Abstract
The antitumor drugs doxorubicin and etoposide, a phodophyllotoxin derivative, are clinically active for the treatment of human malignancies. Because of their extreme effectiveness in the clinic, their modes of actions have been the subject of intense research for over several decades both in the laboratory and in the clinic. It has been found that both doxorubicin and etoposide (VP-16) act on topoisomerase II, induce DNA cleavage, and form double-strand breaks, causing tumor cell death. However, both of these drugs also undergo extensive metabolism in tumor cells and in vivo to various reactive intermediates that bind covalently to cellular DNA and proteins. Moreover, both drugs are metabolized to reactive free radicals that induce lipid peroxidation and DNA damage. However, the role of drug activation in the mechanism of cytotoxicity remains poorly defined. In this review, we critically evaluate the significance of metabolic activation of doxorubicin and etoposide in the mechanism of tumor cytotoxicity.
INTRODUCTION
The clinically active anticancer drugs doxorubicin (also known as adriamycin) and etoposide are used for the treatment of a wide variety of cancers [1]. Doxorubicin and etoposide (VP-16) act as topoisomerase II (Topo II) poisons and induce accumulations of enzyme-linked, double-strand breaks, which are highly cytotoxic to cells [2, 3]. Topoisomerases (I and II) are an important class of nuclear enzymes that are responsible for maintaining the topology of DNA; they are involved in DNA repair, transcription, replication and segregation of chromosomes [4–6]. Inhibition and/or interference with topoisomerase functions leads to cell death. In vivo, the mechanism(s) of tumor cell killing by doxorubicin and VP-16 are extremely complicated in spite of their known action as topo II poisons, and various other mechanisms have been proposed. Cytotoxicity of these drugs could also depend upon other factors, e.g., metabolic activation, as they undergo enzymatic activation to reactive species that either bind to or induce damage to cellular macromolecules. This review examines mechanisms of action based on metabolic activation (bioactivation) of these two anticancer drugs that may be important for their modes of action.
DOXORUBICIN
Doxorubicin was first discovered in early 1970 and has become one of the most important and widely used anticancer drugs for the treatment of both solid and hematological malignancies [1, 7, 8]. It contains an anthraquinone chromophore (Figure 1) and an aminoglycoside. Because of the anthraquinone structure, which contains a quinone-hydroquinone moiety, it is readily reduced by a variety of enzyme systems [9–12], most notably, cytochrome P450-reductase, via one-electron reduction, forming a semiquinone radical (Figure 1). The semiquinone radical of doxorubicin is extremely unstable in the presence of molecular oxygen and undergoes a futile oxidation-reduction cycle forming superoxide radical and, ultimately a highly reactive oxidant, the hydroxyl radical (Figure 2) [9–16]. The formation and reaction of the reactive oxygen radicals with cellular macromolecules is the basis for many of the known toxicological and cytotoxicological effects of doxorubicin. Other enzymatic systems are also known to activate doxorubicin, e.g., xanthine oxidase, DT-diophorase and nitric oxide synthase, to form reactive oxygen species. However, it is believed that the cytochrome P450 reductase system is the main enzymatic reductive pathway for the activation of doxorubicin [10]. There are two main consequences of this reductive activation of doxorubicin: a) generation of covalent binding species and b) formation of free radical species.
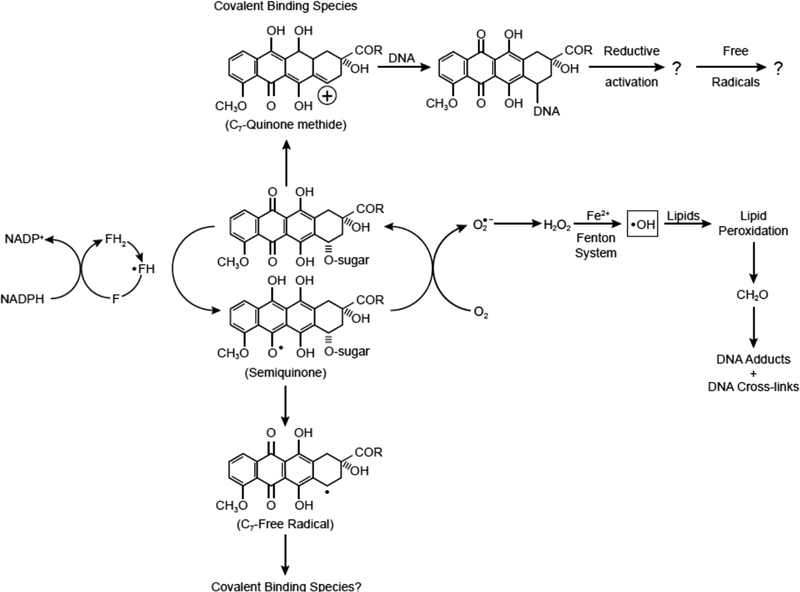
Figure 1:
Proposed formation of alkylating species following enzymatic activation of doxorubicin. Also shown here is the intermediacy of formaldehyde in covalent binding of doxorubicin to DNA and possible redox-cycling of the mono-adduct and generation of hydroxyl radical at the DNA binding site.
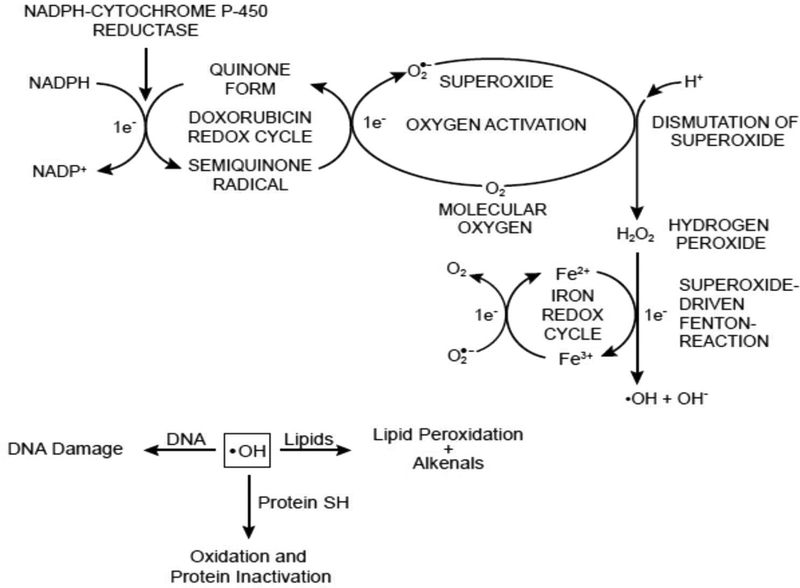
Figure 2:
Formation of reactive oxygen species following reductive activation of doxorubicin and their implications in DNA damage, lipid peroxidation and protein oxidation.
