Abstract
Immunological aging, which encompasses age-associated declines in the immune system (immunosenescence) and increases in inflammation (inflammaging), is associated with morbidity and mortality. A growing body of research suggests stress is one factor that may accelerate immunological aging. This article provides a brief overview of immunological aging, describes key biological pathways acting at multiple lifespan stages linking stress and immunological aging, and reviews recent innovative work characterizing associations between stress in several domains and immunological aging, as well as potential protective and risk factors. Important directions for future research include careful characterizations of the complexities of stress and rigorous measurement of immunological aging processes. Advancing knowledge of stress resilience and healthy immune aging may ultimately slow disease onset and extend healthspan.
Stress and Immunological Aging
Aging is characterized in part by immune deterioration. Dysregulation of the immune system, which provides mechanisms to combat infectious diseases, destroy cancer cells, and respond appropriately to vaccines, increases risk for morbidity and mortality. Stress, broadly conceptualized as exposure to difficult or challenging circumstances (stressors) and the psychological, behavioral, and physiological responses to such circumstances (stress responses) (also see [1]), has emerged as a major factor that may accelerate the rate at which the immune system ages. Older adults in particular may generate exaggerated immune responses to stress that further exacerbate an already weakened immune system due to aging [2, 3]. Chronic stress that is demanding, distressing, and ongoing, may have the most detrimental immune health effects.
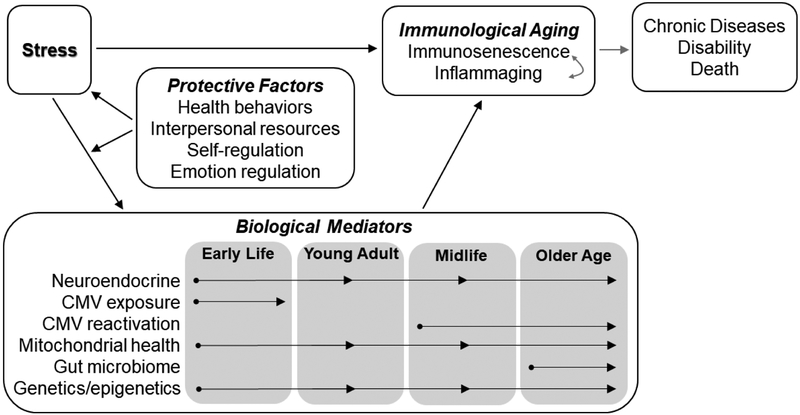
This review article provides a brief overview of immunological aging, describes key biological pathways linking stress and immunological aging, and summarizes recent innovative work characterizing associations between stress and immunological aging as well as potential protective and risk factors (see Figure 1 for a conceptual overview). The review incorporates a lifespan perspective by focusing on stress experienced during and biological mediators acting at multiple developmental stages to influence immunological aging.
Figure 1.
Conceptual model depicting the focus of this review (particularly the associations shown in black arrows). Stress can influence immunological aging (and ultimately health outcomes) via biological mediators that may act at various lifespan stages, but several protective factors may moderate these associations.
Immunological Aging
Normal aging is accompanied by declines in immunity, a process broadly referred to as immunosenescence, and increases in inflammation in the absence of infection, termed inflammaging. Hallmarks of immunosenescence include changes in the adaptive immune system, most prominently in the T cell compartment, responsible for protecting against pathogens residing inside cells or cells that have gone awry (e.g., cancer cells). Changes include decreases in naïve T cells and an accumulation of memory T cells, especially late differentiated senescent or near senescent CD8+ T cells that have shortened telomeres (i.e., protective caps on the ends of chromosomes that prevent deterioration) and that lose the costimulatory molecule CD28 and express maturation marker CD57 [4]. Cells of the innate immune system, such as neutrophils and natural killer (NK) cells that act as the first line of defense against invading pathogens, are also affected by increasing age [5]. Changes include defective activation and lower per-cell cytotoxicity. Inflammaging is characterized by age-related increases in circulating proinflammatory markers such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-α) [6]. Immunosenescence and inflammaging are interrelated; for example, senescent T cells exhibit an inflammatory senescence-associated secretory phenotype (SASP) comprised of cytokines and chemokines that contribute to inflammaging [7]. Although age-related immune changes can be viewed from an evolutionary perspective as an adaptive remodeling [8], these changes are ultimately implicated in impaired vaccine response, greater susceptibility to infectious disease, and the development of age-related diseases (e.g., cardiovascular disease, cancer), disability, and death [4, 5, 9, 10].
