Abstract
Purpose:
To review the advances in radiation therapy for the management of pediatric cancers made by the Children’s Oncology Group (COG) radiation oncology discipline since its inception in 2000.
Methods and Materials:
The various radiation oncology disease site leaders reviewed the contributions and advances in pediatric oncology made through the work of COG. They have presented outcomes of relevant studies and summarized current treatment policies developed by consensus from experts in the field.
Results:
The indications and techniques for pediatric radiation therapy have evolved considerably over the years for virtually all pediatric tumor types, resulting in improved cure rates together with the potential for decreased treatment-related morbidity and mortality.
Conclusions:
The COG radiation oncology discipline has made significant contributions towards the treatment of childhood cancer. Our discipline is committed to continuing research to refine and modernize the use of radiation therapy in current and future protocols with the goal of further improving the cure rates and quality of life of children stricken with cancer.
Introduction
The American Cancer Society estimates that 10,270 new childhood cancer cases and 1190 childhood cancer deaths are expected in 2017.1 In 2000, four NCI-funded cooperative groups charged with studying childhood cancer [the Children’s Cancer Group (CCG), the Pediatric Oncology Group (POG), the Intergroup Rhabdomyosarcoma Study Group (IRSG), and the National Wilms Tumor Study (NWTS)] merged to form the Children’s Oncology Group (COG) – the largest cooperative group dedicated to the study of childhood cancer in the world. Due to the valuable contributions of these different cooperative groups and the excellent accrual of children on prospective national clinical protocols, childhood cancer mortality rates have declined by 66% and 5-year survival has increased from 58% in the past to 83% currently.1 The radiation oncology discipline (including radiation oncologists and medical physicists) has always been an integral part of each of these constituent groups and its seamless assimilation into COG has facilitated the ongoing work of the group in designing, conducting, analyzing and reporting prospective multi-institutional clinical trials for the treatment of childhood cancer. Our discipline was led by Dr. Robert Marcus (2000–2005), Dr. Tom Merchant (2005–2015) and currently Dr. John Kalapurakal (2016 - present). On the occasion of completing the 15th anniversary of the formation of COG, we highlight some of the significant contributions of the radiation oncology discipline and the impact our specialty has made on the treatment of childhood cancer.
CNS Tumors
Medulloblastoma, ependymoma and glioma are common pediatric CNS tumors which require radiotherapy (RT) as part of their treatment.2 Since 1970, the 5-year relative survival rates for children with CNS tumors have improved from 57% to 74%.3 The COG has led clinical advances in radiation treatment planning and delivery, enabling the exposure of RT doses to smaller volumes with fewer side effects (table 1).
Table 1:
Major radiotherapy advancements in pediatric CNS tumors
|
Radiotherapy, combined with surgery and chemotherapy is the cornerstone for the treatment of medulloblastoma. COG studies have been pivotal in delineating many specific treatment strategies that have improved clinical outcomes. Cranio-spinal irradiation (CSI) dose reduction from 36 Gy to 23.4 Gy in patients with average-risk disease yielded inferior event free survival (EFS) when delivered without chemotherapy4 but equivalent EFS if given with concurrent and adjuvant chemotherapy (COG study A9961).5 Compared to post-RT chemotherapy, neoadjuvant chemotherapy had no effect on 5-year EFS (POG study 9031).6 The addition of involved-field RT to post-operative chemotherapy improved EFS in infants not receiving CSI (POG study 9934).7 Lower dose CSI (18 Gy) with concurrent and adjuvant chemotherapy yielded inferior OS and EFS in children ages 3 to 7 years with average-risk medulloblastoma compared to higher doses (23.4 Gy), but reducing boost volumes from whole posterior-fossa to tumor bed plus margin did not compromise outcomes in average risk patients (COG study ACNS0331).8 For high-risk medulloblastoma, giving carboplatin concurrently with CSI as a radiosensitizer was tolerable, and resulted in an encouraging 5-year EFS of 71% (CCG study 99701).9 A trial evaluating concurrent carboplatin and isotretinoin with RT in high risk patients is currently ongoing (COG study ACNS0332). Current and future treatment paradigms incorporate molecular classification. The COG ACNS 1422 and the European SIOP PNET 5 protocols are evaluating 18 Gy CSI without concurrent chemotherapy for patients with WNT-driven non-metastatic medulloblastoma. For higher risk patients (M+ or poor molecular classification) radiosensitization or adjuvant therapy escalation is being considered.
The treatment paradigm for ependymoma consists of gross-total (GTR) or near-total resection (NTR) followed by post-operative RT. The first RT-inclusive COG ependymoma trial closed in 2007(COG study ACNS 0121).10 Children with grade 2 supratentorial tumors with a microscopic GTR were observed, while those who had grade 3 tumors received immediate post-operative RT (54–59.4Gy). Children with posterior fossa tumors who underwent sub-total resection (STR) received two cycles of chemotherapy, possible second-look surgery and RT (54–59.4Gy). Children with posterior fossa tumors who underwent GTR or NTR received immediate post-operative RT (54–59.4Gy). This trial showed that 45% of patients observed after completely resected grade 2 supratentorial ependymoma recurred. Following NTR, cisplatin-based chemotherapy yielded a 40% complete response rate and may have facilitated second look surgery. However, when given to patients with <90% of their tumor resected, chemotherapy increased treatment-related toxicity and provided minimal clinical benefit.11 STR best predicted 5-year EFS (61% vs. 39%), which validated similar benchmark institutional data (81% vs. 41%).10 Immediate post-operative RT for resected posterior fossa ependymoma improved outcomes in children as young as 12 months, and a clinical target volume (CTV) expansion of 1.0 cm was adequate.10 A follow-up COG ependymoma trial ACNS 0831 is currently accruing which randomizes patients with complete or near-complete resection to adjuvant chemotherapy and uses a CTV margin of 0.5cm. In Europe, the SIOP-EP-II protocol also addresses the use of adjuvant chemotherapy in low risk patients in non-randomized patients. For high-risk patients the SIOP-EP II adds an 8 Gy stereotactic boost to residual disease and a randomized evaluation of the use of high dose methotrexate.
COG studies of supra-tentorial high-grade glioma (HGG) have shown improved survival with the addition of temozolomide and lomustine to standard-of-care RT compared to historical controls, with the greatest benefit seen in GBM patients with MGMT overexpression (COG study ACNS0423).12,13 However, concurrent chemotherapy and RT in diffuse-infiltrating pontine gliomas (DIPG) has yielded little success (POG study 9836).14 A study of the G2 checkpoint inhibitor AZD1175 given concurrently with RT in DIPG is ongoing (COG study ADVL1217).
For low grade gliomas, COG study ACNS 0221 protocol studied the efficacy of 54 Gy in unresectable tumors showing no response after at least one cycle of chemotherapy. CT. Reduced RT volumes with a CTV of 5mm were used. Data from this study and the European study SIOP-LGG-2004 for progressive low grade glioma are currently maturing and results are pending.
Rhabdomyosarcoma (RMS) and the Non-Rhabdomyosarcoma Soft Tissue Sarcomas (NR-STS)
The curative approach to soft tissue tumors of children began with the multi-disciplinary management of childhood RMS in the prospective IRSG protocols I-IV. Radiation oncologists have played an essential role in these studies paving the way for current COG studies. IRSG studies established the dose/volume standard guidelines of 50.4 Gy and 41.4 Gy for children with gross and microscopic disease respectively, emphasizing organ preservation, organ function, and quality of life.15–22 (table 2).
