Abstract
Fresh osteochondral allografts are an important treatment option for the repair of full-thickness articular cartilage defects. Viable chondrocytes within the transplanted tissue are considered important to maintaining matrix integrity. The purpose of this study is to determine whether an increase in pH decreases chondrocyte viability during cold storage and whether equilibration of Dulbecco’s modified Eagle’s medium (DMEM) in 5% CO2 normalizes pH and increases chondrocyte survival during storage at 4°C. Freshly isolated bovine articular chondrocytes cultured in alginate beadswere stored for up to 5 days at 4°C or 37°C in DMEM exposedto ambientair or in DMEM equilibrated with 5% CO2. Chondrocyte viability was determined by flow cytometry. Physiologic pH was maintained when DMEM was equilibrated with 5% CO2, while pH increased in ambient air. After 5 days of storage at 4°C, chondrocyte necrosis was higher when stored in ambient air than if equilibrated with 5% CO2. No decrease in chondrocyte viability was observed with storage at 37°C. In addition, chondrocyte viability in bovine cartilage osteochondral cores was examined after storage for 14 days at 4°C in DMEM with and without HEPES, and with and without 5% CO2. Under these conditions, the superficial layer of chondrocytes was more viable when stored in DMEM with HEPES or DMEM equilibrated with 5% CO2 than when stored in DMEM in ambient air. This data shows that an increase in pH decreased bovine chondrocyte viability when refrigerated at 4°C in DMEM, and that optimization of CO2 normalized pH and improved chondrocyte viability during cold storage in DMEM.
Keywords: articular cartilage, chondrocyte viability, osteochondral allograft, cold storage media, slow cytometry
INTRODUCTION
Treatment of full-thickness defects in articular cartilage continues to be a clinical and scientific challenge.1,2 Under normal biological conditions, cartilage allows for smooth and pain-free joint motion, but lacks mechanisms for repair and/or regeneration following injury. Implantation of fresh osteochondral allografts harvested from human cadavers can replace defective cartilage with viable, healthy cartilage.3,4 The use of osteochondral grafts is a reliable option for treatment of both large and small defects in articular cartilage and results in improved pain, function, and range of motion.4–6
Due to clinical requirements for thorough microbiological testing and donor screening, osteochondral tissuesare typically refrigerated for a minimum of 14 days prior to surgery. Viable and functional chondrocytes have been shown to decrease significantly within 14 days of storage at 4°C.7,8 The synthesis of cartilage matrix is dependent on metabolically active chondrocytes, suggesting that the presence of living and functional chondrocytes may be important to longevity of the allograft.
Temperature and pH are two variables that have been implicated in the viability of chondrocytes during long-term storage. Osteochondral tissues have traditionally been stored in Dulbecco’s modified Eagle’s medium (DMEM) with ambient air, which has a bicarbonate buffering system that requires 5% CO2 to maintain a physiological pH near 7.4. Storage conditions lacking sufficient CO2 may contribute to modification of the extracellular and intracellular pH of the chondrocytes, thereby altering extracellular matrix production and resulting in cell death.
The purpose of this study is to test the hypotheses that an increase in pH decreases chondrocyte viability during cold storage and that equilibration of DMEM in 5% CO2 normalizes pH and increases chondrocyte survival during storage at 4°C.
METHODS
Chondrocyte viability was assessed in alginate bead culture over a 5-day period and in osteochondral cores after 14 days. Alginate beads have been established as a reproducible three-dimensional tissue culture model for cartilage by maintaining chondrocyte phenotype, extracellular matrix synthesis, and metabolic regulation.9–11 Using this model, chondrocytes can be isolated from the alginate matrix and undergo quantitative flow cytometry. Osteochondral tissues permit in situ examination of the distribution of viable cells within a full-thickness specimen of articular cartilage.
