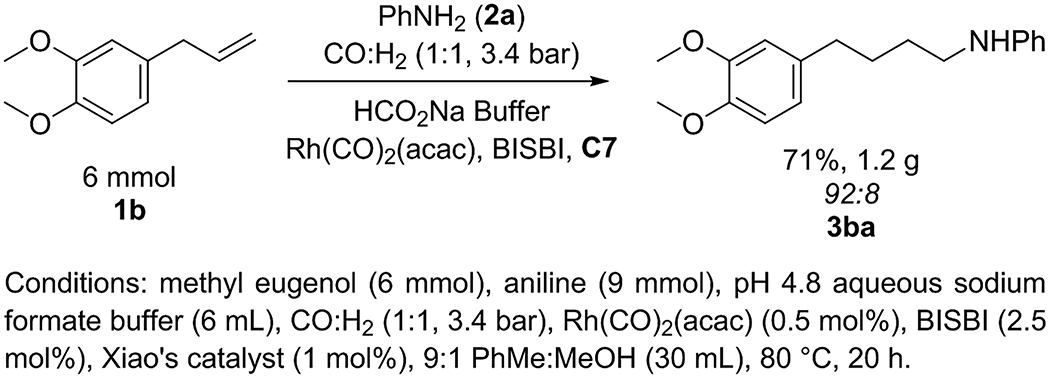Abstract
We report an approach to conducting the hydroaminomethylation of diverse α-olefins with a wide range of alkyl, aryl, and heteroarylamines at low temperatures (70–80 °C) and pressures (1.0–3.4 bar) of synthesis gas. This approach is based on simultaneously using two distinct catalysts that are mutually compatible. The hydroformylation step is catalyzed by a rhodium diphosphine complex, and the reductive amination step, which is conducted as a transfer hydrogenation with aqueous, buffered sodium formate as the reducing agent, is catalyzed by a cyclometallated iridium complex. By adjusting the ratio of CO to H2, we conducted the reaction at one atmosphere of gas with little change in yield. A diverse array of olefins and amines, including hetreroarylamines that do not react under more conventional conditions with a single catalyst, underwent hydroaminomethylation with this new system, and the pharmaceutical ibutilide was prepared in higher yield and under milder conditions than those reported with a single catalyst.
Keywords: hydroaminomethylation, bimetallic, multicatalytic, transfer hydrogenation, hydroformylation, reductive amination
Graphical Abstract

Two cats make HAM: Two distinct, mutually compatible catalysts enable the hydroaminomethylation of alkenes at or near atmospheric pressure of syngas and just 80 °C. The hydroformylation step is catalyzed by a rhodium diphosphine complex, and the reductive amination step, which is conducted as a transfer hydrogenation with aqueous, buffered sodium formate as the reducing agent, is catalyzed by a cyclometallated iridium complex.
Amines are ubiquitous in industrial, biological, and synthetic chemistry. Many industrial products either contain linear amines or are synthesized from amines,[1] and some of the most commonly prescribed pharmaceuticals contain 1-aminoalkyl groups (Scheme 1). Such amines are most commonly synthesized by the amination of alcohols, the reductive amination of aldehydes, or the reduction of amides, nitriles, or nitro compounds.[1] However, the starting materials for these processes are often synthesized from olefins; therefore, a method for the synthesis of amines from olefins would be more direct, less expensive, and more environmentally benign than the multi-step alternatives.
Scheme 1.

APIs containing aminoalkyl groups
The hydroaminomethylation of olefins is an atom-economical and operationally simple reaction involving hydroformylation of an olefin to form an aldehyde and reductive amination of this aldehyde to form an amine.[2] The reaction is commonly conducted with a rhodium-based catalyst ligated by a diphosphine (Scheme 2A). Several limitations, such as the high pressure of synthesis gas (usually around 60 bar) required to achieve acceptable yields,[2a, 2b] have diminished the utility of this reaction. In addition, self-condensation of the aldehyde, hydrogenation of the aldehyde, and hydrogenation of the olefin have been reported to compete with hydroaminomethylation.[2a]
Scheme 2.