COVALENT BINDING
Enzymatic activation of the anthraquinone moiety of doxorubicin results in the formation of highly reactive alkylating species. Moore [17] proposed that the anticancer drugs mitomycin and anthracyclines can be bioreductively activated to reactive species capable of alkylating cellular macromolecules. We were the first to unequivocally show that doxorubicin and its related analog, daunomycin, alkylate DNA and cellular proteins in vitro and in vivo [18–22]. We proposed that following one-electron reduction of doxorubicin, various reactive intermediates are formed (Figure 1) that could alkylate cellular macromolecules [18, 19]. As both reduced glutathione and ethylxanthate inhibited this covalent binding, we proposed that a quinone methide, formed at C7 of the doxorubicin (Figure 1) molecule, was most likely the reactive species that alkylated DNA and proteins [19, 21]. The formation of quinone methide was proposed to result from a two-electron reduction of the quinone function of the doxorubicin. In addition to the one-electron reductases, DT-diophorase, which reduces quinones via a two-electron reduction, also formed this reactive intermediate [21]. In addition, we proposed a C7 radical intermediate that is also generated from the one-electron reduction intermediate, the doxorubicin semiquinone radical [18, 19, 21]. Various investigators have now confirmed our earlier observations on the covalent binding of doxorubicin and daunomycin with DNA [23–27]. The structure of this adduct has also been elucidated [28].
Doxorubicin-DNA adducts are biologically active and have been shown to form at the gpc sites in DNA in vitro or in tumor cells [23, 29]. More interestingly, these doxorubicin-DNA adducts inhibit binding of the transcription factors and DNA polymerase, which may have significant implications for the mechanism of doxorubicin cytotoxicity for tumor cells [30]. Culeen and Phillips [23] also found that DNA adduct formation was significantly enhanced in the presence of Fe2+ ions and suggested the intermediacy of Fe3+-doxorubicin complexes in DNA-doxorubicin adduct formation. Skladanowski and Konopa [31, 32] have shown that doxorubicin and other related anthracyclines form interstrand DNA crosslinks at physiological concentrations and are found to have a close relationship with growth inhibition in a number of tumor cell lines. It is of interest to note that adducts were found to be active in doxorubicin-resistant cells [32], suggesting that the doxorubicin DNA-adducts, once formed, may not be a substrate for p-glycoprotein.
Koch and his collaborators have reported that formaldehyde was a key intermediate in the formation of certain doxorubicin-DNA adducts [33, 34]; these were found to be specific at the gpc sites of DNA and were similar to those observed by Luce et al. [35]. Koch et al. [33, 36] showed that formaldehyde-doxorubicin-DNA adducts are also formed in breast tumor cells. Formation of formaldehyde required induction of oxidative stress, i.e., reductive activation of doxorubicin in the presence of molecular oxygen and peroxidation of cellular membrane [34, 36]. The most important finding for this type of adduct is that they are formed at pharmacological concentrations of doxorubicin and are cytotoxic to tumor cells [34, 37]. Furthermore, preformed adducts (i.e., synthesized in vitro) are taken up and retained in the nuclei of tumor cells by both sensitive and resistant cells more efficiently than the parent drugs [36, 37]. The significance of covalent binding and cross-link formation of doxorubicin and related drugs in cytotoxicity has been reviewed [26].
FREE RADICAL FORMATION
It is well established that doxorubicin (and most anthracyclines) forms a semiquinone radical following its reductive activation both in vitro and in biological systems (Figure 2) [9–12, 16]. The semiquinone radical is reasonably stable under anaerobic conditions and can be detected by electron spin resonance [38, 39]. However, it reacts rapidly with molecular oxygen, generating superoxide anion, hydrogen peroxide, and ultimately hydroxyl radicals in the presence of transition metal ions [13, 15, 16, 38]. Superoxide and hydroxyl radicals are easily detected with EPR using DMPO as a spin- trapping agent [16, 38]. Free radicals, in particular reactive hydroxyl radical, have been shown to induce DNA damage. In addition, hydroxyl radical initiates significant peroxidation of membrane lipids, forming toxic metabolites that alkylate DNA and proteins [40–42]. However, the role of free radical intermediates in the mechanism of cytotoxicity of doxorubicin remains controversial [40, 43–45]. This controversy is due to several factors: First, doxorubicin is effective in vivo at nanomolar concentration (10−7-10−8 M), while the detection of free radicals requires significantly higher (micromolar) concentrations. Second, the formation of hydroxyl radicals requires the presence of free iron (or iron complexed with doxorubicin), and it is believed that there is very little free Fe3+ in vivo (as most of the iron is bound to proteins)
While these are valid arguments against the role of free radicals in doxorubicin cytotoxicity, they can be easily explained on the basis of recent significant supporting information. Detection of free radical species requires a higher concentration of doxorubicin due to the limited sensitivity of EPR detection. When reactive free radicals are generated in cells and tissues, most of them are rapidly destroyed due to reactions with high concentrations of reduced glutathione and other sulfhydryl compounds present in cells and tissues. There are a number of publications that have shown the formation of doxorubicin-GSH conjugates in cells as well as increased formation of oxidized glutathione following doxorubicin treatment. Moreover, in most cellular systems there are significant amounts of superoxide dismutase, catalase and glutathione peroxidase, which eliminate superoxide, hydrogen peroxide and hydroperoxides, respectively, decreasing the possibility of detecting any free radicals. It is not surprising, then, that depletion of glutathione by BSO in most tumor cells, including doxorubicin-resistant tumor cells, significantly enhances hydroxyl radical formation and doxorubicin cytotoxicity, thus implicating free radicals in the mechanism of doxorubicin antitumor activities [46, 47].
