Abstract
Hydrazine derivatives are environmental and food pollutants but are also important because of their use in medicine for the treatment of tuberculosis and cancer. However, hydrazines also pose significant health risks to humans as they are mutagenic and carcinogenic. This review examines various metabolic pathways (enzymatic and non-enzymatic) of hydrazines for the formation of reactive species that bind to cellular macromolecules and lead to cellular dysfunction. It is believed that this biotransformation is responsible for the pharmacology and pathophysiology of hydrazine derivatives.
INTRODUCTION
Many chemicals, either man-made or found in nature, undergo metabolic activation to exert their biological effects. Some of these effects are considered desirable because they are curative, e.g., affect a disease state and lead to a cure. However, there are many chemicals that induce significant undesirable effects (or toxicity) as a result of this metabolic biotransformation, resulting in severe organ toxicity, tumor formation, and in some cases, death [1]. Hydrazine and its derivatives, which are used as high energy rocket fuel, induce a variety of toxic insults, including hypoglycemia, disorders of the CNS, induction of systemic lupus erythematosus, and cancer [2–5]. Hydrazines are also found in tobacco and in edible mushrooms. Isoniazid and iproniazid, monoamine oxidase inhibitors, are in use for the treatment of tuberculosis and, until recently, as an antidepressant, respectively [6, 7]. Hydralazine is a potent arterial vasodilator and plays an important role in the management of hypertension and congestive heart failure [8]. Hydralazine is toxic and induces DNA damage, causes severe forms of systemic lupus erythematosus and has been shown to increase the incidence of lung tumors in mice [5, 9, 10]. Procarbazine is a chemotherapeutic agent used in the treatment of Hodgkin’s disease, malignant melanoma and brain tumors in children [11].
Because of the significance of hydrazine derivatives as environmental pollutants and food contaminants as well as their utility in medicine, significant research has been carried out to elucidate the mechanisms of toxicity of these compounds [2–13]. It has been suggested that metabolic activation of hydrazines leads to their toxicity, and various non-enzymatic and enzymatic systems have been identified [6, 7, 14–17]. Hydrazines undergo acetylation by N-acetyl transferase, in which an acetyl group is transferred from acetyl coenzyme A; the rate of acetylation of hydrazines can be fast or slow depending upon the concentrations of the enzyme and an individual’s phenotype [18]. People who are fast acetylators rapidly convert hydrazine to its acetyl form, thus either increasing or decreasing toxicity depending upon further metabolism of acetylhydrazine to reactive species [6, 7]. Furthermore, cytochrome P450 isozymes (1A1, 1A2, 2B1 and 2E2) have been shown to oxidize hydrazines to toxic intermediates that bind to cellular macromolecules [6, 7, 19]. This review discusses the various aspects of metabolic activations of certain hydrazines, formation of reactive intermediates (carbocations and free radicals), and their roles in in vivo toxicity.
HYDRALAZINE:
Hydralazine, a vasodilator, is one of the most interesting hydrazines in current use in medicine. It is an important drug for the management of high blood pressure and recently has garnered a significant amount of interest for the treatment of cancers, as hydralazine inhibits DNA methyltransferase 1 by inhibiting transfer of a methyl group to DNA in several cancer-silencing/tumor suppressor genes [9, 20, 21]. Hydralazine has also been found to inhibit iron-containing prolyl hydroxylase enzymes, which are important for the induction of hypoxia-induced factor (HIFα) and vascular endothelial growth factor [22]. HIFα is also a critical target in cancer chemotherapy as it is believed to be involved in tumor progression [22]. However, the use of hydralazine in the clinic has been implicated in the development of severe forms of systemic lupus erythematosus in patients who have a slow acetylator-phenotype. Furthermore, hydralazine causes DNA damage, and has been reported to induce some incidence of lung tumors in mice [5, 8].
