Abstract
Introduction:
Pain following herpes zoster (HZ) can persist for months and negatively impact quality of life. To evaluate the effect of zoster vaccine live (ZVL) on progression of pain following HZ, we conducted a prospective cohort study of HZ cases at Kaiser Permanente Southern California.
Methods:
ZVL vaccinated and unvaccinated members aged ≥60 years with laboratory-confirmed HZ from January 18, 2012 to February 26, 2015 were followed up within 5 days of HZ diagnosis, and at 30, 60, and 90 days after diagnosis. Pain was assessed with the Zoster Brief Pain Inventory (ZBPI) on a 0-10 scale, using cut-points of ≥3, ≥5, and ≥7, with postherpetic neuralgia (PHN) defined as pain ≥3 at 90 days. Log binomial regression was used to estimate adjusted risk ratios (aRRs) and 95% confidence intervals (CIs) associated with pain, comparing vaccinated versus unvaccinated HZ patients.
Results:
We interviewed 509 vaccinated and 509 unvaccinated HZ patients. ZVL was associated with significantly lower risks of HZ-related pain at all time-points. The risk of PHN in vaccinated and unvaccinated patients, respectively, was 9.2% and 15.4% (aRR= 0.594, 95% CI: 0.413, 0.854); 2.0% and 4.8% of these patients reported pain ≥7 (aRR=0.332, 95% CI: 0.153, 0.721). Irrespective of vaccination, the risk of PHN was lower in adults aged <70 years versus those ≥70 years and was similar or lower in females versus males.
Conclusion:
We used laboratory confirmation of HZ cases and patient survey to show that aside from preventing HZ, ZVL reduced HZ-related pain and prevented PHN among vaccine recipients who experienced HZ. Observational studies will be needed to evaluate long-term effectiveness of the new recombinant zoster vaccine and its benefits in protecting patients against PHN.
Introduction
Herpes zoster (HZ) is a common condition, affecting more than 1 million people per year in the U.S. prior to the availability of vaccines.[1–3] Elderly persons are most vulnerable to HZ and its consequences. Postherpetic neuralgia (PHN), a debilitating sequela of HZ, occurs in 5% to more than 30% of HZ cases.[4] PHN is widely defined as HZ-related pain persisting 90 days after HZ, although pain of other durations is often reported (e.g. 30 to 180 days after HZ).[5, 6] The pain and discomfort of PHN can last for months to years and can profoundly affect quality of life, interfering with sleep and daily activities, and leading to weight loss, depression and social withdrawal.[7–10]
In 2006, live-attenuated herpes zoster vaccine, ZOSTAVAX® (Merck & Co., Inc., Whitehouse Station, NJ; Zoster Vaccine Live; ZVL) was recommended by the Advisory Committee for Immunization Practices (ACIP) for prevention of HZ in adults aged ≥60 years.[11] The Shingles Prevention Study (SPS) demonstrated that ZVL reduced the risk of HZ by 51.3% and the incidence of PHN by 66.5%,[12] with waning efficacy over time.[13, 14] Similar results have been reported in post-licensure studies across diverse populations.[15–19]
In the SPS, the reduced incidence of PHN in vaccinated participants ages 60-69 years was due mostly to the reduced risk of HZ, as people who did not have HZ could not develop PHN. However, in persons ages ≥70 years, ZVL had an incremental benefit in prevention of PHN in patients who developed HZ despite vaccination.[11, 12, 20] Previously, we conducted a study of ZVL vaccinated and unvaccinated HZ patients using electronic health records (EHR) to assess the risk of PHN. The study found that ZVL was associated with a lower risk of PHN in female HZ patients but not male HZ patients.[21]
Data derived from health care settings, such as EHR, are likely to sensitively capture episodes of HZ, since most adults seek care for this acute condition.[22] On the other hand, such data may not fully capture the progression of HZ to PHN: some patients may not continue to seek care for pain over longer periods of time, particularly since treatments for HZ-related pain may cause side-effects and are often ineffective. Care-seeking behavior for chronic pain may vary between vaccinated and unvaccinated patients, potentially confounding evaluation of vaccine effectiveness against PHN using EHR. Pain assessment using patient survey is not dependent on care-seeking and can capture the severity and duration of HZ-related pain directly reported by the patients. In this study, we interviewed a large cohort of ZVL vaccinated and unvaccinated patients with laboratory-confirmed HZ to assess the progression of HZ-related pain and to evaluate the effect of ZVL on PHN.