Doxorubicin requires reductive activation to generate free radical species. It is possible that certain tumor cells and tissues may not have sufficient amounts of the cytochrome P450-reductase to activate doxorubicin to the semiquinone radical as we found, for example, in human breast tumor cells selected for doxorubicin resistance [48, 49]. Moreover, the activities of the detoxifying enzymes glutathione peroxidase and glutathione transferase are also significantly increased in these resistant cell lines and in nude mouse xenografts [49, 50]. Consequently, significantly fewer doxorubicin-dependent free radicals are detected [16, 49]. In another study, in tumor cells selected for low levels of resistance to doxorubicin, hydroxyl radical formation was found to be similar to that of the sensitive cells; however, a significant decrease in hydroxyl radical formation was observed in the highly resistant variants [51]. These observations, i.e., detectable levels of free radical formed in doxorubicin-sensitive cells but not in resistant cells, support the notion that free radicals do play a role in drug cytotoxicity. Recent advances in florescence detection have further increased sensitivity for the detection of H2O2 in tumor cells and tissues, and now one can easily detect H2O2 formed from sub-micromolar concentrations of doxorubicin [52]. However, interference resulting from high florescence quenching by DNA-bound doxorubicin remains a problem.
Further support for the free radical-mediated cytotoxicity of doxorubicin has come from the very interesting work of Oberley and coworkers, who were the first to show that overexpression of MnSOD, which catalyzes the conversion of superoxide anion radical (O2.-) to hydrogen peroxide, inhibits the growth of tumor cells [53]. When combined with doxorubicin or ionizing radiation, MnSOD enhanced tumor cell killing by both doxorubicin and ionizing radiation [54, 55] and was further enhanced by BCNU, an inhibitor of glutathione reductase. These observations clearly indicate that H2O2 formed from superoxide anion radical is the key intermediate for tumor cell killing by doxorubicin and ionizing radiation [56]. Furthermore, Mimnaugh et al. [57] have reported that the doxorubicin-sensitive human breast MCF-7 cells were significantly more sensitive to H2O2 than the corresponding doxorubicin-resistant MCF-7/ADRR cells, implicating a free radical mechanism in doxorubicin cytotoxicity.
It has been reported that intercalated doxorubicin does not undergo reductive activation and generate free radicals, casting more doubts on the role of free radicals in doxorubicin cytotoxicity as most of doxorubicin in tumor cells is bound to DNA. It should be pointed out that the chromophore of doxorubicin remains intact following covalent binding to DNA (as the semiquinone radical does not bind covalently to DNA); thus, DNA-doxorubicin mono-adduct could be reductively activated in tumors and generate free radical species. In this scenario, any hydroxyl radicals generated would be significantly more damaging to cellular DNA, as they would be formed in close proximity to the DNA, leading to cell death. In this regard, Dikalov et al. [58] have reported that doxorubicin covalently bound to small nucleotides is reductively activated and generates hydroxyl radicals in vitro. While formation of doxorubicin covalently bound to small nucleotides needs to be confirmed in tumors and in vivo, it definitely opens the possibility that covalently bound doxorubicin forms free radicals in vivo and contributes to doxorubicin cytotoxicity.
The role of iron in doxorubicin cytotoxicity is complex; however some conclusions can be derived from various observations. While iron concentration in vivo is low, tumors tend to contain significantly higher concentrations of copper and iron metal ions [59, 60]. Research has shown that cell membranes are not permeable to Fe chelated with doxorubicin; thus, the complex cannot generate or reduce peroxide inside tumor cells (near DNA) to form free radical species and induce cell death. However, it is well known that Fe ions are constantly released from dying cells; these Fe ions could chelate free doxorubicin present in tumor cells and cause free radical-mediated toxicity. Furthermore, the semiquinone radical of doxorubicin has been shown to induce release of iron from ferritin under anaerobic conditions [61], suggesting that doxorubicin-Fe complexes can be formed in tumors and lead to the formation of free radical species.
Further support for doxorubicin free radical-mediated tumor cell kill comes from our recent studies (Sinha et al.; unpublished observations) where topo II catalytic and cleavage activities were inhibited by nitric oxide treatment. In spite of significant inhibition of topo II functions, the cytotoxicity of doxorubicin was not compromised in either human breast MCF-7 or human colon HT-29 cells. In contrast, under identical conditions of nitric oxide treatment, the cytotoxicity and DNA cleavage of VP-16 was significantly reduced in both cell lines. This would suggest an alternate mechanism for doxorubicin, not related to topo II, must be operative and most likely a free radical-dependent mechanism for tumor cell kill by doxorubicin.
ETOPOSIDE (VP-16,213)
VP-16 and its congener, VM-26 (Figure 3), semisynthetic derivatives of epipodophyllotoxin, are active against a wide variety of tumors, most significantly against lymphoma and testicular tumors [62]. While the molecular mechanism of the drug action is not clear, VP-16 induces topo II-mediated single- and double-stranded DNA breaks in tumor cells [4, 5, 63]. It is believed that this VP-16-induced DNA damage ultimately causes cell death. Unlike doxorubicin, VP-16 does not appear to intercalate into DNA. Like doxorubicin, however, VP-16 and VM-26 undergo facile metabolism by a number of enzymatic systems in vitro and in vivo to form reactive products. We were one of the first groups to show that VP-16 is o-demethylated by cytochrome P450, horseradish peroxidase and tyrosinase to o-dihydroxy VP-16 (Figure 3) and to highly reactive o-quinone-VP-16 [64–67]. A number of other laboratories have also confirmed these observations [68, 69]. Formation of these intermediates required one-electron oxidation of the 4-hydroxyl group of VP-16 to form the phenoxyl radical of VP-16, which can be easily detected by EPR [14]. Biologically, it is significant to note that the formation of these metabolites required the presence of the 4’-OH in VP-16, which is also required for the antitumor activity of VP-16, as the o-methylated VP-16 is inactive and does not interact with topo II to induce DNA cleavage [70].
