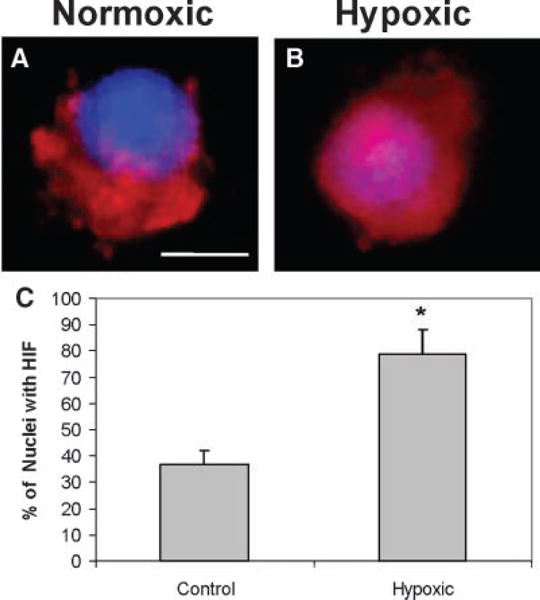
Hypoxia induces nuclear translocation of HIF-1α in chondrocytes grown in alginate beads. Three-dimensional reconstructions of HIF-1α immunofluorescence (shown in red) in (A) normoxic cell with perinuclear HIF-1α staining and (B) hypoxic cell with intranuclear HIF-1α staining. Calibration bar = 2 μm. (C) Percentage of chondrocytes with intranuclear HIF-1α in alginate cultures in normoxia (21% O2) or hypoxia (2% O2). *p < 0.05 versus normoxia.
Histology of Alginate Bead Cultures
Histological sections of alginate beads cultured in normoxic conditions revealed empty lacunae around chondrocytes (Fig. 2A,C,E). Conversely, in cultures grown in 2% oxygen for 17 days, lacunae were seen to be filled with cellular material via H&E staining (Fig. 2B). This material was positively stained by Safranin O and Toluidine blue (Fig. 2D,F).
Figure 2.
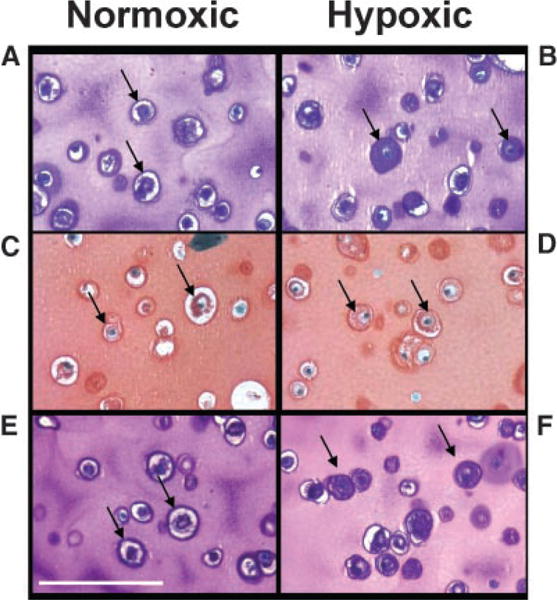
Staining for extracellular matrix components is enhanced in chondrocytes cultured in hypoxia. Alginate bead cultures of chondrocytes after 17 days in normoxia (A,C,E) or hypoxia (B,D,F). Sections were stained with Hematoxylin and Eosin (A,B), Safranin O (C,D), or Toluidine Blue (E,F). Calibration bar = 30 microns.
Proteoglycan Synthesis
After 1 day of culture, the hypoxic chondrocytes exhibited a 44% increase in proteoglycan synthesis compared to those grown in the normoxic environment (p < 0.001). After 3 days in culture, the hypoxic chondrocytes had a 169% increase in proteoglycan synthesis compared to those grown in the normoxic environment (p < 0.05). After 10 days in culture, hypoxic chondrocytes exhibited a 101% increase in proteoglycan synthesis compared to those grown in the normoxic environment (p = 0.001; Fig. 3).