Methods
Study site
We conducted a prospective cohort study at Kaiser Permanente Southern California (KPSC), an integrated health care organization that serves approximately 4.4 million residents of Southern California. Members of KPSC have diverse sociodemographic backgrounds largely representative of the underlying population.[23] KPSC maintains comprehensive EHR, including sociodemographics, diagnosis and procedure codes, vaccinations, medications, and laboratory results. As KPSC is a pre-paid system and recommended vaccines are provided free of charge, members have a strong incentive to receive care within the system, and data captured in EHR are reasonably complete. At the time of the study, ZVL was recommended for prevention of HZ for adults aged ≥60 years. The study was reviewed and approved by the KPSC Institutional Review Board.
Study population
Incident HZ was defined as the first HZ between January 18, 2012 and February 26, 2015, with no HZ diagnosis in the prior year. Patients with incident HZ diagnoses were identified prospectively by International Classification of Diseases, 9th revision (ICD-9) codes 053.xx from outpatient and emergency department encounters within 24 hours of diagnosis. HZ patients aged ≥60 years with a record of ZVL vaccination prior to their HZ diagnosis were defined as vaccinated HZ cases. Vaccinated HZ cases were matched by sex and age (±2 years) to HZ cases with no record of ZVL vaccination (ZVL unvaccinated cases).
Interviews
Upon identification of an incident HZ diagnosis, each HZ patient was contacted via telephone by a trained Research Associate using a standard script to determine eligibility and to set up a face-to-face meeting. Patients were eligible if they could be reached within 10 phone call attempts, could be interviewed face-to-face for collection of skin lesion samples within 5 days of diagnosis, lived within a 3-hour drive, and could competently answer interview questions in English or Spanish.
Informed consent was obtained in-person prior to the baseline interview. Patients were remunerated for their participation. A questionnaire was administered, including information on sociodemographic factors, underlying medical conditions, and the HZ clinical episode. The Zoster Brief Pain Inventory (ZBPI) was used to measure the burden of pain and PHN. The ZBPI is a validated instrument modified by Coplan et al. from the Brief Pain Inventory to specifically measure HZ-related pain and discomfort in adults aged ≥60 years.[24] The ZBPI uses an 11-point Likert scale (0-10) to rate pain in 4 ways (worst, least, average, now) and pain-related interference in 7 functional categories (general activity, mood, walking ability, work, relations with others, sleep, enjoyment of life). As demonstrated by Coplan et al., a ZBPI worst pain score of ≥3 at 90 days is a valid and reliable measure of PHN. The ZBPI was administered at the face-to-face baseline interview, and by telephone at 30 days, 60 days, and 90 days following HZ diagnosis.
Laboratory confirmation of varicella zoster virus (VZV)
At the face-to-face baseline interview, trained RAs collected at least two skin lesion samples according to the protocol prepared by the National Varicella Zoster Virus (VZV) Laboratory at the Centers for Disease Control and Prevention (CDC), which performed the standard polymerase chain reaction (PCR) test to confirm the presence of VZV from the skin specimens.[25] Patients with negative PCR results were excluded from the study.
Statistical analysis
We included all matched and unmatched HZ cases in the analysis to maximize the analytic sample. Descriptive analyses compared patient-reported demographics, baseline comorbidities and clinical characteristics by vaccinated and unvaccinated HZ cases. Chi-square tests or Fisher’s exact tests (for categorical variables) or Kruskal-Wallis tests (for continuous variables) were used to assess the difference between groups. To examine the effect of ZVL vaccination on severity and duration of pain, we present 3 different binary outcomes based on ZBPI scores of worst pain in the past 24 hours of ≥3, ≥5, and ≥7. Risk ratios (RRs) and 95% confidence intervals (CIs) for each of these outcomes at baseline, 30 days, 60 days, and 90 days comparing ZVL vaccinated and unvaccinated cases were estimated using log binomial regression adjusted for age, sex, race/ethnicity; immunosuppression at time of HZ diagnosis; comorbidities in the six months prior to HZ diagnosis; outpatient or emergency department visits in the 30 days prior to HZ diagnosis; and prescription of antivirals at the time of HZ diagnosis. If log binomial regression failed to converge, a COPY method was used to overcome the non-convergence issue.[26] Age- and sex-specific RRs were also estimated, adjusted for the other variables. Analyses were performed using SAS 9.3 (SAS Institute, Inc, Cary, North Carolina).
