ABSTRACT
Histopathology based studies of the pancreas obtained from organ donors are increasing our awareness of islet phenotypic heterogeneity during development and aging, as well as in settings of type 1 diabetes, type 2 diabetes, monogenic diabetes or other forms of this metabolic disease. Islet amyloidosis represents a histopathological feature classically ascribed to patients with type 2 diabetes. Herein, the occurrence of islet amyloidosis and its severity are reported in a child with type 1 diabetes along with histological comparisons of islet amyloidosis in two young adults with recent-onset type 1 diabetes. Islet amyloidosis was infrequent yet widely distributed throughout the pancreas in the child with type 1 diabetes and both adults with type 1 diabetes, with no such pathology seen in matched control donors. Analysis of these cases add to the increasing appreciation of islet heterogeneity in children and young adults with type 1 diabetes. Such knowledge also supports a notion that multiple pathophysiological mechanisms underlie the loss of functional β-cell mass in the spectrum of clinical phenotypes in patients with type 1 diabetes.
KEYWORDS: Histopathology, pancreas, islet amyloid polypeptide, amylin, insulitis, heterogeneity
Introduction
Renewed interest and access to quality human pancreas biospecimens for histopathology studies have led to an increased appreciation for islet heterogeneity in normal subjects as well as in patients with various forms of diabetes. From a historical perspective, islet amyloidosis has widely been considered pathognomonic for type 2 diabetes, developing from extracellular deposition of islet amyloid polypeptide (IAPP).1–3 In this short report, islet amyloidosis is described for the first time to our knowledge, in a child with type 1 diabetes. Two young adults with recent-onset type 1 diabetes are also described with islet amyloidosis with comparisons to matched controls.
Methods
Patients
Pancreas and other tissues were recovered from organ donors through the Network for Pancreatic Organ donors with Diabetes (nPOD) program according to established protocols, with research consent for organ donation secured according to federal and institutional regulations.4 Clinical diagnosis was made by Pediatric endocrinology specialists based on medical history obtained from the terminal hospitalization medical records and laboratory assays including islet-related autoantibodies, HbA1c, C-peptide, and HLA high-risk for type 1 diabetes as previously described (Table 1).4 A family history of diabetes was reported in the organ donor questionnaire from all three patients, yet diabetes type was not specified.
Table 1.
nPOD patient and matched control donor demographic and laboratory assay data.
| Donors with Type 1 Diabetes | Controls | |||||
|---|---|---|---|---|---|---|
| CaseID | 6371 | 6414 | 6362 | 6318 | 6238 | 6339 |
| Age at death, years | 12.5 | 23.1 | 24.9 | 10 | 20 | 23.3 |
| Diabetes duration, years | 2 | 0.43 | 0 | - | - | - |
| Ethnicity | Caucasian | African American | Caucasian | Caucasian | African American | Caucasian |
| Sex | Female | Male | Male | Female | Male | Male |
| BMI (kg/m2) | 16.6 | 28.4 | 28.5 | 17.6 | 21.7 | 25 |
| HbA1c% (mmol/mol) | 9.5 (80) | 14 (130) | 10 (86) | 5.2 (33) | NA | 5.3 (34) |
| C-peptide (ng/mL) | 0.11 | 0.16 | 0.38 | 3.89 | 1.17 | 10.56 |
| Islet autoantibody testinga | GADA+ IA-2A+ ZnT8A+ | GADA+ ZnT8A+ | GADA+ | Negative | Negative | Negative |
| HLA genotype | DRB1*03/13 DQA1*01/05 DQB1*02/06 |
DRB1*03/09 DQA1*05/03 DQB1*02/02 |
DRB1*01/03 DQA1*01/05 DQB1*02/05 |
DRB1*04/11 DQA1*03/05 DQB1*03/03 |
DRB1*03/11 DQA1*04/05 DQB1*03/04 |
DRB1*03/10 DQA1*01/05 DQB1*02/05 |
| Cause of death | Cerebral edema | Anoxia | Head Trauma | Head Trauma | Head Trauma | Head Trauma |
| Insulitis present | Yes | Yes | Yes | No | No | No |
NA, not available.
aIslet autoantibody testing was performed for glutamic acid decarboxylase-65 (GADA), insulinoma-associated protein-2 (IA-2A), and zinc transporter 8 (ZnT8A) by radioimmunoassay as previously reported.3,5 All values were converted to NIDDK units and defined as positive for GADA if ≥20, IA-2A if ≥5, and ZnT8A if ≥0.020.
