Abstract
We have studied the effects of bupivacaine on human and bovine articular chondrocytes in vitro. Time-lapse confocal microscopy of human articular chondrocytes showed > 95% cellular death after exposure to 0.5% bupivacaine for 30 minutes. Human and bovine chondrocytes exposed to 0.25% bupivacaine had a time-dependent reduction in viability, with longer exposure times resulting in higher cytotoxicity. Cellular death continued even after removal of 0.25% bupivacaine. After exposure to 0.25% bupivacaine for 15 minutes, flow cytometry showed bovine chondrocyte viability to be 41% of saline control after seven days. After exposure to 0.125% bupivacaine for up to 60 minutes, the viability of both bovine and human chondrocytes was similar to that of control groups.
These data show that prolonged exposure 0.5% and 0.25% bupivacaine solutions are potentially chondrotoxic.
Chondrolysis is a devastating condition in which widespread chondrocyte death over a relatively short period of time leads to loss of cartilage and rapidly progressive joint degeneration.1 The condition has been reported in the hip, knee, ankle and shoulder. In the hip and knee, it has been traced to exposure of the articular cartilage to toxic substances such as methylmethacrylate and chlorhexidine.2,3 Glenohumeral chondrolysis has been observed in young athletic individuals, typically after treatment of instability of the shoulder.4–6 While the use of thermal probes has been implicated in glenohumeral chondrolysis,4 instruments generating high temperature were not used in all cases.5,6 Recent clinical series and reports have suggested that there is an association between continuous intra-articular infusion of bupivacaine solutions and full-thickness loss of cartilage in teenagers and young adults.5–7 These reports have raised concerns that bupivacaine may have adverse effects on the viability of chondrocytes.
We have shown that the use of 0.5% bupivacaine has a direct cytotoxic effect on bovine articular chondrocytes within both alginate-bead cultures and intact articular cartilage in vitro.8 Although recent clinical reports have suggested a possible association between prolonged continuous intra-articular infusion of bupivacaine and chondrolysis, intra-articular bupivacaine has been used clinically in the form of single injections without apparent ill-effects on articular cartilage. These observations raise the question as to whether bupivacaine is toxic to human chondrocytes. This in vitro study was carried out to test the hypotheses that the toxicity of bupivacaine on articular chondrocytes is dose- and time-dependent, and that bupivacaine has dose- and time-dependent cytotoxic effects on human articular chondrocytes.
Materials and Methods
Fresh human articular cartilage was obtained from tissue donors and macroscopically-healthy areas of osteoarthritic knees of patients undergoing knee replacement according to protocols approved by the Institutional Review Board. Fresh bovine articular cartilage was harvested from the knees of animals within four hours of their slaughter. Fluorescent staining and confocal microscopy9 of human and fresh bovine osteochondral cores were used to study the viability of chondrocytes at 30 minutes after exposure to 0.5%, 0.25%, and 0.125% bupivacaine solutions. Normal (0.9%) saline served as a control because it is the primary component of the bupivacaine solutions which were used. These solutions were preservative-free, commercially-available products (Abbot Laboratories, Abbot Park, Illinois or Hospira, Lake Forest, Illinois), with the exception of the 0.125% solution, which was made by mixing equal amounts of normal saline and commercially-available 0.25% bupivacaine.
Three-dimensional alginate-bead cultures
The articular chondrocytes were isolated and re-suspended in three-dimensional alginate-bead cultures. They were cultured in the alginate beads for seven days, in order to maintain their differentiated phenotype and to allow time for the matrix to form before exposure to bupivacaine.10,11
The fresh human and bovine articular cartilage was harvested as full-thickness slices and minced. Articular chondrocytes were isolated from the cartilage by a two-step enzymic digestion using pronase (EMD Chemicals Inc, San Diego, California) and collagenase P (Roche Applied Sciences, Indianapolis, Indiana). Viability was ascertained by Trypan Blue exclusion. The chondrocytes were then encapsulated in alginate beads at a density of 4 x 106 cells per ml. The beads were incubated at 37ºC in 5% CO2 in chondrocyte growth medium consisting of Dulbecco’s Modified Eagle medium/F12 (1:1) (Invitrogen Corporation, Carlsbad, California), supplemented with 10% fetal bovine serum (Invitrogen Corporation) and 1 % penicillin/streptomycin (Invitrogen Corporation) for one week before exposure to bupivacaine.
Flow cytometry
After one week of culture, the alginate beads were segregated into treatment groups of ten beads each. The experimental groups were immersed in 1 ml of 0.5%, 0.25%, and 0.125% bupivacaine hydrochloride. Control groups were placed in 1 ml of sterile 0.9% saline. The beads were immersed for 15, 30 or 60 minutes and were then washed three times and incubated in chondrocyte growth media. Cell viability was assessed at three time points (one hour, 24 hours and one week) after exposure to bupivacaine. More than 3000 bovine chondrocyte alginate-bead cultures were used in 72 experiments.
