SUMMARY
Objective
Quantitative magnetic resonance imaging (MRI) ultrashort echo time (UTE) T2* is sensitive to cartilage deep tissue matrix changes after anterior cruciate ligament reconstruction (ACLR). This study was performed to determine whether UTE-T2* profile analysis is a useful clinical metric for assessing cartilage matrix degeneration. This work tests the hypotheses that UTE-T2* depthwise rates of change (profile slopes) correlate with clinical outcome metrics of walking mechanics and patient reported outcomes (PRO) in patients 2 years after ACLR.
Design
Thirty-six patients 2 years after ACLR completed knee MRI, gait analysis, and PRO. UTE-T2* maps were generated from MRI images and depthwise UTE-T2* profiles were calculated for weight-bearing cartilage in the medial compartment. UTE-T2* profiles from 14 uninjured subjects provided reference values. UTE-T2* profile characteristics, including several different measures of profile slope, were tested for correlation to kinetic and kinematic measures of gait and also to PRO.
Results
Decreasing UTE-T2* profile slopes in ACLR knees moderately correlated with increasing knee adduction moments (r = 0.41, P < 0.015), greater external tibial rotation (r = 0.44, P = 0.007), and moderately negatively correlated with PRO (r = −0.36, P = 0.032). UTE-T2* profiles from both ACLR and contralateral knees of ACLR subjects differed from that of uninjured controls (P < 0.015).
Conclusions
The results of this study suggest that decreasing UTE-T2* profile slopes reflect cartilage deep tissue collagen matrix disruption in a population at increased risk for knee osteoarthritis (OA). That UTE-T2* profiles were associated with mechanical and patient reported measures of clinical outcomes support further study into a potential mechanistic relationship between these factors and OA development.
Keywords: Cartilage, MRI, UTE-T2* mapping, ACL reconstruction, Gait, PRO
Introduction
Anterior cruciate ligament (ACL) injury greatly increases risk for development of premature osteoarthritis (OA)1,2. While surgical reconstruction of the ACL (ACLR) restores stability to the joint, normal knee biomechanics are not completely restored3–5. Despite high incidence of acute cartilage injury to the lateral side of the knee6, post-ACLR OA predominates in the medial compartment7, leading to a 19-fold increase in the risk to cartilage loss in the medial compartment 7–11 years after ACLR6. Altered knee mechanics following ACLR contribute to increased OA risk by disrupting the articular cartilage matrix8–10, to include the deep cartilage layers adjacent to the subchondral bone8–10. Clinical methods to detect early changes to deep cartilage occurring after ACLR are key to understanding OA pathogenesis and to the development of therapeutic interventions.
Magnetic resonance imaging (MRI) techniques such as T2 and T1rho mapping are useful in depicting changes to the transitional and superficial cartilage layers11,12 where relatively long T2 relaxations (>10 ms) typically dominate. In order to probe collagen fibril integrity and organization in deep cartilage where short T2 relaxations (<10 ms) are abundant13, ultrashort echo time (UTE) imaging is needed. Longitudinal study of ACL injured patients have shown that MRI UTE-enhanced T2* (UTE-T2*) mapping is sensitive to potentially reversible deep cartilage changes reflective of acute injury as well as early degeneration14. Recent studies suggest that UTE-T2* elevations in deep articular cartilage are associated with increased matrix degeneration8,14. However, reports of T2 spatial heterogeneity in damaged and diseased cartilage15,16 have led to the hypothesis that the spatial distribution of UTE-T2* values may be as or more important than the magnitude in reflecting cartilage health status. Previous in vivo assessments of depthwise anisotropy of quantitative MRI metrics in knee cartilage have included zonal analyses8,14,17 and texture analysis12,15,16. Zonal, or “laminar”, analysis suffers from insensitivity to spatial variations within the evaluated region and textural assessments require intensive postprocessing effort and are challenging to interpret because they are often based on second-order statistics. Profile analysis, where a cartilage property is plotted as a function of distance from the subchondral bone, is an attractive alternative to laminar or textural analyses because it permits survey of the full cartilage thickness while resolving depth-wise variations to a simple one-dimensional representation18. In cartilage, the slope of a T2 or UTE-T2* profile may be interpreted as a reflection of the relative degree of laminar organization of the collagen extracellular matrix with higher slope values indicating a more anisotropic laminar collagen ultrastructure, suggesting healthy cartilage, while lower slope values indicate loss of healthy anisotropic laminar structure, suggesting early cartilage breakdown. Previous T2 profile assessments of articular cartilage have demonstrated changes to the laminar structure of the collagen matrix with age, activity and disease11,19,20. The sensitivity of UTE-T2* to cartilage deep tissue signal suggests that profile analyses of UTE data may be useful for detection of early cartilage subsurface degeneration. However, the clinical relevance of cartilage structural changes reflected in UTE-T2* profile assessments has yet to be established.
Joint health is a reflection of the interplay between structure, biology and function21. Gait analysis is a well established clinical metric that provides meaningful objective assessment of joint function. Altered walking mechanics following ACL injury3,5,22 have been suggested as a factor in OA initiation after ACL injury and reconstruction4,23–25. Patient reported outcome (PRO) surveys, by contrast, provide a means to assess patients’ subjective experiences of symptoms and functional limitations. In particular, the Knee Injury and Osteoarthritis Outcome Score (KOOS) is specifically designed to assess symptoms and function in subjects with knee injury and osteoarthritis26. Previous examinations of KOOS in ACL-injured athletes found that worse KOOS scores were reported by soccer players 14 years after injury than by uninjured reference subjects2. In addition, several measures of gait mechanics, including knee center of rotation, adduction and flexion moments measured at 2 years post ACLR, have recently been shown to correlate to patient reported outcomes (PRO) 8 years after ACLR surgery27,28.
Acknowledging the well-established utilities of both gait analyses and PRO for the study of clinical outcomes after ACLR, the goals of this work are: (1) to examine cartilage UTE-T2* profiles in ACLR subjects and uninjured controls, and to compare UTE-T2* profile characteristics to (2) specific kinetic and kinematic measures of walking and (3) PROs in ACLR subjects 2 years after surgery. Specifically, we hypothesize that uninjured controls will exhibit greater depthwise anisotropic laminar structure as reflected in higher UTE-T2* slope values compared to ACLR subjects and that gait and PRO measures will be correlated with greater UTE-T2* profile slope values.
Methods
Thirty-six ACLR subjects (16 female/20 male, 33 ± 11 yrs, 2.19 ± 0.22 yrs post-unilateral ACL-reconstruction) and 14 uninjured controls (6 F/8 Male, 31 ± 9 yrs) participated in these IRB-approved studies. Inclusion criteria for ACLR patients included primary ACLR in either limb (no revision ACLR), 18 ≤ age ≤ 60, body mass index (BMI) < 30 kg/m2, no injections to any joint in previous 6 months, and successful ACLR based on clinical exam (KT-1000 side-to-side difference<5 mm). Subjects with history of serious injury or surgery to either lower limb were excluded (other than ACL tear, meniscus tear, and/or non-operative treatment of MCL tear to the ACLR limb). The average time (mean ± STD) between injury and surgery was 3.5 ± 3.5 months (range 10 days to 15 months). Uninjured controls reported no known or suspected past or current knee injury.
MRI
ACLR subjects underwent 3T MRI examination of both their ACLR and contralateral knees (MR 750, GE Healthcare, Milwaukee, WI) with a transmit-receive eight-channel knee coil. Uninjured subjects underwent MRI examination of one knee (five left/nine right).
UTE-T2* maps were calculated via mono-exponential fitting a series of T2*-weighted MR images acquired with non-uniform echo spacing using a radial-out 3-D Cones acquisition29. The Cones sequence samples MRI data starting at the center of k-space and twisting outwards along conical surfaces in 3-D while allowing for anisotropic field of view (FOV) and resolution. The echo times (TEs) were collected in two serial acquisitions each with four echoes: set 1: 32 μs, 3.6, 7.2,16.0 ms; set 2: 1, 4.7, 9.0, 12.7 ms. Other parameters included: 9° flip angle, 125 kHz bandwidth, 22.5 ms repetition time, 12 cm FOV, 384 acquisition matrix, 3 mm slice thickness. Scantime for each acquisition was 5.25 min for a total acquisition time of 10.5 min per knee. The sagittally oriented imaging volume was centered on the tibiofemoral joint.