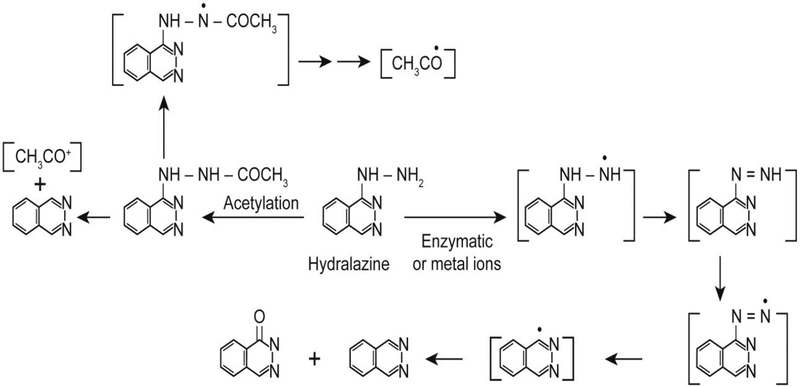
Hydralazine undergoes one-electron oxidation both by metal ions (Cu2+ and Fe3+ ions) and enzymatically (horseradish peroxidase and prostaglandin synthase) to form hydralazyl radical [14–16] which then further decomposes to form various products or reacts with molecular oxygen to generate reactive oxygen-centered radicals (Figure- 1). Hydralazine also has been shown to form DNA radicals following its metabolism in the presence of metal ions [23, 24]. It has been reported by various investigators that oxygen-centered radicals cause DNA strand cleavage and induce oxidative stress [23–29]. Hydralazine has been shown to be a direct-acting mutagen, and the mutagenicity was not increased by inclusions of microsomes or S9-fractions, indicating that metabolic activation was not required for its mutagenicity [8, 30–31]. Direct genotoxicity of hydralazine has also been confirmed in some bacterial systems [30, 31]. However, it is important to point out that if precautions to remove contaminating Fe and Cu ions are not taken, metabolic activation of hydralazine to reactive species may have occurred.
Figure 1:
Structure of hydralazine and formation of various reactive metabolites, catalyzed either by metal ions or enzymes.
The etiology and the mechanisms of hydralazine-induced lupus formation are of significant interest. While various mechanisms have been proposed, they remain poorly understood. It has been suggested that the metabolism of hydralazine may be involved in the induction of lupus as the slow-acetylator phenotype is more at risk than the fast-acetylator [32, 33]. It is reasonable, then, that more hydralazine is available for metabolism in slow acetylators. Reactive species formed from hydralazine that covalently bind to protein have been detected during microsomal metabolism of hydralazine [33]. Formation of phthalazinone (Figure-1) from hydralazine has been implicated in the induction of lupus, and it has been suggested that metabolic differences between slow and fast acetylators may be responsible for lupus formation [33]. In an in vitro study, Dubroff and Reid [34] have shown that hydralazine binds to thymidine and deoxycytidine, forming various modified nucleosides. It is suggested that these modified bases are antigenic and are responsible for lupus [34]. Hydralazine has been shown to intercalate into DNA [35, 36] and stabilize triplex-DNA in the presence of Mg2+ and sperimidine. Interestingly, this triplex-DNA elicited an immunological response, and patients contained antibodies that reacted with this unusual form of DNA [36]. Therefore, it is suggested that formation of antibodies to such triplex DNA may, in part, be responsible for lupus formation. Hydralazine inhibits DNA methyltransferase I, and it has been postulated that this inhibition of DNA methylation leads to activation of certain gene(s) that induce a lupus type syndrome. However, the identity of the gene(s) is not known at this time [37].
ISONIAZID:
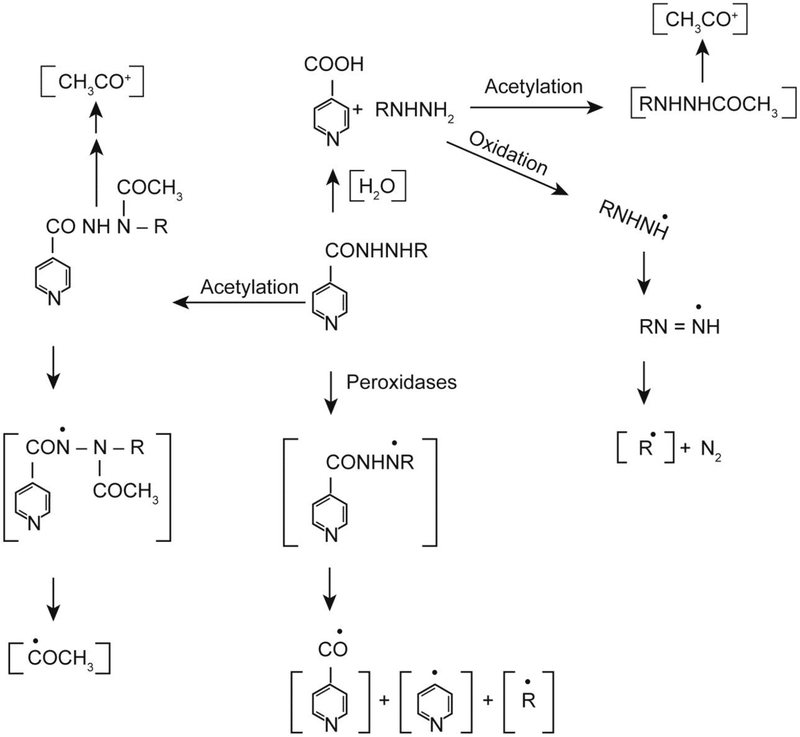
Tuberculosis is a major health problem, especially in poor and underdeveloped countries, and if untreated, tuberculosis remains an important cause of death. Isoniazid is one of the most widely used first-line anti-tuberculosis drugs available today. Unfortunately, isoniazid is also toxic, causing severe hepatotoxicity, and may lead to liver cancer. Metabolism of isoniazid (Figure 2) has been extensively studied, and covalent binding of reactive intermediates to cellular macromolecules has been implicated in its hepatotoxicity [6, 7]. Isoniazid is acetylated in vivo to acetylisoniazid, which is rapidly hydrolyzed to acetylhydrazine. Further metabolism of acetylhydrazine by cytochrome P450 isozymes leads to the formation of reactive acetyl carbocation (CH3CO+), which binds to liver macromolecules [6, 7]. It has been shown that the severity of hepatotoxicity parallels the covalent binding.