Biological Pathways Linking Stress and Immunological Aging
Well-established mediators by which stress may influence immunological aging include disruptions in the hypothalamic-pituitary-adrenal (HPA) axis, specifically altered cortisol levels and glucocorticoid signaling [11]. Additionally, dysregulation of the HPA axis as indicated by greater intraindividual cortisol variability [12] is a newer mechanism for future research that may contribute to inflammaging in older adulthood [13]. HPA axis disruptions act across the lifespan, with emphases during higher-risk developmental periods such as early childhood or older adulthood when stress may disrupt ongoing neurobiological development or further exacerbate glucocorticoid impairment that accompanies normal aging. Infection with persistent viruses, particularly cytomegalovirus (CMV), is another pathway acting in early life and adulthood linking stress and immunological aging. Environmental stressors can increase the probability of CMV infection in childhood; once infected, psychological stress can reactive latent CMV in midlife and older adulthood that, in turn, may drive aspects of immunological aging and age-associated diseases [14–16].
Several other pathways linking stress and immunological aging are garnering interest in psychoneuroimmunology and aging biology. Mitochondria, the cells’ “power plants” that generate energy and metabolic intermediaries needed for cellular function, are increasingly recognized as important players in the stress-immune health link [17]. Circulating cell-free mitochondrial DNA (ccf-mtDNA) released in response to stress (C Trumpff et al., unpublished) may act as damage-associated molecular patterns that trigger inflammaging in older adults [18]. Mitochondrial health likely acts across the lifespan to connect stress and immunological aging; however, older adulthood may be a particularly relevant developmental stage given age-related increases in ccf-mtDNA and accumulated defects that may compromise mitochondrial function. The gut microbiome is another pathway that may act primarily in older adulthood; aging is accompanied by increases in gut permeability (i.e., gut bacteria translocation into the outer area), as well as changes in microbial composition (i.e., less diversity), which is associated with inflammaging in older adults [19]. Moreover, new evidence in humans suggests stress can also increase gut permeability [20]. Epigenetic mechanisms reflected by changes in DNA methylation throughout the epigenome may also play a role in stress-immune aging connections across the lifespan; epigenetic clocks (i.e., molecular age algorithms based on DNA methylation levels) are associated with stress and T cell immunosenescence [21, 22].
Recent Innovative Work in Stress and Immunological Aging
The following sections describe key domains of recent inquiry into associations between stress and immunological aging as well as potential protective and risk factors.
Early Life Adversity
Stress that occurs early in development, in the form of abuse, neglect, separation from parents, or other adverse experience, may have particularly potent effects on immunological aging. Meta-analyses indicate early life adversity is associated with telomere shortening later in life [23, 24], and mitochondrial biology may play an important role in this association [25]. Early life adversity may also increase risk of CMV infection in childhood, which in turn drives T cell immunosenescence in young adulthood [26]. A lifespan approach may be especially useful in studying cumulative effects of stress on immunological aging, as well as identifying whether associations are driven by certain events, types of stressors, and related biological mediators acting in specific sensitive developmental periods [27, 28].
Environmental and Socioeconomic Factors in Adulthood
In adulthood, macro-level environmental and socioeconomic stressors relate to immunological aging. Higher perceived neighborhood problems (e.g., excessive noise, heavy traffic, lack of parks or playgrounds) are associated with shorter leukocyte telomere length in African American women, after controlling for individual-level risk factors [29]. Older adults with more socioeconomic resources, particularly with regard to finances and possessions, display less CD57 expression on NK cells [30]. Conversely, middle-aged adults with lower socioeconomic status display higher levels of T cell immunosenescence, due in part to impaired CMV control [31]. Molecular pathways, including increased gene transcription in immune cells responsible for proinflammatory, antiviral, and stress signaling, may connect socioeconomic stress to immunological aging in older adults [32].