Table 2:
Major radiotherapy advancements in pediatric rhabdomyosarcoma
|
Treatment for RMS is defined by risk of relapse. Low-risk RMS broadly includes favorable site, embryonal/botryoid histology, resected with/without microscopic disease, with specific subgroups having other features as defined by protocol. The D9602 and ARST0331 studies confirmed that patients with embryonal/botryoid histology with only microscopic disease can be effectively controlled with 36 Gy; those with resected lymph node disease N0/N1 with 41.4 Gy.23,24 Patients with orbital primary tumors receiving modest cyclophosphamide doses have excellent 5 year FFS of 87%, and may be treated with 45 Gy, but those with less than a complete response to induction chemotherapy require 50.4 Gy.25 Girls with vaginal RMS require RT by week 12 regardless of response to chemotherapy.26
Intermediate-risk RMS is defined as: non-metastatic patients with alveolar histology; embryonal histology not low risk; and embryonal patients <10 years with metastatic disease. Tumor size is prognostic, as shown in D9803. Patients with group III tumors <5 cm treated with 50.4 Gy have 10% local failure at 5 years, compared to 25% for tumors ≥5 cm.27 Tumor site is also important, but even tumors of unfavorable sites can be controlled with RT, e.g. hand/foot alveolar tumors have 100% local control at 10 years following RT confirming that amputation is not appropriate.28. The current COG intermediate risk RMS study (ARST1431) escalates RT dose to 59.4 Gy for tumors >5cm in size, and tests the addition of temsirolimus to the chemotherapy regimen. ARST1431 is also testing further refinements in RT that may help reduce late effects such as adaptive planning where only the post-chemotherapy volume is targeted after 36 Gy. The European cooperative groups are similarly testing dose escalation for tumors at highest risk of local failure. Current COG protocols provide guidelines for proton therapy and SBRT and the use of these advanced technologies is increasing rapidly. The COG will have a rich repository of dosimetric data available for future evaluation of outcomes with these modalities.
Timing of RT is not critical for most patients with intermediate-risk disease. Less than 2% of patients suffer local disease progression during induction chemotherapy.29 Even patients with parameningeal tumors and features of intracranial disease, cranial nerve palsy, or skull base erosion can defer RT until after induction chemotherapy.30 On the other hand, delayed RT (>24 weeks or omitted completely) is associated with local-regional recurrence, even in low-risk disease.26
High-risk/metastatic disease requires RT for local control of all sites that can be localized, though failure is often systemic. Recommended doses/volumes follow intermediate-risk guidelines.
Diagnostic cross sectional imaging (CT/MRI) and/or delayed surgery cannot reliably predict response but volumetric and metabolic response remain areas of active investigation for both the COG and European groups. However, RT cannot be omitted based upon imaging or pathologic response.31,32 PET/CT can identify in-transit nodal spread.33,34 Precision clinical staging is essential, with biopsy of regional nodes when possible, as nodal disease requires RT. Delayed primary excision has not obviated the need for local-regional RT nor impacted local control over RT alone.35 Radiotherapy quality control is essential; local recurrence is associated with non-compliance with RT guidelines.36 Chemotherapy alone is not sufficient local-regional therapy for RMS; patients with microscopic and gross disease profit from local-regional RT. The only children in whom RT is not routinely recommended are those with low-risk, completely resected disease.
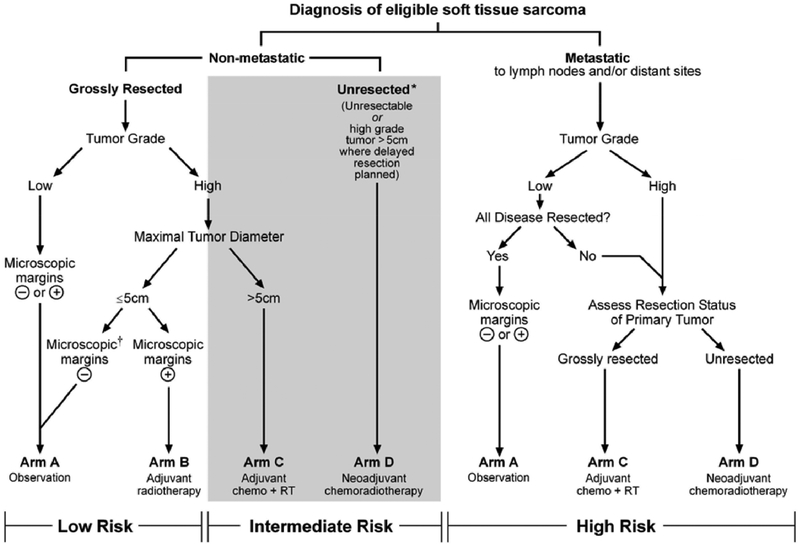
In pediatric non-rhabdo soft tissue sarcoma the role of RT and chemo-RT has been defined by COG study ARST0332. Indications for RT are determined by: localized vs metastatic, surgically resected, margin status, grade, and size (figure 1). When delayed resection is planned, neoadjuvant chemo-RT with 45 Gy is used; microscopic positive margins receive 55.8 Gy, while macroscopic disease needs 64.8 Gy.37 Certain histologic subtypes are chemo-responsive, thus chemo-RT combinations may be useful; other soft tissue sarcomas are successfully managed with RT and surgery alone. The recommended RT technique for all pediatric soft tissue sarcomas is highly conformal RT using image guidance, and either intensity-modulated or particle beam therapy.38 Currently, the COG is conducting a prospective trial for non-rhabdo soft tissue sarcoma in partnership with the NRG cooperative group using a consistent treatment approach for both pediatric and adult patients.
Figure 1:
ARST0332 guidelines for treatment of non-rhabdomyosarcoma soft tissue sarcomas. Management of these tumors is determined by extent of disease, grade, size, and margin status.
Hodgkin Lymphoma
The COG Hodgkin Lymphoma (HL) committee continues to advance the treatment of children with HL by testing new treatment options aimed at improving cure rates while reducing treatment-related toxicity (table 3). The paradigm of response-adapted utilization was demonstrated to be an effective means of refining RT utilization in the COG AHOD0031 study. Selected patients with intermediate-risk HL who had a rapid and complete anatomic response to four cycles of dose-dense ABVE-PC chemotherapy did not have a significant improvement in event-free survival with the addition of adjuvant RT.39 Detailed analysis of the study results further demonstrated that patients with a combination of the clinical features of mediastinal bulk and anemia had significantly better outcome with the addition of RT despite a rapid or complete response to chemotherapy, while those with multiple non-bulky sites did not benefit from RT.40
Table 3:
Major radiotherapy advancements in pediatric Hodgkin lymphoma
|
The management of high-risk HL remains a challenging problem, as the achievement of high cure rates has typically required treatment with significant doses of doxorubicin, alkylating agents and RT. The ongoing COG AHOD1331 trial evaluates the incorporation of brentuximab vedotin, a novel anti-CD 30 antibody-drug conjugate, into the upfront treatment of children with high risk HL. This agent, which has demonstrated remarkable clinical activity in the relapse setting, has the potential to substantially reduce the relapse rate in high-risk patients without the cumulative toxicity of additional conventional treatment. Further development of modern RT in this trial moves away from the large normal tissue volumes required to treat all initially involved nodal sites, and employs involved-site RT, limited to bulky mediastinal adenopathy and nodal areas not achieving a Deauville 3 response after two cycles of chemotherapy.
As COG trials continue to refine the selection of HL patients for RT, it is increasingly recognized that the risk-benefit considerations that drive management decisions are made largely based on studies such as the Childhood Cancer Survivor Study (CCSS) that examined the outcomes of treatments using outdated RT techniques. For example, COG investigators have demonstrated that for patients with stage I/II HL, the radiation dose to the heart and (female) breast tissue in the most recent COG trials are 69% and 84% of the doses received by CCSS subjects.41 Given the linear dose-risk relationship described for many RT-related late effects,42 these findings portend the anticipated reduction in late effects that are expected for survivors of COG trials. In addition, ongoing work continues to aid the refinement of treatment intensity by modeling the outcome of potential changes in RT utilization to provide a more quantitative and less speculative understanding of how to optimize the tradeoff between the risks of HL relapse and late toxicity.