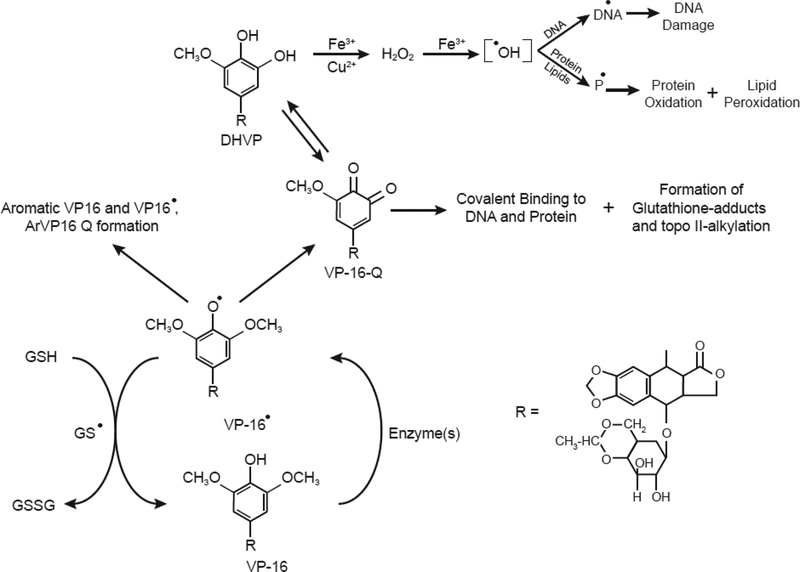
Figure 3:
One-electron oxidation of VP-16 and formation of VP-16 phenoxy radical, o-quinone, and dihydroxy-VP-16. The reactive o-quinone derivative alkylates DNA and proteins, including topoisomerase II. The dihydroxy-VP-16 chelates metal ions and generates reactive oxygen species which induce DNA damage and protein oxidation [71, 72].
Studies from our laboratory have shown that the DHVP and the VP-16-Q derivatives are less cytotoxic to MCF-7 cells and induce less topo II-mediated DNA cleavage than VP-16 [70]. However, when formed in cells or in vivo, the antitumor activities of these metabolites are not known at this time, and the difference observed in MCF-7 cells may simply be due to their high polarity compared to VP-16. Thus, it is possible that these derivatives are not taken up equally by cells or may not reach their critical cellular target(s). In cell-free systems, we have shown that DHVP rapidly chelates both copper and iron ions and, in the presence of H2O2, generates reactive hydroxyl radicals [71, 72]. Hydroxyl radicals formed from DHVP-metal complexes induced significant damage in DNA. Additionally, the DHVP derivative undergoes autoxidation in vitro to produce H2O2 and hydroxyl radical. The rate of hydroxyl radical formation was significantly enhanced in the presence of metal ions or at high pH [14]. In contrast to DHVP, the reactive o-quinone-VP-16 rapidly bound to cellular proteins and DNA [65, 66] and formed glutathione conjugates in cells and in vivo [73]. It has been shown that the o-quinone metabolite also alkylates topo II and inhibits its functions [74–76]. The role of VP-16-Q in drug toxicity is the subject of intense research [74, 76]. It has been suggested that secondary leukemia associated with VP-16 treatment is due to metabolism of VP-16 to the DHVP and VP-16-Q metabolites by cytochrome P-450 CYP3A4, as there was a strong correlation with patients with a polymorphism in the 5’promoter region of CYP3A gene (CYP3A4-W) and secondary leukemia [77, 78]. However, in another study of patients with treatment-related acute leukemia, Reling et al. [79] found no significant association with VP-16 metabolisms.
VP-16 induces oxidation of glutathione to GSSG both in vitro and in vivo. It has been shown that this oxidation results from the reaction of the phenoxy radical of VP-16 with glutathione, forming glutathionyl radical [73]. There are a number of important implications for these observations: (a) cells become vulnerable to oxidative stress due to increased depletion of GSH, which could result in lipid peroxidation and/or oxidation of cellular enzymes critical for cell survival, and (b) lipid peroxidation products (e.g., aldehydes) may alkylate DNA, causing inhibition of DNA synthesis and ultimately cell death. While VP-16 is an inhibitor of lipid peroxidation [80], the DHVP metabolite could induce lipid peroxidation in the presence of free copper or iron ions. This oxidative stress/glutathione depletion caused by the VP-16 radical or VP-16 metabolites may be the basis for the synergistic interactions observed with VP-16 and ionizing radiation or photosensitizers in tumor cells and in the clinic [81, 82].
Metabolism of VP-16 to its dihydroxy and o-quinone derivatives clearly has significant implications for VP-16-dependent toxicity and the induction of secondary leukemia in patients undergoing VP-16-based chemotherapy. However, the role of these metabolites in the cytotoxicity of VP-16 is not understood. While it is true that the metabolites are also capable of forming a topo II-DNA-drug complex, the decreased antitumor activities in tumor cells remains poorly resolved. The VP-16-Q, in vitro, is active against topo II, induces significant DNA cleavage, and binds irreversibly to topo II; it is thus anticipated to be as active as the parent drug. Due to poor uptake and non-specific binding, the antitumor activities of these metabolites cannot be accurately determined in tumor cells. However, it is very reasonable to speculate that if these metabolites are formed near the target, e.g., DNA in nuclei, they may be more active than VP-16. Since it is possible to modulate the metabolic profile of CYP3A-mediated metabolism of VP-16 by using specific inhibitor or stimulators, the antitumor activity and/or toxicity of VP-16 can be studied and the roles of metabolic activation in VP-16 cytotoxicity can be further defined.