To facilitate image registration, 3-D Cones acquisitions underwent cubic interpolation to a 512 matrix (effective pixel size 234 × 234 μm) prior to T2*-curve fitting. Rigid in-plane registration was applied to interpolated images to reduce patient-motion-induced spatial offsets between successive Cones acquisitions. UTE-T2* maps were generated with a mono-exponential pixel-by-pixel T2-fit routine using MRIMapper© (Beth Israel Deaconess and MIT 2006) running on a MATLAB platform. To reduce the impact of noise and partial-voluming of bone or synovial fluid signals on cartilage T2* calculations, UTE-T2* maps were filtered to exclude non-physiologic values. Pixels with T2* values <0 ms or >65 ms were excluded from quantitative analyses. Regions of interest (ROIs) were drawn from the centermost single slice of the medial compartment. In cases where an even number of slices encompassed the medial compartment, the centermost slice with the visually clearest bone–cartilage interface was chosen for analysis. Three ROIs in the central and posterior medial femoral condyle (cMFC and pMFC) and central medial tibial plateau (cMTP), were manually segmented by one individual with 15 years prior segmenting experience (AW) from fitted UTE-T2* maps in accordance with previously illustrated methods4,8,30, Fig. 1(a). This method of slice selection and manual segmentation provides reproducible UTE-T2* values with 8% root-mean-square average coefficients of variation (inter-session repeatability errors) corresponding to absolute precision errors ≤ 1.2 ms for full-thickness femorotibial cartilage30.
Fig. 1.
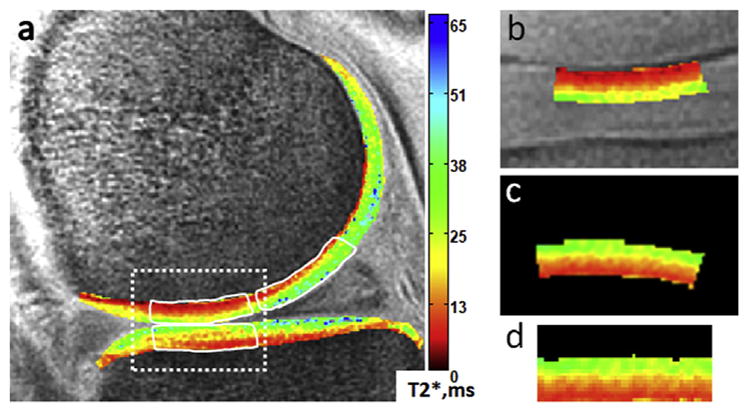
Sample mid-sagittal ultrashort echo time (UTE)-T2* map of an uninjured control subject (a). UTE-T2* profile characteristics were calculated in central medial femoral condyle (cMFC), central medial tibial plateau (cMTP) and posterior medial femoral condyle (pMFC). Briefly, regions of interest were defined such that: the cMFC (a – top left white outline) and cMTP (b – bottom white outline) included femoral or tibial cartilage, respectively, between the interior margins of the anterior and posterior horns of the medial meniscus; the pMFC (a – top right white outline) included femoral cartilage adjacent to the posterior horn of the medial meniscus. Prior to performing profile analysis, the extracted cMFC ROI (a – dashed box, and also b) was rigidly rotated (c) and vertically morphed to flatten (d).
UTE-T2* profile calculation
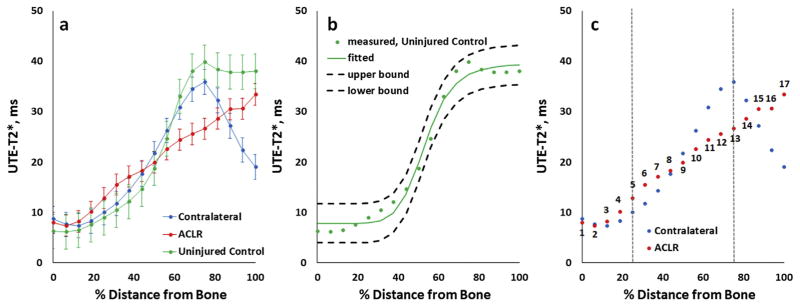
Prior to performing profile analyses, ROIs were individually rigidly-rotated in-plane (≤5°) to minimize angular offsets between the bone–cartilage interface and rows of pixels in UTE-T2* maps, Fig. 1(c). After rotation, segmented cartilage ROIs were vertically morphed to flatten the ROIs such that the cartilage–subchondral plate interface was perpendicular to columns of pixels and parallel to rows of pixels16, Fig. 1(d). A single UTE-T2* profile, from the bone–cartilage interface to the cartilage surface, was computed for each full-thickness cartilage ROI by averaging across the entire width of the ROI, along a line of pixels parallel to the subchondral plate (typically 45–60 pixels), at each row of cartilage depth (typically 11–13 pixels). Profiles were then standardized to tissue thickness by depth-normalizing to 17 points across the cartilage thickness using bilinear interpolation with Matlab’s “imresize” function, Fig. 2(a).
Fig. 2.
(a) Sample ultrashort echo time (UTE)-T2* profile from an uninjured control subject t and from both knees of an anterior cruciate ligament (ACL)-reconstructed subject. Error bars represent ± standard deviation of UTE-T2* values measured along each level of tissue depth parallel to the subchondral plate. Straight lines between measured profile points are provided to facilitate visual appreciation of differences between the profiles. (b) Measured UTE-T2* profile values, the fitted four-parameter sigmoidal curve, and the 95% confidence band of the fitted curve for the same uninjured subject as shown in a demonstrating a good fit of the model to this uninjured control subject’s UTE-T2* profile data. (c) UTE-T2* profile values from both knees of the same ACL-reconstructed subject as shown in a. Profile slopes are calculated in the deepest 0–25% and middle 25–75% of cartilage (by averaging derivatives across points 1 through 5, and 5 through 13, respectively). Percent distance from bone is measured such that 0% indicates bone cartilage interface, 100% indicates synovial surface.
UTE-T2* profile characterization
To compare profile shapes across different knees, a four-parameter logistic equation was employed to describe profiles as sigmoidal curves and to allow properties of and deviations from that shape to be quantitated31. The following model was used:
In this equation, A represents the minimum UTE-T2* at the bone–cartilage interface, D represents the maximum UTE-T2* in superficial cartilage, C is the inflection point marking a transition between increasing and decreasing UTE-T2* values through the depth, and B is a slope factor, that reflects the rate of UTE-T2* change through the depth. Goodness of fit of this model was evaluated by visual assessment of measured data plotted together with the four-parameter fitted curve and 95% confidence intervals, calculated from root mean square error (RMSE)32,33 and Critical t, using 13 residual degrees of freedom, for each uninjured control ROI, Fig. 2(b). Additionally, RMSE was recorded for each profile, averaged for each cohort, and assessed as an informal measure of goodness of fit between cohorts33.
Characterization UTE-T2* profiles across the deepest 0–25% of tissue was additionally assessed by first computing the derivative of the profile at points 1 through 5, then averaging the derivatives. Likewise, the average slope of UTE-T2* profile across the middle 25–75% of tissue depth was calculated by computing the average of the derivatives of profile points 5 through 13, Fig. 2(c). UTE-T2* processing was performed with MATLAB (TheMathWorks, MA).