Histology
Serial paraffin sections (4 μm thick) from each available pancreas region (head, body, tail; N ≥ 1 block/region) were stained by hematoxylin and eosin (H&E) and immunohistochemistry (insulin, glucagon, Ki67, CD3) following standard protocols in all donors (Supplementary Table 1).4 Pancreas head and tail were available from patient 6414, pancreas tail was available from patient 6362, and head, body and tail were available from patient 6371. Stained slides were digitized with an Aperio CS2 slide scanner (Leica/Aperio, Buffalo Grove, IL). Histopathology reviews were conducted on all stained slides. When islet amyloid was detected during initial histopathology reviews, additional pancreas sections (8 μm thick, N = 4–7 sections/patient) were requested for staining using Congo Red (S7441, Cardinal Health, Dublin, OH) to confirm islet amyloidosis. Three age-, BMI-, and sex-matched control donors to the three patients with type 1 diabetes described herein were selected and paraffin sections similarly stained with Congo Red (Table 1).
Image analysis
Congo Red staining within islets was analyzed using HALO software (Indica Labs, Corrales, NM). Amyloid area was automatically quantified using the tissue classifier function in HALO after total tissue, islet count and amyloid positive islet areas were calculated following manual annotations. Islet amyloid prevalence was expressed as amyloid-positive islets/total islets per section (%). Islet amyloid severity was expressed as amyloid-positive area/islet cross-sectional area (%) for islets containing amyloid. Islet amyloid severity was also scaled as follows: 1) minimal (<10% of islet area was amyloid), 2) mild (10–25% of islet area was amyloid), 3) moderate (25–49% of islet area was amyloid), and 4) severe (>50% of islet area was amyloid). Islet amyloid area was also expressed per total tissue sectional area (%).
Statistical analyses
All data are reported as mean ± SD or range with N = number of sections per donor. Data were plotted using Prism 7.0 (GraphPad software, San Diego, CA).
Results
Histopathology reviews of sections stained by H&E revealed islet amyloidosis in the three patients with type 1 diabetes (Figure 1(A–C)). All islets with amyloid had β-cells that were displaced towards the islet periphery or vascular channels in varying degrees by the intra-islet amyloid deposits (Figure 1(D–F)). Congo Red staining showed a wide range in numbers of amyloid-positive islets per section with variable degrees of regional islet amyloidosis in all three patients (Figure 1(G–I)). No amyloid was detected by Congo Red staining in three matched controls (Supplementary Figure 1). Islet amyloid prevalence ranged from 0–9.3%, 0–12.6%, and 0–4.7% in donors 6371, 6414, and 6362, respectively (Figure 1(J)). Islet amyloid severity within only amyloid positive islets per section ranged from 0–41.2%, 0–15.1%, and 0–39.0% in donors 6371, 6414, and 6362, respectively (Figure 1(K)). As expected, the overall amyloid area/total section area (%) was exceedingly small, ranging from 0–0.029%, 0–0.045%, and 0–0.004% in donors 6371, 6414, and 6362, respectively (Figure 1(L)). In donors 6371 and 6362, amyloid-positive islets were distant from each other and rare (0–8 islets/section), with minimal to moderate islet amyloidosis (12 islets with 0.4–19% amyloid area; 7 islets with 25–48% amyloid area) (Supplementary Table 2). Donor 6414 had a higher frequency of amyloid-positive islets (4–35/section) with minimal to moderate amyloidosis (41 islets with 1.5–23% amyloid area, two islets with 25–43% amyloid area, respectively) (Supplementary Table 2). A clustering of amyloid positive islets was observed in two lobules from donor 6414 but not in the other two donors with type 1 diabetes. This lobular pattern of islet amyloid was unevenly distributed across the pancreas, largely being concentrated in the tail with a few foci identified in the head region (Figures 2–3). All three donors with type 1 diabetes had insulitis; however, insulitic islets did not show amyloidosis (Table 1).6
Figure 1.