Both COG and European trials are converging on the use or response-adapted involved-node RT for patients with early stage disease. The appropriate RT dose for patients with persistently PET-avid lesions following chemotherapy is an emerging issue that will require evaluation. European trials have focused on developing quantitative measures of PET-avidity and dose-intensification of chemotherapy as means of using RT more judiciously, whereas COG trials continue to use Deauville scoring of PET response, with substitution of conventional chemotherapy agents with novel agents. Ongoing efforts by COG HL investigators are characterizing the biology of HL treatment response and prognosis. In addition, the routine collection of imaging datasets will facilitate the conduct of radiomic studies that will aid the evaluation of staging and treatment response. Collaborations are also underway with adult oncology trial groups to develop a joint trial for adolescents and young adults with unfavorable early-stage disease, and as a greater proportion of relapsed patients will be radiation-naïve there is increasing attention turning toward the appropriate dose for patients with chemotherapy resistant disease, and how to optimally incorporate RT into protocols for patients with relapsed HL.43
Ewing Sarcoma and Bone Tumors
Advances in chemotherapy combinations as well as improvements in surgical and RT techniques have led to continued improvement in outcomes for patients with localized Ewing sarcoma treated on COG studies (table 4). The addition of ifosfamide and etoposide (IE) to vincristine, doxorubicin and cyclophosphamide (VDC) improved both local control and overall survival while interval compression of VDC alternating with IE chemotherapy improved survival compared to standard 3-week dosing (COG study AEWS0031).44,45 As therapy has improved for localized Ewing sarcoma, nearly 80% of patients are expected to be long-term survivors and functional outcomes are becoming increasingly important. The Ewing study committee has focused on utilizing advances in RT delivery as well as response-adapted therapy to reduce toxicity and improve local control. In the recently completed localized Ewing sarcoma COG study, AEWS1031, a provision for response-adapted post-operative RT as a method to reduce RT volumes for favorable responders was included to reduce risk of musculoskeletal complications after treatment. Specifically, for patients with microscopic positive margins after surgery requiring adjuvant RT, the RT target volume was based on response such that the post-chemotherapy, pre-operative volume was targeted in patients with good pathologic response (>90% necrosis), whereas the larger pre-chemotherapy volume was targeted for poor responders. CTV margins for RT were reduced from 1.5 cm to 1.0 cm with no apparent detriment to local control. In addition, AEWS1031 was the first trial from the COG Bone Tumor Disease Committee to systematically study surgical and RT techniques and volumes in relation to musculoskeletal complications. Although local control for primary tumors exceeds 90% on recent COG trials, local control for patients receiving definitive RT has stagnated at 80%.46 Retrospective analysis of patients treated on the most recent completed COG trials suggest this is primarily due to poorer local control for older age patients (>18) and patients with pelvic tumors.47 Recently presented data from the EuroEwing99 R2Loc study suggests that in high-risk patients (defined primarily as pathologic poor responders but some based on tumor volume >200mL) have improved survival with the addition of busulphan-melphalan (BuMel) high-dose chemotherapy with stem cell rescue (HDSCT).48 The 3-yr EFS for the HDSCT cohort was 67%, compared to 53% in the standard arm. Five year EFS on the most recent COG study using interval-compressed chemotherapy was 73% and it is difficult to match the patient population to compare outcomes for the “high-risk” sub-set. However, surgical patients on COG studies have favorable outcomes and 80% of patients on the R2Loc study had surgery as a component of local therapy. In addition, because of concern of toxicity, central axis tumors were not eligible and pelvic patients had to defer radiotherapy until after high-dose chemotherapy so caution should be taken with adopting this treatment strategy. The upcoming challenges for localized Ewing sarcoma include improving local control for patients with unresectable pelvic tumors and identifying a high-risk population that may benefit from more intensive chemotherapy such as the EuroEwing99 regimen. Analysis of EFS outcomes by size and PET and pathologic response on the recently completed AEWS1031 are currently underway to help answer these questions.
Table 4:
Major radiotherapy advancements in Ewing Sarcoma
|
Although outcomes in patients with localized disease have improved over time, outcomes for patients with metastatic disease remain poor. In the recently reported EuroEwing99 R2Pulm, there was no benefit and increased toxicity for patients treated with BuMel compared to whole lung irradiation (WLI).49 However, 3-yr EFS was over 50%, the best outcomes reported so far for patients with lung-only metastases, confirming the importance of whole-lung irradiation to consolidate patients with lung metastases. European and single institution data suggest that consolidation of all metastatic sites with RT improves EFS for patients with metastatic disease.50 Stereotactic body radiotherapy (SBRT) is an emerging technique that can allow definitive dose RT to be administered in rapid time frame (1 to 5 fractions) and has been used to successfully treat sarcoma metastases in single institution trials.51,52 To improve compliance with the recommendation for RT of all metastatic bony sites, the current metastatic COG Ewing trial, AEWS1221, allows SBRT given in 5 fractions, as opposed to the current standard of 31 fractions. By reducing treatment time and volume we will evaluate whether SBRT will improve compliance with RT to metastatic sites as well as examine local control outcomes with this newer technology.
Renal Tumors
The National Wilms Tumor Study (NWTS) conducted five clinical trials (1969–2002) that successfully reduced the indications for and doses of abdominal RT for children with Wilms tumor. Presently 75% of children with stage I and II tumors do not need RT, and the flank RT dose has been reduced from an age-adjusted regimen (18–40Gy) to just 10Gy for stage III tumors.53 The COG renal tumor committee is the successor of the NWTS. The first generation of COG Wilms tumor (WT) protocols has just been completed (2003–2015) and data analysis is underway (table 5). COG study AREN0321 studied high risk renal tumors including diffuse anaplastic Wilms tumor (DAWT), malignant rhabdoid tumor (MRT) and clear cell sarcomas (CCSK). This study evaluated whether an intensive chemotherapy regimen with cyclophosphamide/ carboplatin/etoposide alternating with vincristine/ doxorubicin/cyclophosphamide improved survival in DAWT and MRT. The RT objectives included delivery of higher dose flank RT (20Gy) for stage III DAWT, the addition of flank RT (10Gy) to regimen DD4A chemotherapy (vincristine, dactinomycin, and doxorubicin) for stage I DAWT, and omission of RT for stage 1 CCSK. Preliminary results indicate that survival for stage II-IV DAWT is superior to NWTS results with a 4 year EFS of 85%, 74% and 46% for stage II, III and IV tumors respectively. Mortality due to treatment-related toxicity, however, was 4.5%.
