ABSTRACT
Immunologic and non-immunologic loss of islet cells upon their transplantation into the liver leads to suboptimal outcomes. Anti-inflammatory agents are used during autologous and allogeneic transplantation. The aim of this qualitative systematic literature review is to evaluate their clinical use and safety. Electronic databases Embase, PubMed, Cumulative Index for Nursing and Allied Health Literature, ClinicalTrials.gov, and EU Clinical Trials Register were searched. Of the 216 unique citations, 10 with tumor necrosis factor (TNF) blockers [etanercept (ETA) or infliximab] and 3 with both TNF blockers and an interluekin-1 receptor antagonist [anakinra (ANA)]) were included. Of these, 12 were in allogeneic and one in autologous transplant. Insulin independence with decreased islet cells and number of transfusions were reported with their use. One infection was reported in a group receiving ETA. Analysis suggested that the use of ETA ± ANA have the potential to improve outcomes in islet cell transplant.
KEYWORDS: Islet cell transplant, inflammation, TNF blockers, anakinra, etanercept, infliximab, auto islet transplant
Introduction
Islet cell transplantation (ICT) is performed either in allogeneic (allo) or autologous (auto) settings for different indications. It prevents brittle diabetes due to improved glycemic control provided by secretion of both insulin as well as glucagon and improves quality of life. Thus, ICT helps to cure type 1 DM in allo settings and prevent brittle type 3c DM in an auto setting.
The liver has demonstrated to be the site of choice for islet transplantation in clinical practice via intra-portal infusion of isolated islet cells. The crucial events occurring in the first hours and days after islet infusion could influence the success of transplantation. During islet infusion, an instant blood-mediated inflammatory reaction (IBMIR) is elicited when islets are exposed to blood and involves coagulation and complement activation.1,2 IBMIR culminates in the disruption of islet morphology by infiltrating leukocytes. Polymorphonuclear cells are the predominant cell type infiltrating the islets, attracted by the upregulation and release of ischemia-induced molecules (i.e. tissue factor, IL-1beta, tumor necrosis factor-alpha, nitric oxide, high-mobility group box 1) and by proinflammatory signals (i.e. monocyte chemoattractant protein, IL-8, IL-6 released from the islets).3-5 Over and above variation in liver pathology itself gives rise to proinflammatory status.6,7
Immunosuppression is needed in an allo setting; however, in both allo and auto ICT, significant islet loss happens due to inflammation. Anti-inflammatory (AI) agents have been used to reduce the harmful effects of proinflammatory cytokines (PIC). AI agents may aid in improving early islet cell function and insulin-independence (II) post-transplant.
Although previous studies have evaluated various strategies including tumor necrosis factor (TNF) blockers and an interleukin-1 receptor antagonist (IL-1RA) in ICTs, no qualitative systematic review has focused on these specific agents. The objective of this systematic literature review was to evaluate the clinical evidence regarding the use and safety of commonly used TNF blockers and IL-1RA in both auto and allo ICT. To our knowledge, this is the most thoroughly searched literature database review on the use of AI agents in clinical ICT.
Results
Literature search results
The searches in PubMed, Embase, and CINAHL yielded a total of 257 citations and an additional 9 citations were found through ClinicalTrials.gov and the EU Clinical Trials Register. The total of 266 citations were exported to Endnote Desktop, and 50 duplicates were removed. This left a total of 216 unique citations found through our search strategy. Out of 216 unique citations, 19 articles were selected by title and abstract screening. In review of those 19 articles, 13 were identified with the use of a TNF blocker with and without IL-1RA (Figure 1).
Figure 1.
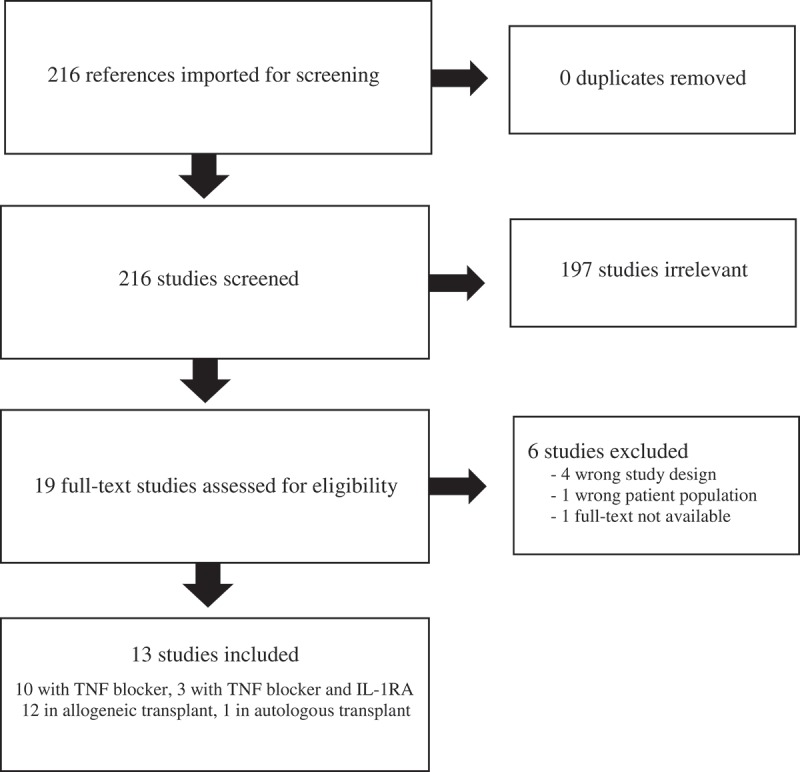
PRISMA flow diagram.