Results
Of 2332 eligible patients contacted, 1111 refused to participate (approximately 45% of vaccinated and 56% of unvaccinated). Of the 1221 (52.4%) who were interviewed, 193 were excluded due to negative PCR test results or inadequate skin lesion samples, 2 were excluded because HZ vaccination could not be confirmed, and 8 were excluded because they were only interviewed at baseline. The final study population included a total of 1018 PCR-confirmed HZ patients (509 ZVL vaccinated patients and 509 ZVL unvaccinated patients, with 497 matched pairs and 12 unmatched patients in each group).
Overall, 68.6% of patients were aged ≥70 years at HZ diagnosis and two-thirds were female (Table 1). Compared to unvaccinated patients, more vaccinated patients were white (68.6% vs. 45.6%), while more unvaccinated patients were Hispanic (28.5% vs. 12.6%). More vaccinated patients than unvaccinated patients had completed a Bachelor’s degree or above (43.0% vs. 32.2%).
Table 1:
Demographic characteristics of zoster vaccine live vaccinated and unvaccinated herpes zoster patients
| Vaccinated N=509 (%) |
Unvaccinated N=509 (%) |
Total N=1018 (%) |
p-value | |
|---|---|---|---|---|
| Age at HZ diagnosis | 0.28 | |||
| Less than 70 years1 | 152 (29.9) | 168 (33.0) | 320 (31.4) | |
| 70 years or more | 357 (70.1) | 341 (67.0) | 698 (68.6) | |
| Mean (standard deviation) | 73.5 (6.7) | 73.1 (6.8) | 73.3 (6.7) | |
| Sex | 0.56 | |||
| Female | 324 (63.7) | 315 (61.9) | 639 (62.8) | |
| Male | 185 (36.3) | 194 (38.1) | 379 (37.2) | |
| Race / ethnicity | <0.0001 | |||
| White | 349 (68.6) | 232 (45.6) | 581 (57.1) | |
| Hispanic | 64 (12.6) | 145 (28.5) | 209 (20.5) | |
| Asian | 43 (8.4) | 58 (11.4) | 101 (9.9) | |
| Black | 26 (5.1) | 49 (9.6) | 75 (7.4) | |
| Multi-Race | 21 (4.1) | 24 (4.7) | 45 (4.4) | |
| Other | 6 (1.2) | 1 (0.2) | 7 (0.7) | |
| Married status2 | 0.07 | |||
| Married/Living with partner | 358 (70.3) | 326 (64.3) | 684 (67.3) | |
| Never married | 8 (1.6) | 18 (3.6) | 26 (2.6) | |
| Separated or divorced | 61 (12.0) | 76 (15.0) | 137 (13.5) | |
| Widowed | 82 (16.1) | 87 (17.2) | 169 (16.6) | |
| Education3 | <0.0001 | |||
| Did not complete high school | 32 (6.3) | 81 (15.9) | 113 (11.1) | |
| High school graduate or equivalent | 78 (15.3) | 109 (21.5) | 187 (18.4) | |
| Some college, vocational, or technical school | 180 (35.4) | 154 (30.3) | 334 (32.8) | |
| Bachelor’s degree or above | 219 (43.0) | 164 (32.2) | 383 (37.7) | |
| Employment4 | 0.15 | |||
| Currently employed | 90 (17.7) | 102 (20.1) | 192 (18.9) | |
| Not employed | 12 (2.4) | 21 (4.1) | 33 (3.2) | |
| Retired | 407 (80.0) | 385 (75.8) | 792 (77.9) | |
HZ – herpes zoster
Three patients were age <60 years (56, 56, and 58 years).
Data were missing from 2 unvaccinated patients.
Data were missing from 1 unvaccinated patient.
Data were missing from 1 unvaccinated patient.
The distribution of comorbidities in the 6 months prior to HZ diagnosis was similar between vaccinated and unvaccinated patients (Table 2), except for higher prevalence of musculoskeletal conditions, depression and lung conditions in vaccinated patients, and higher prevalence of autoimmune disorders and liver or gallbladder conditions in unvaccinated patients. There were no differences between groups in underlying chronic pain prior to HZ symptom onset (32.6% overall) and immunosuppression at time of HZ diagnosis (9.9% overall).