Table 5:
Major radiotherapy advancements in pediatric renal tumors
|
COG study AREN0532 studied very low and standard risk favorable histology (FH) WT. Preliminary results show a 4-year EFS and OS of 90% and 100% respectively for 116 children <2 years old with small tumors, no loss of heterozygosity (LOH) at 1p and 16q, and stage I or II disease after nephrectomy alone.54 For stage I/II tumors with LOH at 1p and 16q, the 4-year EFS after DD4A chemotherapy was 84% compared to 75% with 2 drugs. Among 543 patients with FH stage III tumors without LOH at 1p and 16q the 4 year EFS and OS was 88% and 96% respectively after regimen DD4A and RT. Lymph node involvement was associated with inferior event free survival (83% vs. 95%).55
AREN0533 studied higher risk FHWT to determine if whole lung irradiation (WLI) can be omitted for stage IV tumors with rapid complete resolution of lung metastasis after 6 weeks of regimen DD4A chemotherapy. This study also augmented chemotherapy with regimen M (DD4A, plus cyclophosphamide and etoposide) and WLI for slow incomplete responders. Preliminary results indicate that among 296 patients with lung metastasis 105 (39%) had a complete response at week 6 to regimen DD4A and their 4-year EFS and OS was 78% and 95% respectively without WLI. This was non-significantly inferior to the 85% EFS after WLI and DD4A chemotherapy in NWTS-5.56 Among the slow incomplete responders, the 3 year EFS and OS was 88% and 92% respectively after regimen M and WLI. These outcomes were significantly superior to the estimated event free survival of 75% with regimen DD4A and WLI.57
COG study AREN0534 studied children with bilateral WT. Patients were treated with induction chemotherapy, followed by surgery when possible, and RT based on pathologic findings. Survival data for 208 children shows a 4 year EFS and OS of 82% and 95% respectively compared to 61% and 80% respectively for similar patients on NWTS protocols. After induction chemotherapy, 84% had definitive surgery by 12 weeks and 39% of patients retained at least parts of both kidneys.58
The study committee is planning the next generation of WT studies. In addition to the known clinical, pathologic and molecular markers, gain of chromosome 1q will be used for risk stratification and treatment assignment.59 Novel RT techniques such as IMRT for liver metastases and cardiac-sparing whole lung IMRT utilizing 4D simulation will be implemented for stage IV tumors.60–62 SIOP, on the other hand, continues to use a pre-chemotherapy nephrectomy approach, with flank radiotherapy to a dose of 14.4 Gy reserved for Stage III intermediate-risk tumors and 25.2 Gy for Stage II to III high-risk diffuse anaplasia and Stage III high risk blastemal type Wilms tumor. In the upcoming UMBRELLA SIOP-RTSG 2016 protocol, AP/PA flank fields will not be routinely used; instead the GTV includes only the contact zone of pre-surgical tumor with a CTV anatomically confined expansion of 5 to 10 mm including the para-aortic lymph node chain in the case of lymph node involvement.63
Neuroblastoma
Significant contributions to the treatment of high-risk neuroblastoma have been made by COG radiation oncology (table 6). In the 1990s the CCG 3891 protocol utilized RT only for patients with post-operative gross residual disease, with 10 Gy for abdominal or mediastinal sites and 20 Gy for extra-abdominal sites. Children who were randomized to autologous bone marrow transplantation (ABMT) received an additional 10 Gy in the form of total body irradiation (TBI). Firm conclusions were difficult to draw as not all patients were uniformly treated with RT; nonetheless, results indicated that in combination with RT to the primary tumor, the addition of 10 Gy of TBI with ABMT improved local control compared with chemotherapy without TBI, suggesting a dose–response relationship for local RT.64
Table 6:
Major radiotherapy advancements in neuroblastoma
|
COG study A3973 recommended uniform RT for all patients and showed that RT was an essential component of the multimodality regimen used for high-risk neuroblastoma. However, there was no benefit of extensive lymph node irradiation, irrespective of the extent of surgical resection preceding stem cell transplant.65 In COG study A3973, 21.6 Gy was prescribed to the post-induction chemotherapy, pre-operative primary tumor volume regardless of extent of resection. This approach has become the standard of care for high-risk neuroblastoma.
The subsequent protocol, COG ANBL0532 showed that tandem myeloablative consolidation therapy improved survival in patients with high-risk neuroblastoma especially in the setting of post-consolidative immunotherapy.66 ANBL0532 was also the first neuroblastoma randomized trial in which a RT-related question was posed as a primary objective. The study built on prior data suggesting a dose-response for incompletely resected primary neuroblastoma tumors, and determined whether boosting gross residual primary disease to a total dose of 36 Gy would improve the 3-year local control rate, compared to historical controls. Final results are pending.
COG study ABNL 09P1 was a phase 1 clinical trial evaluating the feasibility of treating high-risk neuroblastoma with I-131 MIBG. The trial closed to accrual in 2015 and preliminary analysis indicates that it is safe to utilize therapeutic I-131 MIBG during the induction phase of high-risk neuroblastoma treatment. Therapeutic I-131 MIBG therapy is an important component of the next randomized phase 3 high-risk neuroblastoma trial, COG study ANBL1531, a five-arm study including three randomized arms. Two of the randomized arms will assess the use of therapeutic I-131 MIBG to improve event-free survival in patients whose tumors have MIBG avidity. This study will open to accrual in the near future. When compared with previous high-risk neuroblastoma protocols there are many differences to the radiation oncology guidelines of ANBL1531 that include more stringent normal tissue constraints, requirement for including the entire vertebral body including the posterior elements within the CTV, and decreasing the CTV margin from 1.5 cm to 1 cm in these young children. Finally, metastatic sites that require RT will be numbered in accordance with the MIBG Curie diagnostic classification.67 This will facilitate assessment of recurrence and aid in re-irradiation treatments.
Future aims of the radiation oncology committee include increasing the RT dose for metastatic disease still present after ASCT and utilizing SBRT for recurrent and metastatic disease.
There are significant differences between the COG approach to the treatment of high risk neuroblastoma and that of the European International Collaboration for Neuroblastoma Research (SIOPEN) approach. Metastatic sites that do not completely respond to induction chemotherapy are not routinely treated in Europe as they are in the United States. Furthermore, SIOPEN treats the primary tumor to 21 Gy in 14 fractions regardless of residual disease after surgical resection (they do not boost to 36 Gy) In North America, under the auspices of the COG, the potential benefit of a boost to gross residual disease is being evaluated as a primary aim in ANBL0532.
Future aims of the COG neuroblastoma radiation oncology committee include evaluating the additive or cooperative effects of therapeutic I-131 MIBG with focal radiotherapy in regard to treatment efficacy and toxicity. This is challenging in the context of both optimal radio-therapeutic dosing to primary sites with residual disease after surgery and metastatic sites that do not completely respond to induction chemotherapy. Additionally, plans are being made to study the role of SBRT in the treatment of recurrent and metastatic disease.
Leukemia
Radiotherapy indications for leukemia have changed significantly over the past few decades, mainly in the direction of reducing the intensity and/or indications based on risk-adapted therapy principles. The risks groups were established based on well-defined criteria validated in multiple clinical trials (Table 7).68,69 COG studies have been instrumental in defining RT indications for newly diagnosed high risk pre-B cell ALL and T-cell acute lymphoblastic leukemia (ALL). Current guidelines for these patients include cranial radiation therapy (CRT) as part of CNS-directed therapy and treating residual disease in testes.70,71 For relapsed disease, RT plays a role for isolated CNS relapses, as well as a conditioning regimen for stem cell transplant delivered as TBI with or without CRT and testicular boost based on specific risk stratifications defined by protocols.72–74 Radiotherapy indications incorporated into recently completed and currently opened COG trials are listed in Table 8. COG RT guidelines emphasize precise integration of radiation into treatment road-maps with particular attention to be paid to its timing with concurrent systemic chemotherapy, as well as evaluation and management of patients’ clinical condition, including concurrent and evolving toxicities. Late effects from cranial RT, testicular RT, and TBI can adversely affect the quality of life and survival of children treated for leukemia and are being evaluated in several ongoing prospective trials.75
Table 7.