At the designated times, the bovine and human beads from each treatment group were removed for labelling using a Vybrant Apoptosis Assay Kit #3 (Molecular Probes Inc., Eugene, Oregon). They were incubated in 1 ml of cold 55 mM sodium citrate (4ºC) to dissolve the alginate and release the chondrocytes, which were collected by centrifugation. In order to stain apoptotic cells and the nuclei of dead cells, without labelling live cells, 5 ml of Alexa Flour 488 Annexin V (Molecular Probes Inc.) which stains apoptotic cells, and 1 ml of propidium iodide (Molecular Probes Inc.), which stains nuclei of dead cells, were added to 100 ml of each suspension. The samples were analysed by flow cytometry.
Time-lapse chondrocyte imaging
Live cell-imaging methods12 were adapted for the observation of the viability of the chondrocytes during exposure to bupivacaine. Bovine and human articular chondrocytes within three-dimensional alginate-bead cultures were labelled with 5 mM 5-chloromethylfluorescein diacetate (CMFDA; Molecular Probes Inc.) and 1.5 mM propidium iodide for 30 minutes and then rinsed in CO2 Independent Media (Invitrogen Corporation) for 30 minutes. The labelled beads were submerged in 0.9% saline (controls), 0.125%, 0.25% or 0.5% bupivacaine and placed in a temperature-controlled live cell-imaging stage (Bioptechs, Butler, Pennsylvania) maintained at 37ºC. Using automated time-lapse confocal microscopy (Olympus IX81 DSU, Olympus America Inc., Center Valley, Pennsylvania) red and green fluorescent channel image sets were acquired at one-minute intervals. The percentage of dead cells was calculated for each image set using Image-Pro Plus software (Media Cybernetics Inc., Bethesda, Maryland) and plotted against time. Overall, 28 experiments, 12 with bovine and 16 with human alginate-bead cultures, were performed.
Evaluation of articular cartilage treated with bupivacaine
A coring device 8 mm in diameter (DePuy Mitek, Inc., Raynham, Massachusetts) was used to harvest osteochondral cores from the trochlear groove of five bovine and three human knees. Overall, 24 bovine osteochondral cores were harvested and assigned to eight groups of three cores each. In four groups, the cores were left intact, while in the other four the most superficial 1 mm of cartilage was removed from the core with a sharp scalpel. All eight groups were then submerged in 0.9% saline, 0.125%, 0.25% or 0.5% bupivacaine solutions for 30 minutes, and were subsequently washed and returned to the chondrocyte growth media for 24 hours. Preliminary evaluations conducted before any treatment revealed the presence of dead cells interspersed with live cells through the full thickness of the articular cartilage in all human tissues. For this reason, the protocol for human tissue was simplified to compare exposure to 0.5% bupivacaine and normal saline only. A total of ten human osteochondral cores were randomly assigned to exposure of either 0.5% bupivacaine or 0.9% saline for 30 minutes each.
Live and dead cells were assessed using confocal microscopy. Specimens were prepared by cutting orthogonal sections approximately 0.5 mm thick and staining them with 5 mM 5-CMFDA and 1.5 mM propidium iodide. Stained sections were washed with phosphate-buffered saline and imaged by fluorescent and confocal microscopy. Using a 10× objective, confocal stacks were obtained from three separate areas of each section, located from 250 μm to 500 μm from the chondral surface. The dimensions of each stack were 250 μm × 250 μm × 25 μm. Image analysis software (Image-Pro Plus, Media Cybernetics) was used to count the red (dead) and green (live) cells in each image series.
Statistical analysis
Data are reported as the mean (SEM). For comparisons between saline and bupivacaine treatments, data were analysed by analysis of variance followed by the Bonferroni t-test. Significance was set at a p-value ≤ 0.05.
Results
Alginate-bead cultures and flow cytometry
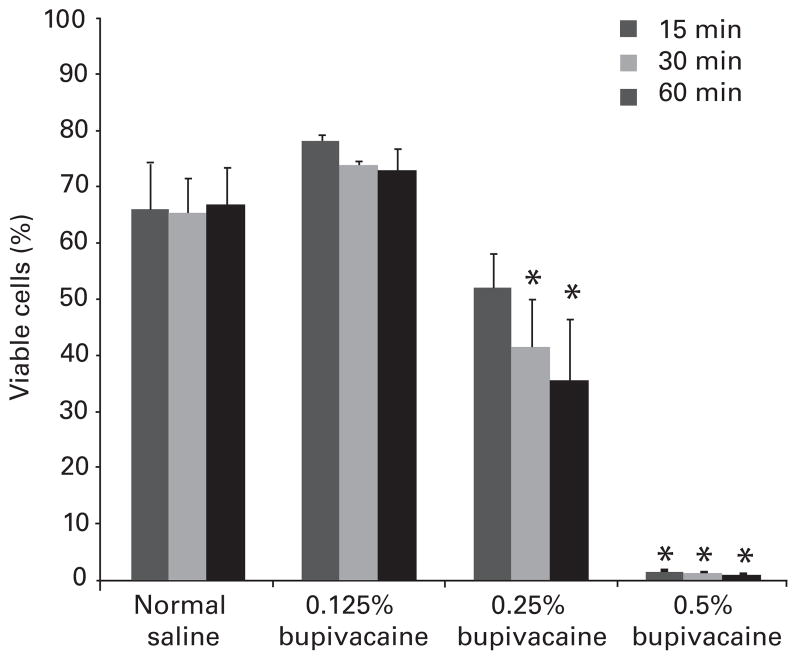
Analysis of data from 72 experiments using more than 3000 bovine chondrocyte alginate beads showed a dose- and time-dependent chondrotoxicity to bupivacaine solutions (Figs 1 to 3). The viability of bovine chondrocytes after exposure to 0.125% bupivacaine was similar to that of the normal saline group (Fig. 1) for 15, 30 or 60 minutes (p > 0.05). Exposure to 0.25% bupivacaine for 15 (52% (SEM 7) (p > 0.05)), 30 (42% (SEM 9) (p = 0.04)) and 60 minutes (36% (SEM 11) (p = 0.02)) resulted in a decreased chondrocyte viability compared with exposure to saline. After exposure to 0.5% bupivacaine, almost complete chondrocyte death/apoptosis was observed at all three time points. This finding is consistent with the results of our previous study.8
Fig. 1.