CONCLUSIONS
It is quite clear from various published data that the mechanisms of cytotoxicity of both doxorubicin and VP-16 in tumor cells and in vivo are not clear, but certain conclusions can be made. Doxorubicin is indeed metabolized by various enzymes in tumors and in vivo, resulting in the formation of the reactive semiquinone radical. Under hypoxic or reduced O2 concentrations, the semiquinone radical is stable and then decomposes to form very reactive species that covalently bind to DNA and proteins. Irrespective of which reactive species are formed (a quinone methide or C7 free radical or species derived from formaldehyde), covalent binding and cross-linking with cellular DNA will cause a myriad of unwanted biological stresses in tumor cells, ultimately causing cell death. This mechanism of tumor cell killing is independent of O2, and as most tumor cells (except for hematologically derived tumors and lung tumors) are hypoxic in nature, the possibility of formation of the covalent binding species is significantly higher. Because doxorubicin accumulates in cellular nuclei and mitochondria due to its equilibrium with doxorubicin bound to DNA, the concentrations of free doxorubicin will be highest in nuclei and in mitochondria, and it will thus be more likely to undergo reductive activation in these cellular compartments. However, bioactivation of the drug would be rate limiting based on the presence or absence of the activating enzymes. Similar situations are also feasible under aerobic conditions; however, the formation of covalently binding species would be significantly limited due to competing reactions of the semiquinone radicals with O2. At high O2 concentrations, a pathway involving oxygen-derived free radical formation (O2.- and .OH) would dominate. However, formaldehyde- or alkenal-derived covalent binding of doxorubicin with DNA is also possible. While DNA-bound doxorubicin is not bioactivated in vitro, free radical species are detected in tumor cells. It should be emphasized that incubations for the detection of free radicals using EPR methods are carried out at micromolar concentrations for a very brief drug exposure time, while the cytotoxicity studies in tumor cells are carried out for days. It is not known at this time whether free radicals are continuously generated in tumor cells at low concentrations of doxorubicin, causing tumor cell death.
Thus, cellular bioactivation is an important mechanism for tumor cell killing by doxorubicin and certainly would depend upon cell type and O2 concentrations. It is suggested that because tumor cell death is caused by doxorubicin at low concentrations, other mechanisms do not contribute significantly; thus, only one mechanism, the topo II-based mechanism, is important for doxorubicin. Furthermore, because oxygen-derived free radical formation requires the presence of O2, and in highly hypoxic cells there is very little O2, it is concluded that these free radicals are not formed. It should be made very clear here that under highly hypoxic conditions, there is little to no tumor cell killing by most drugs, including doxorubicin, irrespective of mechanism. Furthermore, when cells are not dividing, the concentration of topo II is low as topo II is completely cell-cycle dependent, and at low topo II protein levels, doxorubicin will become inactive or tumors will show resistance to it.
The mechanism of tumor cell killing by VP-16 is certainly not as complicated as that of doxorubicin. Unless clear evidence is presented in the future in regard to activation of VP-16 to achieve cell death, the main mechanism remains one that is dependent upon its interactions with topo II and inducing DNA damage. While bioactivation of VP-16 to its dihydroxy and o-quinone derivatives is important, they are also topo II-active and quite possibly contribute to tumor cell killing. With recent observations, it appears that the bioactivation of VP-16 to the o-quinone derivative may contribute towards the known VP-16-induced secondary leukemia formation in patients undergoing VP-16-based chemotherapy.
Acknowledgements:
We thank Dr. Ann Motten and Mrs. Mary Mason for their invaluable help in editing the manuscript. We also thank Drs. Maria Kadiiska and Thomas Van’t Erve for their critical evaluation of the manuscript.
Funding: This research was supported [in part] by the intramural research program of the National Institute of Environmental Health Sciences, NIH. Statements contained herein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the US Government.
References
- [1].Weiss RB, The anthracyclines: will we ever find a better doxorubicin?, Seminars in oncology, 19 (1992) 670–686. [PubMed] [Google Scholar]
- [2].Long BH, Musial ST, Brattain MG, Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16–213 and VM26: a quantitative structure-activity relationship, Biochemistry, 23 (1984) 1183–1188. [DOI] [PubMed] [Google Scholar]
- [3].Nitiss JL, Targeting DNA topoisomerase II in cancer chemotherapy, Nature reviews. Cancer, 9 (2009) 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang JC, DNA topoisomerases, Annual review of biochemistry, 54 (1985) 665–697. [DOI] [PubMed] [Google Scholar]
- [5].Froelich-Ammon SJ, Osheroff N, Topoisomerase poisons: harnessing the dark side of enzyme mechanism, The Journal of biological chemistry, 270 (1995) 21429–21432. [DOI] [PubMed] [Google Scholar]
- [6].Nitiss JL, DNA topoisomerase II and its growing repertoire of biological functions, Nature reviews. Cancer, 9 (2009) 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Carter SK, Adriamycin-a review, Journal of the National Cancer Institute, 55 (1975) 1265–1274. [DOI] [PubMed] [Google Scholar]