ALL Risk Groups
| Standard Risk: All of the following |
|
| High Risk: Any of the following |
|
| And: No VHR features |
| Very High Risk: Any of the following |
|
Table 8:
Radiotherapy in COG leukemia trials
| B-ALL High-risk: |
| CNS 3: CRT 18 Gy (AALL1131) |
| T-ALL: |
| Intermediate and High risk |
| CNS 1 and 2: CRT 12 Gy (AALL0434) |
| CNS 3: CRT 18Gy (AALL0434; AALL1231) |
| Very High risk ALL |
| CNS 1 and 2: CRT 12 Gy (AALL0434) |
| CNS 3: CRT 18 Gy (AALL0434; AALL1231) |
| Relapses: |
| Isolated CNS relapse after> 18 mo from diagnosis: |
| CRT 12 Gy (AALL02P2) |
| CRT 18 Gy for CNS 3 (AALL1331) |
| Early isolated CNS relapse<18 mo from diagnosis (AALL0433): CRT 18 Gy |
| Testicular Radiation: |
| Patients with continued evidence of testicular leukemia at the end of Induction: |
| Testicular RT to 24 Gy (AALL1131; AALL1331) |
| Conditioning regimen for hematopoietic stem cell transplantation: (AALL1331) |
| TBI 12–13.2 Gy |
| Cranial boost 4–6 Gy for CNS 3 disease |
| Testicular boost 6 Gy for persistent disease |
CNS1 = no blast cells in CSF, CNS2 = <5 WBC/microliter CSF with blast cells, CNS3 = >5
WBC/microliter CSF with blast cells, or signs of CNS involvement
Efforts in COG and other international pediatric cooperative groups continue to focus on reducing treatment-related side effects in this highly curable cancer. The cooperative group BFM (Berlin-Frankfurt-Múnster) is an important international platform for promoting research and clinical care for leukemia and lymphoma in children and adolescents in Europe and beyond. The BFM has taken a similar approach to the COG with the elimination of prophylactic CRT in patients with low-risk ALL and the successful stepwise reduction in CRT to 12 Gy in those with intermediate- and high risk ALL.76
Rare Tumors
The COG rare tumors committee has focused on improving outcomes for children with uncommon tumors that by nature require study in a cooperative group setting. For children with retinoblastoma the main focus has been to minimize exposure to radiation therapy in these vulnerable children. In early, stage I, disease COG study ARET12P1 uses intra-arterial therapy, eliminating external beam RT in the up-front setting and incorporating brachytherapy when appropriate. This study recently closed and results will be forthcoming. For eyes that are Stage II or III, the COG ARET0321 trial prescribed multi-agent chemotherapy and 45 Gy RT. For Stage IV disease, chemotherapy and response-based RT was given, followed by ABMT. The EFS for stage 2 and 3 patients was 88% at 3 years and 79% for children with non-CNS metastatic disease.77 These results are significantly improved compared to historical controls.
Nasopharyngeal carcinoma is another rare tumor where RT plays a major role. COG recently completed a prospective trial of response-based RT (COG study ARAR0331) with a goal of delivering lower (61.2 Gy) RT dose for the majority of patients. This trial enrolled 111 patients achieving a 5-yr. EFS of 86%.78 Future studies will continue to refine the intensity of therapy required for chemotherapy-responsive patients.
Late Effects
Despite the remarkable success in improving survival in pediatric oncology, both RT and chemotherapy can cause debilitating or even fatal late effects that are critical to understand, mitigate, or prevent.79,80 The Late Effects radiation oncology subcommittee has contributed to improving the outcomes of pediatric cancer patients in a number of ways. Its function includes (1) review of existing normal tissue RT dose constraints in COG protocols, (2) generating new evidence-based dose constraints for future COG protocols, (3) interfacing with the COG Late Effects Survivorship Guidelines committee for refinement of surveillance recommendations (http://www.survivorshipguidelines.org/), (4) identifying knowledge gaps relating to radiation-associated and combined modality normal tissue toxicities, and (5) training young investigators in RT late effects. The subcommittee has integrated with PENTEC (Pediatric Normal Tissue Effects in the Clinic) to form 18 organ-specific task forces led by COG radiation oncologists. This group will identify RT-associated normal tissue damage as a function of patient age at the time RT was delivered, dose (including parameters such as fraction size), volume, functional organization of the organ exposed, and the impact of ancillary cytotoxic therapy, primarily chemotherapy. The results of these investigations will provide clinicians with the best available data on which to make decisions in treating children for cancer with RT, and inform scientists on necessary future directions for research.81
Quality Assurance and Physics
The Imaging and Radiation Oncology Core (IROC) services at IROC Houston (formerly RPC) and IROC Rhode Island Quality Assurance (formerly QARC) Centers are responsible for providing RT and imaging quality assurance (QA) core support for the COG. Investigators from IROC have been involved with pediatric clinical trials since the origin of both the POG and the CCSG and continue to be part of the quality assurance mission of the COG. IROC works with each study investigator to write protocol guidelines that meet both study objectives and processes established by the NIH, and recommend appropriate credentialing and data management strategies for each study to insure that the goals of the study can be met with the highest quality data possible. IROC Houston is responsible for site qualification and credentialing institutions for participation in clinical trials that use advanced technology RT including all facets of, and maintaining the list of participant institutions and their credentialing status. IROC Rhode Island is responsible for data acquisition, data management and pre and post case review for both imaging and RT objects required for each individual protocol.
The importance of the QA process in maximizing protocol compliance was demonstrated for the conduct of the COG AHOD0031 Hodgkin study mentioned previously. Rapid early review of RT treatment plans by IROC with remedial modifications made when necessary resulted in significantly fewer protocol violations as compared to retrospectively reviewed plans (figure 2).82
Figure 2:
Radiotherapy protocol compliance for AHOD0031. A: Compliance after central rapid review and remediation. B: Compliance in the absence of rapid review.
Perhaps the most significant technical advance in the last decade affecting the QA review of imaging and RT data is the evolution from hardcopy to exclusively digital formats. COG institutions currently submit digital data using a secure FTP site. This is now being transitioned to the American College of Radiology’s Transmission of Imaging and Data (TRIAD) system. The development of digital media transmission tools has permitted real time (same day) evaluation of imaging and RT objects by the QA centers with ad hoc participation by site and study investigators as needed on a simultaneous basis. This evaluation capability has significantly changed protocol development as real time review of objects has permitted secondary and tertiary randomized RT objectives to be imbedded into studies. IROC continues to play an integral role in protocol core support for COG and will continue to be an important component in the success of each clinical trial.
The Physics Subcommittee has made significant contributions to the conduct and quality of RT in COG studies. A survey of portal imaging practices resulted in specific recommendations to reduce imaging radiation doses for pediatric RT.83 A follow-up survey is currently underway. The Physics subcommittee will also review current RT practices in TBI and proton therapy on COG protocols.
Proton Therapy
General consensus has grown within the radiation oncology community that proton therapy can reduce acute and late toxicities in many clinical scenarios of childhood cancer compared to x-ray-based technologies. Objective data demonstrating these benefits are beginning to accrue.84 Improved side effect profiles and quality of life outcomes have been reported for neurocognitive, hearing, and neuro-endocrine function as well as other arenas including second malignancy and acute toxicities.85–94 Furthermore, cost effectiveness studies have found that proton therapy, despite upfront high cost, is cost-effective in the long run when expenses associated with side effect management are factored in.95 COG radiation oncologists and proton physicists have developed specific guidelines for proton RT that are incorporated into COG protocol guidelines and have helped standardize the use of this modality for all tumors in a cooperative group setting. Currently there are approximately 21 proton centers across the country that have been approved by IROC to treat children on COG trials. Thus, COG studies are ideally positioned to help define the role and relative benefits of proton therapy in comparison to photon radiation based on prospective clinical data.
Pediatric Radiation Oncology Education and International Collaboration
COG seeks to improve pediatric radiation oncology education and training in North America and around the world, especially in low and middle income countries. The COG Radiation Oncology Committee includes 300 radiation oncologists and physicists (figure 3) and has an active Young Investigator program. As part of its mission, the committee provides training in state-of-the-art pediatric RT to both established and new pediatric radiation oncologists via dedicated educational sessions, seminars, and on-line resources. An international outreach program has also been established through collaborations with the Pediatric Radiation Oncology Society (PROS) and the International Society of Pediatric Oncology (SIOP). In this regard, a free COG educational platform has been created on the IROC-Rhode Island website (http://www.irocri.qarc.org/). Disease leaders from our discipline also moderate an online discussion forum (https://pediatric-oncology.chartrounds.com/) that aims to improve the quality of childhood cancer care globally through free and timely online discussion of cases with our experts. Such international collaboration facilitates exchange of ideas and helps develop clinical and basic research protocols that will benefit our field in the future.
Figure 3:
COG membership breakdown. Radiation oncology committee members comprise 15% of the COG membership.