Figure 3.

Hypoxia increases proteoglycan synthesis (pmol sulfate incorporated per sample) after 1, 3, or 10 days. Means ± SEM are shown, n = 6 separate experiments using six animals. *p < 0.05 versus normoxia.
Expression of Collagen Immunofluorescence
Indirect immunofluorescence staining for type II and type X collagen showed increased staining in cultures incubated in 2% oxygen compared to 21% oxygen, both in the number of cells showing staining and in the amount of staining around each cell (Fig. 4). Quantitative analysis of staining in seven separate beads showed a 284% increase in type II collagen (p < 0.001) and a 67% increase in type X collagen in 2% oxygen compared to 21% oxygen (p < 0.001; Fig. 5).
Figure 4.
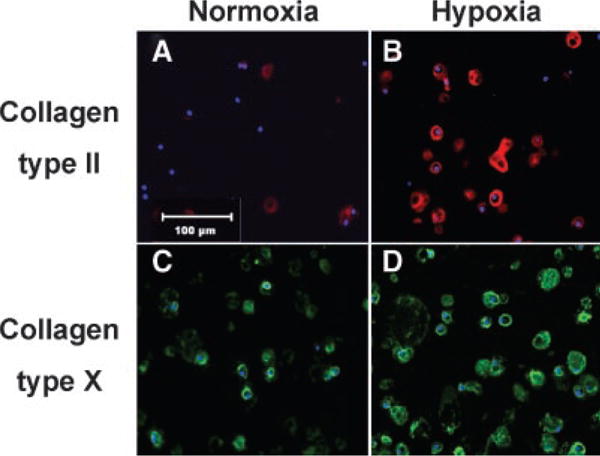
Immunofluorescence of type II and type X collagen in alginate bead cultures incubated for 17 days in normoxia or hypoxia. Type II collagen is shown as red (A,B) and type X collagen (C,D) is shown as green, with nuclei in blue. Both types of collagen are expressed in greater amounts after 17 days of culture in hypoxia (B,D) as compared to normoxia (A,C). Representative areas for each treatment are shown above. Magnification = 40×. Calibration bar = 100 μm.
Figure 5.
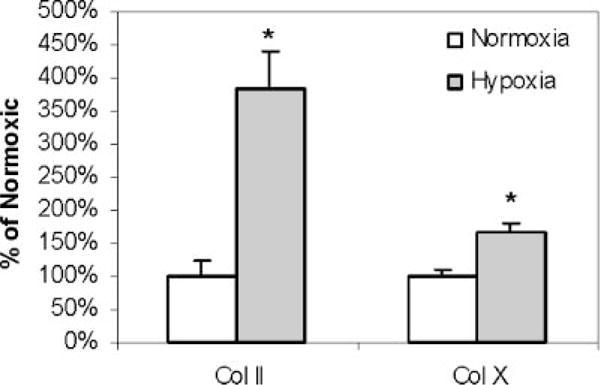
Quantification of type II and type X collagen immunofluorescence. Means and SD for quantitative integration of immunofluorescence in seven beads for each condition are shown. *p < 0.05 versus normoxia.
Collagen II Gene Expression
Real-time quantitative PCR analysis of COL2A1 gene expression showed a 3.2 ± 0.4-fold increase in expression in chondrocytes exposed to 2% O2 (hypoxia) compared with cells from normoxic conditions (1.0 ± 0.4-fold, 21% O2) at 17 days (p < 0.01).
Total Collagen II by ELISA
Analysis of total collagen type II by ELISA showed a 2.3 ± 0.7-fold increase in total collagen II with chondrocytes cultured in hypoxia (2% O2) compared to normoxia at 17 days (21% O2, p < 0.05; Fig. 6).
Figure 6.