Figure 2:
Metabolic activation of isoniazid (R = H) and iproniazid (R = - CH(CH3)2) to various reactive species.
Free radical metabolism, catalyzed by metal ions or myeloperoxidase, also generates reactive intermediates from isoniazid [15, 17]. In this scheme, one-electron oxidation of isoniazid leads to the formation of isoniazidyl radical which can further decompose to form both pyridyl-CO and pyridyl radicals (Figure 2). These radical intermediates are reactive and can readily alkylate proteins and/or initiate lipid peroxidation by the abstraction of a bisallylic hydrogen atom from an unsaturated fatty acid. Furthermore, when acetylhydrazine is formed from the hydrolysis of acetylisoniazid, one-electron oxidation of acetylhydrazine would lead to the generation of the acetyl radical, which has been implicated in covalent binding to cellular macromolecules [6, 7]. Free radical metabolism would occur irrespective of the acetylation status of isoniazid. With slow acetylation, isoniazid would be directly converted to isoniazidyl radical, leading to the formation of reactive species, pyridyl-CO and pyridyl radicals. In contrast, with fast acetylation of isoniazid, acetylhydrazine would be further metabolized either via the free radical pathway or via P450 systems, resulting in reactive intermediates in a tissue/organ specific manner. Free radical pathways would be the main metabolic activation mechanism of isoniazid in tissues/organs rich in peroxidases, and thus, may have implications for the tissue toxicity of isoniazid.
IPRONIAZID:
Iproniazid, a monoamine oxidase inhibitor, was clinically used as an antidepressant. However, it has been withdrawn from clinical use due to its severe hepatotoxicity in humans. Iproniazid is readily hydrolyzed in vivo to isopropylhydrazine (Figure 2), and it has been reported that isopropylhydrazine is rapidly metabolized by cytochrome P450 with the formation of reactive intermediates that covalently bind to proteins [6, 7]. Formation of isopropyl radical has been detected from iproniazid [15], and isopropylhydrazine and isopropyl radical have been identified as the reactive species that covalently bind to cellular proteins [6, 7, 15]. Isopropylhydrazine has also been found to undergo acetylation in vivo and to be further metabolized, resulting in alkylation of proteins [5, 6, 38] (Figure 2).
As discussed for isoniazid, the hydrolysis of iproniazid is not a prerequisite for the formation of isopropyl radical. In the presence of peroxidases and the prostaglandin synthase system, iproniazid is rapidly oxidized to a nitrogen-centered radical (Figure 2), which has been reported to undergo a series of rearrangements to form the isopropyl radical. It is also possible that once formed, acetylisopropylhydrazine could be readily oxidized by peroxidases and prostaglandin synthase to form the carbon-centered radicals CH3CO and isopropyl, both of which could alkylate proteins and lead to toxicity. Thus, in addition to organs and tissues where cytochrome P450-based metabolism of iproniazid is common, organs and tissues rich in peroxidases, e.g., the lung, would be also susceptible to iproniazid toxicity.