Conclusions and Future Plans – Integration of Tumor Biology and Diagnostic Imaging into the Practice of Radiation Oncology
Since its inception 15 years ago the radiation oncology discipline of COG has made many noteworthy contributions aimed at refining the treatment of low-risk patients to decrease treatment-related toxicity and intensifying treatment of high-risk tumors to improve outcomes. We have consistently adopted measures to systematically reduce the radiation exposure of children by: 1) reducing the RT indications for favorable risk tumors (Hodgkin lymphoma, Wilms tumor, CNS tumors, leukemia, retinoblastoma), 2) decreasing the volume of normal tissues irradiated (medulloblastoma, ependymoma, germ cell tumors, Hodgkin lymphoma, rhabdomyosarcoma, Ewing sarcoma, use of proton therapy and IMRT) and 3) reducing the total RT doses for favorable risk tumors (rhabdomyosarcoma, soft tissue sarcoma, medulloblastoma, Wilms tumor) that will reduce late toxicity and enhance the quality of life of childhood cancer survivors. For higher risk tumors we have used higher RT doses together with intensive multi-agent chemotherapy regimens (neuroblastoma, diffuse anaplastic Wilms tumor, rhabdomyosarcoma, Ewing sarcoma) that will hopefully improve tumor control rates.
An inherent difficulty that clinicians face in making treatment decisions regarding any uncommon tumor (which includes most pediatric cancers) is the relative lack of data regarding various treatment approaches, and the slow pace at which new treatment techniques and paradigms can be tested and reported. Given these limitations, clinicians are often tempted to adopt treatment guidelines from ongoing prospective clinical studies as “standard of care” when treating children “off-study”. However, we urge caution when applying untested treatment approaches (e.g., radiation dose reductions or escalation, or changes in radiotherapy treatment volumes or target margins) that are study aims of ongoing investigational trials.
Future work in our discipline will continue to pursue novel ways to enhance the curative potential and reduce the adverse effects of RT. Studies by several groups including the Pediatric Preclinical Testing Program (PPTC) have shown the ability of using mouse models of pediatric cancer to identify novel effective drugs.96,97 However studies of the efficacy of RT alone or in combination with potential radiation sensitizers have lagged behind. It is well-recognized that RT damage to DNA can be repaired by a plethora of enzymatic activities that lead to tumor cell survival or mechanisms that suppress death programs and ultimately resistance.98 Importantly, specific and potent inhibitors of many DNA damage response pathways or small molecules that activate p53-induced apoptotic signaling are in preclinical development, or are under clinical evaluation for treatment of adult malignancies. However, translating these advances to the treatment of childhood cancer presents challenges. Preclinical models that accurately recapitulate the genetics and microenvironment of pediatric cancers may be used to identify those RT/drug combinations that have impact against a majority of models of specific histotypes, or more general broad potentiation of RT effects. Further, with advanced technologies such as nano-formulation, more selective tumor-specific radiation enhancement may be achieved. The use of appropriate preclinical animal models may be able to identify those combinations worthy of advancement to clinical trials and COG radiation oncologists are partnering with basic scientists to identify and translate these treatments into the clinical setting.
Additional areas planned for study include closer collaboration with diagnostic radiology to study the value of imaging modalities such as PET and novel MRI techniques to refine the indications and target volumes for RT, and treatment outcomes of various tumors (Hodgkin lymphoma, rhabdomyosarcomas, Ewing Sarcomas, gliomas, and Wilms tumor). We will also study the potential differences between protons and photons on the brainstem by analyzing post-treatment MRI studies of children with ependymoma and medulloblastoma treated on COG protocols. Thus, the COG radiation oncology discipline is committed to continuing research with a broad range of disciplines to both refine and modernize the use of RT in current and future multidisciplinary protocols with the goal of further improving the cure rates and quality of life of children stricken with cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors of this manuscript have various financial disclosures outside the submitted work. One author is involved with an NIH Grant (CA180803) that coincides with the current work under consideration.
Bibliography
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Constine LS, Tarbell NJ, Halperin EC. Pediatric Radiation Oncology. Philadelphia: Wolters Kluwer; 2016. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 4.Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol 2000;18:3004–11. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 2006;24:4202–8. [DOI] [PubMed] [Google Scholar]
- 6.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol 2013;31:2936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley DM, Merchant TE, Strother D, et al. Induction chemotherapy and conformal radiation therapy for very young children with nonmetastatic medulloblastoma: Children’s Oncology Group study P9934. J Clin Oncol 2012;30:3181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalski J, Janss A, V G, et al. Results of COG ACNS0331: A Phase III Trial of Involved-Field Radiotherapy (IFRT) and Low Dose Craniospinal Irradiation (LD-CSI) with Chemotherapy in Average-Risk Medulloblastoma: A Report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2016;96:937–8. [Google Scholar]
- 9.Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children’s Oncology Group Phase I/II study. J Clin Oncol 2012;30:2648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merchant TE, Li C, Xiong X, Kun LE, Boop FA, Sanford RA. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 2009;10:258–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garvin JH Jr., Selch MT, Holmes E, et al. Phase II study of pre-irradiation chemotherapy for childhood intracranial ependymoma. Children’s Cancer Group protocol 9942: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2012;59:1183–9. [DOI] [PubMed] [Google Scholar]
- 12.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 2011;13:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakacki RI, Cohen KJ, Buxton A, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol 2016;18:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korones DN, Fisher PG, Kretschmar C, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer 2008;50:227–30. [DOI] [PubMed] [Google Scholar]
- 15.Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma--a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 2003;21:78–84. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma--a report from the IRSG. Int J Radiat Oncol Biol Phys 2001;51:718–28. [DOI] [PubMed] [Google Scholar]
- 17.Michalski JM, Meza J, Breneman JC, et al. Influence of radiation therapy parameters on outcome in children treated with radiation therapy for localized parameningeal rhabdomyosarcoma in Intergroup Rhabdomyosarcoma Study Group trials II through IV. Int J Radiat Oncol Biol Phys 2004;59:1027–38. [DOI] [PubMed] [Google Scholar]
- 18.Raney RB, Meza J, Anderson JR, et al. Treatment of children and adolescents with localized parameningeal sarcoma: experience of the Intergroup Rhabdomyosarcoma Study Group protocols IRS-II through -IV, 1978–1997. Med Pediatr Oncol 2002;38:22–32. [DOI] [PubMed] [Google Scholar]
- 19.Smith LM, Anderson JR, Qualman SJ, et al. Which patients with microscopic disease and rhabdomyosarcoma experience relapse after therapy? A report from the soft tissue sarcoma committee of the children’s oncology group. J Clin Oncol 2001;19:4058–64. [DOI] [PubMed] [Google Scholar]
- 20.Wharam MD, Hanfelt JJ, Tefft MC, et al. Radiation therapy for rhabdomyosarcoma: local failure risk for Clinical Group III patients on Intergroup Rhabdomyosarcoma Study II. Int J Radiat Oncol Biol Phys 1997;38:797–804. [DOI] [PubMed] [Google Scholar]
- 21.Wharam MD, Meza J, Anderson J, et al. Failure pattern and factors predictive of local failure in rhabdomyosarcoma: a report of group III patients on the third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 2004;22:1902–8. [DOI] [PubMed] [Google Scholar]
- 22.Wolden SL, Anderson JR, Crist WM, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol 1999;17:3468–75. [DOI] [PubMed] [Google Scholar]