Study characteristics
TNF blockers studied were etanercept (ETA) and infliximab (INF), and IL-1RA studied was anakinra (ANA). These AI agents’ characteristics are highlighted in Table 1. Two included studies were follow-up studies of other included studies, thus duplicating patients and their outcomes, and were assessed separately in this review. Study characteristics of the study and overview of clinical outcomes are in Tables 2 and 3.
Table 1.
| US generic name | Etanercept | Infliximab | Anakinra |
|---|---|---|---|
| US brand name | Enbrel® | Remicade® Inflectra® IXIFI® | Kineret® |
| Drug class | TNF blocker | TNF blocker | IL-1 receptor antagonist |
| Properties | Dimeric human TNF receptor that contains the extraceullular ligand-binding portion of 75 kilodalton (p75)-Fc fusion protein; the Fc component does not contain the CH1 domain of IgG1 | Chimeric IgG1κ monoclonal antibody composed of human constant and murine variable regions specific for human TNF-α | Recombinant, nonglycosylated form of human IL-1 receptor antagonist; it consists of a single methionine residue at its amino terminus, which is different from the native human IL-1 receptor antagonist |
| Mechanism of action | Inhibits binding of both TNF-α and TNF-β | Inhibits TNF-α, but does not neutralize TNF-β | Competitively inhibits IL-1 binding to the IL type 1 receptor |
| Most commonly used dosing in islet cell transplant | 50 mg IV 1 hour pre-transplant, then 25 mg SQ on POD 3, 7, 10 | 5 or 10 mg/kg IV 2 hour pre-transplant | 100 mg IV or SQ on POD 0, then 100 mg SQ daily for 7 days |
| Contraindications | Sepsis | >5 mg/kg in moderate to severe heart failure; previous severe hypersensitivity to inactive components or to any murine proteins | Known hypersensitivity to Escherichia coli-derived proteins or any components of the product |
| Adverse reactions, most common | (>5%) are infections and injection site reactions | (>10%) are infections, headache, abdominal pain, and infusion-related reactions | (≥5%) for adults with RA are injection-site reactions, worsening RA, upper respiratory infections, headache, nausea, diarrhea, sinusitis, arthralgia, flu-like symptoms, and abdominal pain |
Abbreviations: IgG, immunoglobulin G; IL-1, interleukin-1; POD, post-operative day; RA, rheumatoid arthritis; SQ, subcutaneous; TNF, tumor necrosis factor; IV, intravenous
Table 2.
Study characteristics.
| Author and Year | Induction and Maintenance IS Regimen | Regimen Doses/Duration | Comparison Regimen | Patients in AI Agent Arm, N | Total Patients, N | Duration of Follow-up (months) | Primary Outcomes | II Definition |
|---|---|---|---|---|---|---|---|---|
| Allotransplant | ||||||||
| ETA | ||||||||
| Hering 200511 | ATG, DAC, SIR, TAC (MMF substitution at 1y) | ETA 50mg IV 1h pre-txp, 25mg SQ on POD 3, 7, 10 | None | 8 | 8 | 12 | II, safety via severity and duration of procedural complications, infections, or adverse medication or islet events | FBG <126 mg/dL and 2h postprandial BG <180 after insulin discontinuation |
| Bellin 200812 | ATG, CsA, EVR (possible MPA substitution at 1y) | ETA 50mg IV 1h pre-txp, 24mg SQ on POD 3, 7, 10 | None | 6 | 6 | 24 | Safety via CBC, coagulation studies, hepatic panel, creatinine, 24h urine albumin excretion, GFR, medication levels | FBG <127 mg/dL and 2h postprandial BG <180 mg/dL without exogenous inuslin |