Alginate Bead Experiment
Full-thickness slices of cartilage were aseptically removed from freshly slaughtered bovine knees and articular chondrocytes were isolated from cartilage with pronase (EMD Chemicals, San Diego, CA) and then collagenase (Roche Applied Science, Indianapolis, IN) enzymatic digestion. The cells were cultured into alginate beads with a cell density of 4 × 106 chondrocytes/ml alginate as described by Masuda et al.11 A second group of alginate beads were created without chondrocytes to serve as a control group. The beads were stored in 100-mL tissue culture dishes in chondrocyte growth media [1:1 DMEM/Nutrient Mix F-12 (/F-12) +10% fetal bovine serum plus 1% penicillin/streptomycin; Invitrogen, Grand Island, NY] at 37°C/5% CO2 for a period of no longer than 7 days, with media changes every other day. After this initial incubation period, 180 beads with chondrocytes, and 90 beads without chondrocytes were removed and separated in the following fashion:
DMEM/Ambient Air/37°C
Forty-five alginate beads with chondrocytes were placed into one 14-mL round bottom tube with 3 ml chondrocyte growth media (1:1 DMEM/F-12 +10% fetal bovine serum plus 1% penicillin/streptomycin). The loosely capped tube was incubated at room temperature in ambient air for 20 min, and then placed into a 37°C incubator with the cap on tight.
DMEM/5% CO2/37 °C
Three groups of 15 alginate beads with chondrocytes were placed into three 14-mL round bottom tubes with 1 mL chondrocyte growth media (1:1 DMEM/F-12 +10% fetal bovine serum plus 1% penicillin/streptomycin) in each. The tubes were placed into 37°C/5% CO2 incubation with a loose cap and allowed to equilibrate with 5% CO2 for 20 min. The caps were snapped on tight and remained in the 37°C incubator.
DMEM/Ambient Air/4°C
Forty-five beads with chondrocytes were placed into one 14-mL round bottom tube with 3 ml chondrocyte growth media (1:1 DMEM/F-12 +10% fetal bovine serum plus 1% penicillin/streptomycin). One 3-mL aliquot of chondrocyte growth media with 45 chondrocyte-free alginate beads and one 3-mL aliquot of chondrocyte growth media were used as a control. The loosely capped tubes were incubated at room temperature in ambient air for 20 min, and then placed into a 4°C cold room with the cap on tight.
DMEM/5% CO2/4 °C
Three groups of 15 alginate beads with chondrocytes were placed into three 14-mL round bottom tubes with 1 mL chondrocyte growth media (1:1 DMEM/F-12 +10% fetal bovine serum plus 1% penicillin/streptomycin) in each. Three 1-mL aliquots of chondrocyte growth media with 15 chondrocyte-free alginate beads and three 1-mL aliquots of chondrocyte growth media were used as a control. The tubes were placed with loose caps into a 37°C/5% CO2 incubator and allowed to equilibrate with 5% CO2 for 20 min. Then the cap was snapped on tight, and the tube was placed into a 4°C cold room.
After the beads were segregated by temperature and CO2 exposure, the media was not changed again in order to simulate the storage conditions of allografts. The ratio of storage media volume to bead number was standardized with the other groups by storing 15 beads per 1 mL of media.
After 1, 3, and 5 days of storage, 10 beads from each experimental group (warm, ambient air; warm, 5% CO2; cold, ambient air; cold, 5% CO2) were aseptically removed and placed in 55-mM sodium citrate at 4°C to dissolve the alginate and release the chondrocytes. In groups stored with ambient air, the remaining beads were left in the tubes for further storage and collection at subsequent timepoints. After 10 min, the chondrocytes from experimental groups were harvested by centrifugation for 5 min at 600 g and washed twice with phosphate buffered saline (Sigma-Aldrich, St. Louis, MO). Each sample was labeled with a Vybrant® Apoptosis Assay Kit #3 (Invitrogen, Eugene, OR) as directed. One hundred microliters of each suspension was aliquoted into separate microcentrifuge tubes. Five microliters of Alexa Fluor 488 Annexin V and 1 μL of 100 μg/mL propidium iodide working solution was added to each cell suspension and allowed to incubate at room temperature for 15 min while protected from light. Finally, 400 μL of 1 × Annexin Binding Buffer was added to each sample, the samples were placed on ice, and analyzed immediately by flow cytometry. The pH of storage media in each experimental and control group (cold, ambient air; cold, ambient air, alginate; cold, 5% CO2; cold, 5% CO2, alginate) was measured with an Accumet Basic AB 15 pH meter (Fischer Scientific, Pittsburgh, PA) immediately after initial gas equilibration and prior to each assay timepoint.