Approaches to hydroaminomethylation
Hydroaminomethylation is difficult to achieve, in part, because the properties of the most active catalysts for hydroformylation are quite different from those of the most active catalysts for reductive amination.[3] Moreover the reductive amination process must not be strongly inhibited by carbon monoxide. A single catalyst that meets these criteria and catalyzes hydroaminomethylations at low pressures and temperatures has not been identified. In 2003, Beller reported one of the most active and regioselective systems comprising the combination of [Rh(COD)2]BF4 with Xantphos, but these reactions were conducted with 40 bar of syngas at 125 °C (Scheme 2A).[4] Hydroaminomethylations reported by Eilbracht, Alper, Whiteker, Zhang, and others occur under similar conditions.[5] A set of hydroaminomethylations reported by Beller occurred at the lower temperature of 60 °C. However, these reactions were limited to those of vinylarenes, and high pressures (30 bar, 1:5 CO:H2) were still required.[6]
We envisioned a new approach in which two catalysts, one for each step, would react by mechanisms that are distinct and independent from each other, enabling hydroaminomethylation to occur under conditions that are milder than those with a single catalyst.[7] Beller and Luo published hydroaminomethylations conducted with a single phosphine and two metals, but these reactions still required high pressures of synthesis gas and high temperatures (Scheme 2B).[8] Following a different design, Xiao and Han published hydroaminomethylations catalyzed by the combination of a rhodium-based catalyst for the hydroformylation step and a chiral phosphoric acid for the reductive amination step that form enantioenriched, branched amines from α- and β-functionalized olefins (Scheme 2C). However, these systems have not been shown to catalyze linear-selective hydroaminomethylations of unfunctionalized alkenes, and the organic catalyst requires an expensive Hantzsch ester to reduce the imine intermediate.[7a, 7b, 9]
Herein, we report a linear-selective hydroaminomethylation of α-olefins catalyzed by two distinct metal complexes and an approach to the reductive amination step not applied previously to hydroaminomethylation (Scheme 2D). A rhodium-diphosphine complex catalyzes the hydroformylation step, and a phosphine-free, cyclometallated iridium complex catalyzes the reductive amination step by transfer hydrogenation. Key features of this work include the identification of a catalyst for reductive amination that is not poisoned by CO and the use of buffered formic acid for the reduction step. With this system, aromatic, heteroaromatic, and aliphatic amines are formed in high yields and in high regioselectivities with pressures of synthesis gas and temperatures that are significantly lower than those used previously for hydroaminomethylation.
Our strategy for a low-pressure, multicatalytic hydroaminomethylation was based on the hypotheses that the high pressures of hydrogen in existing systems for hydroaminomethylation are required to ensure that reductive amination is faster than self-condensation of the aldehyde and that the reductive amination step of hydroaminomethylation is slow because catalysts for this step tend to be inhibited by carbon monoxide. In this case, a reductive amination catalyst that is unaffected by carbon monoxide could be combined with a suitable hydroformylation catalyst to conduct hydroaminomethylations at low pressures of synthesis gas. A complex that catalyzes reductive amination by transfer hydrogenation might meet this criterion.
To test this reaction design, we studied the hydroaminomethylation of 1-decene (1a) with aniline (2a) in the presence of formic acid, the highly active and selective hydroformylation catalyst generated from Rh(CO)2(acac) and BISBI, and various complexes known to catalyze the transfer hydrogenation of imines and iminium ions (Table 1). The most well-known catalysts for reductive amination by transfer hydrogenation consist of a metal capable of transferring a hydride and a ligand capable of transferring a proton to a polarized multiple bond.[10] However, amine 3aa formed in poor yield from olefin 1a in the presence of ruthenium diamine complexes C1 and C2 (entries 1–4), even though these complexes are known to be active for transfer hydrogenation of imines. The group 9 congeners (catalysts C3-C5) of catalysts C1 and C2 are active for the reductive amination of aldehydes by transfer hydrogenation.[11] However, amine 3aa formed with low regioselectivity and in poor yield in the presence of catalysts C3, C4, and C5 and in the presence of Rh(CO)2(acac) and BISBI (entries 5–7). Control experiments indicated that complexes C3-C5 either degrade into unselective catalysts for hydroformylation or are themselves unselective catalysts for hydroformylation under the conditions in Table 1. This reactivity of the reductive amination catalyst toward hydroformylation leads to low n:iso ratios of the amine product. Amine 3aa also formed in low yields in the presence of catalyst C6 (entry 8). All of the hydroaminomethylations conducted with catalysts C1-C6 (entries 1–8) proceeded to high conversions; the major side products of the reactions formed from self-condensation of undecanal.