| Faradji 200810 | EP | ETA 50mg IV 1h pre-txp, 25mg SQ 2x/wk x 2wk | EP | 4 | 9 | 18 | II following supplemental islet cell infusion | C-peptide positivity without GDF without exogenous insulin |
| Froud 200813 | ATZ, SIR, TAC then SIR, MPA | ETA 50mg IV 1h pre-op, 25mg SQ 2x/wk x 2wk | EP | 3 | 19 | 24 | II, graft function, MMTT, IVGTT | NR |
| Gangemi 20088 | EP | ETA 50mg IV pre-txp, 25mg SQ on POD 3, 7, 10 | EP | 6 | 10 | 15 | II | Without exogenous insulin, FBG >140 mg/dL <4 times/wk and 2h postprandial BG >180 mg/dL <5 times/wk |
| Koh 201014 | DAC or ATG, TAC, SIR or MMF | ETA 50mg IV pre-txp, 25mg SQ on POD 3, 7, 10 | None | 17 | 17 | 19.8 ± 3.2 | II | HbA1c ≤7%, FBG >140 mg/dL <4 times/wk and 2-hr postprandial BG >180 mg/dL <4 times/wk |
| Rickels 20139 | ATG (BAS for 2nd infusion), SIR, TAC | ETA 50mg IV pre-txp, 25mg SQ on POD 3, 7, 10 | EP and normal controls | 11 | 27 | 24 | II, argTT | FBG ≤126 mg/dL and 90 min MMTT BG ≤180 mg/dL, s/p > 1 wk insulin taper |
| INF | ||||||||
| Froud 200515 | EP | INF 5 or 10mg/kg 2h pre-txp | EP | 8 | 16 | 18 | II | FBG <127 mg/dL without exogenous inuslin |
| ETA + ANA | ||||||||
| Matsumoto 201116 | ATG, TAC, MMF | ANA 100mg IV 1h pre-txp, then 100mg SQ daily x 7d; ETA 50mg IV 1h pre-txp, 25mg SQ on POD 3, 6, 10 | ETA + EP | 3 | 6 | 24 | Total insulin required, HbA1c, SUITO index (II defined as >26) | SUITO >26 |
| Takita 201217 | ATG, TAC, MMF | ANA 100mg IV 1h pre-txp then 100mg SQ daily x 7d; ETA 50mg IV 1h pre-txp then 25mg SQ on POD 3, 7, 10 | ETA + EP | 4 | 8 | 12 | Adverse events within 3 months | - |
| ETA Long-term Follow-up | ||||||||
| Bellin 201219 | ATG ± DAC (or TEP alone without ETA) | NR | ATG + TNF blocker; ATG without TNF blocker; IL-2Ra; pancreas transplant | 29 | 946 | 60 | II | No exogenous insulin use for ≥14 consecutive days |
| Qi 201420 | EP | ETA 50mg IV pre-txp, 25mg SQ on POD 3, 7, 10 | EP | 6 | 10 | 60 | II, C-peptide, HbA1c, MMTT, OGTT, IVGTT, GST, Ryan HYPO score | Without exogenous insulin, FBG >140 mg/dL <4 times/wk and 2h postprandial BG >180 mg/dL <5 times/wk |
| Autotransplant | ||||||||
| ETA or ETA + ANA | ||||||||
| Naziruddin 201818 | ATG (other agents NR) | ETA 50mg SQ on POD 0, 25mg SQ on POD 3, 7, 10; in ANA + ETA group, added ANA 100mg SQ daily POD 0–7 | No ETA or ANA (other agents NR) | ETA – 32; ANA + ETA – 59 | 100 | 12 | SUITO index, basal C-peptide, FBG, HbA1c, proinsulin, IL6, IL-8, MCP-1 | - |
Abbreviations: ANA, anakinra; AI, anti-inflammatory; argTT, intravenous arginine tolerance test; ATG, antithymocyte globulin; ATZ, alemtuzumab; BAS, basiliximab; BG, blood glucose; CBC, complete blood count; CsA, cyclosporine; DAC, daclizumab; EP, Edmonton Protocol (DAC, SIR, TAC); ETA, etanercept; EVR, everolimus; FBG, fasting blood glucose; GFR, glomerular filtration rate; GST, glucagon stimulation test; h, hour; HbA1c, hemoglobin A1c; IL-1Ra, interleukin-1 receptor antagonist; IL-6, interleukin-6; IL-8, interleukin-8; II, insulin independence; INF, infliximab; IS, immunosuppression; IV, intravenous; IVGTT, intravenous glucose tolerance test; MCP-1, monocyte chemoattractant protein 1; MMF, mycophenolate mofetil; MMTT, mixed meal tolerance test; MPA, mycophenolic acid; NR, not recorded; OGTT, oral glucose tolerance test; POD, post-operative day; SIR, sirolimus; SQ, subcutaneous; SUITO, secretory unit of islet transplant objects; TAC, tacrolimus; TEP, teplizumab; txp, transplant; y, year
Table 3.