The above procedure was repeated three times with three separate preparations of bovine chondrocytes and alginate beads. Means and standard deviations were calculated and the statistical significance was determined by analysis of variance (ANOVA) followed by a Bonferroni t-test.
Osteochondral Core Tissue Experiment
An 8-mm Mitek coring device was used to harvest 24 osteochondral cores from the trochlear groove of bovine knees obtained within 4 h of slaughter. Isolated cores were washed with Hank’s balanced salt solution (HBSS) and randomly assigned to eight groups of three cores each and placed into 0 or 14 days of cold storage. Cores were prepared as follows:
DMEM/Ambient Air/4°C (Air/DMEM)
Three cores were placed into separate 14-mL round bottom tubes and immersed in chondrocyte growth media (1:1 DMEM/F-12 +10% fetal bovine serum plus 1% penicillin/streptomycin). The loosely capped tubes were incubated at room temperature in ambient air for 20 min, and then placed into a 4°C cold room with the cap on tight until analysis.
DMEM +HEPES/Ambient Air/4°C (Air/HEPES)
Three cores were placed into separate 14-mL round bottom tubes and immersed in chondrocyte growth media (1:1 DMEM/F-12 with 15 mM HEPES buffer and L-glutamine +10% fetal bovine serum plus 1% penicillin/streptomycin). The loosely capped tubes were incubated in ambient air at room temperature for 20 min, and then placed into a 4°C cold room with the cap on tight until analysis.
DMEM/5% CO2/4 °C (CO2/DMEM)
Three cores were placed into 14-mL round bottom tubes and immersed in chondrocyte growth media (1:1 DMEM/F-12 +10% bovine serum plus 1% penicillin/streptomycin). The tubes were incubated at 37°C/5% CO2 with a loose cap and allowed to equilibrate with 5% CO2 for 20 min. The cap was then snapped on tight and placed in the 4°C cold room until analysis. DMEM +HEPES/5% CO2/4 °C (CO2/HEPES) Three cores were placed into separate 14-mL round bottom tubes and immersed in chondrocyte growth media (1:1 DMEM/F-12 with 15 mM HEPES buffer and L-glutamine +10% fetal bovine serum plus 1% penicillin/streptomycin). The tubes were incubated at 37°C/5% CO2 with a loose cap and allowed to equilibrate with 5% CO2 for 20 min. The caps were then snapped on tight and placed in the 4°C cold room until analysis.
After the cores were segregated by CO2 exposure, the media was not changed again in order to simulate the storage conditions of allografts.
At the analysis timepoint (day 0 or day 14), cores were placed at 37°C for 24 h to simulate allograft warming. The cores were cut into orthogonal sections approximately 0.5-mm thick from the bovine cores, and stained with 5-chloromethylfluorescein diacetate (CMFDA; Molecular Probes, Inc., Eugene, OR) and propidium iodide. Stained sections were washed with HBSS and live/dead cells were imaged by confocal microscopy.
To provide consistent image acquisition for counting of live/dead cells from 3-D reconstructed images (150-μm stacked sections), each core was imaged at the surface [superficial cartilage/fluid interface (outer 80 μm)], core midpoint [core midpoint (80-μm region halfway between outer surface and bone interface)], and deep [deep core region (80-μm region halfway between the midpoint and the bone interface)]. Analysis of reconstructed 3-D core sections were counted using Image Pro Plus 6.2 (MediaCybernetics, Inc., Rockville, MD), and data from day 14 was normalized to day 0. Statistical significance was determined by two-way ANOVA followed by a Bonferroni t-test.