Caregiving
Caregiving for a sick or disabled relative (e.g., spouse, child) is frequently examined in psychoneuroimmunology studies of chronic stress effects on immunity. High stress compared to low stress caregivers display lower mitochondrial health function and shifts in T cell composition characteristic of immunosenescence [33, 34]. Other research indicates associations between caregiving burden (but not caregiver status) and increased inflammaging (IL-6 and CRP) in older adults [35]. Associations between duration of caregiving and immunological aging have also been examined in longitudinal research; in older adult women, no significant associations were found between long-term caregiving patterns and leukocyte telomere length at the end of 8 years or changes in telomere length since baseline [36]. In terms of the innate immune system, neutrophil function was preserved in both young and old stressed caregivers, but those with higher psychological stress and other comorbidities displayed poorer neutrophil function [37]. Future research focusing on individual in addition to group differences in chronicity and levels of caregiving stress may further clarify links between chronic stress, age, and immunological aging processes.
Daily Stressors
Daily stressors, or routine challenges of everyday life such as work deadlines, family arguments, and providing care to others, may influence immunological aging. There is support for both the exposure (accumulation over time) and reactivity (heightened psychological and physiological responses) models of daily stress [38, 39]. However, daily stress research has focused exclusively on inflammatory markers (e.g., inflammaging), with one recent exception: greater accumulated daily stress appraisals over an 8-week period were associated with elevated gene expression of cellular senescence signal p16INK4a in middle-aged adults [40]. It remains unknown whether daily stressors in older adulthood influence other indicators or drivers of immunosenescence (e.g., accumulation of late-differentiated T cells or reactivation of latent viruses).
Interpersonal Stressors
Evidence from cross-sectional studies suggests that interpersonal stressors, including loneliness and low social support, are associated with shorter leukocyte telomere length, inflammaging, and T cell immunosenescence [41–44]. These effects may depend in part on CMV reactivation in midlife and older adulthood as well as parasympathetic function [44]. Importantly, interpersonal stressors may affect not only the individuals directly involved, but also other family members in the home exposed to such stress. Among children, greater negative mood reactivity to martial conflict was related to shorter leukocyte telomere length [45]. An important next step will be conducting longitudinal studies that assess how dynamics of interpersonal stressors in childhood and adulthood affect indicators of immunological aging even later in life.
Nonpharmacological Moderators of Resilience and Vulnerability
Health behaviors may attenuate stress effects on immunological aging, potentially acting concurrently in time and mainly in older adulthood. Exercise is one immune-restorative behavior that may decrease stress and slow immunological aging in older adults [46], possibly by reducing proportions of senescent T cells and decreasing inflammaging [47]. Sleep is another restorative health behavior. Inadequate quantity or quality of sleep may exacerbate immunological aging, particularly in the context of stress [48, 49], by activating DNA damage and the proinflammatory SASP in older adults [50]. Lifestyle interventions such as calorie restriction have also been proposed to delay immunological aging. However, initial results in non-obese middle-aged and older adults suggest no clear evidence that long-term calorie restriction slows telomere shortening or T cell immunosenescence [51].
Psychosocial factors may buffer stress effects on immunological aging. Higher subjective and objective measures of social support/integration are associated with lower levels of inflammaging and an immune risk score based upon inflammatory markers and antibody titers for two herpesviruses, including CMV in middle-aged and older adults [52, 53]. Effective emotion regulation strategies and strength of ability to self-regulate may also protect against stressor exposure and reactivity and effects on immunological aging. These factors have yet to be examined in older adults. Identifying ways to effectively promote resilience in the face of stress and immunological aging continues to be an exciting future direction.
Conclusions and Future Directions
Current evidence indicates that stress is associated with immunological aging, including aspects of immunosenescence and inflammaging. Several areas for future direction also emerged from this review. First, defining and measuring “stress” is an ongoing area of investigation [1]. This review highlighted chronic and daily forms of stress in various domains (individual, interpersonal, broader socioeconomic contexts) experienced during different developmental periods (childhood, adulthood). However, individuals may experience several forms of stress simultaneously, and previous and ongoing stressors may influence subsequent stress experiences. Future work that incorporates these complexities into theorizing and research is warranted to determine which aspects of stress most strongly relate to different immunological aging processes.
Second, rigorous and replicable measurement of immunological aging parameters, as well as major drivers of immune aging (e.g., CMV), should be employed. Peripheral blood contains a mixture of several different immune cells and failure to account for the cell-type composition can lead to incorrect interpretations about immunological aging. As one example, unaccounted differences in cell-type composition may lead to pseudo-lengthening of telomeres, whereby measured telomere length appears longer, but only because of changes in the proportion of cells types being measured rather than actual lengthening of telomeres in any given cell type. Sorting cells prior to analyses or focusing on certain cell subsets (e.g., CD8+CD28−) could strengthen validity. Additionally, markers of immunological aging (and biological aging more broadly) may capture different aspects of the aging process; theory and previous research should help guide selection of relevant markers (or alternatively, construction of a composite or algorithm-based measure, see [54]).