PROCARBAZINE:
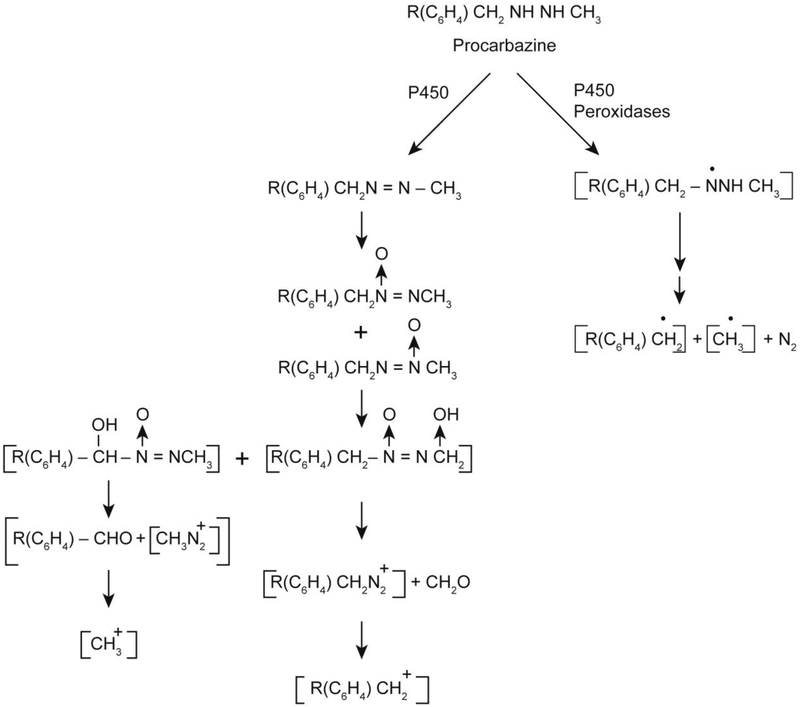
Procarbazine is an anticancer drug used for the treatment of Hodgkin’s lymphoma, malignant melanoma and brain tumors in children [39, 40]. Procarbazine has been shown to be mutagenic in both bacterial and mammalian systems and has been reported to be carcinogenic in mice, rats and monkeys [41, 42]. Procarbazine is a pro-drug and, therefore, requires extensive metabolism for its activity. The metabolism of procarbazine is complex; it has been shown that the methyl group of procarbazine is essential for its antitumor, mutagenic and carcinogenic activities (Figure 3). Procarbazine is rapidly metabolized by cytochrome P450 and monoamine oxidase to its azo derivative and, subsequently, to the azoxy derivative [43, 44] (Figure 3). It has been postulated that the azoxy derivative of procarbazine forms the methyl carbonium ion, which methylates DNA and proteins, inhibiting DNA and protein synthesis for its biological activities [45].
Figure 3:
Metabolic activation of procarbazine to reactive intermediates
Procarbazine is also rapidly oxidized by microsomal P450 systems and peroxidases to free radical species [46]. Using spin-trapping techniques, the formation of various free radical intermediates has been confirmed [46]. The obligatory intermediate for the formation of active methyl and benzyl radicals has been shown to be the formation of a nitrogen-centered radical following one-electron oxidation of procarbazine as shown in Figure 3. The nitrogen-centered radical is postulated to undergo a series of rearrangements to form carbon-centered radicals and nitrogen. The formation of nitrogen has been confirmed during the oxidation of procarbazine.
MISCELLENOUS HYDRAZINES:
Substituted hydrazines are present in the edible mushroom Araricus bisporus. Degradation of Agaritine, N2-[L-(+)-glutamyl]-4- (hydroxymethyl) phenyl hydrazine, the parent hydrazine found in this mushroom, results in the formation of various other substituted hydrazines, e.g., N2-carboxyphenyl hydrazine and p-hydroxymethylphenyl hydrazine derivatives, which are carcinogenic and lead to the formation of tumors in mice [47–50]. A number of studies have clearly shown that these mono-substituted hydrazines undergo extensive metabolism, catalyzed by cytochrome P450, peroxidases and oxyhemoglobin, to free radical species, resulting in covalent binding with proteins and DNA [51–53]. However, there remains some debate in the literature, as substituted hydrazines also form alkyldiazonium ions (Figure 3), which are known to directly alkylate cellular macromolecules and thus have been implicated in the mutagenic and carcinogenic activity of these hydrazines [47–50]. Studies carried out in vitro have also shown that both free radical intermediates and diazonium ions induce DNA strand breaks and covalently alkylate adenine and guanine bases [49, 50]. One of the most interesting conclusions from such studies has been the implication of the formation of both hydroxyl and aryl radicals from the diazonium ions [50]. While the role of the diazonium ions in cytotoxicity of hydrazine derivatives in not known, it is of interest to note that the formation of aryl radicals from substituted hydrazines has been shown to correlate with their cytotoxicity [54]. This would suggest that, irrespective of the metabolic pathway (cytochrome P450 or peroxidases), free radicals would be generated from the activation of hydrazines, as both pathways would produce free radical intermediates, resulting in hydrazine-induced toxicity.