RESULTS
Chondrocyte Viability Analyzed by Flow Cytometry
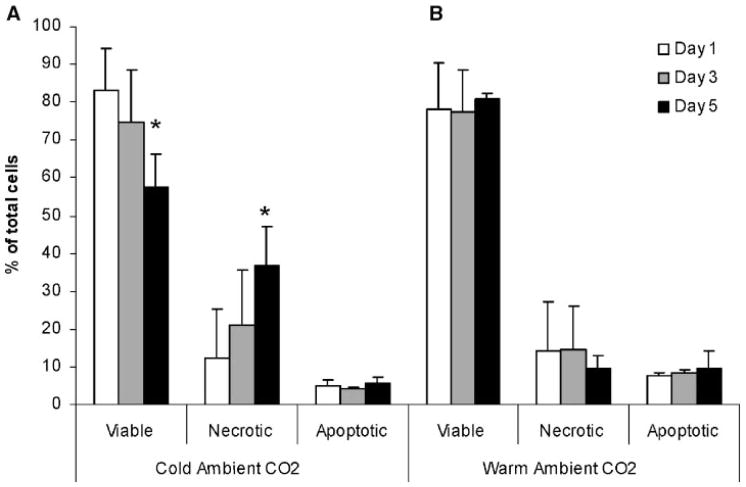
Chondrocytes stored at 4°C (cold), with ambient air showed a progressive loss of viability over time (Fig. 1A). The viability after 1 day was 83% ± 11% and decreased to 57% ± 9% after 5 days (p =0.02). No significant change in the percentage of apoptotic cells was seen over this time period. These conditions were chosen to match those commonly used for storage of osteochondral transplant tissues. Chondrocytes stored at 37°C (warm) in ambient air showed no decrease in viability over the 5-day period staying between 78% ± 12% and 81% ± 1% (Fig. 1B). Again, no significant changes in the percentage of apoptotic cells were noted over this time period.
Figure 1.
Comparison of (A) cold (4°C) versus (B) warm (37°C) storage of chondrocytes in media with ambient air. The percentage of viable cells decreased over time, for cells in cold storage. No change in the percentage of apoptotic cells was seen over 5 days of storage (*p =0.02).
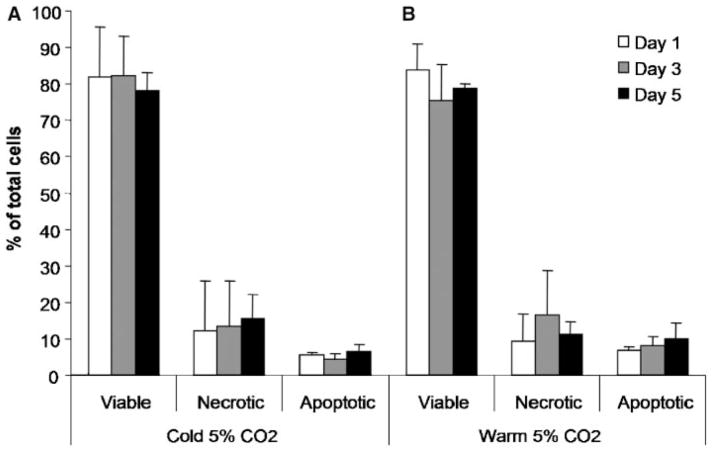
When the storage media was equilibrated with 5% CO2 and stored at 4°C, viability was maintained over the 5-day storage period, starting at 82% ± 14% and ending at 78% ± 5% (Fig. 2A). Storage of cells equilibrated in 5% CO2 at 37°C showed a similar pattern of maintaining viability over 5 days, starting at 83% ± 7% and ending at 79% ± 1% (Fig. 2B).