Gait
All ACLR subjects underwent gait analysis, walking at normal self-selected speed. A 10-camera optoelectronic system (Qualisys, SE) and force plate (Bertec, OH) measured subjects’ motion at 120 Hz. Knee kinematics and kinetics were calculated using Bio-Move software (Stanford University) and the point cluster technique34,35. The foot, shank, and thigh segments’ anatomical reference frames were determined as previously described34 using a standing reference pose collected before the walking trials. Five measures of knee kinetics: adduction moment during loading response (KAM1), late stance (KAM2), and adduction impulse (KAI), maximum extension and flexion moments (KEM, KFM) measured at terminal stance and midstance, respectively36; and three measures of knee kinematics: flexion angle at heel strike (KFA), external rotation at heel strike (KER), and femur anterior displacement at heel strike (FAD) were calculated. Joint moments were normalized to bodyweight and height of each subject (%BW*Ht). These gait variables were selected for analysis based on several prior studies showing the importance of knee kinetics5,36,37 and heel strike kinematics4,36,38,39 within the context of OA and ACL reconstruction.
Patient reported outcomes
PROs were assessed with KOOS; all five KOOS subscales were included: Symptoms, Pain, function in daily living (ADL), function in sports and recreation (Sport/Rec), and knee-related quality of life (QOL)26.
Statistical analyses
Descriptive statistics are presented as means and 95% confidence intervals. Data distributions were assessed for normality with Shapiro Wilk tests for each group (reconstructed, contralateral, uninjured knees). Eight UTE-T2* profile characteristics (maximum, minimum, inflection point, slope factor “B”, RMSE, slope in deepest 25% of tissue, slope in middle 50%, UTE-T2* at mid-thickness) and eight gait metrics (KAM1, KAM2, KAI, KEM, KFM, KFA, KER, FAD) were assessed. Differences in all UTE-T2* profile characteristics between ACLR subject’s reconstructed and contralateral knees were examined with paired t-tests (or Wilcoxin Signed Rank Tests for non-normally distributed data). Differences between uninjured controls and either ACLR subject’s reconstructed or contralateral knee were examined with independent samples assessments t-tests (or Mann–Whitney U Tests). P-values presented are from pooled variances unless the variances were unequal between groups as assessed via F-tests. A statistical significance level of P < 0.05 after Bonferroni adjustment for the three comparisons was used to determine overall significance of differences between groups, and adjusted P values are reported. UTE-T2* profile characteristics for each ROI of ACL-reconstructed knees were correlated to gait metrics of ACL-reconstructed knees using Pearson correlation coefficients (or Spearman’s rho for non-normally distributed data) and were correlated to KOOS PROs using Spearman’s rho correlation coefficients. Due to the paucity of prior research into relationships between depthwise cartilage matrix organization and ambulatory mechanics or PROs, all UTE-T2* profile characteristics were examined for correlation to all gait and PRO metrics. Hence, detection of false positives was controlled in order to reduce the risk of rejecting true correlations in this exploratory study. Correlation results were adjusted for multiple comparisons using a Benjamini–Hochberg procedure with a false discovery rate of 0.15, utilizing eight tests for gait analyses and five tests for PROs40. The false discovery rate was selected to favor sensitivity over possible rejection of true correlations in this exploration. Statistical analyses were performed with SigmaPlot (Systat Software, San Jose, CA) and Excel (Microsoft).
Results
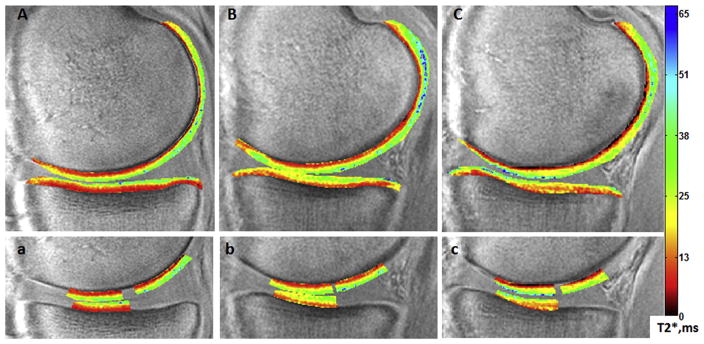
UTE-T2* maps of uninjured controls, uninjured contralateral knees of ACLR subjects, and ACL-reconstructed knees of ACLR subjects exhibit qualitative differences in UTE-T2* distribution in cMFC, pMFC and cMTP cartilage ROIs, Fig. 3. While uninjured control subjects typically show an anisotropic laminar pattern of UTE-T2* values with relatively low (red) values at the bone–cartilage interface increasing to higher values (green-blue) at the synovial surface [Fig. 3(a)], both the contralateral uninjured [Fig. 3(b)] and ACL-reconstructed knees [Fig. 3(c)] of some ACLR subjects demonstrate more variable spatial UTE-T2* distributions.
Fig. 3.
Sample mid-sagittal ultrashort echo time (UTE)-T2* maps of an uninjured control subject (A,a), the contralateral uninjured knee of an anterior cruciate ligament reconstruction (ACLR) subject (B,b), and the anterior cruciate ligament (ACL)-reconstructed knee of the same ACLR subject (C,c). Regions of interest employed in UTE-T2* profile analyses from the ACLR subject’s contralateral (b) and ACL-reconstructed (c) knees demonstrate irregularities from the relatively smooth laminar distribution of UTE-T2* values seen in the uninjured control (a).
UTE-T2* profiles: ACLR vs uninjured controls
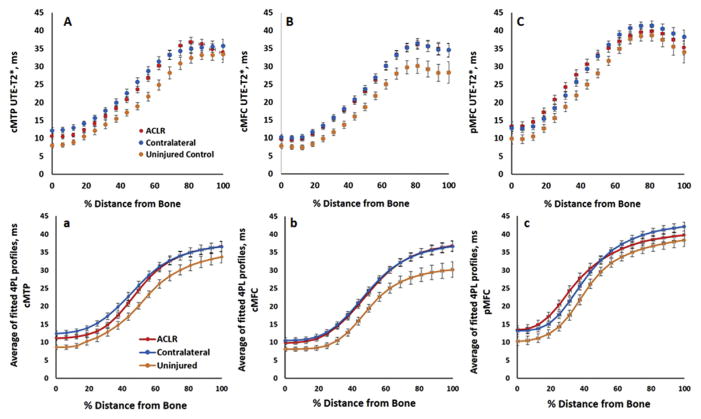
Because none of ACLR subjects in this cohort had substantial cartilage thinning in any of the study regions, data from all subjects were included in profile analyses. Measured depthwise UTE-T2* values, averaged across all individual subjects within a group, show that the ACLR and contralateral knees of ACL-injured subject have similar profiles, and both appear different from uninjured controls [Fig. 4(A)–(C)]. Likewise, the fitted four-parameter sigmoidal curves, averaged across all individual fitted profiles [Fig. 4(a)–(c)] also show similarities between ACLR and contralateral knees, and both differ from uninjured controls. RMSE values observed among ACLR reconstructed knees ranged higher than those of uninjured controls or ACLR subjects’ contralateral knees, Table I. Visual assessments verified that measured UTE-T2* profile data points fell within 95% confidence bands calculated from each subjects’ corresponding fitted sigmoidal model.
Fig. 4.
Mean measured ultrashort echo time (UTE)-T2* profiles (top row) and mean fitted four-parameter sigmoidal curves (bottom row) in the central medial tibial plateau (A,a), central medial femoral condylar (B,b), and posterior medial femoral condylar cartilage (C,c) averaged across 36 anterior cruciate ligament reconstruction (ACLR) subjects and 14 uninjured controls. Measured profiles and calculated sigmoidal curves of uninjured controls appear below those of both knees of anterior cruciate ligament (ACL)-reconstructed suggesting alterations to cartilage matrix organization in both knees of ACLR subjects compared to controls. Percent distance from bone is measured such that 0% indicates bone cartilage interface, 100% indicates synovial surface. Error bars represent ± SEM.
Table I.