Table 1.
Evaluation of conditions for the hydroaminomethylation of 1-decene with aniline

| Entry | DRA Cat.[a] | H2 Source | Hydroformylation Ligand | Yield[b] | n:iso |
|---|---|---|---|---|---|
| 1 | C1 | 5:2 HCO2H:TEA | BISBI | 33 | 97:3 |
| 2 | C1 | HCO2Na buffer[c] | BISBI | 10 | 72:28 |
| 3 | C2 | 5:2 HCO2H:TEA | BISBI | 29 | 98:2 |
| 4 | C2 | HCO2Na buffer[c] | BISBI | 10 | 70:30 |
| 5 | C3 | 5:2 HCO2H:TEA | BISBI | 29 | 59:41 |
| 6 | C4 | 5:2 HCO2H:TEA | BISBI | 34 | 88:12 |
| 7 | C5 | 5:2 HCO2H:TEA | BISBI | 34 | 72:28 |
| 8 | C6 | 5:2 HCO2H:TEA | BISBI | 48 | 85:15 |
| 9 | C7 | 5:2 HCO2H:TEA | BISBI | 30 | 96:4 |
| 10 | C7 | HCO2Na buffer[c] | BISBI | 91 | 98:2 |
| 11 | C7 | HCO2Na buffer[c] | Xantphos | 82 | 98:2 |
| 12 | C7 | HCO2Na buffer[c] | DPEPhos | 75 | 90:10 |
| 13 | C7 | none | BISBI | 0 | N/A |
| 14[d] | C7 | HCO2Na buffer[c] | BISBI | 83 | 99:1 |
| 15[e] | C7 | HCO2Na buffer[c] | BISBI | 76 | 99:1 |
| 16[f] | C7 | HCO2Na buffer[c] | BISBI | 8 | 68:32 |
| 17 | none | HCO2Na buffer[c] | BISBI | 0 | N/A |

Conditions: 1-decene (0.5 mmol), aniline (0.75 mmol), H2 source (500 μL), 9:1 PhMe:MeOH (2.5 mL), Rh(CO)2(acac) (0.5 mol%), hydroformylation ligand (2.5 mol%), reduction catalyst (1 mol%), 80 °C, 20 h.
Direct Reductive Amination catalyst.
Yield of all constitutional isomers of amine 3aa, determined by GC against dodecane as an internal standard.
Aqueous sodium formate buffered to pH 4.8.
0.25 mol% Rh(CO)2(acac), 1.25 mol% BISBI, 0.5 mol% C7.
0.125 mol% Rh(CO)2(acac), 0.625 mol% BISBI, 0.25 mol% C7.
With no Rh(CO)2(acac).
To increase the rate of reductive amination of undecanal relative to that of its self-condensation, we sought catalysts for transfer hydrogenation that might be more stable to carbon monoxide than catalysts C1-C6. Reports that catalyst C7 is more active for the reduction of N-p-anisylketimines than catalyst C4 and the robustness of catalyst C7 endowed by cyclometallation prompted us to attempt hydroaminomethylations with catalyst C7 as a catalyst for reductive amination.[12]
Initial studies with catalyst C7 showed that amine 3aa formed in only 30% yield from olefin 1a, CO, H2, and amine 2a in the presence of catalyst C7 and the combination of Rh(CO)2(acac), and BISBI with a 5:2 HCO2H:Et3N azeotrope as the reducing agent (entry 11). On the basis of prior literature showing that the reductive amination of acetophenone with p-anisidine catalyzed by complex C7 occurs significantly more rapidly with an aqueous sodium formate buffer at pH 4.8 as the reducing agent than with a 5:2 HCO2H:Et3N azeotrope as the reducing agent,[13],[14] we conducted the hydroaminomethylation of olefin 1a with amine 2a with this reducing agent in the presence of C7. Amine 3aa formed in 91% yield under these conditions (entry 10). Amine 3aa formed in lower yields in the presence of XantPhos and DPEPhos than in the presence of BISBI (entries 11–12), and the product did not form in the absence of formate (entry 13). The hydroaminomethylation occurred at lower loadings of the two catalysts with only a small sacrifice in yield (entries 14–15). Amine 3aa formed in 8% yield and 68:32 n:iso in the presence of catalyst C7 with no Rh(CO)2(acac) present (entry 16), indicating that catalyst C7 is only slightly active towards hydroformylation under the developed conditions. Amine 3aa did not form in the absence of a reductive amination catalyst (entry 17).