Bar chart with sem (whiskers) showing the dose-dependent chondro-toxicity of bupivacaine one hour after exposure. The viability of chondrocytes was similar to that of the saline group after exposure to 0.125% bupivacaine (p > 0.05) for 15, 30 or 60 minutes. It decreased significantly after exposure to 0.25% bupivacaine for 30 and 60 minutes. Virtually no viable chondrocytes remained after exposure to 0.5% bupivacaine for 15, 30 or 60 minutes (*p < 0.05 vs normal saline).
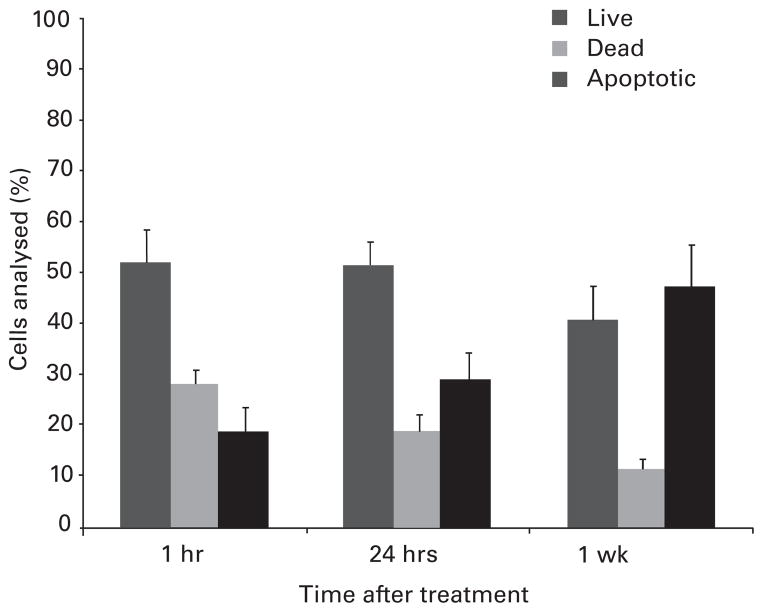
Fig. 3.
Bar chart with sem (whiskers) showing the time-dependent increase in apoptotic cells after exposure to 0.25% bupivacaine, for 15 minutes. There was a progressive increase in the proportion of apoptotic cells from 19% (SEM 5) at one hour to 48% (SEM 8) at one week after exposure.