Root mean square error (RMSE) measures of goodness of fit of the sigmoidal four-parameter logistic model of ultrashort echo time (UTE)-T2* profiles
| cMTP RMSE | cMFC RMSE | pMFC RMSE | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Uninjured | ACLR | Contra | Uninjured | ACLR | Contra | Uninjured | ACLR | Contra | |
| Mean | 2.26 | 2.75 | 2.51 | 2.21 | 2.86 | 2.52 | 3.06 | 2.99 | 2.98 |
| Min | 0.46 | 0.51 | 0.73 | 0.58 | 0.54 | 0.59 | 1.02 | 0.62 | 0.52 |
| Max | 3.73 | 6.45 | 4.77 | 4.72 | 7.89 | 7.52 | 7.42 | 10.49 | 8.14 |
UTE-T2* values measured at mid-thickness (measured at 50% tissue depth, point nine in Fig. 2(c)) differed between both knees of ACLR subjects and uninjured controls, Fig. 4(A) and (B). Mid-thickness UTE-T2* values in ACLR subjects’ ACL reconstructed knees were 23%, 24% higher than those of uninjured controls (cMFC: mean difference = 4.3 ms, 95%CI (1.1, 7.5), P = 0.010; cMTP: mean difference = 4.6 ms, 95%CI (1.5, 7.7), P = 0.005). Mid-thickness UTE-T2* values of contralateral knees were also 27%, 35% higher than those of uninjured controls (cMFC: mean difference = 5.0 ms; MWUT P = 0.012; cMTP: mean difference = 6.7 ms; 95%CI (3.5, 9.9), P = 0.006). UTE-T2* minimum value in cMTP cartilage (“A” from the four-parameter fit equation, Fig. 4(a)) is 45% higher in the contralateral knee of ACL-reconstructed subjects compared to uninjured controls (mean difference = 3.9 ms, 95%CI (2.0, 5.7), P = 0.004). None of the other examined UTE-T2* profile characteristics differed between groups.
UTE-T2* profiles vs walking mechanics
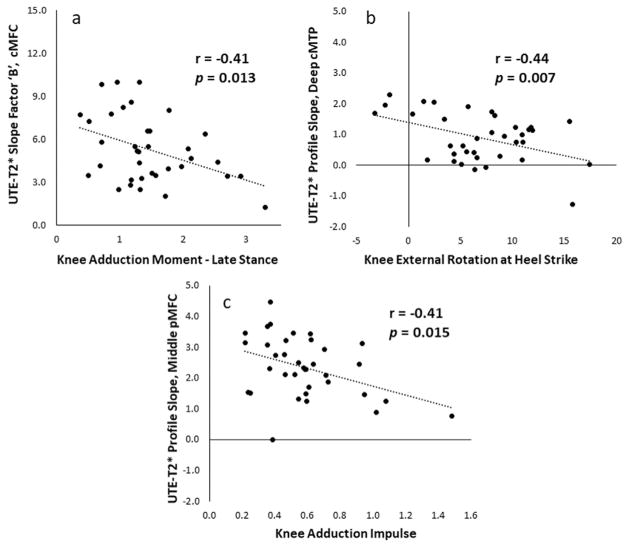
The UTE-T2* profiles characteristics of ACLR subjects, including measures of profile slope, moderately correlated to measures of their knee kinematics and kinetics during walking (Table II). In the reconstructed knees, the “slope factor” (“B”) of the fitted sigmoidal curve correlated to KAM2 [Fig. 5(a)] and significant correlations between UTE-T2* profile slopes (calculated from averaging derivatives) and KER and KAI were observed [Fig. 5(b) and (c)]. Further correlations were detected between UTE-T2* minimum values and KAM2, KAI and KFA; and also between the profile inflection points and KER.
Table II.
Correlations observed between ultrashort echo time (UTE)-T2* profile characteristics and gait metrics within anterior cruciate ligament reconstruction (ACLR) knees
| ROI | UTE-T2* profile characteristic | Gait metric | r | P |
|---|---|---|---|---|
| cMTP | UTE-T2* minimum, L4P | KAM2 | *0.42 | 0.010 |
| KAI | *0.35 | 0.035 | ||
| UTE-T2* maximum, L4P | KER | 0.34 | 0.040 | |
| KEM | 0.34 | 0.046 | ||
| Inflection point, L4P | KER | *0.40 | 0.015 | |
| Slope deepest 25%, AD | KER | −0.44 | 0.007 | |
| cMFC | UTE-T2* minimum, L4P | KFA-HS | *0.41 | 0.013 |
| Slope factor, L4P | KAM2 | −0.41 | 0.013 | |
| pMFC | UTE-T2* minimum, L4P | KER | *0.36 | 0.030 |
| Slope middle 50%, AD | KAM2 | 30.34 | 0.040 | |
| KAI | *−0.41 | 0.015 |
Table includes only correlations with P < 0.05. Results of all comparisons are included in Appendix 1.
L4P indicates parameter calculated from four-parameter logistic sigmoidal curve-fit equation.
AD indicates parameter calculated from average derivatives.
Indicates Spearman’s rho correlation. All others correlations are Pearson.
Bold indicates significant correlation after adjustment for multiple comparisons.
Fig. 5.
Measures of ultrashort echo time (UTE)-T2* profile slope correlate to walking mechanics where a reduction in slope, interpreted as a reflecting a loss of laminar structure, is related to potentially damaging biomechanics. In central medial femoral condyle (cMFC) and posterior medial femoral condyle (pMFC) cartilages (a,c), UTE-T2* slope measures demonstrate moderate negative correlations to external knee adduction moments, suggesting that UTE-T2* profiles are sensitive to disorganization or loosening of the collagen fibril matrix from increased loading. In central medial tibial plateau (cMTP) cartilage (b), the UTE-T2* profile slope in the deepest 25% of cartilage of anterior cruciate ligament (ACL)-reconstructed knees demonstrates a moderate negative correlation to knee external rotation. The negative correlation signals that a reduction in slope was related to increased external rotation, a known outcome of ACL-reconstruction surgery which has previously been suggested as a cause of post-ACLR osteoarthritis development3. Trendlines indicate best linear fits.
UTE-T2* profiles vs patient reported outcomes
KOOS scores demonstrated moderate correlations with UTE-T2* profile characteristics in ACLR subjects, including correlation with a measure of profile slope (Table III). KOOS Symptoms scores correlated to the UTE-T2* profile slope (Fig. 6) and UTE-T2* maximum values. A further correlation was observed between UTE-T2* maximum and KOOS Pain.
Table III.
Correlations observed between ultrashort echo time (UTE)-T2* profile characteristics and patient reported outcomes within anterior cruciate ligament reconstruction (ACLR) knees
| ROI | UTE-T2* profile characteristic | KOOS metric | r | P |
|---|---|---|---|---|
| cMTP | UTE-T2* minimum, L4P | Pain | −0.35 | 0.036 |
| Slope middle 50%, AD | Symptoms | 0.36 | 0.032 | |
| cMFC | UTE-T2* maximum, L4P | Symptoms | 0.43 | 0.008 |
| pMFC | UTE-T2* maximum, L4P | Pain | −0.41 | 0.012 |
Table includes only correlations with P < 0.05. Results of all comparisons are included in Appendix 2.
L4P indicates parameter calculated from four-parameter logistic sigmoidal curve-fit equation.
AD indicates parameter calculated from average derivatives.
Bold indicates significant correlation after adjustment for multiple comparisons.
Fig. 6.