Islets with severe amyloidosis in patients with type 1 diabetes. Representative islets are shown from donors 6371 (A, D, G), 6414 (B, E, H), and 6362 (C, F, I). Serial sections were stained by h&e (A-C), double immunohistochemistry for insulin (red) and Ki67 (brown) (D and F), triple immunohistochemistry for insulin (red), glucagon (yellow) and Ki67 (black) (E), and Congo Red for amyloid (G–I). Islet amyloid prevalence ranged from 0–12.6% (J), and islet amyloid severity within only amyloid positive islets per section ranged from 0–41.2% (K) in the donors with type 1 diabetes. The overall amyloid area/total section area (%) in donors with type 1 diabetes was exceedingly small, ranging from 0–0.045% (L). Three non-diabetic matched control donors (ND 6318, 6238, and 6339) had no amyloid positive islets in any section examined (J–L). Scale bars: 100 µm (A–I). Whole slide images are available through the nPOD Online Pathology Database (https://www.jdrfnpod.org/for-investigators/online-pathology-information/).
Figure 2.
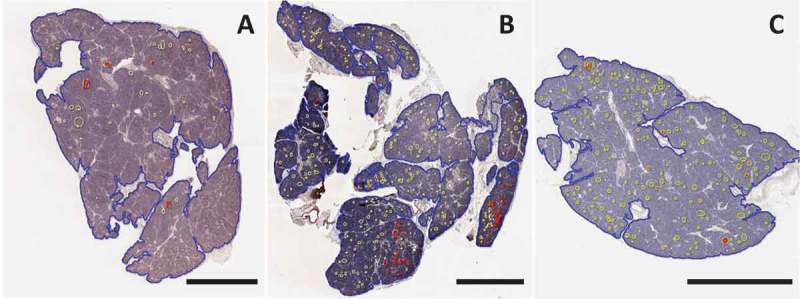
Image analysis of Congo Red staining for islet amyloid in three patients with type 1 diabetes. Sections were stained and analyzed with Congo Red as described in Methods. Whole slide scans were manually annotated for total pancreas area (blue) and islets (yellow). Amyloid area within islets (red) was identified using the tissue classifier within HALO image analysis software. A pancreas body section from case 6371 (A) and pancreas tail from 6362 (C) showed scattered and infrequent islet amyloidosis. A pancreas tail section from case 6414 (B) showed clustering of islet amyloidosis in two lobules (shown in higher magnification in Figure 3). Scale bars: 2 mm. Whole slide images are available through the nPOD Online Pathology Database (https://www.jdrfnpod.org/for-investigators/online-pathology-information/).
Figure 3.
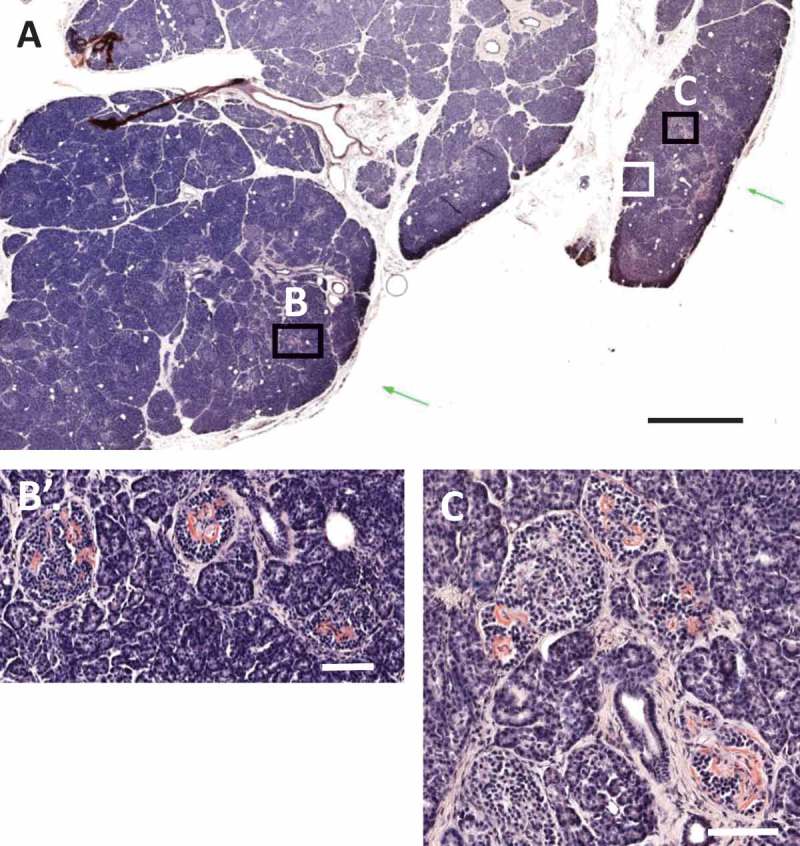
Clustering of islet amyloidosis in type 1 diabetes donor 6414. Islet amyloidosis was observed in two main lobules as shown with pink coloration by Congo Red staining in a block from the tail region (A). Regions with severe islet amyloidosis in both lobules are shown by black boxes in A and in higher magnifications in panels B–C. Scale bars: A: 2 mm, B,C: 100 μm. Whole slide images are available through the nPOD Online Pathology Database (https://www.jdrfnpod.org/for-investigators/online-pathology-information/).