Study results.
| Author and Year | Average (SD) # IC infusions in interventional group vs comparison group | Average IEQ/kg (SD) with infusion interventional vs comparison group |
Difference in IEQ/kg or # of infusions | II | HbA1c (%) | Infection |
|---|---|---|---|---|---|---|
| Allotransplant | ||||||
|
ETA |
|
|
|
|
|
|
| Hering 200511 | 1 (0) | 7271 (1035) | - | 12mo: 100% (8/8); >12mo: 62.5% (5/8) | Pre-txp range 6.6 ± 1.1 to 8.3 ± 1.1 vs post-txp range 5.3 ± 0.5 to 6.0 ± 0.5 | 0/8 |
| Bellin 200812 | 1.9 (0.4) | 6476 (2203.7) | - | 12mo: 83% (5/6); 24mo: 66.7% (4/6) | 24mo: 6.0 ± 0.7; those achieved II: 5.6 ± 0.2 | 0/6 |
| Faradji 200810 | - | SI: 5613 (485) vs 8713 (2123) | - | 18mo: ETA group 100% (4/4) vs non-ETA group 20% (1/5) (p = 0.04); II duration over the 18mo: ETA group >non-ETA group per survival curve comparison (p = 0.025) | 18mo: ETA 6.2 ± 0.08 vs non-ETA 7.05 ± 0.55 | - |
| Froud 200813 | 1.7 (0.6) vs 1.8 (0.4) | 7352.6 (4303.8) vs 7204.9 (1953.1) | Cumulative IEQ/kg: ETA 12,554.3 ± 7173 vs non-ETA 13,058.8 ± 3540 (p = 0.92) | 3mo: ETA 66.7% (2/3) vs non-ETA 87.5% (14/16) | 24mo: ETA 5.1 ± 0.15 vs non-ETA 6.3 ± 0.12 pmol/mL (p < 0.01) | 0/3 |
| Gangemi 20088 | 1.3 (0.5) vs 2.5 (0.6) | 8612.1 (2605.3) vs 9754.1 (4023.2) | Total IEQ to achieve II: ETA 537,495 ± 190,968 with 1 infusion vs non-ETA 1,460,080 ± 418 330 in 2 (n = 2) or 3 (n = 2) infusions (p = 0.028) | 15mo: ETA 66.7% (4/6) vs non-ETA 100% (4/4) | Lower in both groups from baseline (p = 0.001), ETA 7.8 ± 1.0 to 6.1 ± 0.3 (after 1 infusion) vs non-ETA 6.5 ± 0.6 to 5.6 ± 0.2 (after ≥2 infusions) | ETA 1/6 vs non-ETA 0/4 |
| Koh 201014 | 1.1 (0.2) | 6076 (492) vs 9071 (796) | - | ATG + ETA 82.6% (11/13) vs DAC 100% (4/4) (p = 1.) | Pre-SI 7.0 ± 0.2 vs post-SI 6.1 ± 0.2% (p = 0.005) | - |
|
Rickels 20139 |
1.4 (0.5) vs 1.8 (0.4) |
6952.0 (517.7) vs 7951.7 (1242.8) |
Proportion of group with 2 infusions: ETA 36.4% (4/11) vs non-ETA 80% (4/5) (p < 0.05); Total IEQ/kg: ETA 9480 ± 706 vs non-ETA 14313 ± 2237 (p < 0.05) |
12mo: ETA 100% (9/9) vs non-ETA 20% (1/5) |
- |
- |
|
INF |
|
|
|
|
|
|
|
Froud 200515 |
1.9 (0.4) vs 1.8 (0.5) |
6702.4 (2040) vs 7683.8 (1861.2) |
- |
14/14 (100%), 2 did not complete study protocol; II without SI at 12mo: 79% (11/14); II without SI at 18mo: 43% (6/14) |
Normalized in 8 patients with sustained II over 3y |
2/16 including parvovirus anemia, aspiration pneumonia |
|
ETA + ANA |
|
|
|
|
|
|
| Matsumoto 201116 | 1 (0) vs 2 (0) | 11811.8 (1833.2) vs 8119.7 (1018.1) | Total IEQ/infusion: ANA 720522 ± 111826 vs non-ANA 552138 ± 69233 (p = 0.22) | SUITO index at 1mo: ANA 49.6 ± 8.3 vs non-ANA 19.3 ± 6.3 (p < 0.05) | Insulin free: ANA 5.9 ± 0.1 vs non-ANA 5.7 ± 0.3 (p = 0.68) | - |
|
Takita 201217 |
1.25 (0.5) vs 2 (0) |
10953 (1015) vs 8158 (2091) |
ETA+ANA 10953 ± 1015 vs ETA 1st infusion 7881 ± 1753, 2nd infusion 8435 ± 2429 (p = 0.48) |
- |
- |
0/8 opportunistic infection; 2/8 upper respiratory |
|
ETA Long-term Follow-up |
|
|
|
|
|
|
| Bellin 201219 | 1.3 (0.5) vs 2.1 (0.8) | 7298.7 (546.8) vs 6688.9 (343.2) | Cumulative IEQ: Group 1 (intervention group) <Group 2, 3, and 4 (historical CITR groups) (p < 0.01) | II significantly lower at every time point in ATG group without TNF blocker vs ATG with TNF blocker; II and significantly associated variables per adjusted analysis: TNF blocker (p = 0.03) | - | - |
|
Qi 201420 |
1.8 (1.0) vs 2.5 (0.6) |
8237 (2308.1) vs 9754.1 (4023.2) |
Total IEQ/kg: ETA 16414 ± 9423 vs non-ETA 24385 ± 14209 (p = 0.85) |
100% (10/10), ETA needed 1 SI vs non-ETA ≥2 SI to achieve II; 83.3% (5/6) maintained II in ETA vs 25% (1/4) in non-ETA |
<6% within 6mo: 90% (9/10) |
- |
| Autotransplant | ||||||
|
ETA or ETA + ANA |
|
|
|
|||
| Naziruddin 201818 | 1 (0) vs 1 (0) | ETA – 5384 (3104); ANA + ETA – 5352 (3145) vs CTL 5424 (3592) | ETA 5384 ± 3104 vs ANA + ETA 5352 ± 3145 vs CTL 5424 ± 3592 (p = 0.99) | SUITO at 6mo: ANA + ETA>ETA (p = 0.035), ANA + ETA no difference from CTL (p = 0.103) | 6mo: ETA<CTL (p < 0.05), ANA + ETA<CTL (p < 0.05), no difference at other time points | - |
Abbreviations: ANA, anakinra; ATG, antithymocyte globulin; CITR, Collaborative Islet Transplant Registry; CTL, control; DAC, daclizumab; ETA, etanercept; HbA1c, hemoglobin A1c; IC, islet cells; II, insulin independence; mo, months; SD, standard deviation; SI, supplemental infusion; SUITO, secretory unit of islet transplant objects; txp, transplant; y, year
Efficacy
ETA monotherapy