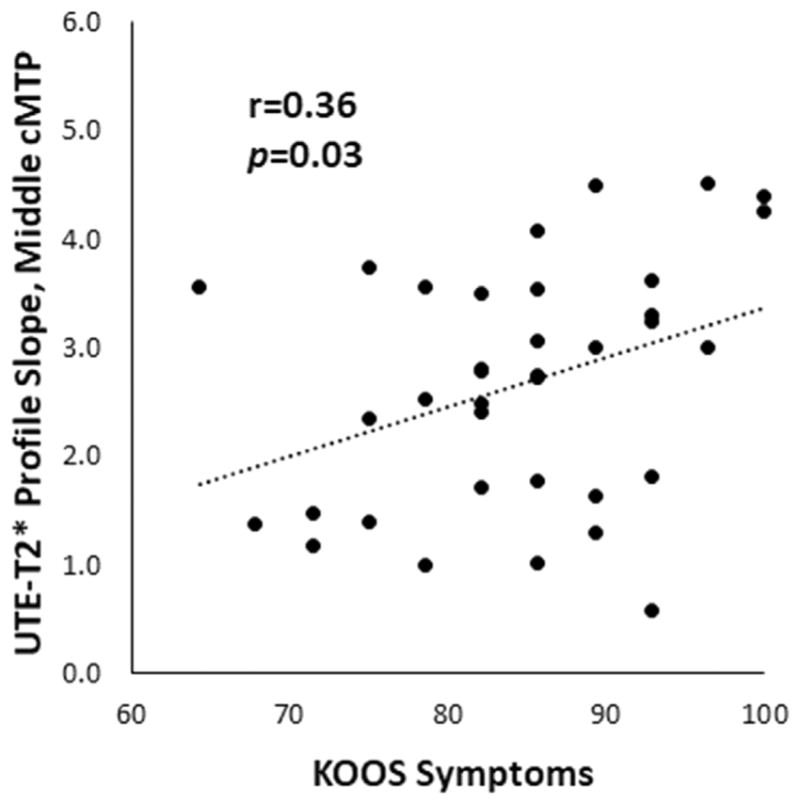
Measures of UTE-T2* profile slope correlate to patient reported outcomes where a reduction in slope, interpreted as a reflecting a loss of laminar structure, is related to worsening of patient reported symptoms. In central medial tibial plateau (cMTP) cartilage, the average slope of the UTE-T2* profile in the middle 50% of cartilage thickness in anterior cruciate ligament (ACL)-reconstructed knees demonstrates a small but significant correlation to Knee Injury and Osteoarthritis Outcome Score (KOOS) Symptoms scores. Trendline indicates best linear fit.
Discussion
This study shows that cartilage MRI UTE-T2* profile characteristics not only differed between uninjured controls and both knees of patients 2 years after ACLR, but that they also correlated with measures of walking mechanics and PROs. UTE-T2* profile slopes in ACLR joints were evaluated as a measure of cartilage extracellular collagen matrix anisotropy where higher UTE-T2* profile slope values were interpreted as indication of relatively more laminar organization and, thus, healthier cartilage. Here, decreasing UTE-T2* profile slope values in ACLR knees moderately correlated with increasing external KAM and external tibial rotation, and demonstrated small but significant correlation to worsening patient reported symptoms. These findings raise the intriguing possibility of a mechanistic relationship between altered knee mechanics, deteriorating PROs and cartilage matrix degeneration following ACLR. Interestingly, elevations in the UTE-T2* minimum and mid-thickness profile values without concurrent decreases in profile slopes suggest that the structural integrity of the cartilage collagen matrix remains largely intact 2 years after ACLR while elevated UTE-T2* values indicate an increase in deep cartilage free water content.
While such findings are consistent with those of cartilage degeneration, it is important to note that the relationship between altered cartilage physiology following ACLR and later development of radiographic OA remains unclear. Specifically, it is not known whether early cartilage degeneration observed in qMRI measures of cartilage are indicative of impending progression to radiographic OA in some or all patients, or if they reflect transient responses to injury, altered mechanics, and healing8,41,42. Long-term radiographic follow-up will be critically important to answering this question.
In the current work, higher KAM seen with lower UTE-T2* profile slopes in deep medial femoral cartilage provide further support for the notion that these profile changes show early cartilage degeneration. Higher KAM reflective of greater loading of the medial compartment has been associated with progression of medial knee OA37. Furthermore, longitudinal studies of knee cartilage following subjects with osteoarthritis for up to 5 years have detected decreases in the medial to lateral cartilage thickness ratio with larger KAM at baseline37. While it is not possible from this work to determine a causal connection between early loss of collagen anisotropic organization and later loss of cartilage thickness, associations of both to alterations in KAM in ACLR knees are consistent with numerous studies showing that supraphysiologic loading of knee cartilage leads to cartilage degeneration37,43,44.
Relatively small kinematic changes in the knee following ACLR, particularly to tibial external rotation and knee extension, may be clinically relevant in accelerating cartilage loss and the development of OA45,46. It is well established that ACLR knees exhibit greater tibial external rotation during ambulation3,47. Prior research indicates that up to 30% of ACLR subjects experience loss of knee extension by 2 years after ACLR4 and that the deficit persists a decade or longer46. In this work, the observed negative correlation between tibial external rotation and UTE-T2* profile slope in medial tibial cartilage suggests that greater external rotation is moderately associated with greater loss of cartilage anisotropic laminar structure. Similarly, in older adults, an increase in knee flexion angle (a loss of extension) was observed in subjects with more severe OA36. In the current study, an increase in knee flexion angle moderately correlated with elevated minimum UTE-T2*. These data in patients just 2 years after ACLR further substantiates the hypothesis that UTE-T2* elevations signal early cartilage deep tissue matrix degeneration.
The potential clinical relevance of changes to UTE-T2* profile characteristics is also reflected in PROs. A previous comparison of quantitative cartilage MRI metrics and PROs following ACL reconstruction found that side-to-side differences in cartilage T1rho values measured prior to surgery were predictive of all KOOS outcomes except for sports function at 1 year following surgery48. In a larger study, prolonged T1rho and T2 values were associated with worse KOOS Symptoms, Pain and Activities of Daily Living measured 6 months post-surgery49. The observed small but significant correlation between decreased UTE-T2* profile slope and worse KOOS Symptoms at just 2 years after ACLR in the current study similarly shows a potential relationship between cartilage structural changes and clinical outcomes.
The finding that UTE-T2* profiles of ACL-reconstructed and contralateral uninjured knees both differed from UTE-T2* profiles of healthy control subjects provides further support for the observations of bilateral changes to joint function over time following unilateral ACL injury50. Interestingly, a recent study by Pedoia et al. also detected qMRI evidence of changes to the biochemical composition consistent with early cartilage degeneration in the contralateral knee over the first 6 months following ACL reconstruction42. However, resolution of the observed T2 and T1rho elevations toward normal levels between 6 months and 12 months led the authors to speculate that the matrix changes were only transient and that contralateral cartilage recovered as patients returned to normal activities42. By contrast, a prior gait study in patients with unilateral ACLR found differences in joint loading between healthy controls and both the ACL-reconstructed and contralateral uninjured knees of ACLR subjects 2–3 years following reconstruction5. Because the current study lacked a control cohort for the gait and PRO measures, it was not possible to directly gauge the degree of mechanical dysfunction or subjective functional status in either knee of the ACLR cohort compared to healthy controls.
This study had several limitations. First, the relatively small numbers for this initial evaluation of UTE-T2* profile analysis in ACLR subjects limits the ability to fully characterize the clinical utility of this new metric, especially in a population where variation in cartilage UTE-T2* status is expected8. Because the cessation of post-surgical rehabilitation programs and return to normal activity following ACL reconstruction is variable across patients, the 2-year follow-up period may be too soon to assess chronically altered gait mechanics and their effects on cartilage health in some patients. In addition, the use of FDR to correct for multiple comparisons may have led to detection of false positives among the many correlations examined. Nevertheless, the observed moderate correlations between UTE-T2* profile analyses and clinical metrics of gait parameters and PROs strongly support progression to well-powered studies with larger cohorts and more targeted examination of fewer UTE-T2* profile and gait metrics.
Second, UTE-T2* profiles presented in this work were calculated from a single slice in the center of the medial condyle and do not reflect focal UTE-T2* variations existing in other cartilage regions. Future studies may benefit from assessment of larger ROIs encompassing a larger portion of weight-bearing cartilage. Furthermore, because the longest echo acquired with 3-D Cones was only 16 ms, long T2 signals may not have been well-captured with this sequence potentially causing an underestimation of measured profile values, particularly in superficial cartilage where longer T2 signals are expected18. This may also have led to an underestimation of differences between cohorts and decreased sensitivity to correlations between profile characteristics and gait metrics.