Discussion
Islet amyloidosis is such a common pathological feature of the pancreas in type 2 diabetes, it is widely considered a pathologic feature for this disease.7 Islet amyloid is derived from IAPP (amylin) and is co-produced/co-secreted with insulin from β-cells. When misfolded and mistrafficked within β-cells, it has the propensity to aggregate and form intermediate toxic oligomers or non-toxic fibrils.8 The membrane-permeant IAPP oligomers may act as non-selective ion channels, further compounding β-cell stress that may ultimately lead to cellular apoptosis. Conversely, the IAPP fibrils are relatively inert, depositing as amyloid following the cell’s demise.8
In 1978, Westermark and Wilander performed a hallmark study providing a detailed analysis of islet amyloid deposits across the pancreas in a cohort of control subjects (mean age 74.8 ± 7.9 years, n = 15) and subjects having type 2 diabetes (73.8 ± 3.8; diabetes duration 6.5 ± 3.8 years, n = 12).9 Using Congo Red to identify amyloid deposits, they found that the percentage of amyloid-containing islets increased from the head to the tail region in subjects with type 2 diabetes (head: 30.1 ± 22.9%, range 3–81%; body: 49.2 ± 28.3%, range 10–97%; tail: 71.5 ± 17.3%, range 46–96%). In contrast, islets with amyloid were infrequent in the control subjects (mean in head 0%, body 1.3%, and tail 1.5%). Beyond its association with type 2 diabetes, islet amyloid has been reported as an incidental finding during the autopsy of the elderly and in the analysis of transplanted islets, insulinomas, cystic-fibrosis related diabetes, and monogenic diabetes cases.10–15 Interestingly, islet amyloid forms rapidly in islets transplanted into patients with type 1 diabetes and is thought to play a role in islet graft inflammation and dysfunction (reviewed in16).
More recently, Westermark et al. reported 10% islet amyloidosis by Congo Red staining in two of six patients (range 18–35 years old) with recent-onset type 1 diabetes who underwent pancreatic biopsy.17 The amyloid was detected both intra-and extracellularly and in association with dying β-cells, possibly indicating that amyloid first accumulates intracellularly in stressed β-cells in type 1 diabetes. This observation provides an intriguing parallel between the pathological processes in type 1 and type 2 diabetes, adding support to the concept that common metabolic derangements likely occur in β-cells in both diseases.
This novel report of amyloidosis occurring in a child with type 1 diabetes adds new appreciation for the ability of islet amyloidosis to occur in type 1 diabetes, even in the young. Our further finding of islet amyloid in two young adults with recent onset type 1 diabetes is in keeping with the report by Westermark et al.17 The child with type 1 diabetes showed a very similar pattern to the two young adults in terms of islet amyloid prevalence and severity. Notably, however, the child and one of the young adults (6362) showed a scattered distribution of amyloid-containing islets whilst, in the other young adult (6414), lobular clustering of amyloid-containing islets was seen. This lobular clustering of amyloid was also noted in the 2 subjects with recent onset type 1 diabetes in the Westermark report.17 Islet amyloid prevalence was similar in our 3 donors and the 2 subjects reported by Westermark et al.; this is interesting given that only biopsies of pancreatic tail were available in the latter report, whereas we were able to study the entire pancreas. Islet amyloid severity in the affected islets was not quantitated in the Westermark report; however, based upon the Figure provided, it appears that the amyloid tended to be less severe in their cases.
Overall, the presence of amyloid in pancreatic islets in type 1 diabetes, as well as heterogeneity in amyloid distribution and severity, indicate that further investigation is needed to gain an improved understanding for the role of islet amyloidosis as a potential pathogenic feature in this disease.
Funding Statement
This work was supported by the JDRF under grant numbers 25-2013-268, 17-2012-3, and 25-2012-516.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
MLB, LMJ, and AEB researched the data and reviewed/edited the manuscript. MAA reviewed and edited the manuscript. MCT conceived of the study and reviewed/edited the manuscript. High resolution HLA typing for donor 6414 was provided by Dr. Maki Nakayama and Dr. Sally Kent. The authors acknowledge Dr. Amanda Posgai for editorial assistance and the nPOD staff members and Organ Procurement Organizations that partner with nPOD to recover organ donors. Additional donor details and whole slide scans of stained sections reported in this study are available through the nPOD Online Pathology database (https://www.jdrfnpod.org/for-investigators/online-pathology-information/).