Table 2:
Comorbidities in zoster vaccine live vaccinated and unvaccinated herpes zoster patients
| Vaccinated N=509 (%) |
Unvaccinated N=509 (%) |
Total N=1018 (%) |
p-value | |
|---|---|---|---|---|
| Comorbidities in the 6 months prior to HZ diagnosis: | ||||
| Heart or circulatory | 410 (80.6) | 407 (80.0) | 817 (80.3) | 0.81 |
| Musculoskeletal | 305 (59.9) | 270 (53.0) | 575 (56.5) | 0.03 |
| Endocrine (thyroid disorders, diabetes) | 177 (34.8) | 185 (36.3) | 362 (35.6) | 0.60 |
| Allergies or asthma | 141 (27.7) | 121 (23.8) | 262 (25.7) | 0.15 |
| Depression | 80 (15.7) | 58 (11.4) | 138 (13.6) | 0.04 |
| Kidney or bladder | 62 (12.2) | 61 (12.0) | 123 (12.1) | 0.92 |
| Skin | 59 (11.6) | 50 (9.8) | 109 (10.7) | 0.36 |
| Stomach or intestinal | 52 (10.2) | 53 (10.4) | 105 (10.3) | 1.0 |
| Cancer | 44 (8.6) | 32 (6.3) | 76 (7.5) | 0.15 |
| Lung | 46 (9.0) | 29 (5.7) | 75 (7.4) | 0.04 |
| Neurological | 29 (5.7) | 27 (5.3) | 56 (5.5) | 0.78 |
| Autoimmune (lupus, rheumatoid arthritis) | 15 (2.9) | 36 (7.1) | 51 (5.0) | <0.01 |
| Liver or gallbladder | 4 (0.8) | 11 (2.2) | 15 (1.5) | 0.07 |
| Chronic pain before onset of HZ symptoms1 | 173 (34.0) | 159 (31.4%) | 332 (32.6) | 0.57 |
| Immunosuppression at time of HZ diagnosis2 | 54 (10.6) | 47 (9.2) | 101 (9.9) | 0.46 |
HZ, – herpes zoster
Data were missing for 2 unvaccinated patients.
Patient reported having been told by a health care provider that their immune system was weak at the time of HZ diagnosis due to underlying condition, medical treatment, or medication.
Among vaccinated patients, the median time between ZVL vaccination and rash onset was 4.4 years (interquartile range: 2.8, 5.6). In the month prior to rash onset, there were no differences between vaccinated and unvaccinated patients in prodromal pain or discomfort (48.1% overall), other prodromal symptoms (27.4%), or doctor or emergency department visits (12.2%) (Table 3). Most patients were prescribed an antiviral at time of diagnosis.
Table 3:
Clinical characteristics of herpes zoster in zoster vaccine live vaccinated and unvaccinated patients
| Vaccinated N=509 (%) |
Unvaccinated N=509 (%) |
Total N=1018 (%) |
p-value | |
|---|---|---|---|---|
| Prodromal pain or discomfort in month prior to rash onset1 | 0.63 | |||
| Yes | 241 (47.3) | 248 (48.8) | 489 (48.1) | |
| No | 268 (52.7) | 260 (51.2) | 528 (51.9) | |
| Other prodromal symptoms (fever, headache, body aches, fatigue, or confusion) in month prior to rash onset2 | 0.98 | |||
| Yes | 137 (27.3) | 139 (27.4) | 276 (27.4) | |
| No | 364 (72.7) | 368 (72.6) | 732 (72.6) | |
| Doctor or emergency department for pain or discomfort in month prior to rash onset3 | 0.10 | |||
| Yes | 53 (10.5) | 70 (13.9) | 123 (12.2) | |
| No | 451 (89.5) | 434 (86.1) | 885 (87.8) | |
| Prescribed antiviral at time of diagnosis4 | 0.23 | |||
| Yes | 474 (93.5) | 478 (95.2) | 952 (94.4) | |
| No | 33 (6.5) | 24 (4.8) | 57 (5.6) | |
| Days from rash onset to health care visit or beginning antiviral treatment5 | 0.12 | |||
| Same day | 65 (13.7) | 76 (15.9) | 141 (14.8) | |
| 1-3 days | 220 (46.4) | 246 (51.6) | 466 (49.0) | |
| 4-6 days | 117 (24.7) | 99 (20.8) | 216 (22.7) | |
| >6 days | 72 (15.2) | 56 (11.7) | 128 (13.5) | |
Data were missing for 1 unvaccinated patient.