- [8].Wiernik PH, Dutcher JP, Clinical importance of anthracyclines in the treatment of acute myeloid leukemia, Leukemia, 6 Suppl 1 (1992) 67–69. [PubMed] [Google Scholar]
- [9].Bachur NR, Gordon SL, Gee MV, Anthracycline antibiotic augmentation of microsomal electron transport and free radical formation, Molecular pharmacology, 13 (1977) 901–910. [PubMed] [Google Scholar]
- [10].Bachur NR, Gordon SL, Gee MV, Kon H, NADPH cytochrome P-450 reductase activation of quinone anticancer agents to free radicals, Proceedings of the National Academy of Sciences of the United States of America, 76 (1979) 954–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kalyanaraman B, Morehouse KM, Mason RP, An electron paramagnetic resonance study of the interactions between the adriamycin semiquinone, hydrogen peroxide, iron-chelators, and radical scavengers, Archives of biochemistry and biophysics, 286 (1991) 164–170. [DOI] [PubMed] [Google Scholar]
- [12].Sato S, Iwaizumi M, Handa K, Tamura Y, Electron spin resonance study on the mode of generation of free radicals of daunomycin, adriamycin, and carboquone in NAD(P)H-microsome system, Gan, 68 (1977) 603–608. [PubMed] [Google Scholar]
- [13].Doroshow JH, Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase, Cancer research, 43 (1983) 4543–4551. [PubMed] [Google Scholar]
- [14].Kalyanaraman B, Nemec J, Sinha BK, Characterization of free radicals produced during oxidation of etoposide (VP-16) and its catechol and quinone derivatives. An EPR Study, Biochemistry, 28 (1989) 4839–4846. [DOI] [PubMed] [Google Scholar]
- [15].Sinha BK, Katki AG, Batist G, Cowan KH, Myers CE, Adriamycin-stimulated hydroxyl radical formation in human breast tumor cells, Biochemical pharmacology, 36 (1987) 793–796. [DOI] [PubMed] [Google Scholar]
- [16].Sinha BK, Katki AG, Batist G, Cowan KH, Myers CE, Differential formation of hydroxyl radicals by adriamycin in sensitive and resistant MCF-7 human breast tumor cells: implications for the mechanism of action, Biochemistry, 26 (1987) 3776–3781. [DOI] [PubMed] [Google Scholar]
- [17].Moore HW, Bioactivation as a model for drug design bioreductive alkylation, Science, 197 (1977) 527–532. [DOI] [PubMed] [Google Scholar]
- [18].Sinha BK, Chignell CF, Binding mode of chemically activated semiquinone free radicals from quinone anticancer agents to DNA, Chemico-biological interactions, 28 (1979) 301–308. [DOI] [PubMed] [Google Scholar]
- [19].Sinha BK, Binding specificity of chemically and enzymatically activated anthracycline anticancer agents to nucleic acids, Chemico-biological interactions, 30 (1980) 67–77. [DOI] [PubMed] [Google Scholar]
- [20].Sinha BK, Sik RH, Binding of [14C]-adriamycin to cellular macromolecules in vivo, Biochemical pharmacology, 29 (1980) 1867–1868. [DOI] [PubMed] [Google Scholar]
- [21].Sinha BK, Gregory JL, Role of one-electron and two-electron reduction products of adriamycin and daunomycin in deoxyribonucleic acid binding, Biochemical pharmacology, 30 (1981) 2626–2629. [DOI] [PubMed] [Google Scholar]
- [22].Sinha BK, Trush MA, Kennedy KA, Mimnaugh EG, Enzymatic activation and binding of adriamycin to nuclear DNA, Cancer research, 44 (1984) 2892–2896. [PubMed] [Google Scholar]
- [23].Cullinane C, Phillips DR, Induction of stable transcriptional blockage sites by adriamycin: GpC specificity of apparent adriamycin-DNA adducts and dependence on iron(III) ions, Biochemistry, 29 (1990) 5638–5646. [DOI] [PubMed] [Google Scholar]
- [24].Cullinane C, van Rosmalen A, Phillips DR, Does adriamycin induce interstrand cross-links in DNA?, Biochemistry, 33 (1994) 4632–4638. [DOI] [PubMed] [Google Scholar]
- [25].Cullinane C, Cutts SM, Panousis C, Phillips DR, Interstrand cross-linking by adriamycin in nuclear and mitochondrial DNA of MCF-7 cells, Nucleic acids research, 28 (2000) 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cutts SM, Nudelman A, Rephaeli A, Phillips DR, The power and potential of doxorubicin-DNA adducts, IUBMB life, 57 (2005) 73–81. [DOI] [PubMed] [Google Scholar]
- [27].van Rosmalen A, Cullinane C, Cutts SM, Phillips DR, Stability of adriamycin-induced DNA adducts and interstrand crosslinks, Nucleic acids research, 23 (1995) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zeman SM, Phillips DR, Crothers DM, Characterization of covalent adriamycin-DNA adducts, Proceedings of the National Academy of Sciences of the United States of America, 95 (1998) 11561–11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cutts SM, Swift LP, Rephaeli A, Nudelman A, Phillips DR, Sequence specificity of adriamycin-DNA adducts in human tumor cells, Molecular cancer therapeutics, 2 (2003) 661–670. [PubMed] [Google Scholar]
- [30].Cutts SM, Parsons PG, Sturm RA, Phillips DR, Adriamycin-induced DNA adducts inhibit the DNA interactions of transcription factors and RNA polymerase, The Journal of biological chemistry, 271 (1996) 5422–5429. [DOI] [PubMed] [Google Scholar]
- [31].Skladanowski A, Konopa J, Relevance of interstrand DNA crosslinking induced by anthracyclines for their biological activity, Biochemical pharmacology, 47 (1994) 2279–2287. [DOI] [PubMed] [Google Scholar]
- [32].Skladanowski A, Konopa J, Interstrand DNA crosslinking induced by anthracyclines in tumour cells, Biochemical pharmacology, 47 (1994) 2269–2278. [DOI] [PubMed] [Google Scholar]
- [33].Taatjes DJ, Gaudiano G, Resing K, Koch TH, Alkylation of DNA by the anthracycline, antitumor drugs adriamycin and daunomycin, Journal of medicinal chemistry, 39 (1996) 4135–4138. [DOI] [PubMed] [Google Scholar]