As the guarantor of this work, Dr. Martha Campbell-Thompson assumes full responsibility for the ethical acquisition and presentation of the data as well as the decision to submit and publish the manuscript.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Clark A. Nilsson MR: islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157–169. doi: 10.1007/s00125-003-1304-4. [DOI] [PubMed] [Google Scholar]
- 2.Westermark P, Andersson A, Westermark GT.. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 3.Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Aston-Mourney K, Carr DB, Westermark P, Westermark GT, et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011;178:2632–2640. doi: 10.1016/j.ajpath.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell-Thompson M, Wasserfall C, Kaddis J, Albanese-O‘Neill A, Staeva T, Nierras C, Moraski J, Rowe P, Gianani R, Eisenbarth G, Atkinson M, et al. Network for pancreatic organ donors with diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wasserfall C, Montgomery E, Yu L, Michels A, Gianani R, Pugliese A, Nierras C, Kaddis JS, Schatz DA, Bonifacio E, Atkinson MA. Validation of a rapid type 1 diabetes autoantibody screening assay for community-based screening of organ donors to identify subjects at increased risk for the disease. Clin Exp Immunol. 2016;185:33–41. doi: 10.1111/cei.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell-Thompson M, Fu A, Kaddis JS, Wasserfall C, Schatz DA, Pugliese A, Atkinson MA. Insulitis and beta-cell mass in the natural history of Type 1 diabetes. Diabetes. 2016;65:719–731. doi: 10.2337/db15-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 8.Costes S, Langen R, Gurlo T, Matveyenko AV, Butler PC. Beta-cell failure in type 2 diabetes: a case of asking too much of too few? Diabetes. 2013;62:327–335. doi: 10.2337/db12-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–421. doi: 10.1007/BF01219652. [DOI] [PubMed] [Google Scholar]
- 10.Vanga RR, Dhingra S, Patel K. Insulin expressing pancreatic neuroendocrine tumor associated with intratumor amyloidosis. Clin Gastroenterol Hepatol. 2017;15:A35–A36. doi: 10.1016/j.cgh.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Westermark GT, Westermark P, Berne C, Korsgren O. Transplantation NNfCI: widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 12.Sempoux C, Guiot Y, Dubois D, Moulin P, Rahier J. Human type 2 diabetes: morphological evidence for abnormal beta-cell function. Diabetes. 2001;50(Suppl 1):S172–177. doi: 10.2337/diabetes.50.2007.S172. [DOI] [PubMed] [Google Scholar]
- 13.O‘Brien TD, Butler AE, Roche PC, Johnson KH, Butler PC. Islet amyloid polypeptide in human insulinomas. Evidence for intracellular amyloidogenesis. Diabetes. 1994;43:329–336. doi: 10.2337/diab.43.2.329. [DOI] [PubMed] [Google Scholar]
- 14.Hull RL, Gibson RL, McNamara S, Deutsch GH, Fligner CL, Frevert CW, Ramsey BW, Sanda S. Islet interleukin-1β immunoreactivity is an early feature of cystic fibrosis that may contribute to β-cell failure. Diabetes Care. 2018;41:823–830. doi: 10.2337/dc18-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanyoura M, Jacobsen L, Carmody D, Del Gaudio D, Alkorta-Aranburu G, Arndt K, Hu Y, Kobiernicki F, Kusmartseva I, Atkinson MA, et al. Pancreatic histopathology of human monogenic diabetes due to causal variants in KCNJ11, HNF1A, GATA6, and LMNA. J Clin Endocrinol Metab. 2018;103:35–45. doi: 10.1210/jc.2017-01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denroche HC. Verchere CB: IAPP and type 1 diabetes: implications for immunity, metabolism and islet transplants. J Mol Endocrinol. 2018;60:R57–R75. doi: 10.1530/JME-17-0138. [DOI] [PubMed] [Google Scholar]
- 17.Westermark GT, Krogvold L, Dahl-Jørgensen K, Ludvigsson J. Islet amyloid in recent-onset type 1 diabetes-the DiViD study. Ups J Med Sci. 2017;122:201–203. doi: 10.1080/03009734.2017.1359219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