Finally, advances in biomarker assays and increasing sophistication in research measurement and analysis provide support and motivation for well-powered studies of diverse samples that test complex models of stress and immunological aging, including links to clinically relevant health outcomes, as proposed in Figure 1. Longitudinal studies of stress, immunological aging, and biological mechanisms across different time scales and lifespan stages will be especially informative. Innovative inquiry into stress effects and the roles of protective factors and mediators in immunological aging may assist in slowing the onset of disease and extending healthspan – the portion of life spent in good health.
Highlights.
Aging is accompanied by declines in immune function and increases in inflammation.
Stress in several domains is associated with immunological aging.
Biological pathways connecting stress and immunological aging are reviewed.
Protective factors include health behaviors, intra- and inter-personal resources.
Future work on stress resilience and healthy immune aging may extend healthspan.
Acknowledgments
This work was supported by the National Institute on Aging (AG056635-K99).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Nothing declared.
References and Recommended Reading
Papers of particular interest, published within the period of review, have been highlighted as:
*of special interest
**of outstanding interest
References
- 1.Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB: More than a feeling: a unified view of stress measurement for population science. Front Neuroendocrinol 2018, 49:146–169.**A thorough conceptual model of stress and stress measurement that integrates several perspectives (epidemiological, affective, and psychophysiological) across life course stages and stressor domains. A Stress Typology is proposed that provides a common language to describe important conceptual dimensions of stress.
- 2.Casaletto KB, Staffaroni AM, Elahi F, Fox E, Crittenden PA, You M, Neuhaus J, Glymour M, Bettcher BM, Yaffe K, Kramer JH: Perceived stress is associated with accelerated monocyte/macrophage aging trajectories in clinically normal adults. The Am J Geriatr Psychiatry 2018, 26:952–963.*A longitudinal investigation (up to 8 visits at ~15-month intervals) of the effect of baseline perceived stress on changes over time in cytokine markers associated with monocyte/macrophage activation in community-dwelling older adults. Higher perceived stress at baseline was associated with steeper age-related elevations in cytokines over time. Moreover, increases in monocyte/macrophage cytokine levels were associated with declines in executive function over time.
- 3.Vitlic A, Lord JM, Phillips AC: Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. Age 2014, 36:1169–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pera A, Campos C, López N, Hassouneh F, Alonso C, Tarazona R, Solana R: Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015, 82:50–55. [DOI] [PubMed] [Google Scholar]
- 5.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T: Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012, 24:331–341. [DOI] [PubMed] [Google Scholar]
- 6.Ferrucci L, Fabbri E: Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018, 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callender LA, Carroll EC, Beal RW, Chambers ES, Nourshargh S, Akbar AN, Henson SM: Human CD 8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 2018, 17:e12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C: Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 2018, 8:1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S: The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med 2018, 5:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawelec G: Immune parameters associated with mortality in the elderly are context-dependent: lessons from Sweden, Holland and Belgium. Biogerontology 2017. 10.1007/s10522-017-9739-z [DOI] [PubMed] [Google Scholar]
- 11.Bauer ME: Glucocoritcoids and dehydroepiandrosterone: a role in immunosenescence? In Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Edited by Fulop T, Franceschi C, Hiroakwa K, Pawelec G. Springer; 2018:1–29. [Google Scholar]
- 12.Segerstrom SC, Sephton SE, Westgate PM: Intraindividual variability in cortisol: approaches, illustrations, and recommendations. Psychoneuroendocrinology 2017, 78:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herriot H, Wrosch C, Gouin JP, Miller GE: Intra-individual cortisol variability and low-grade inflammation over 10 years in older adults. Psychoneuroendocrinology 2017, 77:141–149. [DOI] [PubMed] [Google Scholar]
- 14.Aiello AE, Chiu YL, Frasca D: How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? Geroscience 2017, 39:261–271.**A review of the influence of CMV on age-related alterations in immune function and chronic diseases such as type 2 diabetes, cancer, and cardiovascular disease. Socioeconomic factors and psychosocial stressors that influence CMV infection and IgG antibody response are also discussed.