- [34].Taatjes DJ, Gaudiano G, Resing K, Koch TH, Redox pathway leading to the alkylation of DNA by the anthracycline, antitumor drugs adriamycin and daunomycin, Journal of medicinal chemistry, 40 (1997) 1276–1286. [DOI] [PubMed] [Google Scholar]
- [35].Luce RA, Sigurdsson ST, Hopkins PB, Quantification of formaldehyde-mediated covalent adducts of adriamycin with DNA, Biochemistry, 38 (1999) 8682–8690. [DOI] [PubMed] [Google Scholar]
- [36].Taatjes DJ, Fenick DJ, Koch TH, Nuclear targeting and nuclear retention of anthracycline-formaldehyde conjugates implicates DNA covalent bonding in the cytotoxic mechanism of anthracyclines, Chemical research in toxicology, 12 (1999) 588–596. [DOI] [PubMed] [Google Scholar]
- [37].Taatjes DJ, Koch TH, Growth inhibition, nuclear uptake, and retention of anthracycline-formaldehyde conjugates in prostate cancer cells relative to clinical anthracyclines, Anticancer research, 19 (1999) 1201–1208. [PubMed] [Google Scholar]
- [38].Kalyanaraman B, Perez-Reyes E, Mason RP, Spin-trapping and direct electron spin resonance investigations of the redox metabolism of quinone anticancer drugs, Biochimica et biophysica acta, 630 (1980) 119–130. [DOI] [PubMed] [Google Scholar]
- [39].Schreiber J, Mottley C, Sinha BK, Kalyanaraman B, Mason RP, One-electron reduction of daunomycin, daunomycinone, and 7-deoxydaunomycine by the xanthine xanthine-oxidase system - Detection of semiquinone free-radicals by electron-spin-resonance, J. Am. Chem. Soc, 109 (1987) 348–351. [Google Scholar]
- [40].Myers CE, Mimnaugh EG, Yeh GC, Sinha BK, Biochemical Mechanisms of Tumor Cell Kill by the Anthracyclines, in: Lown JW (Ed.) Anthracycline and Anthracenedione-based Anticancer Agents, Elsevier, New York, 1988, pp. 527–569. [Google Scholar]
- [41].Sinha BK, Free radicals in anticancer drug pharmacology, Chemico-biological interactions, 69 (1989) 293–317. [DOI] [PubMed] [Google Scholar]
- [42].Sinha BK, Mimnaugh EG, Free radicals and anticancer drug resistance: oxygen free radicals in the mechanisms of drug cytotoxicity and resistance by certain tumors, Free radical biology & medicine, 8 (1990) 567–581. [DOI] [PubMed] [Google Scholar]
- [43].Doroshow JH, Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones, Proceedings of the National Academy of Sciences of the United States of America, 83 (1986) 4514–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H, Doxorubicin (adriamycin): a critical review of free radical-dependent mechanisms of cytotoxicity, Pharmacology & therapeutics, 47 (1990) 219–231. [DOI] [PubMed] [Google Scholar]
- [45].Gewirtz DA, A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin, Biochemical pharmacology, 57 (1999) 727–741. [DOI] [PubMed] [Google Scholar]
- [46].Kramer RA, Zakher J, Kim G, Role of the glutathione redox cycle in acquired and de novo multidrug resistance, Science, 241 (1988) 694–697. [DOI] [PubMed] [Google Scholar]
- [47].Dusre L, Mimnaugh EG, Myers CE, Sinha BK, Potentiation of doxorubicin cytotoxicity by buthionine sulfoximine in multidrug-resistant human breast tumor cells, Cancer research, 49 (1989) 511–515. [PubMed] [Google Scholar]
- [48].Cowan KH, Batist G, Tulpule A, Sinha BK, Myers CE, Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats, Proceedings of the National Academy of Sciences of the United States of America, 83 (1986) 9328–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mimnaugh EG, Fairchild CR, Fruehauf JP, Sinha BK, Biochemical and pharmacological characterization of MCF-7 drug-sensitive and AdrR multidrug-resistant human breast tumor xenografts in athymic nude mice, Biochemical pharmacology, 42 (1991) 391–402. [DOI] [PubMed] [Google Scholar]
- [50].Batist G, Tulpule A, Sinha BK, Katki AG, Myers CE, Cowan KH, Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells, The Journal of biological chemistry, 261 (1986) 15544–15549. [PubMed] [Google Scholar]
- [51].Benchekroun MN, Sinha BK, Robert J, Doxorubicin-induced oxygen free radical formation in sensitive and doxorubicin-resistant variants of rat glioblastoma cell lines [corrected and republished erratum originally printed in FEBS Lett 1993 May 17;322(3):295–8], FEBS letters, 326 (1993) 302–305. [DOI] [PubMed] [Google Scholar]
- [52].Ubezio P, Civoli F, Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells, Free radical biology & medicine, 16 (1994) 509–516. [DOI] [PubMed] [Google Scholar]
- [53].Weydert CJ, Waugh TA, Ritchie JM, Iyer KS, Smith JL, Li L, Spitz DR, Oberley LW, Overexpression of manganese or copper-zinc superoxide dismutase inhibits breast cancer growth, Free radical biology & medicine, 41 (2006) 226–237. [DOI] [PubMed] [Google Scholar]
- [54].Sun W, Kalen AL, Smith BJ, Cullen JJ, Oberley LW, Enhancing the antitumor activity of adriamycin and ionizing radiation, Cancer research, 69 (2009) 4294–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sun WG, Weydert CJ, Zhang Y, Yu L, Liu J, Spitz DR, Cullen JJ, Oberley LW, Superoxide Enhances the Antitumor Combination of AdMnSOD Plus BCNU in Breast Cancer, Cancers, 2 (2010) 68–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Oberley LW, Mechanism of the tumor suppressive effect of MnSOD overexpression, Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 59 (2005) 143–148. [DOI] [PubMed] [Google Scholar]
- [57].Mimnaugh EG, Dusre L, Atwell J, Myers CE, Differential oxygen radical susceptibility of adriamycin-sensitive and -resistant MCF-7 human breast tumor cells, Cancer research, 49 (1989) 8–15. [PubMed] [Google Scholar]
