ABSTRACT
F-box protein is a substrate-receptor of SKP1-Cullin1-F-box protein (SCF) E3 ligase, but the function of many family members remains elusive. We found that F-box and WD-repeat domain-containing 2 (FBXW2) inhibits proliferation and invasion of lung cancer cells by targeting S phase kinase-associated protein 2 (SKP2) and β-catenin for degradation. Lower FBXW2 predicts a worse patient survival. Thus, FBXW2 appears a lung tumor suppressor.
KEYWORDS: Lung cancer, FBXW2, SKP2, β-catenin, proliferation and invasion, ubiquitylation and degradation
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer which is the leading cancer-related death in the world. The overall 5-year survival rate for NSCLC patients is 11–17% due to late-stage diagnosis, tumor metastasis and recurrence1. Thus, the understanding of the molecular mechanism that drives the initiation, progression, and metastasis of NSCLC is imperative and significant, which would provide novel therapeutic targets for this deadly disease.
F-box proteins are the substrate recognition receptors of the SCF (SKP1-Cullin1-F-box protein) E3 ubiquitin ligase complexes with 69 family members found in human genome2. Among them, only three members are well studied. F-box and WD-repeat domain-containing 7 (FBXW7) is a well-known tumor suppressor that promotes ubiquitylation and degradation of various oncogenic proteins, including c-JUN, c-MYC, Cyclin E, MCL1, Notch1. The other two well-studied F-box proteins are S phase kinase-associated protein 2 (SKP2) and β-transducin repeats-containing protein (β-TrCP). While SKP2 is a typical oncoprotein that promotes degradation of tumor suppressive substrates such as p21 and p27, β-TrCP acts as an oncoprotein or tumor suppressor in a manner dependent on its substrates3,4. Alterations in these three F-box proteins were found in many human cancers with a well correlation of patient survival (worse for SKP2 overexpression or FBXW7 downregulation)3,4. Although increasing numbers of F-box proteins have their substrates being identified and functions being elucidated,5 most of the F-box proteins are still in their orphan stages and their functional characterization awaits for the identification of the corresponding substrates. Furthermore, whether an active cross-talk exists among the F-box proteins under physiological or stress conditions remains largely unknown.
To this end, we performed the immunoprecipitation screen for F-box proteins that interact with β-TrCP, and identified F-box and WD-repeat domain-containing 2 (FBXW2), a poorly characterized F-box protein with only one known substrate, glial cell missing 1 (GCM1), which is a transcription factor that promotes placental cell migration and invasion6. Further characterization revealed FBXW2 is a new substrate of β-TrCP for targeted ubiquitylation and degradation upon being phosphorylated by vaccinia related kinase 2 (VRK2) kinase7. We further found that FBXW2 binds to yet another F-box protein SKP2 under physiological condition and promotes SKP2 ubiquitylation and degradation7. Thus, we established a negative cascade among these three F-box proteins with β-TrCP at the upstream, FBXW2 in the middle, and SKP2 at the downstream. This negative cascade operates during cell cycle progression. Specifically, when cells enter the cell cycle upon growth factor stimulation, the level of VRK2 and β-TrCP increases to reduce the level of FBXW2 (by promoting its degradation), leading to accumulation of SKP2 (by inhibiting its degradation) to degrade cell cycle inhibitors, such as p21 and p27, to ensure proper cell cycle progression7.
Biologically, FBXW2, in contrast to oncogenic β-TrCP1 and SKP2, acts as a tumor suppressor to inhibit growth and survival of lung cancer cells, mainly by targeting SKP2 for degradation. We have extended this observation to human lung cancer tissues and found that FBXW2 is down-regulated in cancer tissues and few of loss-of or gain-of-function mutations were identified and characterized. Importantly, high FBXW2 levels predict a better survival for lung cancer patients, further suggesting that FBXW2 is a tumor suppressor in the lung. Taken together, an F-box protein cascade in a form of the oncogene-tumor suppressor-oncogene (β-TrCP-FBXW2-SKP2) axis may operate during lung tumor development7.