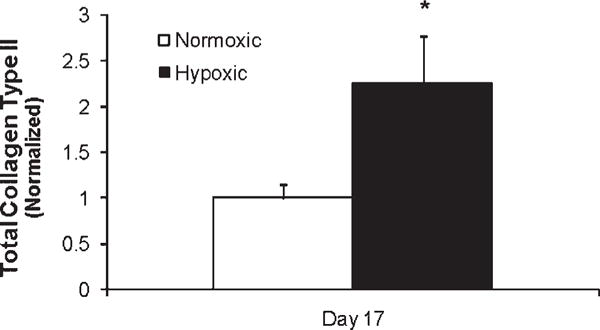
Quantification of total type II collagen by ELISA. Hypoxia increases total collagen type II accumulation 17 days postexposure. Mean and SEM normalized to the normoxic condition. *p < 0.05 versus normoxia.
Viability of Recovered Chondrocytes from Alginate
Live/dead staining and spinning-disk confocal imaging of chondrocytes (normoxic and hypoxic) in intact alginate beads showed greater than 95% cell viability in the outer bead region at 14 days. However, recovery of total chondrocytes cultured in normoxic condition (control) resulted in 67 ± 14% cell viability after 14 days, while 92 ± 4% cell viability was found with hypoxia (p < 0.05). Interestingly, culture in 2% oxygen enhanced the viability of recovered chondrocytes.
DISCUSSION
These studies show that sustained exposure of bovine articular chondrocytes in three-dimensional alginate culture to an environment of 2% oxygen enhanced chondrocyte matrix production and viability over a 17 day period. This enhancement was seen at the protein and gene levels, including an increase in proteoglycan synthesis and increased deposition and gene expression of type II collagen and increased deposition of type X collagen. Increased matrix synthesis was seen after only 1 day of sustained hypoxia, as assessed by proteoglycan synthesis, and continued throughout the course of the 17 day study period. These studies were conducted in a constant, rigorously maintained hypoxic environment, with pre-equilibration of media with 2% oxygen prior to media changes, eliminating periods of reoxygenation. The 2% oxygen used for hypoxic treatment in these experiments increased the number of chondrocytes in which the hypoxia-responsive transcription factor, HIF-1α, was translocated to the nucleus, confirming chondrocyte adaptation to the experimental hypoxic conditions.
An increase in type II collagen has been observed in several studies where chondrocytes were exposed to widely varying degrees of hypoxia thereby raising questions concerning the role of hypoxia in collagen II expression.11,14–16,21,26–28 Using a glove box for precise control of oxygen tensions, these data show that sustained hypoxia increases collagen II gene expression and collagen II deposition as well as chondrocyte viability and proteoglycan synthesis in three-dimensional alginate bead culture. To date, there has been no direct link between HIF-1α and collagen II, such as the discovery of a hypoxia response element on the Col2a1 gene, so mechanisms for the induction of collagen genes with hypoxia remain unclear. However, the DEC1 and DEC2 genes, which encode transcription factors that are involved in chondrocyte differentiation, contain such hypoxia response elements.29 These nuclear regulators could provide a potential link between hypoxia and increased collagen II production and gene expression. In addition, there was improved viability of chondrocytes cultured in hypoxic conditions compared with chondrocytes in normoxia. This suggests that the hypoxic environment, with chondrocytes encapsulated in alginate beads, provides a better environment for longer term cell maintenance when compared to normoxic culture.
This study differs from others, probing the effects of hypoxia in cartilage by utilizing a hypoxic chamber that maintains continuous low oxygen levels with culture for up to 17 days. Many varied methods have been utilized to simulate hypoxia, with the use of triple gas incubators or sealed environmental chambers flushed with premixed gases. These devices are good for maintaining fixed oxygen levels when sealed. However, with long-term experimentation, media exchange is required, thus exposing chondrocytes to temporary periods of reoxygenation and normoxia.