We attribute the high yields and regioselectivities of the reaction in entry 10 to several factors. First, Xiao’s cyclometallated catalyst is not significantly inhibited by low pressures of CO; control experiments indicated that the yield of the reductive amination of undecanal catalyzed by complex C7 was the same in the absence of CO as in the presence of 1.7 bar of CO. In contrast, complexes C1, C2 and C6 were poisoned by CO, even at this low pressure (see the supporting information for details). Second, Xiao’s catalyst is compatible with the aqueous HCO2Na buffer, a mild, inexpensive hydrogen surrogate. Finally, Xiao’s catalyst does not hydrogenate imines by a metal-ligand bifunctional mechanism.[12] Instead, Xiao’s catalyst transfers a hydride to an iminium ion that is formed by protonation of an imine or enamine in an acidic medium.[12]
The scope of olefins that undergo this hydroaminomethylation is illustrated by the examples in Table 2. Phenols (3ca), malonates (3da), nitriles (3ea), enolizable ketones (3fa), allylic acetates (3ga), allylic alcohols (3ha), and disubstituted olefins (3ka) were all tolerated. Olefins bearing electron-withdrawing groups in the allylic position reacted with excellent regioselectivities (1b, 1c, 1d, 1g, 1h); such β-functionalized olefins often undergo hydroformylations with low n:iso ratios, due to their tendencies to isomerize to internal olefins. Sterically hindered α-olefins (1g, 1i, 1j, 1k) also underwent hydroaminomethylation. As expected, the regioselectivities of the reactions of these alkenes were higher than those of reactions of less hindered alkenes. In addition, the double hydroaminomethylation of olefin 1l proceeded in high yield and with high regioselectivity.
Table 2.
Hydroaminomethylations of various olefins with aniline
 |
The scope of arylamines that undergo hydroaminomethylation was studied with the olefin methyl eugenol (1b) as the coupling partner. The results are given in Table 3. Both electron-poor (2b, 2c, 2e) and electron-rich (2d) anilines underwent hydroaminomethylation. Anilines bearing ortho substituents (2f) and those bearing ketones (2e) also underwent hydroaminomethylation.
Table 3.
Hydroaminomethylations of methyl eugenol with various arylamines
 |
The scope of heteroarylamines that undergo hydroaminomethylation with olefin 1b is also shown in Table 3. Heteroarylamines are widespread in pharmaceuticals and agrochemicals, but hydroaminomethylations with such regents are limited.[15] For reference, we conducted the reaction of olefin 1b with 2-aminopyridine (2g) under standard conditions for hydroaminomethylation (125 °C, 40 bar 1:5 CO:H2) with the single catalyst formed from the combination of [RhCOD2]BF4 and Xantphos. Only trace amounts of amine 3bg formed; the major species present in the crude reaction mixture was the starting olefin 1b.[16] In contrast, the reactions of methyl eugenol with heteroarylamines, including aminopyridines (2g, 2h), aminoindoles (2i), aminoquinolines (2j, 2k), and aminodibenzofurans (2l) occurred in good yield under the conditions we developed with two catalysts. Even heteroarylamines capable of chelating metal catalysts (2k) reacted. This contrast in reactivity demonstrates the unusual compatibility of the new system for hydroaminomethylation with biologically important heteroarenes.
Alkylamines might be expected to be quenched by the acidic buffer containing formic acid, but both primary and secondary alkylamines underwent hydroaminomethylation under the conditions we developed with two catalysts and a pH 4.8 formate buffer (Table 4). Both cyclic (2m, 2n, 2o, 2p) and acyclic (2q, 2r, 2s) secondary amines underwent the hydroaminomethylation. Sterically hindered aliphatic amines were less reactive towards hydroaminomethylation than unhindered aliphatic amines, presumably due to slow reduction of sterically hindered iminium ions. Primary aliphatic amines (2t) also underwent hydroaminomethylation to form secondary amines without the formation of tertiary amines.
Table 4.