Data were missing for 8 vaccinated and 2 unvaccinated patients.
Data were missing for 5 vaccinated and 5 unvaccinated patients.
Data were missing for 2 vaccinated and 7 unvaccinated patients.
Data were missing for 35 vaccinated and 32 unvaccinated patients.
At baseline, the risks of worst pain in the past 24 hours by the 3 ZBPI worst pain score cut-points (≥3, ≥5, and ≥7) were not significantly different for vaccinated and unvaccinated patients; worst pain ≥3 was reported by 75.4% of vaccinated patients and 78.7% of unvaccinated patients, while worst pain ≥7 was reported by 40.9% and 46.5%, respectively (Figure 1 and Supplementary Table 1). By 30 days, the percentages of vaccinated and unvaccinated patients reporting pain had dropped sharply, and the risks of pain by all 3 ZBPI cut-points were significantly lower in vaccinated versus unvaccinated patients. The percentages reporting pain further declined by 60 days and 90 days and continued to be lower in vaccinated versus unvaccinated patients. The risk of worst pain >3 at 90 days (PHN) was 9.2% in vaccinated patients and 15.4% in unvaccinated patients (aRR= 0.594, 95% CI: 0.413, 0.854). The risk of worst pain ≥7 at 90 days was 2.0% in vaccinated patients and 4.8% in unvaccinated patients (aRR=0.332, 95% CI: 0.153, 0.721). The point estimates of aRR for all 3 ZBPI worst pain score cut-points were lower at 90 days than point estimates of aRR at 30 days or 60 days, suggesting stronger associations of ZVL with more prolonged and severe levels of pain.
Figure 1:
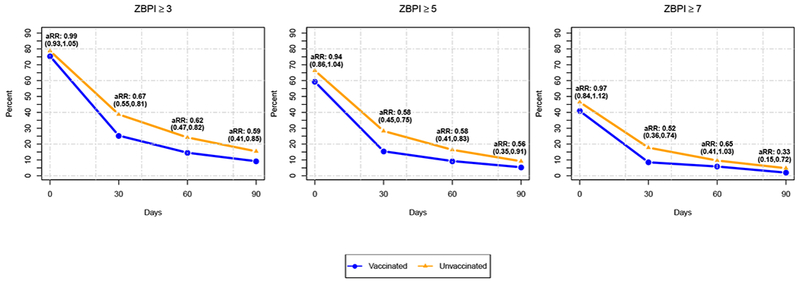
Patient-reported pain by Zoster Brief Pain Inventory (ZBPI) score cut-offs for worst pain in past 24 hours of ≥3, ≥5, and ≥7 at herpes zoster (HZ) diagnosis (baseline) and 30 days, 60 days, and 90 days in zoster vaccine live (ZVL) vaccinated and unvaccinated patients
Stratified analyses were conducted at 90 days to assess any effect modification by age or sex (Table 4); denominator size, and thus statistical power and precision, varied across different cells, but the patterns were instructive. The risks of pain at 90 days by ZBPI worst pain score cut-points of ≥3 and ≥5 were significantly lower for vaccinated versus unvaccinated patients aged ≥70 years, and in men. For severe pain (ZBPI ≥7 cut-point), a statistically significant lower risk was observed in vaccinated versus unvaccinated patients aged ≥70 years and in women. The risk of worst pain ≥3 at 90 days (PHN) was 8.8% in vaccinated women, 14.1% in unvaccinated women, 9.8% in vaccinated men, and 17.6% in unvaccinated men. The risk of PHN increased with age and was 4.6% in vaccinated patients aged <70 years, 11.1% in unvaccinated patients aged <70 years, 11.2% in vaccinated patients aged ≥70 years, and 17.6% in unvaccinated patients aged ≥70 years.