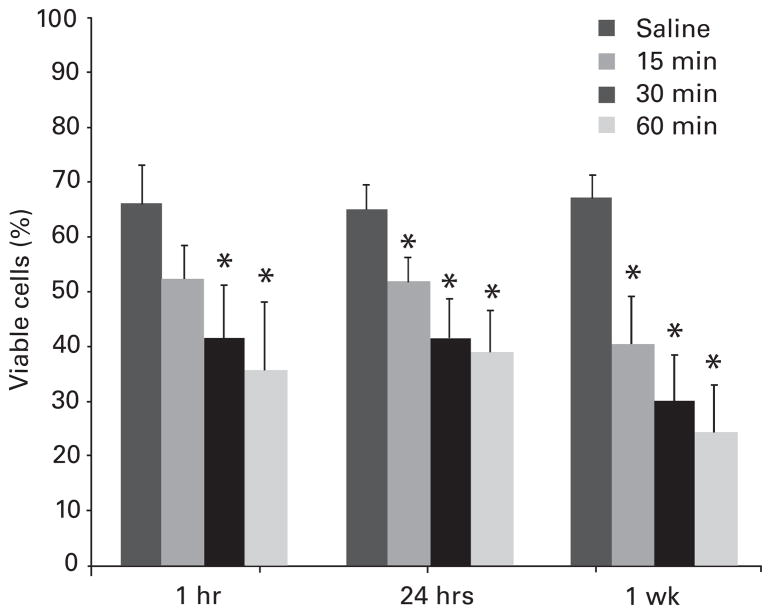
Bovine cultures exposed to 0.25% bupivacaine showed higher viability than that of cultures exposed to 0.5% bupivacaine at all time points (p < 0.001), but had a time-dependent decrease in viability. This decrease after exposure to 0.25% bupivacaine was observed both as a function of the time of exposure (15, 30 or 60 minutes) and of time after exposure to bupivacaine (one hour, 24 hours, or one week; Fig. 2). When recovered one hour after exposure to 0.25% bupivacaine for 15 minutes, viability was 79% (SEM 11) of that of the saline control, but this was not significant (p > 0.05). With increasing time after bupivacaine exposure, a sustained cytotoxic effect was observed, with viability declining to 52% (SEM 4) (p = 0.047) and 41% (SEM 7) (p = 0.004) at 24 hours and one week, respectively. For longer exposure times (30 and 60 minutes) to 0.25% bupivacaine, chondrotoxicity was detected by one hour after bupivacaine exposure, with chondrocyte viabilities respectively declining to 42% (SEM 9) (p = 0.038) and 36% (SEM 11) (p = 0.024), respectively, compared with that of exposure to saline. At one week after exposure of 30 and 60 minutes to 0.25% bupivacaine, the viability declined to 31% (SEM 8) (p < 0.001) and 25% (SEM 9) (p < 0.001) of the controls, respectively (Fig. 2).
Fig. 2.
Bar chart with sem (whiskers) showing the time-dependent chondrotoxicity of 0.25% bupivicaine at one hour, 24 hours and one week after exposure to 0.25% bupivacaine for 15, 30 and 60 minutes. There was a progressive decrease in the viability of chondrocytes (*p < 0.05 vs normal saline).
Analysis of necrotic and apoptotic cells at one hour, 24 hours, and one week after exposure to 0.25% bupivacaine showed a progressive dose-dependent increase in favour of the apoptotic cells. The fraction of apoptotic chondrocytes after exposure for 15 minutes to 0.25% bupivacaine, increased from 19% (SEM 5) of non-viable cells, when examined one hour after exposure, to 29% (SEM 6) of non-viable cells at 24 hours, and to 48% (SEM 8) at one week after exposure to bupivacaine (Fig. 3).
Confocal microscopy
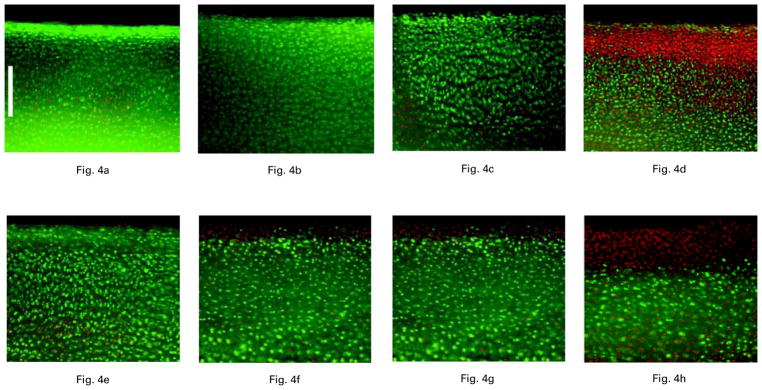
In 20 bovine osteochondral specimens confocal microscopy showed a dose-dependent chondrotoxicity of bupivacaine (Fig. 4). As described in our previous report,8 exposure to 0.5% bupivacaine for 30 minutes resulted in increased rates of chondrocyte death in intact bovine articular cartilage (Fig. 4d) compared with saline. Cellular death rates were even more pronounced in those specimens which had the top 1 mm of cartilage removed (Fig. 4h).
Fig 4.
The effects on fluorescent micrographs one hour after exposure of bovine articular cartilage to saline (a, e), 0.125% bupivacaine (b, f), 0.25% bupivacaine (c, g) and 0.5% bupivacaine (d, h) for 30 minutes. Cartilage with intact surfaces (a to d) shows increased chondrocyte surface death after exposure to 0.5% bupivacaine only whereas that with the superficial 1 mm removed (e to h) shows increased surface chondrocyte death with exposure to both 0.25% and 0.5% bupivacaine. No difference in the viability of chondrocytes was seen after exposure to 0.125% bupivacaine (fluorescent nuclei stained red; viable chondrocytes stained green, calibration bar 250 μm).
Analysis of the articular cartilage at one day after exposure for 30 minutes to 0.25% bupivacaine similarly showed a protective effect of the intact articular surface. In bovine tissues with intact articular surfaces, exposure to 0.25% bupivacaine for 30 minutes (Fig 4c) left 57% (SEM 7) of cells still viable compared with 76% (SEM 5) in the control group (Fig 4a). This difference was not statistically significant (p > 0.05). In bovine tissue explants with the top 1 mm of the articular cartilage removed, increased cell death was observed (Fig. 4g), with the rate of chondrocyte viability declining to 33% (SEM 4) (p = 0.01) compared with the control group (Fig. 4e). At one day after exposure to 0.125% bupivacaine, viability rates of bovine cartilage with and without intact articular surfaces were 85% (SEM 4) and 84% (SEM 3), respectively (Figs 4b and 4f), showing no signficant difference compared with the control group (p > 0.05) (Figs 4a and 4e).