CONCLUSIONS:
It is clear that one of the main metabolic pathways for hydrazine derivatives leads to the formation of various free radical species in vitro and in vivo. As discussed with individual hydrazines, the metabolism is catalyzed by cytochrome P450, monoamine oxidase and various peroxidases, including the prostaglandin/AA system. Oxyhemoglobin [55, 56], neutrophils [57] and trace metal ions, e.g., Fe and Cu, are also known to activate hydrazines to free radical species and have been shown to induce DNA damage but there remains uncertainty about whether this oxidation of hydrazines contributes to hydrazine toxicity in humans. This concern is primarily based on the fact that metal ions such as iron and copper are not free in vivo. It should be pointed out that bound iron in hemoglobin is a well-known oxidant for hydrazines, e.g., phenyl hydrazine and hydralazine, and induces formation of reactive intermediates which cause DNA damage. Copper is an essential trace element that is widely distributed throughout the body and forms the essential redox–active reaction center in a variety of metalloenzymes. Copper concentration is significantly altered in tumors, and that serum concentrations are correlated with tumor incidence, progression and recurrence in a number of human tumors. Thus, the oxidative metabolism of hydrazines by copper and iron and consequent formation of reactive species may contribute significantly to the pathophysiology of hydrazines in humans.
It is now well documented that free radical species are very reactive and bind irreversibly to cellular macromolecules, causing inhibition of cellular functions and inducing profound cellular damage. Primary free radicals, e.g., alkyl radicals, react with molecular O2, leading to the formation of reactive oxygen-derived species, superoxide anion radical (O2.-), hydrogen peroxide (H2O2) and, ultimately, to the highly reactive hydroxyl radical (.OH). Alternatively, primary radicals can abstract hydrogen atoms from membrane lipids, inducing peroxidation and decomposition of lipid membranes and compromising cellular functions. Formation of reactive oxygen species has been shown to induce “oxidative stress” where the production of oxidant overwhelms antioxidant defense mechanisms. Oxidative stress is known to deplete reduced glutathione in cells, compromising cellular integrity [58, 59]. Hydrazine derivatives have been shown to deplete glutathione and cause oxidative stress [28]. Thus, formation of free radical species during the biotransformation of hydrazines may be very significant in the toxicity and pathophysiology of hydrazines. The formation of oxygen radicals and metal/peroxo species from hydralazine has been implicated in DNA strand scission, and it has been suggested that these reactive species may also form oxidation products of guanosine bases in DNA [24, 60]. The formation of 8-oxo-7,8-dihydroguanine has been shown to alter binding of methyltransferase 1 to DNA, resulting in the inhibition of this enzyme [61–63]. At this time, however, the role of free radical metabolism of hydralazine in the induction of a lupus-type syndrome in patients is not known.
It is noteworthy that Rehse and Shahrouri (64) have reported that certain hydrazine derivatives generate nitric oxide (.NO) in the presence of hydrogen peroxide, and this formation of nitric oxide parallels their platelet aggregation and antithrombotic effects. It is known that nitric oxide formation leads to generation of other reactive species, e.g., peroxynitrite and NO2 radicals, which induce DNA damage and cause nitrosylation of proteins and enzymes, resulting in compromised cellular functions. This pathway, if confirmed with hydrazine derivatives in vivo, would also then contribute to the known toxicity of hydrazines in vivo. However, a cautionary note: hydrazines are extremely reducing, and any nitric oxide formed might be rapidly reduced by excess hydrazines.
The contributions of various metabolic pathways to hydrazine toxicity are difficult to ascertain at this time in humans. It should be pointed out that the metabolism of hydrazines by cytochrome P450 in the liver would contribute significantly to the known hepatotoxicity of hydrazines. However, this metabolic pathway for hydrazine is also known to lead to binding of reactive species to the active site of cytochrome p450 isozymes, leading to their inactivation (suicidal inactivation) and inhibiting further metabolism. Under this scenario, P450-based metabolism of other drugs which require activation for their biological activity would also be severely compromised and could render them ineffective. This could pose a significant health risk to humans where a drug is administered in combination with hydrazines. Peroxidative metabolism would be similar to the P450-based metabolism of hydrazines in that reactive intermediates formed during metabolism would irreversibly bind to peroxidases, leading to inactivation of the enzymes. Nevertheless, metabolic activation of hydrazines and subsequent binding/damage to cellular biomolecules is clearly the main mechanism for hydrazine toxicity in vivo.
ACKNOWLEDGMENTS
The authors thank Dr. Ann Motten, Ms Jean Corbett and Mrs. Mary Mason for critically editing the manuscript.
Funding
This research was supported [in part] by the intramural research program of the NIH, National Institute of Environmental Health Sciences. Statements contained herein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the U.S. Government.