Finally, the four-parameter logistic equation was chosen in an attempt to find a simple but robust set of metrics to characterize an S-shape curve that reflects physical variations in cartilage properties through the depth measured by UTE-T2* including minimum and maximum UTE-T2* values at the bone–cartilage and cartilage–synovium interfaces, the rate of UTE-T2* change through the depth, and the transition point between increasing and decreasing depthwise UTE-T2* variation. Given the S-shape characteristic of UTE-T2* profiles, alternative descriptions, such as fourth order polynomials or principle component analyses, may not provide parameters as easily interpreted in physical terms. Goodness of fit evaluations of the sigmoidal four-parameter logistic equation, including RMSE magnitude and range and visual appearance of fitted curves relative to measured data, suggest that this model provides a reliable estimate of UTE-T2* profiles in uninjured control subjects. Moreover, the observation of larger RMSE values in some ACLR subjects indicates that the sigmoidal model fits some ACLR subjects worse than others, potentially due to a difference in the depthwise organization of their cartilage collagen matrices compared to uninjured controls. Nonetheless, the data show promise in employing depthwise profile analysis to benefit from the ability of UTE-T2* to capture cartilage deep tissue signal in the evaluation of early cartilage subsurface changes in a population at high risk for post-traumatic OA.
Conclusion
This study shows changes to cartilage UTE-T2* profile characteristics in patients just 2 years after ACLR that moderately correlate to both walking mechanics and PROs. These findings suggest that UTE-2* profile analyses may be useful for discerning early cartilage degeneration. Further longitudinal study of these metrics in larger cohorts are needed to determine whether UTE-T2* profile characteristics and gait mechanics may be predictive of future OA development.
Acknowledgments
Role of the funding source
The study sponsors had no involvement in study design, collection, analysis and interpretation of data; in writing of the manuscript; or in the decision to submit the manuscript for publication.
The authors thank Dr Jayme Koltsov, PhD, for her guidance regarding statistical analyses. We also acknowledge funding support from NIH RO1 AR052784 (CR Chu) and GE Healthcare for MRI time and sequence support.
Appendix 1
All tested correlations between ultrashort echo time (UTE)-T2* profile characteristics and gait metrics within anterior cruciate ligament reconstruction (ACLR) knees.
| Walking Gait Metric | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROI | UTE-T2* Profile Characteristic | KFA | FAD | KER | KFM | KEM | KAM1 | KAM2 | KAI | |
|
| ||||||||||
| cMTP | UTE-T2* Minimum, L4P | Correlation Coefficient | −0.024 | 0.055 | 0.097 | 0.025 | 0.164 | 0.194 | 0.424 | 0.352 |
| Sig. (2-tailed) | 0.891 | 0.751 | 0.573 | 0.885 | 0.338 | 0.257 | 0.010 | 0.035 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | −0.106 | −0.047 | 0.344 | −0.090 | 0.335 | 0.207 | 0.096 | 0.120 | |
| Sig. (2-tailed) | 0.538 | 0.787 | 0.040 | 0.600 | 0.046 | 0.225 | 0.579 | 0.486 | ||
| Inflection Point, L4P | Correlation Coefficient | −0.051 | −0.196 | .401* | −0.018 | 0.154 | 0.271 | 0.031 | 0.068 | |
| Sig. (2-tailed) | 0.769 | 0.252 | 0.015 | 0.918 | 0.371 | 0.109 | 0.856 | 0.695 | ||
| Slope Factor, L4P | Correlation Coefficient | 0.027 | −0.081 | 0.176 | 0.044 | 0.189 | 0.077 | 0.033 | −0.002 | |
| Sig. (2-tailed) | 0.876 | 0.637 | 0.305 | 0.800 | 0.271 | 0.653 | 0.850 | 0.992 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | 0.111 | 0.196 | −0.443 | 0.159 | −0.293 | −0.166 | −0.184 | −0.046 | |
| Sig. (2-tailed) | 0.518 | 0.251 | 0.007 | 0.354 | 0.083 | 0.333 | 0.283 | 0.789 | ||
| Slope Middle 50%, AD | Correlation Coefficient | −0.053 | −0.114 | 0.247 | −0.105 | 0.323 | 0.013 | −0.125 | −0.185 | |
| Sig. (2-tailed) | 0.758 | 0.507 | 0.146 | 0.543 | 0.055 | 0.941 | 0.466 | 0.279 | ||
|
| ||||||||||
| cMFC | UTE-T2* Minimum, L4P | Correlation Coefficient | 0.409 | −0.068 | 0.208 | 0.228 | 0.027 | 0.192 | 0.179 | 0.169 |
| Sig. (2-tailed) | 0.013 | 0.692 | 0.222 | 0.181 | 0.878 | 0.262 | 0.295 | 0.325 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | −0.048 | 0.192 | 0.219 | 0.212 | 0.202 | 0.149 | 0.296 | 0.198 | |
| Sig. (2-tailed) | 0.783 | 0.261 | 0.200 | 0.214 | 0.238 | 0.386 | 0.079 | 0.248 | ||
| Inflection Point, L4P | Correlation Coefficient | −0.043 | 0.156 | 0.120 | 0.236 | 0.046 | −0.010 | 0.121 | −0.006 | |
| Sig. (2-tailed) | 0.805 | 0.364 | 0.487 | 0.166 | 0.791 | 0.955 | 0.481 | 0.971 | ||
| Slope Factor, L4P | Correlation Coefficient | −0.008 | −0.187 | −0.192 | −0.042 | −0.172 | −0.203 | −0.408 | −0.240 | |
| Sig. (2-tailed) | 0.963 | 0.276 | 0.262 | 0.806 | 0.315 | 0.234 | 0.013 | 0.159 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | 0.010 | −0.013 | 0.247 | 0.083 | 0.076 | 0.257 | 0.295 | 0.095 | |
| Sig. (2-tailed) | 0.953 | 0.939 | 0.147 | 0.630 | 0.659 | 0.130 | 0.080 | 0.578 | ||
| Slope Middle 50%, AD | Correlation Coefficient | −0.052 | −0.088 | 0.106 | 0.058 | 0.253 | 0.030 | 0.073 | −0.087 | |
| Sig. (2-tailed) | 0.766 | 0.612 | 0.540 | 0.736 | 0.137 | 0.861 | 0.674 | 0.614 | ||
|
| ||||||||||
| pMFC | UTE-T2* Minimum, L4P | Correlation Coefficient | 0.258 | −0.117 | 0.363 | 0.168 | −0.079 | 0.244 | 0.069 | 0.125 |
| Sig. (2-tailed) | 0.128 | 0.495 | 0.030 | 0.329 | 0.646 | 0.152 | 0.688 | 0.468 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | 0.131 | −0.084 | 0.029 | 0.036 | 0.268 | −0.037 | 0.001 | −0.126 | |
| Sig. (2-tailed) | 0.447 | 0.628 | 0.866 | 0.836 | 0.114 | 0.829 | 0.994 | 0.464 | ||
| Inflection Point, L4P | Correlation Coefficient | −0.055 | −0.151 | −0.057 | 0.019 | −0.092 | 0.003 | −0.145 | −0.122 | |
| Sig. (2-tailed) | 0.752 | 0.381 | 0.742 | 0.911 | 0.592 | 0.988 | 0.398 | 0.477 | ||
| Slope Factor, L4P | Correlation Coefficient | 0.206 | −0.072 | 0.093 | −0.038 | −0.119 | 0.259 | −0.206 | −0.019 | |
| Sig. (2-tailed) | 0.229 | 0.676 | 0.588 | 0.827 | 0.489 | 0.126 | 0.228 | 0.912 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | −0.082 | 0.038 | 0.031 | −0.024 | 0.251 | −0.170 | 0.209 | 0.046 | |
| Sig. (2-tailed) | 0.633 | 0.828 | 0.857 | 0.891 | 0.140 | 0.322 | 0.221 | 0.790 | ||
| Slope Middle 50%, AD | Correlation Coefficient | 0.198 | −0.082 | 0.118 | 0.092 | 0.030 | −0.148 | −0.344 | −0.403 | |
| Sig. (2-tailed) | 0.247 | 0.637 | 0.491 | 0.595 | 0.864 | 0.390 | 0.040 | 0.015 | ||
Shaded grey indicates Pearson correlation; No shading (white) indicates Spearman’s Rho correlation
Shaded orange indicates P<0.05.