- 15.Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, Stalder T, Hoffman K, Fischer JE, Bosch JA: Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun 2014, 38:133–141. [DOI] [PubMed] [Google Scholar]
- 16.Reed RG, Greenberg RN, Segerstrom SC: Cytomegalovirus serostatus, inflammation, and antibody response to influenza vaccination in older adults: the moderating effect of beta blockade. Brain Behav Immun 2017, 61:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard M, McEwen BS, Epel E, Sandi C: An energetic view of stress: focus on mitochondria. Front Neuroendocrinol 2018, 49:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T: Circulating mitochondrial DNA increases with age and is a familiar trait: implications for “inflammaging”. Eur J Immunol 2014, 44:1552–1562. [DOI] [PubMed] [Google Scholar]
- 19.Buford TW, Carter CS, VanDerPol WJ, Chen D, Lefkowitz EJ, Eipers P, Morrow CD, Bamman MM: Composition and richness of the serum microbiome differ by age and link to systemic inflammation. Geroscience 2018, 40:257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Wilson SJ, Bailey ML, Andridge R, Peng J, Jaremka LM, Fagundes CP, Malarkey WB, Laskowski B, Belury MA: Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology 2018, 98:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA: An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10:573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, Ashley-Koch AE, Garrett M, Kimbrel NA, Lori A, Workgroup VM: Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology 2018, 92:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanssen LM, Schutte NS, Malouff JM, Epel ES: The relationship between childhood psychosocial stressor level and telomere length: a meta-analysis. Health Psychol Res 2017, 5:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR: Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 2018, 23:858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridout KK, Khan M, Ridout SJ: Adverse childhood experiences run deep: early life stress, telomeres, and mitochondrial DNA copy number, the biological markers of cumulative stress. Bioessays 2018, 40:e1800077. [DOI] [PubMed] [Google Scholar]
- 26.Elwenspoek M, Sias K, Hengesch X, Schaan VK, Leenen FA, Adams P, Mériaux SB, Schmitz S, Bonnemberger F, Ewen A, Schächinger H: T cell immunosenescence after early life adversity: association with cytomegalovirus infection. Front Immunol 2017, 8:1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, Epel ES: Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci USA 2016, 113:E6335–E6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennyson RL, Gettler LT, Kuzawa CW, Hayes MG, Agustin SS, Eisenberg DT: Lifetime socioeconomic status and early life microbial environments predict adult blood telomere length in the Philippines. Am J Hum Biol 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gebreab SY, Riestra P, Gaye A, Khan RJ, Xu R, Davis AR, Quarells RC, Davis SK, Gibbons GH: Perceived neighborhood problems are associated with shorter telomere length in African American women. Psychoneuroendocrinology 2016, 69:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segerstrom SC, Al-Attar A, Lutz CT: Psychosocial resources, aging, and natural killer cell terminal maturity. Psychol Aging 2012, 27:892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiello AE, Feinstein L, Dowd JB, Pawelec G, Derhovanessian E, Galea S, Uddin M, Wildman DE, Simanek AM: Income and markers of immunological cellular aging. Psychosom Med 2016, 78:657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine ME, Crimmins EM, Weir DR, Cole SW: Contemporaneous social environment and the architecture of late-life gene expression profiles. Am J Epidemiol 2017, 186:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, Burelle Y, Epel ES: A mitochondrial health index sensitive to mood and caregiving stress. Biol Psychiatry 2018, 84:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prather AA, Epel ES, Parra EP, Coccia M, Puterman E, Aiello AE, Dhabhar FS: Associations between chronic caregiving stress and T cell markers implicated in immunosenescence. Brain Behav Immun 2018, 73:546–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potier F, Degryse JM, de Saint-Hubert M: Impact of caregiving for older people and pro-inflammatory biomarkers among caregivers: a systematic review. Aging Clin Exp Res 2017, 30:119–132. [DOI] [PubMed] [Google Scholar]
- 36.Chang SC, Crous-Bou M, Prescott J, Rosner B, Simon NM, Wang W, De Vivo I, Okereke OI: Relation of long-term patterns in caregiving activity and depressive symptoms to telomere length in older women. Psychoneuroendocrinology 2018, 89:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vitlic A, Lord JM, Taylor AE, Arlt W, Bartlett DB, Rossi A, Arora-Duggal N, Welham A, Heald M, Oliver C, Carroll D: Neutrophil function in young and old caregivers. Br J Health Psychol 2016, 21:173–189. [DOI] [PubMed] [Google Scholar]