REFERENCES
- 1.Nelson SD (1982) Metabolic activation and drug toxicity. J Med Chem 25: 753–765. [DOI] [PubMed] [Google Scholar]
- 2.Trohalaki S, Zellmer RJ, Pachter R, Hussian SM, Frazier JM (2002) Risk assessment of high-energy chemicals by in vitro toxicity screening and quantitative structure-activity relationships. Toxicol Science 68: 498–507. [DOI] [PubMed] [Google Scholar]
- 3.Fortney SR (1966) Effect of hydrazine on liver glycogen, arterial glucose, lactate, pyruvate and acid-base balance in the anesthetized dog. J Pharmcol Expt Ther 153: 562–568. [PubMed] [Google Scholar]
- 4.Toth B (1978) Tumorigenic effect of 1-hydrazinophthalazine hydrochloride in mice. J Natl Cancer Inst 61: 1363–1365. [DOI] [PubMed] [Google Scholar]
- 5.Parodi S, De Flora S, Cavanna M, Pino A, Bennicecelli C, et al. (1981) DNA-damaging activity in vivo and bacterial mutagenicity of sixteen hydrazine derivatives as related quantitatively to their carcinogenicity. Cancer Res 41: 1469–1482. [PubMed] [Google Scholar]
- 6.Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timberell, et al. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann Intern Med 84: 181–192. [DOI] [PubMed] [Google Scholar]
- 7.Nelson SD, Mitchell JR, Timberell JA, Snodgrass WR, et al. (1976) Isoniazid and iproniazid-activation of metabolites to toxic intermediates in man and rat. Science 193: 901– 903. [DOI] [PubMed] [Google Scholar]
- 8.De Flora S, Zanacchi P, Bennicelli C, Camoirano A, Cavanna M, et al. (1982) In vivo and in vitro genotoxicity of three antihypertensive hydrazine derivatives (hydralazine, dihydralazine, and endralazine). Environ Mutagen 4: 605–619. [DOI] [PubMed] [Google Scholar]
- 9.Arce C, Segura-Pacheco B, Perez-Cardenas E, Taja-Chayeb L, Candelaria M, et al. (2006) Hydralazine targets: from blood vessels to epigenome. J Transl Med 4: 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timbrell JA, Facchini V, Harland SJ, Mansilla-Tinoco R (1984) Hydralazine-induced Lupus: is there a toxic metabolic pathway? Eur J Clinc Pharmacol 27:555–559. [DOI] [PubMed] [Google Scholar]
- 11.Martz G, Dalessandri A, Keel HJ, Bollag W (1963) Preliminary clinical results with a new antitumor agent RO-4–6467 (NSC-77213). Cancer Chemother Rep 33: 5–14. [PubMed] [Google Scholar]
- 12.Toth B (1980) Actual new cancer-causing hydrazines, hydrazides, and hydrazones. J Cancer Res Clin Oncol 97: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freese E, Sklarow S, Fresse EB (1968) DNA damage caused by antidepressant hydrazines and related drugs. Mutat Res 5: 343–348. [DOI] [PubMed] [Google Scholar]
- 14.Sinha BK, Motten AG (1982) Oxidative metabolism of hydralazine. Evidence for nitrogen centered radicals formation. Biochim Biophys Res Commun 105: 1044–1051. [DOI] [PubMed] [Google Scholar]
- 15.Sinha BK (1983) Enzymatic activation of hydrazine derivatives. A spin-trapping study. J Biol Chem 258: 796–801. [PubMed] [Google Scholar]
- 16.Kalyanaraman B, Sinha BK (1985) Free radical-mediated activation of hydrazine derivatives. Environ Health Prespect 64: 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranguelova K, Suarez J, Magliozzo RS, Mason RP (2008) Spin trapping investigations of peroxide- and isoniazid-induced radicals in Mycobacterium tuberculosis catalase-peroxidase. Biochemistry 47: 11377–11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramachandran G, Swaminathan S (2012) Role of pharmacogenomics in the treatment of tuberculosis: a review. Pharmacogenomics Pers Med 5: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaney J, Timbrell JA (1995) Role of cytochromeP450 in hydrazine toxicity in isolated hepatocytes in vitro. Xenobiotica 25: 1399–1410. [DOI] [PubMed] [Google Scholar]
- 20.Cruz-Hernandez E, Perez-Cardena E, Taja-Chayeb L, Chavez- Blanco, Trejo-Becerril C, et al. (2011) DNA demethylating activity of hydralazine in cancer cell lines. Life Sci Med Res 2011: 2–8. [Google Scholar]
- 21.Segura-Pacheo B, Trejo-Becerril C, Perez-Cardenas E, Taja-Chayeb L, Mariscal I, et al. (2003) Reactivation of tumor suppressor genes by the cardiovascular drugs hydralazine and procainamide and their potential use in cancer therapy. Clini Cancer Res 9: 1596–1603. [PubMed] [Google Scholar]