To further elucidate the mechanism of FBXW2 anticancer activity, we used affinity purification-mass spectrometry method and identified oncogenic protein β-catenin as yet another FBXW2 substrate. β-catenin, an Armadillo protein, acts both as a component of cell-cell adhesion structure by interacting with the cytoplasmic domain of E-cadherin and as a cellular signaling molecule involved in the regulation of gene expression following activation by Wnt or epidermal growth factor (EGF)8. Upon activation, β-catenin translocates to the nucleus where it interacts with transcription factors, the T cell factor/lymphoid enhancer factor-1 (TCF/LEF-1) family to transactivate the expression of downstream target genes including MYC, CCND1, MMP2, MMP7, and MMP9, thus regulating cell proliferation, differentiation, migration, and invasion8.
β-catenin has been well characterized as a substrate of β-TrCP9. In the absence of Wnt, cytoplasmic β-catenin forms a complex with axin/conductin, casein kinase 1 (CK1), glycogen synthase kinase-3β (GSK-3β), and the adenomatous polyposis coli protein (APC). CK1 and GSK-3β sequentially phosphorylate β-catenin at N-terminal Ser and Thr residues, resulting in its ubiquitylation and proteasomal degradation by β-TrCP9. However, the ubiquitin ligase responsible for β-catenin degradation following EGF stimulation is unknown. Our most recent work showed that FBXW2 binds to β-catenin upon AKT1-mediated phosphorylation at the C-terminus in response to growth factor stimulation, followed by targeted ubiquitylation and degradation of β-catenin. Thus, FBXW2 negatively regulates the β-catenin levels and transcriptional activity10.
What is the biological significance of FBXW2-mediated β-catenin degradation and what is the difference from β-TrCP-mediated β-catenin degradation? It has been previously shown that upon EGF stimulation, EGFR activates MMPs expression to promote migration and invasion in malignant diseases, but the underlying mechanism remains elusive. Our work showed that this process is mediated by the AKT1-FBXW2-β-catenin-MMPs axis. Specifically, AKT1-phosphorylated β-catenin preferentially binds to the promoters of the MMP-2, −7 and −9 via TCF4M/S, whereas Wnt activated β-catenin has a preference to bind to the promoters of MYC and CCND1 via TCF4E. Thus, FBXW2-promoted β-catenin degradation and consequent MMP down-regulation would inhibit migration and invasion of lung cancer cells, whereas Wnt-controlled β-TrCP-β-catenin axis mainly regulates cell proliferation. In clinical samples, we found a general tendency of inverse correlation of the protein levels between FBXW2 and β-catenin in lung cancer tissues. Moreover, the expression of FBXW2 was significantly reduced in the tumors with lymph-node metastasis as compared to those without lymph node metastasis, while the expression of β-catenin was just the opposite. Finally, lung cancer patients with low FBXW2/high β-catenin had a significantly poorer survival, whereas high FBXW2/low β-catenin had a better survival.
In summary, our study showed that FBXW2 is a substrate of β-TrCP for targeted ubiquitylation and degradation after being phosphorylated by VRK2. On the other hand, FBXW2 is an E3 ligase for SKP2 and β-catenin. Upon phosphorylation of SKP2 likely by a yet-to-be-identified kinase or of β-catenin by AKT1, FBXW2 promotes their ubiquitylation and degradation, to inhibit proliferation and invasion of lung cancer cells. FBXW2, therefore, acts as a tumor suppressor in the lung (Figure 1).
Figure 1.