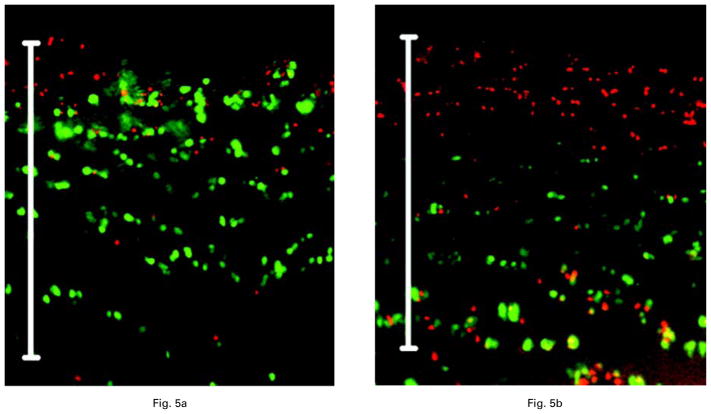
As with the bovine cells, the results of exposure of intact human articular cartilage to 0.5% bupivacaine showed an increased chondrocyte death rate compared with that of exposure to saline. Analysis of the data from ten human osteochondral specimens showed predominantly dead cells whereas most cells in saline-exposed cores were viable (Fig 5a). After exposure to 0.5% bupivacaine for 30 minutes, increased cellular necrosis was observed in the top 0.25 μm to 0.5 μm of human articular cartilage (Fig. 5b). Quantitative analysis of confocal reconstructions obtained at 100 μm below the articular surface showed a 1.7 (SEM 0.09)-fold increase in dead chondrocytes compared with saline-exposed human articular cartilage (p < 0.001).
Fig. 5.
Confocal microscopic images of intact human articular cartilage obtained 24 hours after exposure to a) saline and b) 0.5% bupivacaine for 30 minutes. There is increased cell death after exposure to 0.5% bupivacaine (p < 0.05) (fluorescent nuclei stained red; viable chondrocytes stained green; calibration bar 500 μm).
Time-lapse chondrocyte imaging
For both human and bovine alginate-bead cultures, the data from 28 experiments (12 with bovine and 16 with human tissue) showed a dose-dependent chondrotoxicity after continuous exposure to 0.5% and 0.25% bupivacaine solutions. No differences in the rates of chondrocyte death were seen for either bovine or human chondrocytes in alginate-bead cultures after one hour of exposure to 0.125% bupivacaine.
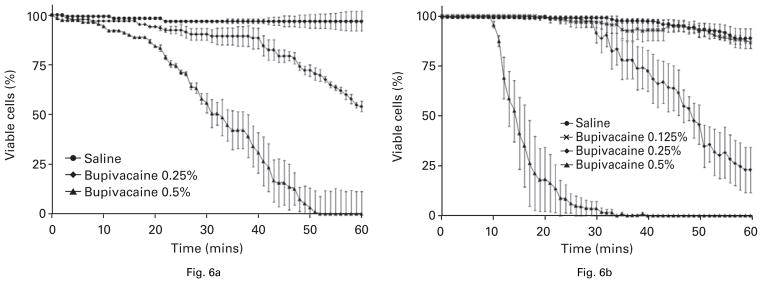
Exposure of bovine alginate-bead cultures to 0.5% bupivacaine solutions resulted in 90% (SEM 6) cell viability after 15 minutes, 56% (SEM 13) after 30 minutes, and no living chondrocytes after 60 minutes (Fig. 6a). By contrast, the rates of living bovine chondrocytes after exposure to 0.25% bupivacaine were 97% (SEM 1), 90% (SEM 4), and 54% (SEM 12) after 15, 30 and 60 minutes, respectively (p < 0.01) (Fig. 6a). For bovine alginate-bead cultures immersed in saline, 97% (SEM 1) of cells survived the 60-minute experiments. A separate 60-minute experiment comparing exposure of bovine chondrocytes to saline and to 0.125% bupivacaine showed no difference in viability (p > 0.05).
Fig. 6.
Graphs of time-lapse confocal microscopy showing the dose- and time-dependent toxic effect of bupivacaine on bovine and human chondrocytes within alginate-bead cultures. Figure 6a – bovine chondrocyte survival curves showing 50% cellular death after 34 minutes in 0.5% bupivacaine and after 60 minutes in 0.25% bupivacaine. Figure 6b – survival curves of human articular chondrocytes showing cellular death rates of 50% after 13 minutes in 0.5% bupivacaine and 47 minutes in 0.25% bupivacaine. After 60 minutes, > 89% (SEM 5) of human chondrocytes exposed to saline remained viable; no difference was observed between exposure to saline or 0.125% bupivacaine.