Hydroaminomethylations of methyl eugenol with various aliphatic amines
 |
To demonstrate further the applicability of this work to the preparation of medicinally relevant amines, we synthesized amine 3mt, the active ingredient in a drug sold under the generic name ibutilide (Scheme 3). Under the conditions in Scheme 3, olefin 1m underwent hydroaminomethylation in 76% yield. Previous examples of this hydroaminomethylation occurred in significantly lower yields and required pressures of syngas that are much higher than those in the current work.[5e]
Scheme 3.

Synthesis of Ibutilide
Finally, the hydroaminomethylations reported in this work can be conducted easily on large scales and at atmospheric pressure of syngas. The hydroaminomethylati on of 6 mmol of methyl eugenol with aniline gave 1.24 g of amine 3ba (71% yield, Scheme 4). By adjusting the ratio of CO to H2 to 1:2 CO:H2, the reaction of 1-decene (1a) with aniline (2a) formed N-undecyl aniline (3aa) in 65% yield at a total pressure of only 1 atm at 70 °C (Scheme 5).
Scheme 4.
Hydroaminomethylation on a 6-mmol scale
Scheme 5.

Hydroaminomethylation at atmospheric pressure
In summary, we have developed a scalable, linear-selective, dual-catalytic hydroaminomethylation of α-olefins occurring at low temperature and pressure by exploiting the combination of a catalyst for hydroformylation and a catalyst for reductive amination that is active under carbon monoxide and that reduces imines by transfer hydrogenation. The pressures and temperatures of the reaction are the lowest reported for linear-selective hydroaminomethylations, and the reactions can even be conducted at atmospheric pressure of synthesis gas. The reaction occurs with a broad range of olefins and amines and is uniquely suitable for the preparation of a wide range of medicinally relevant heteroarylamines. Efforts to further increase the activity of dual catalysts and the scope of reactants are ongoing.
Supplementary Material
Acknowledgements
This work was supported by the Director, Office of Science, U.S. Department of Energy, under contract No. DE-AC02–05CH1123. Jeffrey C. Holder thanks the National Institutes of Health for sup-port through 5F32GM112312.
References
- [1].a) Hayes KS, Appl. Catal., A 2001, 221, 187–195; [Google Scholar]; b) Roose P, Eller K, Henkes E, Rossbacher R, Höke H in Amines, Aliphatic, 2015. [Google Scholar]
- [2].a) Crozet D, Urrutigoity M, Kalck P, ChemCatChem 2011, 3, 1102–1118; [Google Scholar]; b) Chen CD, Zhang X-Q, X., Org. Chem. Front 2016, 3, 1359–1370; [Google Scholar]; c) Kalck P, Urrutigoïty M, Chem. Rev 2018, 118, 3833–3861. [DOI] [PubMed] [Google Scholar]
- [3].Hartwig JF in Organotransition Metal Chemistry: From Bonding to Catalysis, 1 ed., University Science Books, Mill Valley, California, 2009, 774. [Google Scholar]
- [4].Ahmed M, Seayad AM, Jackstell R, Beller M, J. Am. Chem. Soc 2003, 125, 10311–10318. [DOI] [PubMed] [Google Scholar]
- [5].a) Seayad A, Ahmed M, Klein H, Jackstell R, Gross T, Beller M, Science 2002, 297, 1676–1678; [DOI] [PubMed] [Google Scholar]; b) Ahmed M, Bronger RPJ, Jackstell R, Kamer PCL, van Leeuwen PWNM, Beller M, Chem. Eur. J 2006, 12, 8979–8988; [DOI] [PubMed] [Google Scholar]; c) Vieira TO, Alper H, Chem. Commun 2007, 2710–2711; [DOI] [PubMed] [Google Scholar]; d) Liu G, Huang K, Cai C, Cao B, Chang M, Wu W, Zhang X, Chem. Eur. J 2011, 17, 14559–14563; [DOI] [PubMed] [Google Scholar]; e) Briggs JR, Klosin J, Whiteker GT, Org. Lett 2005, 7, 4795–4798; [DOI] [PubMed] [Google Scholar]; f) Whiteker GT, Top. Catal 2010, 53, 1025–1030; [Google Scholar]; g) Liu G, Huang K, Cao B, Chang M, Li S, Yu S, Zhou L, Wu W, Zhang X, Org. Lett 2012, 14, 102–105; [DOI] [PubMed] [Google Scholar]; h) Wu L, Fleischer I, Jackstell R, Beller M, J. Am. Chem. Soc 2013, 135, 3989; [DOI] [PubMed] [Google Scholar]; i) Liu G, Li Z, Geng H, Zhang X, Catal. Sci. Technol 2014, 4, 917–921; [Google Scholar]; j) Liu J, Kubis C, Franke R, Jackstell R, Beller M, ACS Catalysis 2016, 6, 907–912; [Google Scholar]; k) Subhani MA, Mueller K-S, Eilbracht P, Adv. Synth. Catal 2009, 351, 2113–2123; [Google Scholar]; l) Schmidt A, Marchetti M, Eilbracht P, Tetrahedron 2004, 60, 11487–11492. [Google Scholar]
- [6].Routaboul L, Buch C, Klein H, Jackstell R, Beller M, Tetrahedron Lett. 2005, 46, 7401–7405. [Google Scholar]
- [7].For multicatalytic strategies for the combination of hydroformylation with the hydrogenation of aldehydes, see the following publications: Takahashi K, Yamashita M, Nozaki K, J. Am. Chem. Soc 2012, 134, 18746–18757; Kohei T, Makoto Y, Takeo I, Koji N, Kyoko N, Angew. Chem. Int. Ed 2010, 49, 4488–4490; Yuki Y, Takahashi K, Tanaka Y, Nozaki K, J. Am. Chem. Soc 2013, 135, 17393–17400.