Figure 2.
Comparison of (A) cold (4°C) versus (B) warm (37°C) storage of chondrocytes in media equilibrated with 5% CO2. There was no change in the percentage of viable, necrotic, or apoptotic cells over 5 days of storage.
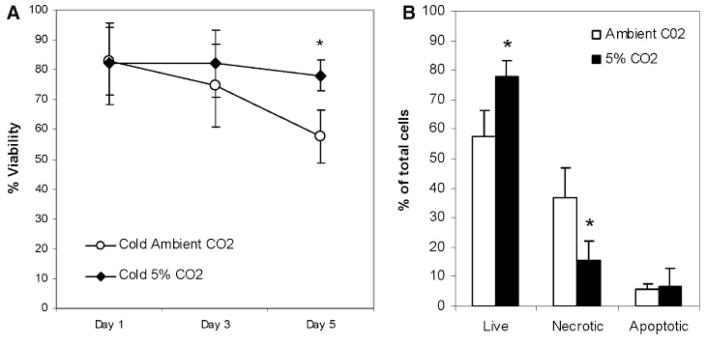
Comparing the 4°C storage conditions, the group equilibrated in 5% CO2 maintained a significantly higher degree of viability than the group in ambient air (p =0.02, Fig. 3A).
Figure 3.
Comparison of cold storage conditions. (A) A decrease in the number of viable cells was seen over time. (B) On day 5, the chondrocytes in media equilibrated with 5% CO2 showed a significantly higher percentage of viable and a lower percentage of nonviable cells (*p =0.02).
Measurement of pH
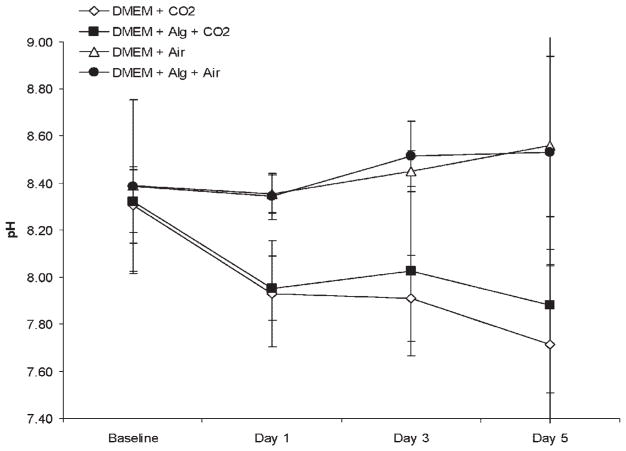
Among control groups stored without chondrocytes, the pH of DMEM stored at 4°C/5%CO2 with and without alginate demonstrated a decrease in pH from a baseline of 8.3 in both groups, to a similar pH of 7.9 and 7.7 (p =0.51) at day 5, respectively. In contrast, the pH of DMEM stored at 4°C/ambient air with and without alginate demonstrated an increase in pH from a baseline of 8.4 in both groups, to a similar alkaline pH of 8.5 and 8.6 (p =0.84) at day 5, respectively (Fig. 4).
Figure 4.
pH of storage media in 4°C in control experiment. In groups stored with ambient air, the pH increased to a statistically similar basic pH at day 5 (p =0.84), regardless of alginate addition. In groups stored with 5% CO2, the pH changed to a similar pH (p = 0.51) at day 5, regardless of alginate addition. A significant increase in pH was shown in media stored with alginate and ambient air compared to media stored with alginate and 5% CO2 (p < 0.05).