Time-lapse imaging of human chondrocytes (Fig. 6b) within alginate beads showed that exposure to 0.5% bupivacaine solutions resulted in a cell viability of 41% (SEM 18) after 15 minutes, 4% (SEM 4) after 30 minutes and no living chondrocytes after 60 minutes. Exposure of human chondrocytes to 0.25% bupivacaine showed viability of 100% (SEM 1), 92% (SEM 5) and 23% (SEM 12) after 15, 30 and 60 minutes, respectively (Fig. 6b). By contrast, 89% (SEM 5) of human chondrocytes within alginate beads immersed in saline survived the 60-minute experiment (Fig. 6). There were no differences in the viability rates between human chondrocyte beads exposed to 0.125% bupivacaine and saline (p > 0.05).
Death occurred at a faster rate in human than in bovine chondrocytes exposed to 0.5% and 0.25% bupivacaine (Fig. 6). Exposure to 0.5% bupivacaine resulted in the death of 50% of bovine chondrocytes in 34 minutes and 50% of human chondrocytes in 13 minutes. When immersed in 0.25% bupivacaine, the 50% death rate for the bovine chondrocytes was observed in 60 minutes, but 47 minutes for human chondrocytes.
Discussion
Our results show that 0.5% and 0.25% bupivacaine solutions are toxic to human and bovine articular chondrocytes in vitro. Furthermore, bupivacaine chondrotoxicity was dose- and time-dependent. While 0.5% bupivacaine has been shown to have a profound cytotoxic effect on chondrocytes, the chondrotoxicity of 0.25% bupivacaine increased with increasing duration of exposure as well as with the time after exposure. The viability of bovine and human chondrocytes after exposure to 0.125% bupivacaine for up to 60 minutes was similar to that after exposure to saline for all experiments.
These findings provide critical new information relating to current concerns on the clinical use of bupivacaine. Our results may provide an explanation for the absence of clinical reports on the association between chondrolysis and the use of intra-articular bupivacaine before the introduction of continuous-infusion pumps.5,7 In addition to systemic absorption and to the potential counteraction of the expected dilutional effects of joint fluid, continuous intra-articular infusion protocols may prolong the exposure of chondrocytes to bupivacaine. Eventually, the threshold for chondrolysis may be reached, as has been suggested clinically.5,7
Our study also showed that sustained exposure of cartilage to bupivacaine may lead to increased rates of chondrocyte death within the surface layers of intact human articular cartilage, a finding consistent with the previous study on bovine cartilage.8 Further work on bovine tissue also indicated a dose-dependent chondrotoxicity of bupivacaine and confirmed the protective role of an intact articular surface reported previously.8 In the present study, exposure to the medium dose (0.25%) of bupivacaine for 30 minutes did not cause significant toxicity to intact bovine articular cartilage, but did increase chondrocyte death in injured bovine articular cartilage. At the lowest dose (0.125%) of bupivacaine no significant difference in the viability of chondrocytes was seen compared with saline-exposed controls. This dose-dependent result is in agreement with the in vivo study by Dogan et al13 in rabbits which showed increased “articular cartilage inflammation” within 24 hours after a single intra-articular dose of 0.5% bupivacaine. The partially protective role of an intact articular surface and the reduced chondrotoxicity of 0.25% bupivacaine may also explain why previous studies using cartilage explants showed no significant histological or metabolic changes in the articular cartilage exposed to 0.25% bupivacaine.14–16
Despite these observations, prolonged exposure to 0.25% bupivacaine cannot be considered benign. In bovine articular cartilage without an intact articular surface exposed to 0.25% bupivacaine for 30 minutes, increased chondrocyte death was seen. A time-dependent increase in bovine chondrocyte death and rates of apoptosis were detected using both flow cytometry and time-lapse confocal microscopy. The longer the duration of exposure to 0.25% bupivacaine, the more pronounced were cellular death and apoptosis. Data from flow cytometry at one and seven days after exposure to 0.25% bupivacaine showed progressive bovine chondrocyte death and apoptosis, indicating a sustained deleterious effect of 0.25% bupivacaine, even after its withdrawal (Fig. 3). This observation is consistent with reports implicating apoptosis as a mechanism for bupivacaine-induced cytotoxicity.17 The finding of time-dependent cytotoxicity of 0.25% bupivacaine to articular chondrocytes is consistent with a recent in vivo study, which showed a chondrotoxic effect of continuous infusion of 0.25% bupivacaine into the shoulders of rabbits.18
In order to investigate the effects of continuous exposure of articular chondrocytes to bupivacaine solutions, we used time-lapse confocal microscopy to monitor the viability of cells directly. The imaging showed dose- and time-dependent cytotoxic effects of 0.5% and 0.25% bupivacaine. Cellular death occurred faster with 0.5% than with 0.25% bupivacaine for both human and bovine specimens. We additionally observed a faster decrease in the viability of human, compared with bovine, chondrocytes. These findings may be attributed either to an inherent greater susceptibility of human chondrocytes to the effects of bupivacaine or to a reduced vigour of the human chondrocytes used. While each experiment was carefully controlled and the alginate bead-culture technique allowed for uniform distribution and selection of viable cells, one of the limitations of our study was that human cells could not be considered to be as healthy as bovine cells. The human material used in our study was either from fresh osteochondral specimens obtained several days after donor death or from grossly normal-appearing areas of articular cartilage resected from osteoarthritic knees during joint replacement. By contrast, bovine chondrocytes were harvested from the knees of healthy animals and used within four hours after slaughter. Diseased chondrocytes could be more susceptible to the effects of cytotoxic agents.19 Our data, however, clearly show a dose- and time-dependent distinctive toxicity of bupivacaine in human articular chondrocytes.
