A novel epigenetic regulator Jarid1b (KDM5B) that contributes to increasing vascular soluble epoxide hydrolase (sEH) protein expression and impairing endothelial function was identified by Vasconez et al1 in this edition of Act Physiologica.
Elevated angiotensin II levels contribute to several cardiovascular diseases and endothelial dysfunction in hypertension.2 Endothelial dysfunction in angiotensin II-dependent hypertension is partly because of increased sEH activity.3,4 Increases in vascular sEH expression and activity result in the degradation of endothelial-derived hyperpolarizing epoxyeicosatrienoic acids (EETs).3,4 EPHX2 is the gene that encodes the sEH protein. Experimental studies in animal models as well as human studies have clearly demonstrated that increased EPHX2 mRNA expression, sEH protein expression, or sEH activity contribute to endothelial dysfunction and cardiovascular disease.3 Interest in sEH as an important contributor to endothelial dysfunction in cardiovascular diseases has been further ignited by recent evidence in humans demonstrating that sEH inhibition improves endothelial-dependent forearm blood flow responses to bradykinin in subjects with chronic obstructive pulmonary disease (COPD).5 A major gap that remains is how elevated angiotensin II levels results in increased sEH expression and endothelial dysfunction.
Angiotensin II-dependent upregulation of sEH has been demonstrated in animal models of hypertension and in cultured endothelial cells.3 Kidney and renal microvascular sEH expression increase in angiotensin II hypertension.4 Likewise, sEH inhibitors are antihypertensive and improve endothelial function in angiotensin II-dependent hypertension.4 Cultured endothelial cells exposed to angiotensin II have increased EPHX2 mRNA expression and sEH protein expression.6 Previous findings revealed that angiotensin II regulation of the transcriptional factor AP-1 contributed to increased endothelial cell EPHX2 and sEH levels.6 More recently, epigenetics like histone modifications have been demonstrated to make significant contributions to cardiovascular diseases.7 Although EPHX2 polymorphisms contribute to cardiovascular disease, epigenetic EPHX2 regulation has not been explored.3 The findings by Vasconez et al in this Acta Physiologica edition provide novel evidence that a histone demethylase Jarid1b (KDM5B) mediates angiotensin II-induced endothelial dysfunction by increasing sEH expression.1
Endothelial dysfunction is an indicative of poor cardiovascular health and a strong predictor for future cardiovascular events.8 Vasconez et al evaluated the contribution of the histone demethylase, Jarid1b, on angiotensin II-mediated endothelial dysfunction.1 Angiotensin II-induced endothelial dysfunction has previously been demonstrated to be because of decreased nitric oxide (NO) availability and EETs.3,4 Increased sEH protein expression in angiotensin II hypertension is responsible for the decrease in EETs.4 A contribution for Jarid1b to angiotensin II-mediated endothelial NO-independent aortic and mesenteric resistance artery impaired relaxation was identified by Vasconez et al utilizing Jarid1b knockout mice and Jarid1b pharmacological inhibition.1 NO-independent endothelial vasorelaxation was improved by sEH or Jarid1b inhibition. Confirmatory data demonstrated that the sEH inhibitor, AUDA did not improve vasorelaxation in Jarid1b knockout mice. These data provide convincing evidence that Jarid1b deletion or inhibition improves endothelial function in mice exposed to elevated angiotensin II levels.
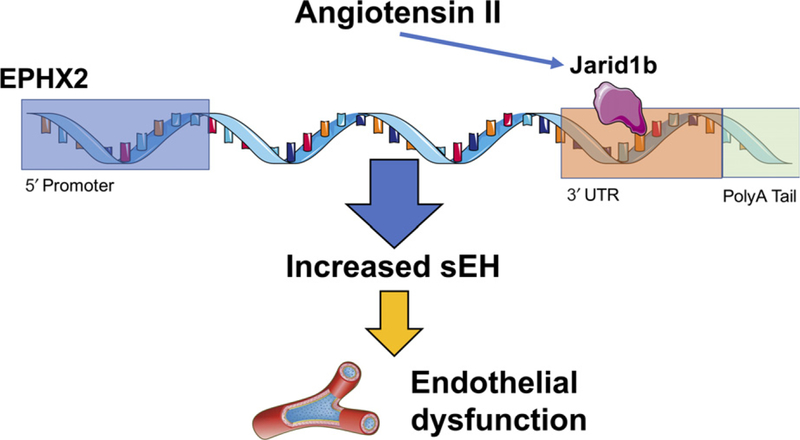
Next, the mechanism responsible for histone demethylase Jarid1b induced increases in sEH expression and endothelial cell dysfunction were explored. Additional evaluation was conducted in cultured lung endothelial cells from wild-type and Jarid1b knockout mice. Angiotensin II increased sEH expression in cultured endothelial cells and Jarid1b-deficient cells or Jarid1b inhibition decreased sEH expression. Further investigation into the Jarid1b mechanism of action was conducted. Jarid1b epigenetic actions have previously been demonstrated to be because of demethylation on histone H3K4me3.9 Histone demethylation by Jarid1b would be anticipated to suppress sEH expression. Thus, the contribution for Jarid1b to increase endothelial cell sEH expression must occur via another mechanism. Aortic and cultured human microvascular smooth muscle cell chromatin immunoprecipitation studies determined that Jarid1b binds to the EPHX2 3’UTR region to maintain UTR length and increase the stability of EPHX2 mRNA (Figure 1). Likewise, Jarid1b inhibition in cultured vascular smooth muscle cells resulted in a greater level of EPHX2 3’UTR in the nucleus compared to cytoplasm that would result in decreased sEH protein expression and improved endothelial function.