There were seven studies included assessing ETA as part of an allo ICT protocol.8-14 The definition and time points for assessment of II varied amongst the studies. Assessments of II at time points ≥1 year and Kaplan-Meier survival analyses suggest favorable long-term (≥1 year) maintenance of II with ETA. Faradji et al reported both II and duration of II improved II at 18 months with ETA versus non-ETA (p = 0.04 and 0.025 respectively).10 Gangemi et al reported all patients achieving II with lower IEQ in the ETA group (p = 0.028); however, this study reported less II at 15 months in the ETA group versus the comparison group.8 Koh et al reported no statistical difference in II achievement (p = 1.000) or duration of II (p = 0.247) within its cohort between those receiving daclizumab (DAC) versus anti-thymocyte globulin (ATG) + ETA. II in 10 patients lasted longer after receipt of a supplemental infusion (SI) with ETA compared to their course after initial transplantation without ETA (p = 0.009).14 HbA1c was shown to be significantly lower after islet cell transplant (average reduced to ≤6 to 6.2%) with ETA administration.8,10,13,14
Mixed meal tolerance test (MMTT) was assessed in four of these studies either by C-peptide response or by mixed meal stimulation index (MMSI), and two studies assessed duration and durability of MMTT response between groups. Faradji et al reported MMSI was increased throughout the study period after SI with ETA administration (p < 0.01), whereas significant increases in MMSI in the control group were seen after SI only at 3–9 months (p < 0.03).10 Froud et al reported higher peak C-peptide stimulation in the 12 month study period except at 3 months compared to controls (p < 0.05), and MMSI was similarly higher except at 3 and 12 months (p < 0.01).13 MMTT response was characterized as diabetic or non-diabetic by Gangemi et al with the ETA group having 80% (4/5) vs non-ETA group having 100% (4/4) non-diabetic response (p = NS).8 Koh et al found no difference in its MMTT assessment between ETA and non-ETA administered patients (p > 0.05).14
Oral glucose tolerance test (OGTT) and intravenous glucose tolerance test (IVGTT) were each assessed in a total of three of the studies.8,10-12 Outcomes varied and not all studies had a comparative group. Two studies evaluated intravenous arginine tolerance test.9,11 Rickels et al evaluated acute insulin response to glucose-potentiated arginine and found it was greater in the ETA group than in the control group.9
Gangemi et al found less cumulative IEQ were needed to achieve II with ETA administration (one infusion per patient) versus the comparison group (two to three infusions per patient) (p = 0.028).8 Rickels et al reported less total IEQ/kg required with ETA administration (p < 0.05) with fewer patients requiring two infusions (p < 0.05).9 Thus, there is some evidence an ETA-based protocol may be associated with a lower IEQ requirement. In two studies, no difference was found between groups with respect to IEQ/kg/infusion or cumulative IEQ/kg.10,13
INF monotherapy
The only prospective, randomized study included in this review assessed infliximab in addition to the Edmonton protocol and did not find clinical benefit with the use of INF (Table 3) nor differences in OGTT or IVGTT.15 Only one dose of infliximab was given prior to transplant, and the dose was increased during the study from 5 mg/kg to 10 mg/kg.15
ETA ± ANA combination therapy
Two studies assessed ETA + ANA combination therapy compared to ETA with the use of the Edmonton protocol in allo ICT.16,17 Only one of these studies assessed II, defining it as secretory unit of islet transplant objects (SUITO) index >26. The SUITO index at one month in ETA + ANA group was greater than in the comparison group after the first infusion (p < 0.05).16 There was no difference in SUITO index between the groups after the second infusion.16
One study evaluated the use of AI agents in auto ICT.18 The administration of ETA + ANA maintained baseline IL-6 level and significantly reduced the levels of IL-8 and MCP-1 back to baseline levels. Use of ETA alone resulted in marginally reduced levels of IL-8 and MCP-1 compared to control group.18 High-mobility group box 1 (HMGB1) was significantly reduced in the ANA + ETA group compared to ETA and the control group. There was no difference between ETA alone when compared with the control group.18 The SUITO index was significantly higher in the ANA + ETA group compared with ETA at 6 months; however, there was no difference in comparison with the no AI group.18
ETA follow-up studies
There were two follow-up studies including patients and results from previous studies.19,20