- 22.Knowles HJ, Tian YM, Mole DR, Harris AL (2004) Novel mechanism of action of hydralazine. Induction of hypoxia-inducible factor-I α, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ Res 95: 162–169. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Kawanishi S (1991) Free radical production and site-specific DNA damage induced by hydralazine in the presence of metal ions or peroxidase/hydrogen peroxide. Biochem Pharmacol 41: 905–914. [DOI] [PubMed] [Google Scholar]
- 24.Sinha BK, Leinisch F, Bhattacharjee S, Mason RP (2014) DNA cleavage and detection of DNA radicals formed from hydralazine and copper (II) by ESR and immuno-spin trapping. Chem Res Toxicol 27: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aruoma OI, Halliwell B, Gajewski E, Dizdaroglu M (1991) Copper-ion-dependent damage to the bases in DNA in the presence of hydrogen peroxide. Biochem J 273: 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha BK, Antholine WM, Kalyanaraman B, Eliot HM (1990) Copper ion-dependent oxy-radical mediated DNA damage from dihydroxy derivative of etoposide. Biochim Biophys Acta 1096: 81–83. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Kawanishi S (1989) Hydroxyl free radical is not the main active species in site-specific DNA damage induced by copper (II) ion and hydrogen peroxide. J Biol Chem 264: 15435– 15440. [PubMed] [Google Scholar]
- 28.Halliwell B, Gutteridge JM, Cross CE (1992) Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med 119: 598–620. [PubMed] [Google Scholar]
- 29.Hussain SM, Frazier JM (2002) Cellular toxicity of hydrazine in primary rat hepatocytes. Toxicol Sciences 69: 424–432. [DOI] [PubMed] [Google Scholar]
- 30.Williams GM, Mazue G, McQueen CA, Shimada T (1980) Genotoxicity of the antihypertensive drugs hydralazine and dihydralazine. Science 210: 329–330. [DOI] [PubMed] [Google Scholar]
- 31.McQueen CA, Way BM, Queener SM (1993) Mutagenicity of hydralazine in mammalian cells and bacteria. Toxicol Appl Pharmacol 118: 135–138. [DOI] [PubMed] [Google Scholar]
- 32.Lunde PKM, Frishid K, Hansteen V (1977) Disease and acetylation polymorphism. Clin Pharmacokinet 2: 182–197. [DOI] [PubMed] [Google Scholar]
- 33.Streeter AJ, Timbrell JA (1983) Studies on the in vivo metabolism of hydralazine in the rat. Drug Metabol Dispos 11: 184–189. [PubMed] [Google Scholar]
- 34.Dubroff LM, Reid RJ (1980) Hydralazine-pyrimidine interactions may explain hydralazine-induced lupus erythematosus. Science 208: 404–406. [DOI] [PubMed] [Google Scholar]
- 35.Sinha BK, Patterson MA (1983) Free radical metabolism of hydralazine. Binding and degradation of nucleic acids. Biochem Pharmacol 32: 3279–3284. [DOI] [PubMed] [Google Scholar]
- 36.Thomas TJ, Seibold JR, Adams LE, Hess EV (1995) Triplex-DNA stabilization by hydralazine and the presence of anti-(triplex DNA) antibodies in patients treated with hydralazine. Biochem J 311: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, et al. (1988) Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J. Immunology 140: 2197–2200. [PubMed] [Google Scholar]
- 38.Nelson SD, Mitchell JR, Snodgrass WR, Timbrell JA (1978) Hepatotoxicity and metabolism of iproniazid and isopropylhydrazine. J Pharmacol Therap 206: 574–585. [PubMed] [Google Scholar]
- 39.De Vita VT, Serpick AA, Carbone PP (1970) Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann Intern Med 73: 881–895. [DOI] [PubMed] [Google Scholar]
- 40.Spivak SD (1974) Drugs five years later: Procarbazine Ann Intern Med 81: 795–800. [DOI] [PubMed] [Google Scholar]
- 41.Malek FA, Moritz K-U, Fanghanel J (2003) Effects of prenatal procarbazine administration on intrauterine development in rats. Ann Anat 185: 117–119. [DOI] [PubMed] [Google Scholar]
- 42.Armand J-P, Ribrag V, Harrousseau J-L, Abrey L (2007) Reappraisal of the use of procarbazine in the treatment of lymphomas and brain tumors. Therap Clinc Risk Manag 3: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunn DL, Lubet RA, Prough RA (1979) Oxidative metabolism of N-isopropyl-α -(2-methylhydrazino)-p-toluamide hydrochloride (Procarbazine) by rat liver microsomes. Cancer Res 39: 4555–4563. [PubMed] [Google Scholar]