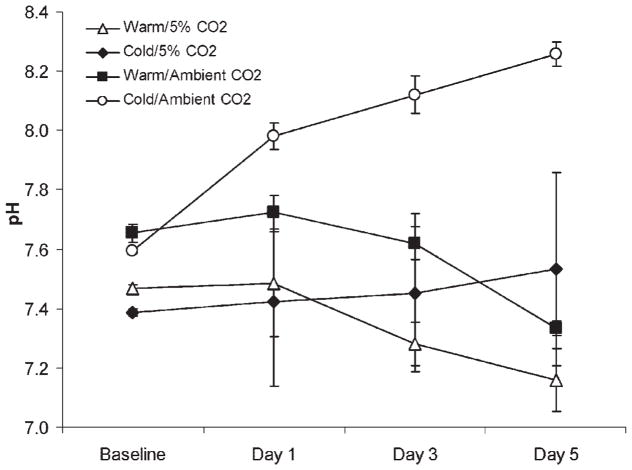
Also at day 5, DMEM with alginate/5%CO2 had a pH of 7.9, whereas DMEM with alginate/ambient air had a pH of 8.5 (p <0.05) (Fig. 4). Among experimental groups stored with chondrocytes, the initial pH of storage media which had been equilibrated in 5% CO2 was lower than that in ambient air (Fig. 5). The pH of both of the warm storage groups declined slightly over the 5-day incubation period (Fig. 5). The warm, ambient air group decreased from pH 7.7 to pH 7.3 and the warm group with 5% CO2 decreased from pH 7.5 to pH 7.2 (p <0.05) by day 5. In contrast to the warm storage groups, the pH of the cold groups diverged. The pH for cold media with 5% CO2 went from pH 7.4 to pH 7.5. However, the cold group with ambient air increased significantly from pH 7.6 to pH 8.3. This increase in media alkalinity was associated with an increase in necrotic cells after 5 days (Fig. 3B).
Figure 5.
pH of chondrocyte growth media in each storage group. The pH increased significantly over time with cells stored at 4°C with ambient air (p <0.05).
Osteochondral Core Tissue Experiment
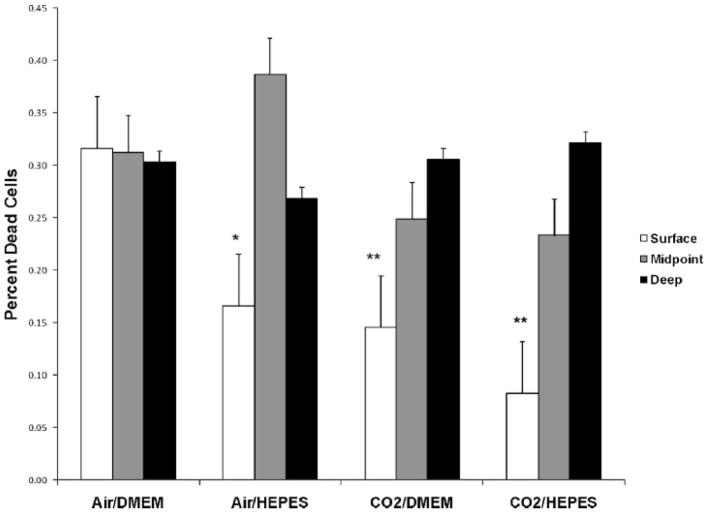
Following 14 days of storage at 4°C and rewarming for 24 h at 37°C, the deep and middle regions of osteochondral cores had similar viability; >30% of remaining cells were dead (Fig. 6). Within the superficial layers, CO2/DMEM (15 ± 6%) had fewer dead chondrocytes when compared with Air/DMEM (32 ± 3%, p <0.001). Further, when comparing the superficial region of osteochondral cores between Air/DMEM (32 ± 3%) and Air/HEPES (17 ± 5%), storage with the addition of HEPES improved chondrocyte viability (p <0.01). Chondrocyte viability in CO2/HEPES (8 ± 5%) also exhibited fewer dead cells within the superficial core regions when compared to Air/DMEM (32 ± 3%, p <0.05). While there was a trend towards increased chondrocyte viability in the groups equilibrated with CO2 (Fig. 6), no statistical difference in the percent of dead chondrocytes was observed between Air/HEPES, CO2/DMEM, and CO2/HEPES in the superficial osteochondral core regions.