Using quantitative techniques, we did not observe any differences in the viability rates of human or bovine chondrocytes after exposure to 0.125% bupivacaine or saline. Our study has shown that human chondrocytes may tolerate exposure periods to 0.125% bupivacaine of up to one hour. Because of the expected dilutional effects of synovial absorption, intra-articular effusion and arthroscopic lavage fluids, these results give insight into why the long-term clinical use of bupivacaine as a single intra-articular injection has not been associated with chondrolysis. While synovial absorption of bupivacaine is relatively inefficient,20,21 studies have shown that bupivacaine is absorbed from large joints over several hours with peak absorption occurring within the first hour.22,23 Therefore, our findings for 0.125% bupivacaine suggest that a combination of systemic absorption and either lavage fluid or effusion sufficient to dilute the injected bupivacaine to a concentration of 0.125% or less reduces the potential for chondrotoxicity.
It is important to note that in vitro assessments do not account for dilutional effects or in vivo reparative processes. Rather, the in vitro studies provide a reproducible, quantitative means of assessing the viability of cells in a controlled fashion. This cannot be accomplished in vivo, either in animals or in clinical studies. These advanced laboratory techniques have shown a dose- and time-dependent toxic effect of bupivacaine on articular chondrocytes. As has been reported previously,8 intact articular cartilage is partially protected from the cytotoxic effects of bupivacaine, suggesting that the in vivo effects may be less pronounced than these in vitro. While in vitro results cannot be directly extrapolated to clinical practice, they provide important information regarding the intra-articular use of bupivacaine. It is widely used as a local anaesthetic agent for arthroscopic surgery because of its long-lasting properties, thereby effectively supplementing general or regional anaesthesia. These properties, however, may also account for the cytoxic effects of 0.5% bupivacaine and the progressive death and apoptosis of chondrocytes observed in our study after exposure to 0.25% bupivacaine. These data suggest that intra-articular bupivacaine should be used at the lowest dosage and for the shortest period of time necessary. This data also suggest that prolonged, continuous intra-articular use of 0.25% and 0.5% bupivacaine may increase the potential for chondrotoxicity.
Footnotes
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributor Information
C. R. Chu, Cartilage Restoration Laboratory, Department of Orthopaedic Surgery University of Pittsburgh School of Medicine, 3471 Fifth Ave, Suite 911, Pittsburgh, Pennsylvania, 15213, USA.
N. J. Izzo, Cartilage Restoration Laboratory, Department of Orthopaedic Surgery University of Pittsburgh School of Medicine, 3471 Fifth Ave, Suite 911, Pittsburgh, Pennsylvania, 15213, USA.
C. H. Coyle, Cartilage Restoration Laboratory, Department of Orthopaedic Surgery University of Pittsburgh School of Medicine, 3471 Fifth Ave, Suite 911, Pittsburgh, Pennsylvania, 15213, USA.
N. E. Papas, Cartilage Restoration Laboratory, Department of Orthopaedic Surgery University of Pittsburgh School of Medicine, 3471 Fifth Ave, Suite 911, Pittsburgh, Pennsylvania, 15213, USA.
A. Logar, Research Specialist Department of Pediatrics Children’s Hospital, 3460 Fifth Avenue, Pittsburgh, Pennsylvania 15213, USA.