Table 4:
Zoster Brief Pain Inventory scores for worst pain in past 24 hours at 90 days after herpes zoster diagnosis in zoster vaccine live vaccinated and unvaccinated cases, stratified by age and sex
| Vaccinated N=5011 n (%) |
Unvaccinated N=4801 n (%) |
Unadjusted Risk Ratio (95% CI) |
Adjusted2 Risk Ratio (95% CI) |
|
|---|---|---|---|---|
| ZBPI ≥3 | ||||
| Age | ||||
| Less than 70 years3 | 7 (4.6) | 18 (11.1) | 0.417 (0.179, 0.971) | 0.466 (0.198, 1.098) |
| 70 years or more | 39 (11.2) | 56 (17.6) | 0.629 (0.429, 0.922) | 0.627 (0.419, 0.938) |
| Sex | ||||
| Female | 28 (8.8) | 42 (14.1) | 0.633 (0.403, 0.993) | 0.751 (0.473, 1.192) |
| Male | 18 (9.8) | 32 (17.6) | 0.537 (0.310, 0.932) | 0.466 (0.263, 0.827) |
| Total | 46 (9.2) | 74 (15.4) | 0.591 (0.417, 0.837) | 0.594 (0.413, 0.854) |
| ZBPI ≥5 | ||||
| Age | ||||
| Less than 70 years3 | 5 (3.3) | 6 (3.7) | 0.894 (0.279, 2.869) | 0.845 (0.240, 2.970) |
| 70 years or more | 22 (6.3) | 38 (12.0) | 0.537 (0.325, 0.887) | 0.514 (0.303, 0.871) |
| Sex | ||||
| Female | 16 (5.0) | 25 (8.4) | 0.607 (0.331, 1.115) | 0.718 (0.383, 1.344) |
| Male | 11 (6.0) | 19 (10.4) | 0.585 (0.287, 1.195) | 0.450 (0.217, 0.930) |
| Total | 27 (5.4) | 44 (9.2) | 0.596 (0.376, 0.947) | 0.562 (0.347, 0.910) |
| ZBPI ≥7 | ||||
| Age | ||||
| Less than 70 years | 1 (0.7) | 3 (1.9) | 0.358 (0.038, 3.401) | 0.156 (0.011, 2.280) |
| 70 years or more | 9 (2.6) | 20 (6.3) | 0.417 (0.193, 0.903) | 0.328 (0.144, 0.750) |
| Sex | ||||
| Female | 5 (1.6) | 16 (5.4) | 0.297 (0.110, 0.799) | 0.299 (0.108, 0.828) |
| Male | 5 (2.7) | 7 (3.9) | 0.722 (0.234, 2.233) | 0.440 (0.134, 1.451) |
| Total | 10 (2.0) | 23 (4.8) | 0.422 (0.203, 0.878) | 0.332 (0.153, 0.721) |
HZ – herpes zoster
CI – confidence interval
ZBPI – Zoster Brief Pain Inventory
Data from the ZBPI at 90 days were missing from 8 vaccinated and 29 unvaccinated cases.
Risk ratios were adjusted for race/ethnicity, immunosuppression at the time of HZ diagnosis, comorbidities in the 6 months prior to HZ diagnosis (autoimmune disorders, musculoskeletal conditions, lung conditions, liver/gallbladder conditions, depression), doctor or emergency department visits in the 30 days prior to HZ, and antiviral prescription at the time of HZ diagnosis. Models stratified by age group also adjusted for sex, and models stratified by sex also adjusted for age group.
The model was adjusted for the same covariates except liver/gallbladder conditions (the rarest condition among the adjusted covariates).
Discussion
This large, prospective cohort study of HZ patients used laboratory methods to confirm all cases, and used patient survey, which is not biased by health-seeking behavior, to directly assess HZ-related pain and PHN. Our study indicates that ZVL vaccination reduces HZ-related pain duration and severity and provides incremental benefits beyond protection against HZ. It also adds to our understanding of the likelihood with which HZ progresses to PHN, and how this progression is influenced by age and sex.
The risk of PHN in this study was similar to that in the SPS, which also used survey of confirmed HZ patients to assess the progression of pain directly reported by patients as a function of ZVL.[12] The SPS actively identified HZ cases among enrolled participants, whereas we started with medically-attended HZ, which could be more clinically-relevant. In the SPS, the risk of PHN was 8.6% in vaccinated HZ patients and 12.5% in unvaccinated patients, compared to 9.2% and 15.4%, respectively, in our study. In both our study and the SPS, the risk of PHN was higher in male versus female HZ patients and HZ patients aged ≥70 years versus younger patients.