Bellin et al reported group 1 (ATG and ETA, with or without DAC) compared to group 2 (historical comparator group from the Collaborative Islet Transplant Registry (CITR) receiving a TNF blocker) had showed similar rates of II at three years (p > 0.05) and at five years (p > 0.05). However, both cohorts at these time points exhibited significantly higher rates of II than other comparator groups from CITR that did not receive any TNF blocker.19 Bellin et al also conducted an adjusted analysis to determine the relative impact of induction immunosuppression when accounting for potential confounding variables and found TNF blocker use was significantly associated with II regardless of other patient, donor, graft, or immunosuppression variables (p = 0.03).19
Qi et al found MMTT response was 100% (5/5) with ETA vs 50% (1/2) in the comparator group at 1 year and 83.3% (4/5) vs 50% (1/2) at 5 years, respectively.20 No difference was found in this study between OGTT and IVGTT.20 Bellin et al 2012 reported cumulative IEQ requirements were lower with ETA compared to the other groups (p < 0.01) while Qi 2014 found no difference, but reported fewer transfusions required to achieve II with ETA use.
Safety
Of the 23 patients who received ETA, only one patient had a reported serious infection. This patient was diagnosed with diabetic jaw myonecrosis with associated muscle and bone infection (Table 3). No findings regarding safety were reported to be specifically related to the use of an AI agent.
Discussion
AI agents play an important role in preservation of islet cell function at the time of infusion and post-transplant. Although significant differences in II were inconsistently reported with the use of an AI agent, the reduced need for subsequent transfusions and the overall improved islet cell survival post-infusion were consistent. Further, the 10th Annual Report of the CITR reports on the use of a TNF blocker to be associated with improved and favorable clinical outcomes, which concurs with our review.21
TNF blockers neutralize TNF-α, a potent pro-inflammatory cytokine. They have demonstrated a reduction in inflammatory markers in mice models and have demonstrated a continued impact on autoimmunity.22-25 However, not all TNF blockers have similar pharmacological properties. ETA, unlike INF, binds and neutralizes lymphotoxin-α3 and lymphotoxin-α2β1.26-28 The PIC, IL-1β, results in β-cell destruction, and ANA decreases the effects of PIC with respect to NO production, necrosis, apoptosis, glucose stimulated insulin secretion, and mitochondrial dysfunction, thus, reducing IBMIR.29-32
Reported infections with the use of AI agents were minimal; however, it is challenging to assess overall infection risk contributed by AI agents when patients are also receiving induction and maintenance immunosuppression. Although no reported infections were noted when using ETA + ANA, the combined use does have a warning for serious infection risk, including opportunistic infections and tuberculosis.17,33,34 Due to the shortened duration of its use compared to their FDA indications for other diseases, the risk of infection appears to be significantly less. It is still important to test for latent tuberculosis before TNF blocker use and during therapy, and for treatment, if necessary, to be initiated prior to its use. Prior history and risk of invasive fungal infection can assist in determining if empiric therapy is warranted for individuals during use of AI agents. Further, testing for hepatitis B prior to its use, during therapy, and several months after therapy should be completed due to risk of reactivation in patients previously infected with hepatitis B.33-35
This qualitative review encompasses the most updated and extensive literature search clinically evaluating the use of AI agents and summarizes their safety and outcomes beyond II. It is important to consider though that no statistical analysis was completed due to the heterogeneity of the study designs. The non-randomized control trials were included without a comparative group and there was an inconsistency throughout all the studies in definitions of primary outcomes. The difference in induction agents, ATG or DAC, along with changes in the maintenance immunosuppression regimen have the potential to alter the outcomes regardless of the use of an AI agent and is a confounding variable. The studies included in this review comprised those with historical cohorts, which made it challenging to assess if overall practices in both groups were similar. The small number of subjects evaluated, the limited number of centers publishing on this topic, and some discrepancy in reported medication doses were further limitations. At the same time it argues for the large multicenter single protocol prospective study on the subject.