The intermittent alterations in oxygen tension during culture in a hypoxic environment can have unknown effects on chondrocyte biology, as reoxygenation can be harmful to cells. Martin et al.21 have shown that changing the oxygen level from 5 to 20% results in changes in the DNA binding of NF-κB and AP-1 and alterations in the regulation of genes downstream from these transcription factors such as MMP-3 and TIMP-2. In addition, Schneider et al.30 have shown that reoxygenation results in the release of free radicals from articular synoviocytes. While they did not see a similar response from articular chondrocytes, they utilized primary chondrocytes acutely after isolation and not during prolonged tissue culture or after a recovery phase. The effect of reactive oxygen species on chondrocyte metabolism has been well documented.25,31,32 Other reactive oxygen species can be released from mitochondria under severely anoxic conditions.33,34 However, we used an oxygen tension of 2%, which was low enough to cause a translocation of HIF-1α, yet above the level that decreases ATP production in chondrocytes.13,14
The 2% oxygen used for hypoxic treatment in this study increased the number of chondrocytes with HIF-1α nuclear expression, thus confirming chondrocyte adaptation to experimental hypoxic conditions after 6 h. Previous publications have demonstrated up-regulation of HIF-1α expression in the nucleus with chondrocyte exposure to hypoxia.9,13
Immunolocalization of HIF-1α may be useful for identifying cells and tissue experiencing sustained hypoxia. Further, hypoxia and HIF-1α in the growth plate have been shown to play a role in proliferation and cell survival by modulating the rate of glycolysis and the expression of cell cycle regulators.35 Oxygen concentration regulates the stability of HIF-1α protein.7 The oxygen-dependent HIF-1α prolyl hydroxylases (PHD1, PHD2, and PHD3)5,36 coactivate HIF-1α by binding to PHD enzymes, preventing them from catalyzing the modifications to HIF-1α, which target it for degradation.36 The stabilized HIF-1α is thus free to translocate to the nucleus. In chondrocytes, hypoxia has been shown to increase the transcription of a different prolylhydroxylase, which is involved in the collagen triple helix formation,16 possibly providing another potential link between increased matrix production and HIF-1α translocation. The transactivation step required for HIF-1α activity is dependent on a hypoxia-dependent arginylhydroxylase.5,9
Several roles have been identified for hypoxia in the biology of chondrocytes. The chondrocytic differentiation factors DEC1 and DEC2 are up-regulated in hypoxia.29,37 Also, the expression of chondrocyte-specific genes in mesenchymal cells is dependent on hypoxia-regulated expression of SOX-9.38 In addition to the role of HIF-1α in development and signaling, altered oxygen regulation could contribute to the pathology of osteoarthritis. Previous studies have shown altered matrix production and HIF-1α translocation, observed experimentally, in osteoarthritic chondrocytes.39–41
Disruption of the articular surface through chondral injury or degenerative fissuring likely alters the normal oxygen gradient and can expose chondrocytes to inconsistent, abnormal physiologic oxygen tensions. Chondrocyte repair responses and cartilage homeostasis may be disrupted by the introduction of synovial fluid and collapse of the normal oxygen gradient resulting in oxygen levels outside the threshold for optimal matrix production. These studies demonstrate that chondrocyte matrix production, gene expression, and chondrocyte viability are enhanced with sustained hypoxia (2% O2), similar to the physiological conditions of deep articular chondrocytes. An improved understanding of the effects of oxygen tension on chondrocyte metabolism may lead to new strategies to restore cartilage metabolic homeostasis following chondrocyte injury and contribute to future clinical interventions.
Acknowledgments
The authors would like to thank Lesa M. Lewis Werkmeister, BS and Deanna M. Didiano, BS for their technical assistance.
Footnotes
The authors have no conflicts of interest.