Figure 6.
Percentage of dead chondrocytes on day 14 (normalized to day 0) in osteochondral cores stored at 4°C. Equilibration of DMEM in CO2 and/or the addition of HEPES to DMEM led to a significant decrease in chondrocyte death compared to DMEM with cold storage (4°C). There was no significant difference in cell death between Air/HEPES, CO2/DMEM, and CO2/HEPES. Significant difference at *p <0.01 and **p <0.001 versus Air/DMEM.
DISCUSSION
Transplantation of fresh osteochondral allografts is an important clinical option for the treatment of full-thickness cartilage defects.3–6 Fresh osteochondral allografts have traditionally been stored at 4°C in basal media. This study shows that: 1) an increase in pH decreases the viability of bovine chondrocytes stored at 4°C in DMEM; and that 2) optimization of CO2 normalizes pH and improves bovine chondrocyte viability during cold storage. These results provide information potentially important to improving chondrocyte viability during cold storage of osteochondral tissues and to improved understanding of chondrocyte homeostasis.
Fresh osteochondral allografts offer a potential clinical advantage over frozen osteochondral tissues due to the presence of live chondrocytes to maintain the cartilage matrix. Aubin et al. in a cohort study showed 85% graft survival 10 years after osteochondral allograft transplantation in the knee.12 Beaver et al. reported 64% graft survival at 10-years posttransplant.13 In both these studies, the grafts were used within 48 h after asystole. Currently, fresh osteochondral allografts are not commonly available until 14 days after tissue harvest with uncertain effects on chondrocyte viability.7,8 In a recent study of baboon knee allograft transplants, Malinin et al. suggested that storing the allograft in 4°C for more than 21 days prior to transplant was directly related with poor graft appearance grossly and histologically among allografts that were examined in vivo 6 weeks after transplant.14
Many studies have shown a substantial decrease in chondrocyte viability with increasing duration of cold storage.15–18 Our data shows that a progressive loss of chondrocyte viability seen over 5 days when the chondrocyte cultures were stored in DMEM at 4°C under ambient air was accompanied by an increase in pH. In contrast, when the pH was normalized by equilibration with 5% CO2, chondrocyte survival during 5 days of cold storage improved. This data provides a possible explanation for the observed decrease in chondrocyte viability following cold storage in containers sealed in ambient air. Tissue culture media, such as DMEM is usually buffered with a bicarbonate solution that requires an atmosphere with 5% CO2 to maintain an optimal physiological pH. Without adequate CO2, the pH becomes basic, as was measured in our alginate bead experiments.
When stored in DMEM at 37°C in ambient air or with 5% CO2, the pH of the media for cell-seeded alginate beads remained close to the physiologic range throughout the study period. Maintenance of pH at 37°C was likely due to cell metabolism. In contrast, our data showing that the pH of the media for cell-seeded alginate beads stored in DMEM in ambient air at 4°C was comparable to similarly stored cell-free alginate beads indicate that chondrocytes in cold storage likely had decreased metabolic activity consistent with the expected effect of refrigeration.
Results of the osteochondral core studies using DMEM buffered with HEPES, a CO2 independent buffering agent, support that an increase in pH contributed to increased chondrocyte cell death during cold storage. The addition of HEPES to DMEM improved superficial chondrocyte cell viability following 14 days of storage at 4°C. A trend towards increasing superficial chondrocyte viability in osteochondral cores stored at 4°C in DMEM equilibrated with 5% CO2, suggests that both CO2 equilibration and use of HEPES buffer may be valuable strategies to reduce chondrocyte death in osteochondral tissues during cold storage in DMEM.