- 23.Breneman J, Meza J, Donaldson SS, et al. Local control with reduced-dose radiotherapy for low-risk rhabdomyosarcoma: a report from the Children’s Oncology Group D9602 study. Int J Radiat Oncol Biol Phys 2012;83:720–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walterhouse DO, Meza JL, Breneman JC, et al. Local control and outcome in children with localized vaginal rhabdomyosarcoma: a report from the Soft Tissue Sarcoma committee of the Children’s Oncology Group. Pediatr Blood Cancer 2011;57:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ermoian RP, Breneman J, Walterhouse DO, et al. 45 Gy is not sufficient radiotherapy dose for Group III orbital embryonal rhabdomyosarcoma after less than complete response to 12 weeks of ARST0331 chemotherapy: A report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer 2017;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol 2014;32:3547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolden SL, Lyden ER, Arndt CA, et al. Local Control for Intermediate-Risk Rhabdomyosarcoma: Results From D9803 According to Histology, Group, Site, and Size: A Report From the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2015;93:1071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.La TH, Wolden SL, Su Z, et al. Local therapy for rhabdomyosarcoma of the hands and feet: is amputation necessary? A report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2011;80:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minn AY, Lyden ER, Anderson JR, et al. Early treatment failure in intermediate-risk rhabdomyosarcoma: results from IRS-IV and D9803--a report from the Children’s Oncology Group. J Clin Oncol 2010;28:4228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: an analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys 2013;87:512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke M, Anderson JR, Kao SC, et al. Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: the Intergroup Rhabdomyosarcoma Study-IV experience--a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. J Clin Oncol 2007;25:4909–13. [DOI] [PubMed] [Google Scholar]
- 32.Rodeberg DA, Stoner JA, Hayes-Jordan A, et al. Prognostic significance of tumor response at the end of therapy in group III rhabdomyosarcoma: a report from the children’s oncology group. J Clin Oncol 2009;27:3705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casey DL, Wexler LH, Fox JJ, et al. Predicting outcome in patients with rhabdomyosarcoma: role of [(18)f]fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 2014;90:1136–42. [DOI] [PubMed] [Google Scholar]
- 34.La TH, Wolden SL, Rodeberg DA, et al. Regional nodal involvement and patterns of spread along in-transit pathways in children with rhabdomyosarcoma of the extremity: a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2011;80:1151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodeberg DA, Wharam MD, Lyden ER, et al. Delayed primary excision with subsequent modification of radiotherapy dose for intermediate-risk rhabdomyosarcoma: a report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Int J Cancer 2015;137:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Million L, Anderson J, Breneman J, et al. Influence of noncompliance with radiation therapy protocol guidelines and operative bed recurrences for children with rhabdomyosarcoma and microscopic residual disease: a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2011;80:333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spunt SL, Million L, Anderson J, Donaldson SS, Hawkins DS. Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. J Clin Oncol 2014;32:10008. [Google Scholar]
- 38.Lin C, Donaldson SS, Meza JL, et al. Effect of radiotherapy techniques (IMRT vs. 3D-CRT) on outcome in patients with intermediate-risk rhabdomyosarcoma enrolled in COG D9803--a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2012;82:1764–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman DL, Chen L, Wolden S, et al. Dose-intensive response-based chemotherapy and radiation therapy for children and adolescents with newly diagnosed intermediate-risk hodgkin lymphoma: a report from the Children’s Oncology Group Study AHOD0031. J Clin Oncol 2014;32:3651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charpentier AM, Friedman DL, Wolden S, et al. Predictive Factor Analysis of Response-Adapted Radiation Therapy for Chemotherapy-Sensitive Pediatric Hodgkin Lymphoma: Analysis of the Children’s Oncology Group AHOD 0031 Trial. Int J Radiat Oncol Biol Phys 2016;96:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou R, Ng A, Constine LS, et al. A Comparative Evaluation of Normal Tissue Doses for Patients Receiving Radiation Therapy for Hodgkin Lymphoma on the Childhood Cancer Survivor Study and Recent Children’s Oncology Group Trials. Int J Radiat Oncol Biol Phys 2016;95:707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol 2009;27:3901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dharmarajan KV, Friedman DL, Schwartz CL, et al. Patterns of relapse from a phase 3 Study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): a report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys 2015;92:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med 2003;348:694–701. [DOI] [PubMed] [Google Scholar]
- 45.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol 2012;30:4148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DuBois SG, Krailo MD, Gebhardt MC, et al. Comparative evaluation of local control strategies in localized Ewing sarcoma of bone: a report from the Children’s Oncology Group. Cancer 2015;121:467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed S Local control in localized Ewing sarcoma: A report from the Children’s Oncology Group Connective Tissue Oncology Society Annual Meeting; 2016; Portugal. [Google Scholar]
- 48.Whelan J, Le Deley M, Dirksen U, et al. Efficacy of busulfan-melphalan high dose chemotherapy consolidation (BuMel) in localized high-risk Ewing sarcoma (ES): Results of EURO-EWING 99-R2 randomized trial (EE99R2Loc). Journal of Clinical Oncology 2016;34:11000-. [Google Scholar]
- 49.Dirksen M, Le Deley M, Brennan B, et al. Efficacy of busulfan-melphalan high dose chemotherapy consolidation (BuMel) compared to conventional chemotherapy combined with lung irradiation in ewing sarcoma (ES) with primary lung metastases: Results of EURO-EWING 99-R2pulm randomized trial (EE99R2pul). Journal of Clinical Oncology 2016;34:11001-. [Google Scholar]
- 50.Haeusler J, Ranft A, Boelling T, et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 2010;116:443–50. [DOI] [PubMed] [Google Scholar]
- 51.Liu AK, Stinauer M, Albano E, Greffe B, Tello T, Maloney K. Local control of metastatic sites with radiation therapy in metastatic Ewing sarcoma and rhabdomyosarcoma. Pediatr Blood Cancer 2011;57:169–71. [DOI] [PubMed] [Google Scholar]
- 52.Brown LC, Lester RA, Grams MP, et al. Stereotactic body radiotherapy for metastatic and recurrent ewing sarcoma and osteosarcoma. Sarcoma 2014;2014:418270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalapurakal JA, Dome JS, Perlman EJ, et al. Management of Wilms’ tumour: current practice and future goals. Lancet Oncol 2004;5:37–46. [DOI] [PubMed] [Google Scholar]
- 54.Fernandez CV, Perlman E, Mullen EA, xx, yy. Clinical outcome and biological predictors of relapse following nephrectomy only for very low risk Wilms tumor (VLR WT): A report from Children’s Oncology Group AREN0532. J Clin Oncol 2015;33 suppl:10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez CV, Mullen EA, Ehrlich P, xx, yy. Outcome and prognostic factors in stage III favorable histology Wilms tumor (FHWT): A report from the Children’s Oncology Group (COG) study AREN0532. J Clin Oncol 2015;33 suppl:10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dix DB, Gratias EJ, Seibel N, xx, yy. Omission of lung radiation in patients with stage IV favorable histology Wilms Tumor (FHWT) showing complete lung nodule response after chemotherapy: A report from Children’s Oncology Group study AREN0533. J Clin Oncol 2015;33 suppl. [Google Scholar]
- 57.David B, Dix DB, Gratias EJ, xx, yy. Treatment of stage IV favorable histology Wilms tumor with incomplete lung metastasis response after chemotherapy: A report from Children’s Oncology Group study AREN0533. J Clin Oncol 2014;32:10001. [Google Scholar]