Bold indicates significant after adjustment for multiple comparisons
Reported p values are unadjusted for FDR.
Appendix 2
All tested correlations between ultrashort echo time (UTE)-T2* profile characteristics and patient reported outcomes within anterior cruciate ligament reconstruction (ACLR) knees, Spearman’s rho correlations.
| KOOS | |||||||
|---|---|---|---|---|---|---|---|
| ROI | UTE-T2* Profile Characteristic | Symptoms | Pain | ADL | Sports/Rec | QOL | |
|
| |||||||
| cMTP | UTE-T2* Minimum, L4P | Correlation Coefficient | −.056 | −.351* | −.197 | −.275 | −.273 |
| Sig. (2-tailed) | .748 | .036 | .251 | .104 | .107 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | .266 | .048 | .050 | .119 | .162 | |
| Sig. (2-tailed) | .116 | .782 | .773 | .488 | .345 | ||
| Inflection Point, L4P | Correlation Coefficient | .042 | −.022 | .061 | .128 | .223 | |
| Sig. (2-tailed) | .807 | .897 | .722 | .457 | .191 | ||
| Slope Factor, L4P | Correlation Coefficient | .190 | −.012 | .170 | .028 | .023 | |
| Sig. (2-tailed) | .266 | .943 | .322 | .872 | .892 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | .062 | .178 | −.090 | −.094 | .039 | |
| Sig. (2-tailed) | .719 | .298 | .600 | .584 | .819 | ||
| Slope Middle 50%, AD | Correlation Coefficient | .358* | .196 | .316 | .163 | .178 | |
| Sig. (2-tailed) | .032 | .252 | .061 | .344 | .299 | ||
|
| |||||||
| cMFC | UTE-T2* Minimum, L4P | Correlation Coefficient | −.104 | −.018 | .050 | −.279 | −.099 |
| Sig. (2-tailed) | .548 | .918 | .770 | .099 | .566 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | .434** | .078 | .095 | .175 | .136 | |
| Sig. (2-tailed) | .008 | .652 | .581 | .306 | .427 | ||
| Inflection Point, L4P | Correlation Coefficient | .166 | .054 | .055 | .138 | .017 | |
| Sig. (2-tailed) | .332 | .753 | .749 | .422 | .922 | ||
| Slope Factor, L4P | Correlation Coefficient | −.180 | −.066 | −.074 | −.146 | −.060 | |
| Sig. (2-tailed) | .293 | .703 | .668 | .396 | .730 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | .319 | .164 | .038 | .123 | .099 | |
| Sig. (2-tailed) | .058 | .338 | .824 | .474 | .566 | ||
| Slope Middle 50%, AD | Correlation Coefficient | .286 | −.079 | .083 | .034 | −.148 | |
| Sig. (2-tailed) | .091 | .646 | .629 | .842 | .389 | ||
|
| |||||||
| pMFC | UTE-T2* Minimum, L4P | Correlation Coefficient | −.301 | −.109 | −.096 | −.273 | −.094 |
| Sig. (2-tailed) | .074 | .527 | .577 | .107 | .586 | ||
| UTE-T2* Maximum, L4P | Correlation Coefficient | −.109 | −.414* | .094 | −.043 | −.122 | |
| Sig. (2-tailed) | .528 | .012 | .584 | .804 | .478 | ||
| Inflection Point, L4P | Correlation Coefficient | .054 | .008 | .094 | .092 | .124 | |
| Sig. (2-tailed) | .755 | .961 | .586 | .595 | .473 | ||
| Slope Factor, L4P | Correlation Coefficient | −.191 | .153 | −.227 | −.101 | −.083 | |
| Sig. (2-tailed) | .265 | .373 | .184 | .557 | .632 | ||
| Slope Deepest 25%, AD | Correlation Coefficient | .052 | −.186 | −.027 | .005 | −.042 | |
| Sig. (2-tailed) | .763 | .277 | .877 | .979 | .806 | ||
| Slope Middle 50%, AD | Correlation Coefficient | .212 | −.184 | −.089 | .062 | −.178 | |
| Sig. (2-tailed) | .215 | .282 | .606 | .719 | .298 | ||
Shaded orange indicates P<0.05.
Bold indicates significant after adjustment for multiple comparisons
Reported p values are unadjusted for FDR.
Footnotes
Author contributions
Ashley Williams contributed to conception of the study, designed the algorithms to analyze the MRI data, contributed to interpretation of MRI data, drafted and critically revised the manuscript, and approved final submission. Matthew Titchenal acquired the MRI, gait and PRO data, analyzed the gait and PRO data, contributed to interpretation of MRI, gait and PRO data, revised the article critically, and approved final submission. Thomas Andriacchi contributed to MRI processing scheme design and interpretation of MRI and gait data, revised the article critically, and approved final submission. Constance R. Chu contributed to conception of the study and interpretation of MRI, gait and PRO data, critically revised the manuscript, and approved the final submission.
Competing interests statement
The authors have no conflicts of interest.
References
- 1.Oiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009 Jul;37(7):1434–43. doi: 10.1177/0363546509338827. [DOI] [PubMed] [Google Scholar]
- 2.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004 Mar;63(3):269–73. doi: 10.1136/ard.2003.008136. Epub 2004/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. J Biomech. 2010 Jun 18;43(9):1817–22. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanlan SF, Favre J, Andriacchi TP. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J Biomech. 2013 Mar 15;46(5):849–54. doi: 10.1016/j.jbiomech.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 5.Zabala ME, Favre J, Scanlan SF, Donahue J, Andriacchi TP. Three-dimensional knee moments of ACL reconstructed and control subjects during gait, stair ascent, and stair descent. J Biomech. 2013 Feb 1;46(3):515–20. doi: 10.1016/j.jbiomech.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012 Feb;40(2):276–85. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 7.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014 May;42(5):1049–57. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 8.Chu CR, Williams AA, West RV, Qian Y, Fu FH, Do BH, et al. Quantitative magnetic resonance imaging UTE-T2* mapping of cartilage and meniscus healing after anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2014 Aug;42(8):1847–56. doi: 10.1177/0363546514532227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyde A, Riggs CM, Bushby AJ, McDermott B, Pinchbeck GL, Clegg PD. Cartilage damage involving extrusion of mineralisable matrix from the articular calcified cartilage and subchondral bone. Eur Cell Mater. 2011 May 28;21:470–8. doi: 10.22203/ecm.v021a35. discussion 8. [DOI] [PubMed] [Google Scholar]
- 10.Su F, Hilton JF, Nardo L, Wu S, Liang F, Link TM, et al. Cartilage morphology and T1rho and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage. 2013 Aug;21(8):1058–67. doi: 10.1016/j.joca.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage. 2007 Feb;15(2):198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med. 2009 Jun;61(6):1310–8. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Takahashi AM, Chung CB. Ultrashort TE spectroscopic imaging (UTESI): application to the imaging of short T2 relaxation tissues in the musculoskeletal system. J Magn Reson Imag. 2009 Feb;29(2):412–21. doi: 10.1002/jmri.21465. Epub 2009/01/24. [DOI] [PubMed] [Google Scholar]
- 14.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010 Apr;18(4):539–46. doi: 10.1016/j.joca.2010.02.001. Epub 2010/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urish KL, Keffalas MG, Durkin JR, Miller DJ, Chu CR, Mosher TJ. T2 texture index of cartilage can predict early symptomatic OA progression: data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2013 Oct;21(10):1550–7. doi: 10.1016/j.joca.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams A, Winalski CS, Chu CR. Early articular cartilage MRI T2 changes after anterior cruciate ligament reconstruction correlate with later changes in T2 and cartilage thickness. J Orthop Res. 2017 Mar;35(3):699–706. doi: 10.1002/jor.23358. Epub 2016 Jul 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011 Apr;65(4):1184–94. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 18.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2–preliminary findings at 3 T. Radiology. 2000 Jan;214(1):259–66. doi: 10.1148/radiology.214.1.r00ja15259. Epub 2000/01/22. [DOI] [PubMed] [Google Scholar]