In conclusion, this is the most detailed systematic qualitative analysis performed in literature. It demonstrated the addition of ETA or ETA + ANA to the medication regimen during ICT is associated with the potential for improved clinical outcomes compared to those regimens without an AI agent. A higher infection risk was not seen with the addition of one or more AI agents.
Methods
Search methodology
Available literature on TNF blockers and IL-1RA in auto and allo ICT were systematically reviewed and clinically appraised. A systematic search of Embase, PubMed, Cumulative Index for Nursing and Allied Health Literature (CINAHL), ClinicalTrials.gov, and EU Clinical Trials Register was conducted from date of database inception to October 8, 2018. Search strategies for the concepts of ICT, TNF blockers, and IL-1RA were created using a combination of subject headings and keywords and were used to search PubMed, Embase, and CINAHL. In PubMed and Embase, the Cochrane human filter was used to exclude animal and cell studies, exclusion Boolean language was used to remove conference abstracts and proceedings, and an English language search limit was used. In CINAHL, database-supplied limits for humans, English language, and the journal article publication type were used. Keywords for all the search terms were also used to search ClinicalTrials.gov and EU Clinical Trials Register. The full list of searched databases and the search strategies are listed in the supplementary materials. The systematic literature review completed a dual reviewer methodology.
Study selection and data extraction
Studies were included if the participants were ≥18 years-old undergoing auto or allo ICT receiving a TNF blocker and/or an IL-1RA in the medication regimen and was evaluating patient-centered outcomes. If there was no comparative group or historical cohort, the overall AI regimen needed to be reported as the same for all subjects. Those studies included with a historical cohort or comparative group required the two groups to have different AI regimens. Review articles, case reports, case series, case studies, and abstracts were excluded. Studies with a focus on prior pancreas transplant and/or enriched bone marrow cells were excluded. Prospective and retrospective studies with full text available were evaluated.
Risk of bias assessment
Risk of Bias Assessment tool for Non-randomized Studies (RoBANS) and the Cochrane Bias assessment for randomized studies were used.29 These assessments of publications are included in the supplementary materials.
Disclosure of potential conflicts of interest
No potential conflict of interest were disclosed.
Compliance with ethics guidelines
This article is based on previously conducted studies. Articles in this review do not contain those performed by any of the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. [DOI] [PubMed] [Google Scholar]
- 2.Barshes NR, Wyllie S, Goss JA.. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Luekoc Biol. 2005;77(5):587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 3.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. [DOI] [PubMed] [Google Scholar]
- 4.Johansson U, Olsson A, Gabrielsson S, Nilsson B, Korsgren O. Inflammatory mediators expressed in human islets of Langerhans: implications for islet transplantation. Biochem Biophys Res Commun. 2003;308:474–479. [DOI] [PubMed] [Google Scholar]
- 5.Bertuzzi F, Marzorati S, Maffi P, Piemonti L, Melzi R, de Taddeo F, Valtolina V, D’Angelo A, Di Carlo V, Bonifacio E, et al. Tissue factor and CCL2/monocyte chemoattractant protein-1 released by human islets affect islet engraftment in type 1 diabetic recipients. J Clin Endocrinol Metab. 2004;89(11):5724–5728. doi: 10.1210/jc.2004-0659. [DOI] [PubMed] [Google Scholar]
- 6.Desai CS, Khan KM, Ma X, Li H, Wang J, Fan L, Chen G, Smith JP, Cui W. Effect of liver histopathology on islet cell engraftment in the model mimicking autologous islet cell transplantation. ISLETS. 2017;9(6):140–149. doi: 10.1080/19382014.2017.1356558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desai CS, Khan KM, Megawa FB, Rilo H, Jie T, Gruessner A, Gruessner R. Influence of liver histopathology on transaminitis following total pancreatectomy and autologous islet transplantation. Dig Dis Sci. 2013;58(5):1349–1354. doi: 10.1007/s10620-012-2264-7. [DOI] [PubMed] [Google Scholar]
- 8.Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, Qi M, Wang Y, Pallan P, Owens C, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. 2008;8(6):1250–1261. doi: 10.1111/j.1600-6143.2008.02234.x. [DOI] [PubMed] [Google Scholar]