- [8].a) Zimmermann B, Herwig J, Beller M, Angew. Chem. Int. Ed 1999, 38, 2372–2375; [DOI] [PubMed] [Google Scholar]; b) Wang Y, Zhang C, Luo M, Chen H, Li XJ, Arkivoc 2008, xi, 165–174. [Google Scholar]
- [9].a) Villa-Marcos B, Xiao J, Chinese J. Catal 2015, 36, 106–112; [Google Scholar]; b) Han JM, Xing-Han L, Zhi Y, Org. Lett 2017, 19, 1076. [DOI] [PubMed] [Google Scholar]
- [10].a) Wang D, Astruc D, Chem. Rev 2015, 115, 6621–6686; [DOI] [PubMed] [Google Scholar]; b) Takao Ikariya MS in Bifunctional Molecular Catalysis, Vol. 37, Springer-Verlag; Berlin Heidelberg, 2011, 31–53; [Google Scholar]; c) Gordon PAD, John C, Dalton Trans. 2016, 45, 6741–7180. [Google Scholar]
- [11].Blacker J, Martin J, in Asymmetric Catalysis on Industrial Scale, Wiley-VCH Verlag GmbH & Co. KGaA, 2004, pp. 201–216. [Google Scholar]
- [12].a) Wang C, Pettman A, Bacsa J, Xiao J, Angew. Chem 2010, 122, 7710–7714; [Google Scholar]; b) Talwar D, Salguero NP, Robertson CM, Xiao J, Chem. Eur. J 2014, 20, 245–252; [DOI] [PubMed] [Google Scholar]; c) Xiao WT, Chunho L, Xiaofeng W, Jianliang, Synlett 2013, 25, 81–84. [Google Scholar]
- [13].Lei Q, Wei Y, Talwar D, Wang C, Xue D, Xiao J, Chem. Eur. J 2013, 19, 4021–4029. [DOI] [PubMed] [Google Scholar]
- [14].The precise pH of the buffer strongly influences the outcomes of reductive aminations with Xiao’s catalyst. Under basic conditions, neither imines nor ketones are protonated, and the reduction does not occur. In contrast, under highly acidic conditions, amines, ketones, and imines are protonated and both the chemoselectivity for the reduction of C=N bonds and the rate of the reductive amination are low. See reference 14.
- [15].Beller has shown that 1,1-diphenylethylene undergoes hydroaminomethylations with piperidines bearing remote pyridyl and pyrimidyl groups, but this olefin undergoes hydroaminomethylation with 4-aminopyridine in low yield.; Ahmed M, Buch C, Routaboul L, Jackstell R, Klein H, Spannenberg A, Beller M, Chem. Eur. J 2007, 13, 1594–1601. [DOI] [PubMed] [Google Scholar]
- [16].Reaction Conditions: 0.5 mmol methyl eugenol, 0.5 mmol 2-aminopyridine, 40 bar CO:H2 (1:5), [Rh(COD)2]BF4 (0.1 mol%), Xantphos (0.4 mol%), 1.5 mL PhMe:MeOH (1:1), 125 °C, 20 h
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.