- 19.Cha JG, Lee JC, Kim HJ, Han JK, Lee EH, Kim YD, et al. Comparison of MRI T2 relaxation changes of knee articular cartilage before and after running between young and old amateur athletes. Korean J Radiol. 2012 Sep-Oct;13(5):594–601. doi: 10.3348/kjr.2012.13.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosher TJ, Liu Y, Yang QX, Yao J, Smith R, Dardzinski BJ, et al. Age dependency of cartilage magnetic resonance imaging T2 relaxation times in asymptomatic women. Arthritis Rheum. 2004 Sep;50(9):2820–8. doi: 10.1002/art.20473. Epub 2004/10/01. [DOI] [PubMed] [Google Scholar]
- 21.Chu CR, Andriacchi TP. Dance between biology, mechanics, and structure: a systems-based approach to developing osteoarthritis prevention strategies. J Orthop Res. 2015 Jul;33(7):939–47. doi: 10.1002/jor.22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med. 2009 May;43(5):366–70. doi: 10.1136/bjsm.2008.052522. [DOI] [PubMed] [Google Scholar]
- 23.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009 Feb;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samaan MA, Facchetti L, Pedoia V, Tanaka MS, Link TM, Souza RB, et al. Cyclops lesions are associated with altered gait patterns and medial knee joint cartilage degeneration at 1 year after ACL-reconstruction. J Orthop Res. 2017 Oct;35(10):2275–81. doi: 10.1002/jor.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng HL, Wu D, Su F, Pedoia V, Souza RB, Ma CB, et al. Gait characteristics associated with a greater increase in medial knee cartilage T1rho and T2 relaxation times in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017 Sep 01; doi: 10.1177/0363546517723007. [DOI] [PubMed]
- 26.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcome. 2003 Nov 03;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Gait mechanics 2 years after anterior cruciate ligament reconstruction are associated with longer-term changes in patient-reported outcomes. J Orthop Res. 2017 Mar;35(3):634–40. doi: 10.1002/jor.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titchenal MR, Chu CR, Erhart-Hledik JC, Andriacchi TP. Early changes in knee center of rotation during walking after anterior cruciate ligament reconstruction correlate with later changes in patient-reported outcomes. Am J Sports Med. 2017 Mar;45(4):915–21. doi: 10.1177/0363546516673835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006 Mar;55(3):575–82. doi: 10.1002/mrm.20796. [DOI] [PubMed] [Google Scholar]
- 30.Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011 Jan;19(1):84–8. doi: 10.1016/j.joca.2010.10.018. Epub 2010/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeLean A, Munson P, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radio-ligand assay, and physiological dose-response curves. Am J Physiol. 1978;235(2):E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- 32.Brown AM. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput Meth Progr Biomed. 2001 Jun;65(3):191–200. doi: 10.1016/s0169-2607(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 33.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. Faseb J. 1987 Nov;1(5):365–74. [PubMed] [Google Scholar]
- 34.Dyrby CO, Andriacchi TP. Secondary motions of the knee during weight bearing and non-weight bearing activities. J Orthop Res. 2004 Jul;22(4):794–800. doi: 10.1016/j.orthres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Alexander EJ, Andriacchi TP. Correcting for deformation in skin-based marker systems. J Biomech. 2001 Mar;34(3):355–61. doi: 10.1016/s0021-9290(00)00192-5. [DOI] [PubMed] [Google Scholar]
- 36.Favre J, Erhart-Hledik JC, Andriacchi TP. Age-related differences in sagittal-plane knee function at heel-strike of walking are increased in osteoarthritic patients. Osteoarthritis Cartilage. 2014 Mar;22(3):464–71. doi: 10.1016/j.joca.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chehab EF, Favre J, Erhart-Hledik JC, Andriacchi TP. Baseline knee adduction and flexion moments during walking are both associated with 5 year cartilage changes in patients with medial knee osteoarthritis. Osteoarthritis Cartilage. 2014 Nov;22(11):1833–9. doi: 10.1016/j.joca.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Favre J, Erhart-Hledik JC, Chehab EF, Andriacchi TP. Baseline ambulatory knee kinematics are associated with changes in cartilage thickness in osteoarthritic patients over 5 years. J Biomech. 2016 Jun 14;49(9):1859–64. doi: 10.1016/j.jbiomech.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 39.Scanlan SF, Donahue JP, Andriacchi TP. The in vivo relationship between anterior neutral tibial position and loss of knee extension after transtibial ACL reconstruction. Knee. 2014 Jan;21(1):74–9. doi: 10.1016/j.knee.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B. 1995;57:289–300. [Google Scholar]
- 41.Palmieri-Smith RM, Wojtys EM, Potter HG. Early cartilage changes after anterior cruciate ligament injury: evaluation with imaging and serum biomarkers-a pilot study. Arthroscopy. 2016 Jul;32(7):1309–18. doi: 10.1016/j.arthro.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 42.Pedoia V, Su F, Amano K, Li Q, McCulloch CE, Souza RB, et al. Analysis of the articular cartilage T1rho and T2 relaxation times changes after ACL reconstruction in injured and contralateral knees and relationships with bone shape. J Orthop Res. 2017 Mar;35(3):707–17. doi: 10.1002/jor.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brisson NM, Wiebenga EG, Stratford PW, Beattie KA, Totterman S, Tamez-Pena JG, et al. Baseline knee adduction moment interacts with body mass index to predict loss of medial tibial cartilage volume over 2.5 years in knee osteoarthritis. J Orthop Res. 2017 Nov;35(11):2476–83. doi: 10.1002/jor.23564. [DOI] [PubMed] [Google Scholar]
- 44.Sowers M, Karvonen-Gutierrez CA, Palmieri-Smith R, Jacobson JA, Jiang Y, Ashton-Miller JA. Knee osteoarthritis in obese women with cardiometabolic clustering. Arthritis Rheum. 2009 Oct 15;61(10):1328–36. doi: 10.1002/art.24739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clin Orthop Relat Res. 2006 Jan;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 46.Shelbourne KD, Gray T. Minimum 10-year results after anterior cruciate ligament reconstruction: how the loss of normal knee motion compounds other factors related to the development of osteoarthritis after surgery. Am J Sports Med. 2009 Mar;37(3):471–80. doi: 10.1177/0363546508326709. [DOI] [PubMed] [Google Scholar]
- 47.Webster KE, Feller JA. Alterations in joint kinematics during walking following hamstring and patellar tendon anterior cruciate ligament reconstruction surgery. Clin Biomech. 2011 Feb;26(2):175–80. doi: 10.1016/j.clinbiomech.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Su F, Pedoia V, Teng HL, Kretzschmar M, Lau BC, McCulloch CE, et al. The association between MR T1rho and T2 of cartilage and patient-reported outcomes after ACL injury and reconstruction. Osteoarthritis Cartilage. 2016 Jul;24(7):1180–9. doi: 10.1016/j.joca.2016.01.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amano K, Li AK, Pedoia V, Koff MF, Krych A, Link TM, et al. Effects of surgical factors on cartilage can be detected using quantitative magnetic resonance imaging after anterior cruciate ligament reconstruction. Am J Sports Med. 2017 Apr;45(5):1075–84. doi: 10.1177/0363546516677794. [DOI] [PubMed] [Google Scholar]
- 50.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Longitudinal changes in knee gait mechanics between 2 and 8 years after anterior cruciate ligament reconstruction. J Orthop Res. 2017 Oct 06; doi: 10.1002/jor.23770. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]