- [58].Dikalov SI, Rumyantseva GV, Weiner LM, Sergejev DS, Frolova EI, Godovikova TS, Zarytova VF, Hydroxyl radical generation by oligonucleotide derivatives of anthracycline antibiotic and synthetic quinone, Chemico-biological interactions, 77 (1991) 325–339. [DOI] [PubMed] [Google Scholar]
- [59].Cohen Y, Epelbaum R, Haim N, McShan D, Zinder O, The value of serum copper levels in non-Hodgkin’s lymphoma, Cancer, 53 (1984) 296–300. [DOI] [PubMed] [Google Scholar]
- [60].Carpentieri U, Myers J, Thorpe L, Daeschner CW 3rd, Haggard ME, Copper, zinc, and iron in normal and leukemic lymphocytes from children, Cancer research, 46 (1986) 981–984. [PubMed] [Google Scholar]
- [61].Monteiro HP, Vile GF, Winterbourn CC, Release of iron from ferritin by semiquinone, anthracycline, bipyridyl, and nitroaromatic radicals, Free radical biology & medicine, 6 (1989) 587–591. [DOI] [PubMed] [Google Scholar]
- [62].O’Dwyer PJ, Leyland-Jones B, Alonso MT, Marsoni S, Wittes RE, Etoposide (VP-16–213). Current status of an active anticancer drug, The New England journal of medicine, 312 (1985) 692–700. [DOI] [PubMed] [Google Scholar]
- [63].Sinha BK, Topoisomerase inhibitors. A review of their therapeutic potential in cancer, Drugs, 49 (1995) 11–19. [DOI] [PubMed] [Google Scholar]
- [64].Haim N, Roman J, Nemec J, Sinha BK, Peroxidative free radical formation and O-demethylation of etoposide(VP-16) and teniposide(VM-26), Biochemical and biophysical research communications, 135 (1986) 215–220. [DOI] [PubMed] [Google Scholar]
- [65].Haim N, Nemec J, Roman J, Sinha BK, Peroxidase-catalyzed metabolism of etoposide (VP-16–213) and covalent binding of reactive intermediates to cellular macromolecules, Cancer research, 47 (1987) 5835–5840. [PubMed] [Google Scholar]
- [66].Haim N, Nemec J, Roman J, Sinha BK, In vitro metabolism of etoposide (VP-16–213) by liver microsomes and irreversible binding of reactive intermediates to microsomal proteins, Biochemical pharmacology, 36 (1987) 527–536. [DOI] [PubMed] [Google Scholar]
- [67].Usui N, Sinha BK, Tyrosinase-induced free radical formation from VP-16,213: relationship to cytotoxicity, Free radical research communications, 10 (1990) 287–293. [DOI] [PubMed] [Google Scholar]
- [68].van Maanen JM, de Vries J, Pappie D, van den Akker E, Lafleur VM, Retel J, van der Greef J, Pinedo HM, Cytochrome P-450-mediated O-demethylation: a route in the metabolic activation of etoposide (VP-16–213), Cancer research, 47 (1987) 4658–4662. [PubMed] [Google Scholar]
- [69].Fan Y, Schreiber EM, Giorgianni A, Yalowich JC, Day BW, Myeloperoxidase-catalyzed metabolism of etoposide to its quinone and glutathione adduct forms in HL60 cells, Chemical research in toxicology, 19 (2006) 937–943. [DOI] [PubMed] [Google Scholar]
- [70].Sinha BK, Politi PM, Eliot HM, Kerrigan D, Pommier Y, Structure-activity relations, cytotoxicity and topoisomerase II dependent cleavage induced by pendulum ring analogues of etoposide, European journal of cancer, 26 (1990) 590–593. [DOI] [PubMed] [Google Scholar]
- [71].Sinha BK, Antholine WM, Kalyanaraman B, Eliot HM, Copper ion-dependent oxy-radical mediated DNA damage from dihydroxy derivative of etoposide, Biochimica et biophysica acta, 1096 (1990) 81–83. [DOI] [PubMed] [Google Scholar]
- [72].Sinha BK, Eliot HM, Kalayanaraman B, Iron-dependent hydroxyl radical formation and DNA damage from a novel metabolite of the clinically active antitumor drug VP-16, FEBS letters, 227 (1988) 240–244. [DOI] [PubMed] [Google Scholar]
- [73].Katki AG, Kalyanaraman B, Sinha BK, Interactions of the antitumor drug, etoposide, with reduced thiols in vitro and in vivo, Chemico-biological interactions, 62 (1987) 237–247. [DOI] [PubMed] [Google Scholar]
- [74].Bender RP, Ham AJ, Osheroff N, Quinone-induced enhancement of DNA cleavage by human topoisomerase IIalpha: adduction of cysteine residues 392 and 405, Biochemistry, 46 (2007) 2856–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Jacob DA, Mercer SL, Osheroff N, Deweese JE, Etoposide quinone is a redox-dependent topoisomerase II poison, Biochemistry, 50 (2011) 5660–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Smith NA, Byl JA, Mercer SL, Deweese JE, Osheroff N, Etoposide quinone is a covalent poison of human topoisomerase IIbeta, Biochemistry, 53 (2014) 3229–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pendleton M, Lindsey RH Jr., Felix CA, Grimwade D, Osheroff N, Topoisomerase II and leukemia, Annals of the New York Academy of Sciences, 1310 (2014) 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ezoe S, Secondary leukemia associated with the anti-cancer agent, etoposide, a topoisomerase II inhibitor, International journal of environmental research and public health, 9 (2012) 2444–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, Pui CH, Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia, Leukemia, 12 (1998) 346–352. [DOI] [PubMed] [Google Scholar]
- [80].Sinha BK, Trush MA, Kalyanaraman B, Microsomal interactions and inhibition of lipid peroxidation by etoposide (VP-16, 213): implications for mode of action, Biochemical pharmacology, 34 (1985) 2036–2040. [DOI] [PubMed] [Google Scholar]
- [81].Haddock MG, Ames MM, Bonner JA, Assessing the interaction of irradiation with etoposide or idarubicin, Mayo Clinic proceedings, 70 (1995) 1053–1060. [DOI] [PubMed] [Google Scholar]
- [82].Gantchev TG, Brasseur N, van Lier JE, Combination toxicity of etoposide (VP-16) and photosensitisation with a water-soluble aluminium phthalocyanine in K562 human leukaemic cells, British journal of cancer, 74 (1996) 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]