This study found higher risk of PHN by patient survey than our previous study at KPSC of HZ patients followed through encounters in the EHR, in which the risk of PHN was 4.9% in vaccinated patients and 8.7% in unvaccinated patients. In the EHR study, the risk of PHN was higher in female versus male unvaccinated HZ patients (10.4% versus 5.8%), but not in vaccinated HZ patients (4.1% in females and 6.0% in males). The association of ZVL with a lower risk of PHN in sex-specific strata also differed between this study using patient survey and the previous EHR study; the latter observed a reduced risk of PHN in vaccinated versus unvaccinated female HZ patients but not males, whereas the survey results reported here suggest ZVL may offer some protection against the progression and severity of HZ-related pain in both men and women who develop HZ despite vaccination.
One explanation for the differences between patient survey and EHR may be variations in care-seeking behavior. HZ patients experiencing pain lasting weeks to months may tire of presenting for health care without obtaining relief and may drop out of care. Others may retain more hopefulness or gain therapeutic benefits and continue to seek care. These individual characteristics and behaviors vary widely in the population and are likely influenced by many measurable and unmeasurable factors. EHR capture HZ-related pain from health care encounters, but exclusive reliance on these data may underestimate the risk of progression of HZ to PHN. This phenomenon, which has been inadequately considered in the medical literature, may similarly affect assessments of other types of pain conditions as they become chronic.
These data have implications for shingles vaccine uptake as providers and patients may be more likely to consider vaccination if they know the vaccine will protect against severe pain even if the protection against HZ is not perfect. The newly licensed zoster vaccine, Shingrix® (GlaxoSmithKline, Research Triangle Park, NC; recombinant zoster vaccine; RZV) is indicated for the prevention of HZ in adults aged ≥50 years[27] and was preferentially recommended over ZVL by the ACIP [28], The high efficacy of RZV against HZ and PHN in clinical trials precluded observation of an incremental impact of RZV on PHN beyond prevention of HZ.[29, 30] Long-term observational studies will be required to assess this phenomenon and other unanswered questions on RZV long-term effectiveness and safety.
This study had several potential limitations. Patients who agreed to participate in the study might have differed from non-participants. For example, participants could have greater severity of HZ-related pain than non-participants, or greater interest in the disease. However, this participation bias was likely similar between ZVL vaccinated and unvaccinated patients. While our analyses accounted for sociodemographic characteristics, comorbidities, and prior health care utilization, there may have been other factors associated with receipt of ZVL that were unrecognized and unaccounted for. In addition, a small portion of the patients did not respond to attempts for one or more of the 30, 60, or 90-day interviews. It is possible that these patients had less pain than those who did respond. To assess potential bias, sensitivity analyses for PHN were conducted by assuming missing patients had ZBPI worst pain scores of 0 (no pain) in their subsequent response or assuming their pain stayed the same as reported in the most recently available interview. The aRR estimated from sensitivity analyses did not vary significantly, suggesting bias due to non-response was likely minimal. Finally, the sample size for some subgroup analyses were low. Therefore, although the point estimates of aRR were less than one for all age- or sex- subgroups, the confidence intervals for some of them were wide.
In conclusion, this study describes follow up of laboratory-confirmed HZ cases and provides evidence that ZVL protects against PHN even when HZ occurs despite vaccination. Our study also provides insights on the natural history of HZ and demonstrates that survey data relating to chronic pain can differ considerably from EHR-encounter data, even in the same health care settings - a methodological issue that can benefit from further analysis. As RZV is increasingly used over ZVL, it will be imperative to evaluate the incremental benefit of RZV in preventing HZ-related pain.
Supplementary Material
Acknowledgements
We thank Kimberly Holmquist for project management; Fernando Barreda, Ana Espinosa-Rydman, Nidia Golla, Hong Hua, Peggy Hung, Bibiana Martinez, Magdalena Pomichowski, Melena Taylor, Leticia Vega-Daily, and Sandra Zakai for data collection; Songyue Chen for programming contributions; and the HZ patients who participated in the study.