References
- 1.Brighton CT, Heppenstall RB. Oxygen tension in zones of the epiphyseal plate, the metaphysis and diaphysis. An in vitro and in vivo study in rats and rabbits. J Bone Joint Surg Am. 1971;53:719–728. [PubMed] [Google Scholar]
- 2.Silver IA. Measurement of pH and ionic composition of pericellular sites. Philos Trans R Soc Lond B Biol Sci. 1975;271:261–272. doi: 10.1098/rstb.1975.0050. [DOI] [PubMed] [Google Scholar]
- 3.Poole AR, Rizkalla G, Ionescu M, et al. Osteoarthritis in the human knee: a dynamic process of cartilage matrix degradation, synthesis and reorganization. Agents Actions Suppl. 1993;39:3–13. doi: 10.1007/978-3-0348-7442-7_1. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruick RK, McKnight SL. Transcription. Oxygen sensing gets a second wind. Science. 2002;295:807–808. doi: 10.1126/science.1069825. [DOI] [PubMed] [Google Scholar]
- 6.Gopfert T, Gess B, Eckardt KU, et al. Hypoxia signalling in the control of erythropoietin gene expression in rat hepatocytes. J Cell Physiol. 1996;168:354–361. doi: 10.1002/(SICI)1097-4652(199608)168:2<354::AID-JCP14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Jiang BH, Semenza GL, Bauer C, et al. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 8.Hofer T, Desbaillets I, Hopfl G, et al. Dissecting hypoxia-dependent and hypoxia-independent steps in the HIF-1alpha activation cascade: implications for HIF-1alpha gene therapy. FASEB J. 2001;15:2715–2717. doi: 10.1096/fj.01-0546fje. [DOI] [PubMed] [Google Scholar]
- 9.Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- 10.Pfander D, Swoboda B, Cramer T. The role of HIF-1alpha in maintaining cartilage homeostasis and during the pathogenesis of osteoarthritis. Arthritis Res Ther. 2006;8:104. doi: 10.1186/ar1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfander D, Cramer T, Schipani E, et al. HIF-1alpha controls extracellular matrix synthesis by epiphyseal chondrocytes. J Cell Sci. 2003;116:1819–1826. doi: 10.1242/jcs.00385. [DOI] [PubMed] [Google Scholar]
- 12.Pfander D, Kobayashi T, Knight MC, et al. Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development. 2004;131:2497–2508. doi: 10.1242/dev.01138. [DOI] [PubMed] [Google Scholar]
- 13.Brucker PU, Izzo NJ, Chu CR. Tonic activation of hypoxia-inducible factor 1alpha in avascular articular cartilage and implications for metabolic homeostasis. Arthritis Rheum. 2005;52:3181–3191. doi: 10.1002/art.21346. [DOI] [PubMed] [Google Scholar]
- 14.Grimshaw MJ, Mason RM. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthritis Cartilage. 2001;9:357–364. doi: 10.1053/joca.2000.0396. [DOI] [PubMed] [Google Scholar]
- 15.Domm C, Schunke M, Christesen K, et al. Rediffer-entiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002;10:13–22. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- 16.Grimmer C, Balbus N, Lang U, et al. Regulation of Type II Collagen Synthesis during Osteoarthritis by Prolyl-4-Hydroxylases: Possible Influence of Low Oxygen Levels. Am J Pathol. 2006;169:491–502. doi: 10.2353/ajpath.2006.050738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardingham T. Proteoglycans: their structure, interactions and molecular organization in cartilage. Biochem Soc Trans. 1981;9:489–497. doi: 10.1042/bst0090489. [DOI] [PubMed] [Google Scholar]
- 18.Heinegard D, Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989;3:2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- 19.Bruckner P, van der Rest M. Structure and function of cartilage collagens. Microsc Res Tech. 1994;28:378–384. doi: 10.1002/jemt.1070280504. [DOI] [PubMed] [Google Scholar]
- 20.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Martin G, Andriamanalijaona R, Grassel S, et al. Effect of hypoxia and reoxygenation on gene expression and response to interleukin-1 in cultured articular chondrocytes. Arthritis Rheum. 2004;50:3549–3560. doi: 10.1002/art.20596. [DOI] [PubMed] [Google Scholar]