- 58.Ehrlich P, Chi YY, Chintagumpala MM, et al. Results of the first prospective multi-institutional treatment study in children with bilateral Wilms tumor (AREN0534): A report from the Children’s Oncology Group. Ann Surg 2017;266:470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gratias EJ, Dome JS, Jennings LJ, et al. Association of Chromosome 1q Gain With Inferior Survival in Favorable-Histology Wilms Tumor: A Report From the Children’s Oncology Group. J Clin Oncol 2016;34:3189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalapurakal JA, Gopalakrishnan M, Walterhouse D. Final report of a prospective clinical tria of cardiac sparing whole-lung intensity modulated radiation therapy in patients with metastatic pediatric tumors. Int J Radiat Oncol Biol Phys 2016;96 suppl:118–9. [Google Scholar]
- 61.Kalapurakal JA, Pokhrel D, Gopalakrishnan M, Zhang Y. Advantages of whole-liver intensity modulated radiation therapy in children with Wilms tumor and liver metastasis. Int J Radiat Oncol Biol Phys 2013;85:754–60. [DOI] [PubMed] [Google Scholar]
- 62.Kalapurakal JA, Zhang Y, Kepka A, et al. Cardiac-sparing whole lung IMRT in children with lung metastasis. Int J Radiat Oncol Biol Phys 2013;85:761–7. [DOI] [PubMed] [Google Scholar]
- 63.van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, et al. Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol 2017;14:743–52. [DOI] [PubMed] [Google Scholar]
- 64.Haas-Kogan D, Swift PS, Selch MT, et al. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int J Radiat Oncol Biol Phys 2003;56:28–39. [DOI] [PubMed] [Google Scholar]
- 65.Haas-Kogan D, London WB, Van Ryn C, et al. Extent of lymph node radiation coverage in high-risk neuroblastoma does not affect clinical outcome: A report from the COG A3973 study. Submitted to Int J Radiat Oncol Biol Phys. [Google Scholar]
- 66.Park JR, Kreissman SG, London WB, xx, yy. A phase 3 randomized clinical trial of tandem myeloablative autologous stem cell transplant using peripherl blood stem cells a consolidation therapy for high-risk neuroblastoma: A Children’s Oncology Group study. J Clin Oncol 2016;34:abstract LBA3. [Google Scholar]
- 67.Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method--application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer 1995;31A:256–61. [DOI] [PubMed] [Google Scholar]
- 68.Schultz KR, Pullen DJ, Sather HN, xx, yy. Risk and response-based classification of childhood B-precursor acute lymphoblastic leukemia: A combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cnacer Group (CCG). Blood 2007;109:926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith M, Arthur D, Camitta B, xx, yy. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 1996;14:18–24. [DOI] [PubMed] [Google Scholar]
- 70.Richards S, Pui CH, Gaynon P, xx, yy. Systeatic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 2013;60:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vora A, Andreano A, Pui CH, xx, yy. Influence of cranial radiotherapy on outcome in children with acute lymphblastic leukemia treated with contemporary therapy. J Clin Oncol 2016;34:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen K, Devidas M, Cheng SC, xx, yy. Factors influencing survival after relapse from acute lymphoblastic leukemia: A Children’s Oncology Group study. Leukemia 2008;22:2142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eapen M, Raetz E, Zhang MJ, xx, yy. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acture lymphoblastic leukemia in a second remission: A collaborative study of the Children’s Oncology Group and the Center for International Blook and Marrow Transplant Research. Blood 2006;107:4961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gassas A, Sung L, Saunders EF, xx, yy. Comparative outcome of hematopeietic stem cell transplantation for pediatric acutre lymphoblastic leukemia following cyclophosphamide and total body irradiation or VP16 and total body irradiation conditioning regimens. Bone Marrow Transplant 2006;38:739–43. [DOI] [PubMed] [Google Scholar]
- 75.Hijiya N, Hudson MM, Lensing S. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007;297:1207–15. [DOI] [PubMed] [Google Scholar]
- 76.Moricke A, Zimmermann M, Reiter A, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 2010;24:265–84. [DOI] [PubMed] [Google Scholar]
- 77.Dunkel IJ, Krailo MD, Chantada GL, et al. Intensive multi-modality therapy for extra-ocular retinoblastoma (RB): A Children’s Oncology Group (COG) trial (ARET0321). J Clin Oncol 2017;35:Abstr 10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Galindo C, Krailo MD, Krasin MJ, et al. Treatment of childhood nasopharyngeal carcinoma (cNPC) with neoadjuvant chemotherapy (NAC) and concomitant chemoradiotherapy (CCRT): Results of the Children’s Oncology Group ARAR0331 study. J Clin Oncol 2016;34:abstract 10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ehrhardt JJ, Sandlund JT, Zhang N, xx, yy. Late outcomes of adult survivors of childhood non-Hodgkin lymphoma: a report from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yeh JM, Nekhlyudov L, Goldie SJ, Mertens AC, Diller L. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med 2010;152:409–17, W131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhatia S, Armenian SH, Armstrong GT, et al. Collaborative Research in Childhood Cancer Survivorship: The Current Landscape. J Clin Oncol 2015;33:3055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dharmarajan KV, Friedman DL, FitzGerald TJ, et al. Radiotherapy quality assurance report from children’s oncology group AHOD0031. Int J Radiat Oncol Biol Phys 2015;91:1065–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olch AJ, Geurts M, Thomadsen B, Famiglietti R, Chang EL. Portal imaging practice patterns of children’s oncology group institutions: Dosimetric assessment and recommendations for minimizing unnecessary exposure. Int J Radiat Oncol Biol Phys 2007;67:594–600. [DOI] [PubMed] [Google Scholar]
- 84.Mohan R, Grosshans D. Proton therapy - Present and future. Adv Drug Deliv Rev 2017;109:26–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cochran DM, Yock TI, Adams JA, Tarbell NJ. Radiation dose to the lens during craniospinal irradiation-an improvement in proton radiotherapy technique. Int J Radiat Oncol Biol Phys 2008;70:1336–42. [DOI] [PubMed] [Google Scholar]
- 86.Cotter SE, Herrup DA, Friedmann A, et al. Proton radiotherapy for pediatric bladder/prostate rhabdomyosarcoma: clinical outcomes and dosimetry compared to intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:1367–73. [DOI] [PubMed] [Google Scholar]
- 87.Eaton BR, Esiashvili N, Kim S, et al. Endocrine outcomes with proton and photon radiotherapy for standard risk medulloblastoma. Neuro Oncol 2016;18:881–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eaton BR, MacDonald SM, Yock TI, Tarbell NJ. Secondary Malignancy Risk Following Proton Radiation Therapy. Front Oncol 2015;5:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lester-Coll NH, Morse CB, Zhai HA, et al. Preserving Fertility in Adolescent Girls and Young Women Requiring Craniospinal Irradiation: A Case Report and Discussion of Options to Be Considered Prior to Treatment. J Adolesc Young Adult Oncol 2014;3:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Min CH, Paganetti H, Winey BA, et al. Evaluation of permanent alopecia in pediatric medulloblastoma patients treated with proton radiation. Radiat Oncol 2014;9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early Cognitive Outcomes Following Proton Radiation in Pediatric Patients With Brain and Central Nervous System Tumors. Int J Radiat Oncol Biol Phys 2015;93:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Song S, Park HJ, Yoon JH, et al. Proton beam therapy reduces the incidence of acute haematological and gastrointestinal toxicities associated with craniospinal irradiation in pediatric brain tumors. Acta Oncol 2014;53:1158–64. [DOI] [PubMed] [Google Scholar]
- 93.Yock TI, Bhat S, Szymonifka J, et al. Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiother Oncol 2014;113:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yock TI, Yeap BY, Ebb DH, et al. Long-term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016;17:287–98. [DOI] [PubMed] [Google Scholar]
- 95.Mailhot Vega R, Kim J, Hollander A, et al. Cost effectiveness of proton versus photon radiation therapy with respect to the risk of growth hormone deficiency in children. Cancer 2015;121:1694–702. [DOI] [PubMed] [Google Scholar]
- 96.Jones L, Carol H, Evans K, et al. A review of new agents evaluated against pediatric acute lymphoblastic leukemia by the Pediatric Preclinical Testing Program. Leukemia 2016;30:2133–41. [DOI] [PubMed] [Google Scholar]
- 97.Kurmasheva RT, Houghton PJ. Identifying novel therapeutic agents using xenograft models of pediatric cancer. Cancer Chemother Pharmacol 2016;78:221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kennedy RD, D’Andrea AD. DNA repair pathways in clinical practice: lessons from pediatric cancer susceptibility syndromes. J Clin Oncol 2006;24:3799–808. [DOI] [PubMed] [Google Scholar]