References
- 1.Sanders TG, Zlatkin MB, Paruchuri BN, Higgins RW. Chondrolysis of the gleno-humeral joint after arthroscopy: findings on radiography and low-field-strength MRI. AJR Am J Roentgenol. 2007;188:1094–8. doi: 10.2214/AJR.05.1477. [DOI] [PubMed] [Google Scholar]
- 2.Leclair A, Gangi A, Lacaze F, et al. Rapid chondrolysis after an intra-articular leak of bone cement in treatment of a benign acetabular subchondral cyst: an unusual complication of percutaneous injection of acrylic cement. Skeletal Radiol. 2000;29:275–8. doi: 10.1007/s002560050607. [DOI] [PubMed] [Google Scholar]
- 3.van Huyssteen AL, Bracy DJ. Chlorhexidine and chondrolysis in the knee. J Bone Joint Surg [Br] 1999;81-B:995–6. doi: 10.1302/0301-620x.81b6.9719. [DOI] [PubMed] [Google Scholar]
- 4.Good CR, Shindle MK, Kelly BT, Wanich T, Warren BF. Glenohumeral chondrolysis after shoulder arthroscopy with thermal capsulorrhaphy. Arthroscopy. 2007;23:797. doi: 10.1016/j.arthro.2007.03.092. [DOI] [PubMed] [Google Scholar]
- 5.Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1628–34. doi: 10.1177/0363546507304136. [DOI] [PubMed] [Google Scholar]
- 6.Petty DH, Jazrawi LM, Estrada LS, Andrews JR. Glenohumeral chondrolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32:509–15. doi: 10.1177/0363546503262176. [DOI] [PubMed] [Google Scholar]
- 7.Bojescul JA, Wilson G, Taylor DC. Idiopathic chondrolysis of the ankle. Arthroscopy. 2005;21:224–7. doi: 10.1016/j.arthro.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Chu CR, Izzo NJ, Papas NE, Fu H. In vitro exposure to 0. 5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693–9. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Chu CR, Monosov AZ, Amiel D. In situ assessment of cell viability within biodegradable polylactic acid polymer matrices. Biomaterials. 1995;16:1381–4. doi: 10.1016/0142-9612(95)96873-x. [DOI] [PubMed] [Google Scholar]
- 10.Benya PD. Modulation and reexpression of the chondrocyte phenotype: mediation by cell shape and microfilament modification. Pathol Immunopathol Res. 1988;7:51–4. doi: 10.1159/000157093. [DOI] [PubMed] [Google Scholar]
- 11.Masuda K, Sah RL, Hejna MJ, et al. A novel two-step method for the formation of tissue-engineered cartilage by matrure bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139–48. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 12.St Croix CM, Stitt MS, Watkins SC, Pitt BR. Fluorescence resonance energy transfer-based assays for the real-time detection of nitric oxide signaling. Methods Enzymol. 2005;396:317–26. doi: 10.1016/S0076-6879(05)96026-6. [DOI] [PubMed] [Google Scholar]
- 13.Dogan N, Erdem AF, Erman Z, Kizilkaya M. The effects of bupivacaine and neostigmine on articular cartilage and synovium in the rabbit knee joint. J Int Med Res. 2004;32:513–19. doi: 10.1177/147323000403200509. [DOI] [PubMed] [Google Scholar]
- 14.Fulkerson JP, Winters TF., Jr Articular cartilage response to arthroscopic surgery: a review of current knowledge. Arthroscopy. 1986;2:184–9. doi: 10.1016/s0749-8063(86)80065-2. [DOI] [PubMed] [Google Scholar]
- 15.Jaureguito JW, Wilcox JW, Thisted RA, et al. The effects of morphine on human articular cartilage of the knee: an in vitro study. Arthroscopy. 2002;18:631–6. doi: 10.1053/jars.2002.32587. [DOI] [PubMed] [Google Scholar]
- 16.Nole R, Munson NM, Fulkerson JP. Bupivacaine and saline effects on articular cartilage. Arthroscopy. 1985;1:123–7. doi: 10.1016/s0749-8063(85)80042-6. [DOI] [PubMed] [Google Scholar]
- 17.Park CJ, Park SA, Yoon TG, et al. Bupivacaine induces apoptosis via ROS in the Schwann cell line. J Dent Res. 2005;84:852–7. doi: 10.1177/154405910508400914. [DOI] [PubMed] [Google Scholar]
- 18.Gomoll AH, Kang RW, Williams JM, Bach BR, Cole BJ. Chondrolysis after continuous intra-articular bupivacaine infusion: an experimental model investigating chondrotoxicity in the rabbit shoulder. Arthroscopy. 2006;22:813–19. doi: 10.1016/j.arthro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Best AJ, Nixon MF, Taylor GI. Brief exposure of 0. 05% chlorhexidine does not impair non-osteoarthritic human cartilage metabolism. J Hosp Infect. 2007;67:67–71. doi: 10.1016/j.jhin.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Liguori GA, Chimento GF, Borow L, Figgie M. Possible bupivacaine toxicity after intraarticular injection for postarthroscopic analgesia of the knee: implications of the surgical procedure. Anesth Analg. 2002;94:1010–13. doi: 10.1097/00000539-200204000-00044. [DOI] [PubMed] [Google Scholar]
- 21.Wasudev G, Smith BE, Limbird TJ. Blood levels of bupivacaine after arthroscopy of the knee joint. Arthroscopy. 1990;6:40–2. doi: 10.1016/0749-8063(90)90095-u. [DOI] [PubMed] [Google Scholar]
- 22.Debruyne D, Moulin MA, Carmes C, Bequin JA, Locker B. Monitoring serum bupivacaine levels during arthroscopy. Eur J Clin Pharmacol. 1985;27:733–5. doi: 10.1007/BF00547058. [DOI] [PubMed] [Google Scholar]
- 23.Kaeding CC, Hill JA, Katz J, Benson L. Bupivacaine use after knee arthroscopy pharmacokinetics and pain control study. Arthroscopy. 1990;6:33–9. doi: 10.1016/0749-8063(90)90094-t. [DOI] [PubMed] [Google Scholar]