- 22.Masuda K, Sah RL, Hejna MJ, et al. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura K, Chu CR, Sobajima S, et al. Adenoviral-mediated transfer of TGF-beta1 but not IGF-1 induces chondrogenic differentiation of human mesenchymal stem cells in pellet cultures. Exper Hematol. 2005;33:865–872. doi: 10.1016/j.exphem.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gannon JM, Walker G, Fischer M, et al. Localization of type X collagen in canine growth plate and adult canine articular cartilage. J Orthop Res. 1991;9:485–494. doi: 10.1002/jor.1100090404. [DOI] [PubMed] [Google Scholar]
- 25.Studer RK, Levicoff E, Georgescu H, et al. Nitric oxide inhibits chondrocyte response to IGF-I: inhibition of IGF-IRbeta tyrosine phosphorylation. Am J Physiol Cell Physiol. 2000;279:C961–969. doi: 10.1152/ajpcell.2000.279.4.C961. [DOI] [PubMed] [Google Scholar]
- 26.Hansen U, Schunke M, Domm C, et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941–949. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 27.Kurz B, Domm C, Jin M, et al. Tissue engineering of articular cartilage under the influence of collagen I/III membranes and low oxygen tension. Tissue Eng. 2004;10:1277–1286. doi: 10.1089/ten.2004.10.1796. [DOI] [PubMed] [Google Scholar]
- 28.Risbud MV, Albert TJ, Guttapalli A, et al. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: implications for cell-based transplantation therapy. Spine. 2004;29:2627–2632. doi: 10.1097/01.brs.0000146462.92171.7f. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki K, Kawamoto T, Tanimoto K, et al. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J Biol Chem. 2002;277:47014–47021. doi: 10.1074/jbc.M204938200. [DOI] [PubMed] [Google Scholar]
- 30.Schneider N, Mouithys-Mickalad AL, Lejeune JP, et al. Synoviocytes, not chondrocytes, release free radicals after cycles of anoxia/re-oxygenation. Biochem Biophys Res Commun. 2005;334:669–673. doi: 10.1016/j.bbrc.2005.06.147. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto H, Silverton SF, Debolt K, et al. Superoxide dismutase and catalase activities in the growth cartilage: relationship between oxidoreductase activity and chondrocyte maturation. J Bone Miner Res. 1991;6:569–574. doi: 10.1002/jbmr.5650060607. [DOI] [PubMed] [Google Scholar]
- 32.Schalkwijk J, van den Berg WB, van de Putte LB, et al. Hydrogen peroxide suppresses the proteoglycan synthesis of intact articular cartilage. J Rheumatol. 1985;12:205–210. [PubMed] [Google Scholar]
- 33.Dawson TL, Gores GJ, Nieminen AL, et al. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am J Physiol. 1993;264:C961–967. doi: 10.1152/ajpcell.1993.264.4.C961. [DOI] [PubMed] [Google Scholar]
- 34.Yang W, Block ER. Effect of hypoxia and reoxygenation on the formation and release of reactive oxygen species by porcine pulmonary artery endothelial cells. J Cell Physiol. 1995;164:414–423. doi: 10.1002/jcp.1041640222. [DOI] [PubMed] [Google Scholar]
- 35.Schipani E, Ryan HE, Didrickson S, et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 37.Iwata T, Kawamoto T, Sasabe E, et al. Effects of overexpression of basic helix-loop-helix transcription factor Dec1 on osteogenic and adipogenic differentiation of mesenchymal stem cells. Eur J Cell Biol. 2006;85:423–431. doi: 10.1016/j.ejcb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Robins JC, Akeno N, Mukherjee A, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 39.Coimbra IB, Jimenez SA, Hawkins DF, et al. Hypoxia inducible factor-1 alpha expression in human normal and osteoarthritic chondrocytes. Osteoarthritis Cartilage. 2004;12:336–345. doi: 10.1016/j.joca.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Pfander D, Cramer T, Swoboda B. Hypoxia and HIF-1alpha in osteoarthritis. Int Orthop. 2005;29:6–9. doi: 10.1007/s00264-004-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yudoh K, Nakamura H, Masuko-Hongo K, et al. Catabolic stress induces expression of hypoxia-inducible factor (HIF)-1 alpha in articular chondrocytes: involvement of HIF-1 alpha in the pathogenesis of osteoarthritis. Arthritis Res Ther. 2005;7:R904–914. doi: 10.1186/ar1765. [DOI] [PMC free article] [PubMed] [Google Scholar]