In conclusion, maintaining chondrocyte viability during cold storage of fresh osteochondral tissues prior to clinical transplantation is considered important to long-term survival and performance of the allograft. Our data showing that an increase in media pH during cold storage at 4°C reduces chondrocyte survival in DMEM, and that equilibration of DMEM with CO2 improves chondrocyte viability under the same conditions. This provides information important to both the study of methods to optimize chondrocyte survival of osteochondral grafts during prolonged ex vivo storage and to understanding factors important to chondrocyte homeostasis.
Acknowledgments
The authors thank Lesa M. Lewis Werkmeister and Nicole Papas for their technical assistance. The authors have no professional or financial affiliations that may be perceived to have biased this presentation.
References
- 1.Scopp JM, Mandelbaum BR. Cartilage restoration: overview of treatment options. J Knee Surg. 2004;17:229–233. doi: 10.1055/s-0030-1248227. [DOI] [PubMed] [Google Scholar]
- 2.Scopp JM, Mandelbaum BR. A treatment algorithm for the management of articular cartilage defects. Orthop Clin North Am. 2005;36:419–426. doi: 10.1016/j.ocl.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Chu CR, Convery FR, Akeson WH, et al. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;360:159–168. [PubMed] [Google Scholar]
- 4.Ghazavi MT, Stockley I, Yee G, et al. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg [Br] 1997;79:1008–1013. doi: 10.1302/0301-620x.79b6.7534. [DOI] [PubMed] [Google Scholar]
- 5.Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67–75. doi: 10.1016/s0278-5919(05)70130-7. [DOI] [PubMed] [Google Scholar]
- 6.Jamali AA, Emmerson BC, Chung C, et al. Fresh osteochondral allografts. Clin Orthop Relat Res. 2005;437:176–185. [PubMed] [Google Scholar]
- 7.Allen RT, Robertson CM, Pennock AT, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sports Med. 2005;33:1479–1484. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 8.Rohde RS, Studer RK, Chu CR. Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clin Orthop Relat Res. 2004;427:226–233. doi: 10.1097/01.blo.0000138955.27186.8e. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Izzo NJ, Papas NE, et al. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693–699. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Hauselmann HJ, Aydelotte MB, Schumacher BL, et al. Synthesis and turnover of proteoglycans by human and bovine adult articular chondrocytes cultured in alginate beads. Matrix. 1992;12:116–129. doi: 10.1016/s0934-8832(11)80053-3. [DOI] [PubMed] [Google Scholar]
- 11.Masuda K, Sah RL, Hejna MJ, et al. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139–148. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 12.Aubin PP, Cheah HK, Davis AM, et al. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;391(Suppl):S318–S327. doi: 10.1097/00003086-200110001-00029. [DOI] [PubMed] [Google Scholar]
- 13.Beaver RJ, Mahomed M, Backstein D, et al. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg [Br] 1992;74:105–110. doi: 10.1302/0301-620X.74B1.1732235. [DOI] [PubMed] [Google Scholar]
- 14.Malinin T, Temple HT, Buck BE. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg [Am] 2006;88:762–770. doi: 10.2106/JBJS.D.02991. [DOI] [PubMed] [Google Scholar]
- 15.Csonge L, Bravo D, Newman-Gage H, et al. Banking of osteochondral allografts, part II. Preservation of chondrocyte viability during long-term storage. Cell Tissue Bank. 2002;3:161–168. doi: 10.1023/A:1023687419152. [DOI] [PubMed] [Google Scholar]
- 16.Pearsall AWT, Tucker JA, Hester RB, et al. Chondrocyte viability in refrigerated osteochondral allografts used for transplantation within the knee. Am J Sports Med. 2004;32:125–131. doi: 10.1177/0095399703258614. [DOI] [PubMed] [Google Scholar]
- 17.Sammarco VJ, Gorab R, Miller R, et al. Human articular cartilage storage in cell culture medium: guidelines for storage of fresh osteochondral allografts. Orthopedics. 1997;20:497–500. doi: 10.3928/0147-7447-19970601-04. [DOI] [PubMed] [Google Scholar]
- 18.Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg [Am] 2003;85-A:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]