- 38.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J: Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol 2012, 31:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sin NL, Graham-Engeland JE, Almeida DM: Daily positive events and inflammation: findings from the National Study of Daily Experiences. Brain Behav Immun 2015, 43:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rentscher KE, Carroll JE, Repetti RL, Cole SW, Reynolds BM, Robles TF. Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a. Psychoneuroendocrinology 2019, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch JA, Fischer JE, Fischer JC: Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav Immun 2009, 23:527–534. [DOI] [PubMed] [Google Scholar]
- 42.Carroll JE, Roux AV, Fitzpatrick AL, Seeman T: Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis (MESA). Psychosom Med 2013, 75:171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nersesian PV, Han HR, Yenokyan G, Blumenthal RS, Nolan MT, Hladek MD, Szanton SL: Loneliness in middle age and biomarkers of systemic inflammation: findings from Midlife in the United States. Soc Sci Med 2018, 209:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson SJ, Woody A, Padin AC, Lin J, Malarkey WB, Kiecolt-Glaser JK: Loneliness and telomere length: immune and parasympathetic function associations with accelerated aging. Ann Behav Med 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robles TF, Carroll JE, Bai S, Reynolds BM, Esquivel S, Repetti RL: Emotions and family interactions in childhood: associations with leukocyte telomere length. Psychoneuroendocrinology 2016, 63:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puterman E, Weiss J, Lin J, Schilf S, Slusher A, Johansen KL, Epel ES: Aerobic exercise lengthens telomeres and reduces stress in family caregivers: a randomized controlled trial-Curt Richter Award Paper 2018. Psychoneuroendocrinology 2018*A unique randomized controlled trial of aerobic exercise training in a high-stress sample (caregivers). Compared to the waitlist control group, telomere length appeared significantly longer and stress decreased in caregivers who participated in 24 weeks of aerobic exercise training. However, the apparent telomere lengthening effects were not due to changes in telomerase levels.
- 47.Bigley AB, Baker FL, Spielmann G, Simpson RJ: Aging, immunity, and the impact of physical exercise In Handbook of Immunosenescence: Basic Understanding and Clinical Implications. Edited by Fulop T, Franceschi C, Hiroakwa K, Pawelec G. Springer; 2018:1–57. [Google Scholar]
- 48.Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T, Horvath S: Epigenetic aging and immune senescence in women with insomnia symptoms: findings from the Women’s Health Initiative Study. Biol Psychiatry 2017, 81:136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prather AA, Gurfein B, Moran P, Daubenmier J, Acree M, Bacchetti P, Sinclair E, Lin J, Blackburn E, Hecht FM, Epel ES: Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav Immun 2015, 47:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll JE, Cole SW, Seeman TE, Breen EC, Witarama T, Arevalo JM, Ma J, Irwin MR: Partial sleep deprivation activates the DNA damage response (DDR) and the senescence-associated secretory phenotype (SASP) in aged adult humans. Brain Behav Immun 2016, 51:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomiyama AJ, Milush JM, Lin J, Flynn JM, Kapahi P, Verdin E, Sinclair E, Melov S, Epel ES: Long-term calorie restriction in humans is not associated with indices of delayed immunologic aging: a descriptive study. Nutr Healthy Aging 2017, 4:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elliot AJ, Heffner KL, Mooney CJ, Moynihan JA, Chapman BP: Social relationships and inflammatory markers in the MIDUS Cohort: the role of age and gender differences. J Aging Health 2018, 30:904–923. [DOI] [PubMed] [Google Scholar]
- 53.Hasselmo K, Mehl MR, Tackman AM, Carey AL, Wertheimer AM, Stowe RP, Sbarra DA: Objectively measured social integration is associated with an immune risk phenotype following martial separation. Ann Behav Med 2018, 52:130–45.*An empirical study of the differential effects of subjective and objective measures of social support on an immune risk composite. An innovative objective measure of social support was used, namely the Electronically Activated Recorder (EAR), an observational, real-time, ecological data capture method that records audio snippets from daily life.
- 54.Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A: Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol 2017, 187:1220–1230.*An empirical study that compared several methods to quantify biological aging and assessed their associations with aging-related outcomes (i.e., physical functioning, cognitive decline, and subjective signs of aging). Contrary to expectation, there was low agreement among the different biological aging measures. Biomarker composites and one epigenetic clock were consistently related to aging-related outcomes, but effect sizes were modest.