- 9.Rickels MR, Liu C, Shlansky-Goldberg RD, Soleimanpour SA, Vivek K, Kamoun M, Min Z, Markmann E, Palangian M, Dalton-Bakes C, et al. Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes. 2013;62:2890–2897. doi: 10.2337/db12-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, Mineo D, Baidal DA, Cure P, Ponte G, et al. Long term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658–1665. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, Matsumoto I, Ihm S, Zhang H, Parkey J, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 12.Bellin M, Kandaswamy R, Parkey J, Zhang H, Liu B, Ihm S, Ansite J, Witson J, Bansal-Pakala P, Balamurugan AN, et al. Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant. 2008;8(11):2463–2470. doi: 10.1111/j.1600-6143.2008.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Froud T, Baidal DA, Faradji R, Cure P, Mineo D, Selvaggi G, Kenyon NS, Ricordi C, Alejandro R. Islet transplantation with alemtuzumab induction and calcineurin-free maintenance immunosuppression results in improved short- and long-term outcomes. Transplantation. 2008;86(12):1695–1701. doi: 10.1097/TP.0b013e31819025e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh A, Imes S, Kin T, Dinyari P, Malcolm A, Shapiro TC, Senior A. Supplemental islet infusions restore insulin independence after graft dysfunction in islet transplant recipients. Transplantation. 2010;89(3):361–365. doi: 10.1097/TP.0b013e3181bcdbe8. [DOI] [PubMed] [Google Scholar]
- 15.Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: miami experience. Am J Transplant. 2005;5(8):2037–2046. doi: 10.1111/j.1600-6143.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, SoRelle J, Onaca N, et al. Improving efficacy of clinical islet transplantation with iodixanol-based islet purification, thymoglobulin induction, and blockage of IL-1β and TNF-α. Cell Transplant. 2011;20(10):1641–1647. doi: 10.3727/096368910X564058. [DOI] [PubMed] [Google Scholar]
- 17.Takita M, Matsumoto S, Shimoda M, Chujo D, Itoh T, SoRelle JA, Purcell K, Onaca N, Naziruddin B, Levy MF. Safety and tolerability of the t cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation. Clin Transplant. 2012;26(5):E471–484. doi: 10.1111/ctr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naziruddin B, Kanakm MA, Chang CA, Takita M, Lawrence MC, Dennison AR, Onaca N, Levy MF. Improved outcomes of islet autotransplant after total pancreactomy by combined blockade of IL-1β and TNFα. Am J Transplant. 2018;18(9):2322–2329. doi: 10.1111/ajt.14961. [DOI] [PubMed] [Google Scholar]
- 19.Bellin MD, Barton FB, Heitman A, Alejandro R, Hering BJ. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576–1583. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi M, Kinzer K, Danielson KK, Martellotto J, Barbaro B, Wang Y, Bui JT, Gaba RC, Knuttinen G, Gracia-Roca R, et al. Five-year follow-up of patients with type 1 diabetes transplanted with allogeneic islets: the UIC experience. Acta Diabetol. 2014;51(5):833–843. doi: 10.1007/s00592-014-0627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eggerman T, Arreaza-Rubin G, Hering B. Collaborative Islet Transplant Registry Tenth Annual Report (2017). Rockville (MD): Collaborative Islet Transplant Registry (US); 2017. January 6 [accessed 2018 December6]. http://citregistry.org/system/files/10th_AR.pdf. [Google Scholar]
- 22.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187(5):2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 23.Farney AC, Xenos E, Sutherland DER, Widmer M, Stephanian E, Field MJ, Kaufman DB, Stevens RB, Blazer B, Platt J, et al. Inhibition of pancreatic islet beta cell function by tumor necrosis factor is blocked by a soluble tumor necrosis factor receptor. Transplant Proc. 1993;25:865–866. [PubMed] [Google Scholar]
- 24.Xenos ES, Farney AC, Widmer MB, Casanova D, Stevens RB, Blazer BR, Sutherland DE, Gores PF. Effect of tumor necrosis factor alpha and of the soluble tumor necrosis factor receptor on insulin secretion of isolated islets of Langerhans. Transplant Proc. 1992;24:2863–2864. [PubMed] [Google Scholar]
- 25.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71(1):152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- 26.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, Salfeld J, Sasso EH. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. 2009;131(2):308–316. doi: 10.1016/j.clim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Scallon B, Cai A, Solowski N, Rosenberg A, Song XY, Shealy D, Wagner C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301:418–426. [DOI] [PubMed] [Google Scholar]
- 29.Park J, Lee Y, Seo H, Jang B, Son H, Kim S, Shin S, Hahn S. Risk of bias assessment tool for non-randomized studies (RoBANS): development and validation of a new instrument. Abstracts of the 19th Cochrane Colloquium; 2011;19-22; Madrid, Spain: John Wiley & Sons. [Google Scholar]
- 30.McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Trnasplant. 2012;12(2):322–329. doi: 10.1111/j.1600-6143.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 31.Sahraoui A, Kloster-Jensen K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell Transplant. 2014;23(10):1199–1211. doi: 10.3727/096368913X667529. [DOI] [PubMed] [Google Scholar]
- 32.Schwarznau A, Hanson MS, Sperger JM, Schram BR, Danobeitia JS, Greenwood KK, Vijayan A, Fernandez LA. IL-1 beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009;220(2):341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enbrel® [package insert]. Thousand Oaks (California): Amgen Inc; 2015. [Google Scholar]
- 34.Kineret® [package insert]. Stockholm (Sweden): Swedish Oprhan Biovitrum AB; 2012. [Google Scholar]
- 35.Remicade® [package insert]. Horsham (Pennsylvania): Janssen Biotech, Inc; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