FIGURE 1.
Epigenetic mechanism for angiotensin II–mediated increase in soluble epoxide hydrolase (sEH) expression and endothelial dysfunction. Angiotensin II increases transcription factor Jarid1b (KDM5B) resulting in maintenance of EPHX2 3’ untranslated region (3’UTR) length and mRNA stability to increase sEH protein expression and subsequent endothelial dysfunction. EPHX2, sEH gene
The findings in this study have exciting potential for developing therapeutics for cardiovascular diseases. In contrast, there are unanswered questions about Jarid1b regulation of sEH in cardiovascular disease. One interesting aspect was the finding that Jarid1b regulated sEH expression in endothelial and vascular smooth muscle cells. The importance for sEH regulation in endothelial vs vascular smooth muscle cells in cardiovascular diseases remains poorly understood. Future studies to evaluate Jarid1b and sEH in cardiovascular diseases that have impaired vascular smooth muscle cell function could provide insight. Another avenue that is worthy of additional investigation is heart, brain, liver, and kidney organ damage associated with metabolic and cardiovascular diseases. The contribution for increased sEH as a contributing factor to endoplasmic reticulum stress, apoptosis, inflammation, and fibrosis to result in organ damage has been clearly established.3 Not known is the contribution for Jarid1b on sEH regulation in these diseases. Limited insight into Jarid1b anti-inflammatory effects was provided in the current study. This limited data demonstrated that Jarid1b pharmacological inhibition decreased vascular mRNA expression of the inflammatory mediator tumour necrosis factor alpha (TNF). Additional studies are required to determine the full anti-inflammatory actions with pharmacological Jarid1b inhibition or in Jarid1b knockout mice. Another key question is whether Jarid1b regulation of sEH expression is limited to diseases associated with elevated angiotensin II levels. Accordingly, there remains significant exploration to determine the importance for the ability of Jarid1b to increase sEH protein expression and contribute to cardiovascular and other diseases.
In conclusion, the findings in this scientific article provide convincing evidence that Jarid1b inhibitors are a novel therapeutic target for cardiovascular diseases.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases DK103616 and Dr. Ralph and Marian Falk Medical Research Trust Bank of America, N.A., Trustee. Servier Medical Art was used to generate Figure and is licensed by Servier under a Creative Commons Attribution 3.0 Unported License.
Footnotes
CONFLICT OF INTEREST
No conflicts to declare.
REFERENCES
- 1.Vasconez AE, Janetzko P, Oo JA, et al. The histone demethylase Jarid1b mediates angiotensin II-induced endothelial dysfunction by controlling the 3’UTR of soluble epoxide hydrolase. Acta Physiol (Oxf) 2018;e13168 [Epub ahead of print]. 10.1111/apha.13168. [DOI] [PubMed]
- 2.Ferrario CM . Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J Renin Angiotensin Aldosterone Syst 2006;7:3–14. [DOI] [PubMed] [Google Scholar]
- 3.Imig JD. Prospective for cytochrome P450 epoxygenase cardiovascular and renal therapeutics. Pharmacol Ther [Epub ahead of print] Review. 10.1016/j.pharmthera.2018.06.015 [DOI] [PMC free article] [PubMed]
- 4.Zhao X, Yamamoto T, Newman JW, et al. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol 2004;15:1244–1253 [PubMed] [Google Scholar]
- 5.Yang L, Cheriyan J, Gutterman DD, et al. Mechanisms of vascular dysfunction in COPD and effects of a novel soluble epoxide hydrolase inhibitor n smokers. chest 2017;151: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ai D, Fu Y, Guo D, et al. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA 2007;104:9018–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordovás JM, Smith CE. Epigenetics and cardiovascular disease. Nat Rev Cardiol 2010;7:510–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract 2014;2014:291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Y, Zhu Z, Han G, et al. JARID1B is a histone H3 lysine 4 demethylase up-regulated in prostate cancer. Proc Natl Acad Sci USA 2007;104:19226–19231. [DOI] [PMC free article] [PubMed] [Google Scholar]