Funding
This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Health (NIH). Funding Number: 5R01AI089930
Conflict of interest
Katia Bruxvoort reports receiving research support from GlaxoSmithKline (GSK), Dynavax, and Seqirus. Lei Qian reports receiving research support from GSK, Dynavax, and Novavax. Lina S. Sy reports receiving research support from GSK, Novavax, and Dynavax. Yi Luo reports receiving research support from GSK, Novavax, and Seqirus. Hung Fu Tseng reports receiving research support from GSK, Novavax, and Seqirus. All other authors report no conflicts of interest.
Abbreviations
- ACIP
Advisory Committee for Immunization Practices
- CI
confidence interval
- EHR
electronic health records
- HZ
herpes zoster
- KPSC
Kaiser Permanente Southern California
- PCR
polymerase chain reaction
- PHN
postherpetic neuralgia
- RR
risk ratio
- RZV
recombinant zoster vaccine
- SPS
Shingles Prevention Study
- VZV
varicella zoster virus
- ZBPI
Zoster Brief Pain Inventory
- ZVL
zoster vaccine live
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the NIAID, NIH, or the Centers for Diseases Control and Prevention (CDC).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Johnson BH, Palmer L, Gatwood J, Lenhart G, Kawai K, Acosta CJ. Annual incidence rates of herpes zoster among an immunocompetent population in the United States. BMC Infect Dis. 2015;15:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing Incidence of Herpes Zoster Over a 60-year Period From a Population-based Study. Clin Infect Dis. 2016;63:221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4:e004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157:30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Drolet M, Brisson M, Schmader KE, Levin MJ, Johnson R, Oxman MN, et al. The impact of herpes zoster and postherpetic neuralgia on health-related quality of life: a prospective study. CMAJ. 2010;182:1731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–6. [DOI] [PubMed] [Google Scholar]
- [9].Dworkin RH, Gnann JW Jr., Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9:S37–44. [DOI] [PubMed] [Google Scholar]
- [10].Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis. 2004;39:342–8. [DOI] [PubMed] [Google Scholar]
- [11].Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices Centers for Disease C, Prevention. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- [12].Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. [DOI] [PubMed] [Google Scholar]
- [13].Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–6. [DOI] [PubMed] [Google Scholar]
- [16].Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marin M, Yawn BP, Hales CM, Wollan PC, Bialek SR, Zhang J, et al. Herpes zoster vaccine effectiveness and manifestations of herpes zoster and associated pain by vaccination status. Hum Vaccin Immunother. 2015;11:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Izurieta HS, Wernecke M, Kelman J, Wong S, Forshee R, Pratt D, et al. Effectiveness and Duration of Protection Provided by the Live-attenuated Herpes Zoster Vaccine in the Medicare Population Ages 65 Years and Older. Clin Infect Dis. 2017;64:785–93. [DOI] [PubMed] [Google Scholar]
- [19].Baxter R, Bartlett J, Fireman B, Marks M, Hansen J, Lewis E, et al. Long-Term Effectiveness of the Live Zoster Vaccine in Preventing Shingles: A Cohort Study. Am J Epidemiol. 2018;187:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zostavax. Prescribing information [package insert]. 2017.
- [21].Tseng HF, Lewin B, Hales CM, Sy LS, Harpaz R, Bialek S, et al. Zoster Vaccine and the Risk of Postherpetic Neuralgia in Patients Who Developed Herpes Zoster Despite Having Received the Zoster Vaccine. J Infect Dis. 2015;212:1222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hales CM, Harpaz R, Bialek SR. Self-reported herpes zoster, pain, and health care seeking in the Health and Retirement Study: implications for interpretation of health care-based studies. Ann Epidemiol. 2016;26:441–6 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koebnick C, Langer-Gould AM, Gould MK, Chao CR, Iyer RL, Smith N, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–56. [DOI] [PubMed] [Google Scholar]
- [25].Leung J, Harpaz R, Baughman AL, Heath K, Loparev V, Vazquez M, et al. Evaluation of laboratory methods for diagnosis of varicella. Clin Infect Dis. 2010;51:23–32. [DOI] [PubMed] [Google Scholar]
- [26].Petersen MR, Deddens JA. A revised SAS macro for maximum likelihood estimation of prevalence ratios using the COPY method. Occup Environ Med. 2009;66:639. [DOI] [PubMed] [Google Scholar]
- [27].Shingrix. Prescribing information [package insert]. 2017.
- [28].Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375:1019–32. [DOI] [PubMed] [Google Scholar]
- [30].Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372:2087–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