- 44.Prough RA, Brown MI, Dannan GA, Guengerich FP (1984) Major isozymes of rat liver microsomal cytochrome P-450 involved in the N-oxidation of N-isopropyl-α-(2-methylazo)-p-toluamide, the azo-derivative of procarbazine. Cancer Res 44: 543–548. [PubMed] [Google Scholar]
- 45.Erickson JM, Tweedie DJ, Ducore JM, Prough RA (1989) Cytotoxicity and DNA damage caused by the azoxy metabolite of procarbazine in L1210 tumor cells. Cancer Res 49: 127–133. [PubMed] [Google Scholar]
- 46.Sinha BK (1984) Metabolic activation of procarbazine: evidence for carbon-centered radical intermediates. Biochem Pharmacol 33: 2777–2781. [DOI] [PubMed] [Google Scholar]
- 47.Kelly RB, Daniels EG, Hinmann JW (1962) Agaritine: isolation, degradation, and synthesis. J Org Chem 27: 3229–3231. [Google Scholar]
- 48.Chauhan YS, Toth B (1984) Synthesis of N2-[y-L-(+)-glutamyl]-4-carboxyphenylhydrazine, a postulated precursor of agaritine of Agaricus bispporus. J Agric Food Chem 32: 676–678. [Google Scholar]
- 49.Chin A, Hung M-H, Stock LM (1981) Reactions of benzenediazonium ions with adenine and its derivatives. J Org Chem 46: 2203–2207. [Google Scholar]
- 50.Lawson T, Gannet PM, Yau W-M, Dalal NS, Toth B (1995) Different patterns of mutagenicity of arenediazonium ions in V79 cells and Salmenella typhimurium TA102: evidence for different mechanisms of action. J Agric Food Chem 43: 2627–2635. [Google Scholar]
- 51.Moloney SJ, Snider BJ, Prough RA (1984) The interactions of hydrazine derivatives with rat hepatic cytochrome P-450. Xenobiotica 14: 803–814. [DOI] [PubMed] [Google Scholar]
- 52.Erickson JM, Prough RA (1986) Oxidative metabolism of some hydrazine derivatives by rat liver and lung tissue fractions. J Biochem Toxicol 1: 41–52. [DOI] [PubMed] [Google Scholar]
- 53.Tomasi A, Albano E, Botti B, Vannini V (1987) Detection of free radical intermediates in the oxidative metabolism of carcinogenic hydrazine derivatives. Toxicol Pathol 15: 178–183. [DOI] [PubMed] [Google Scholar]
- 54.Gamberini M, Cidade MR, Valotta LA, Armelin MCS, Leite LCC (1998) Contribution of hydrazines-derived alkyl radicals to cytotoxicity and transformation induced in normal c-myc-overexpressing mouse fibroblasts. Carcinogenesis 19: 147–155. [DOI] [PubMed] [Google Scholar]
- 55.Augusto A, Faljoni-Alario A, Leite LCC, Nobrega FG (1984) DNA strand scission by the carbon radical derived from 2-phenylethylhydrazine metabolism. Carcinogenesis 5: 781–784. [DOI] [PubMed] [Google Scholar]
- 56.Runge-Morris M, Wu N, Novak RF (1994) Hydrazine-mediated DNA damage: role of hemoprotein, electron transport, and organic free radicals. Toxicol Applied Pharmacol 125: 123–132. [DOI] [PubMed] [Google Scholar]
- 57.Kalyanaraman B, Sohnle PG (1985) Generation of free radical intermediates from foreign compounds by neutrophil-derived oxidants. J Clin Invest 75: 1618–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kidd PM (1997) Glutathione: Systemic protectant against oxidative and free radical damage. Alt Med Rev 2: 155–176. [Google Scholar]
- 59.Vaziri ND, Wang XQ, Oveisi F, Rad B (2000) Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 36: 142–146. [DOI] [PubMed] [Google Scholar]
- 60.Cadet J, Douki T, Ravanat J-L (2008) Oxidatively generated damage to the guanine moiety of DNA: mechanistic aspects and formation in cells. Acc Chem Res 41: 1075–1083. [DOI] [PubMed] [Google Scholar]
- 61.Turk PW, Laayoun A, Smith SS, Weitzman SA (1995) DNA adduct 8-hydroxyl-2’-deoxyguanosine (8-hydroxyguanine) affects function of DNA methyltransferase. Carcinogenesis 16: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 62.Cerda S, Weitzman SA (1997) Influence of oxygen radical injury on DNA methylation. Mutat Res 386: 141–152. [DOI] [PubMed] [Google Scholar]
- 63.Maltseva DV, Baykov AA, Jeltsch A, Gromova ES (2009) Impact of 7,8-dihydro-8-oxoguanine on methylation of CpG sites by Dnmt3a. Biochemistry 48: 1361–1368. [DOI] [PubMed] [Google Scholar]
- 64.Rehs K, Shahrouri T (1998) New NO donors with antithrombotic and vasodilating activities, part 24. Hydrazine derivatives. Archiv Der Pharmazie 331: 308–312. [DOI] [PubMed] [Google Scholar]