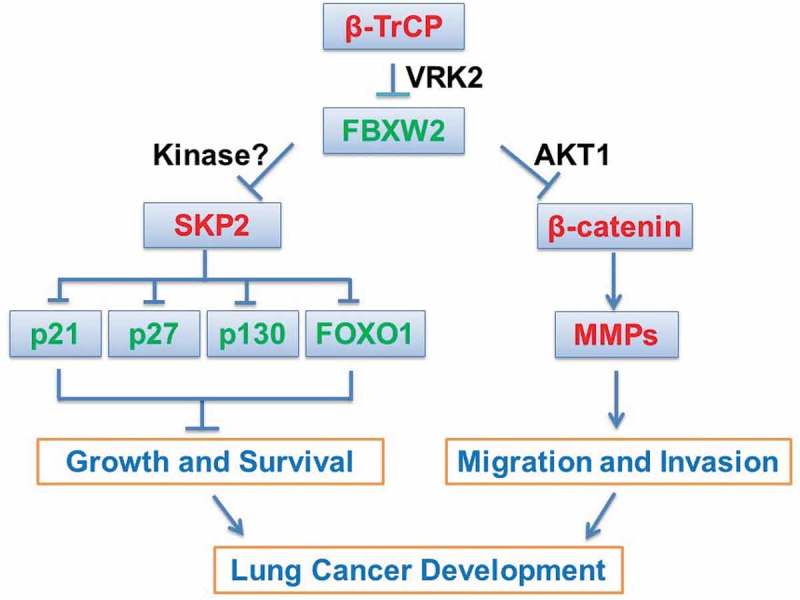
Legend: mechanisms of tumor suppression by FBXW2.
F-box and WD-repeat domain-containing 2 (FBXW2) is a novel substrate of β-transducin repeats-containing protein (β-TrCP), but an active E3 ligase for S phase kinase-associated protein 2(SKP2) and β-catenin. Specifically, FBXW2 is phosphorylated by vaccinia related kinase 2(VRK2) during cell cycle progression, and then binds to β-TrCP and being degraded. While SKP2 is likely phosphorylated by a yet-to-be-identified kinase, β-catenin is phosphorylated by AKT1 in response to growth factor. Phosphorylated SKP2 and β-catenin are then recognized by FBXW2, followed by targeted ubiquitylation and degradation. Depletion of SKP2 and β-catenin causes accumulation of tumor suppressor substrates of SKP2 (e.g., p21 and p27), and inactivation of transcription of the MMPs, leading to suppression of growth and survival, and migration and invasion of lung cancer cells. Thus, FBXW2 appears to be a tumor suppressor in the lung.
Funding Statement
This work was supported by the National Cancer Institute (NCI) grant (RO1CA156744) to Y.S.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skaar JR, Pagan JK, Pagano M. SCF ubiquitin ligase-targeted therapies. Nat Rev Drug Discov. 2014;13(889–903). doi: 10.1038/nrd4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14(233–247). doi: 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randle SJ, Laman H. F-box protein interactions with the hallmark pathways in cancer. Semin Cancer Biol. 2016;36(3–17). doi: 10.1016/j.semcancer.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Chiang M-H, Liang F-Y, Chen C-P, Chang C-W, Cheong M-L, Wang L-J, Liang C-Y, Lin F-Y, Chou -C-C, Chen H. Mechanism of hypoxia-induced GCM1 degradation: implications for the pathogenesis of preeclampsia. J Biol Chem. 2009;284(26):17411–17419. doi: 10.1074/jbc.M109.016170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J, Zhou W, Yang F, Chen G, Li H, Zhao Y, Liu P, Li H, Tan M, Xiong X, Sun Y. The beta-TrCP-FBXW2-SKP2 axis regulates lung cancer cell growth with FBXW2 acting as a tumour suppressor. Nat Commun. 2017;8(14002). doi:10.1038/ncomms14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169(985–999). doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Stamos JL, Weis WI. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(a007898). doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F, Xu J, Li H, Tan M, Xiong X, Sun Y. FBXW2 suppresses migration and invasion of lung cancer cells via promoting β-catenin ubiquitylation and degradation. Nat Commun. 2019;10(1). doi: 10.1038/s41467-019-09289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


