Abstract
Background: Tranexamic acid (TXA) is efficiently used to control blood loss during total knee arthroplasty (TKA). The role of intraarticular epinephrine needs further clarification. Limited data exist, concerning the combined use of intravenous and intraarticular TXA plus epinephrine in the intraoperative management of blood loss in patients undergoing TKA.
Methods: This study aimed to evaluate the safety and efficacy of intravenous and intraarticular TXA plus epinephrine in the intraoperative blood management in primary TKA. In this case-control study, 204 patients undergoing primary cemented TKA were enrolled. One hundred two patients received one gr TXA intravenously and intraarticular injection of a mixture containing 500 mg TXA and 0.6 mg epinephrine. They compared to a historical control group comprised of 102 patients that received the same drug combination without epinephrine. The two groups were comparable concerning age, sex, the grade of osteoarthritis, and preoperative hemoglobin and hematocrit.
Results: The epinephrine group had significantly higher postoperative hemoglobin (11.70 vs 10.75, p <0.001) and hematocrit (35.70 vs 32.25, p <0.001) compared to the control group at the first postoperative day. The epinephrine group received fewer transfusions, not reaching statistical significance (p =0.110), compared to the control group during hospitalization. The rate of complications was similar between the groups. The combined use of TXA and epinephrine was positively associated with a smaller postoperative hemoglobin drop.
Conclusion: The combination of intravenous and intraarticular TXA plus epinephrine was safe and reduced the drop of hemoglobin at the first postop day but not significantly the rate of transfusions, in patients undergoing primary TKA. Future higher-level of evidence studies are needed to validate these results. HIPPOKRATIA 2018, 22(2): 86-90.
Keywords: Knee arthroplasty, blood loss, tranexamic acid, epinephrine, intraarticular injections, adrenaline
Introduction
The expected hemoglobin drop after total knee arthroplasty (TKA) can range from 2.8 to 4.8 g/dl; the subsequent need for transfusion could be as high as 20 %1,2. Trauma caused during TKA and the tourniquet mediated hypoxia of the lower extremity promotes the release of tissue plasminogen activator from the vascular endothelium that temporarily promotes fibrinolysis and local blood circulation3,4. Therefore, the post-deflation period with the sudden vein expansion induces local hemorrhage3,4.
The reduction of blood loss and transfusions following TKA is crucial to achieving favorable outcomes5. Intra-operative blood-saving techniques have a pivotal role in the blood management of patients undergoing TKA6. Tranexamic acid (TXA) and epinephrine (EP) are currently used drugs to control blood loss7. TXA is a synthetic analog of the amino acid lysine. It can inhibit fibrinolysis by blocking the lysine binding site of plasminogen and plasminogen binding to fibrin3,8. It can be used in different doses, routes, and timing for several types of operations9. EP is a potent peripheral vasoconstrictor, transported in the wound area through the action of α2 adrenoreceptors, that augments the platelet aggregation10. It also promotes the release of several coagulation factors through β-adrenergic activation10. The efficacy of diluted EP in the management of blood loss in TKA has not been fully clarified10,11.
The most effective method of intraoperative management of the blood loss in TKA remains controversial6. The combined administration of TXA during TKA efficiently reduces blood loss and need for transfusion12-15. The simultaneous intraarticular administration of EP and TXA was proven to be safe16 and superior to TXA alone, in blood conservation following TKA17-19. To the best of our knowledge, no published studies evaluate the safety and efficacy of the combined use of intravenous and intraarticular TXA plus EP for patients undergoing TKA.
In the current study, patients undergoing primary cemented TKA were administered intravenous TXA preoperatively and intraarticular drug mixture containing TXA and EP. We aimed to compare the hemoglobin drop at the first postop day and the safety in terms of postoperative complications, between two groups of TKA patients; the first group received intravenous and intraarticular TXA plus EP (EP group), and the control group received only intravenous and intraarticular TXA.
Materials and Methods
This single-center case-control study took place in a tertiary academic hospital unit. The study was approved by the Institution’s Scientific Research Board and was conducted according to the World Medical Association Declaration of Helsinki of 1964 as revised in 1975 and 2000. All patients were informed about their participation in the study and gave informed consent.
Inclusion/exclusion criteria
Patients scheduled for primary cemented unilateral TKA for end-stage knee osteoarthritis (OA) with age >18 years were included in the study. The exclusion criteria comprised revision and bilateral knee arthroplasty, uncemented or hybrid TKA, trauma, inflammatory arthritis, history of a thromboembolic event, coagulation disease, and hypersensitivity to the used drugs.
The standard care of blood conservation in our institution was the intraoperative intravenous and intraarticular administration of TXA till the end of 2016. The historical control group was recruited from all patients undergoing unilateral TKA by the chief surgeon during 2016 after removing those that did not fulfill inclusion criteria. In total, 102 out of 192 patients that were operated by the surgeon during 2016, were eligible as controls. Thirty-six were excluded due to revision, twenty-two due to inflammatory or posttraumatic arthritis, fourteen had a prior history of a thromboembolic event, and twenty received a different implant. From April 2017 until May 2018, all patients undergoing primary unilateral TKA received the standard care plus intraarticular EP (EP group). For the EP group, 127 patients were screened from April 2017 to May 2018. Twenty-five patients were excluded; 12 patients for revision surgery, seven for inflammatory, and six for posttraumatic arthritis. Recruitment stopped when the groups reached an equal number of patients.
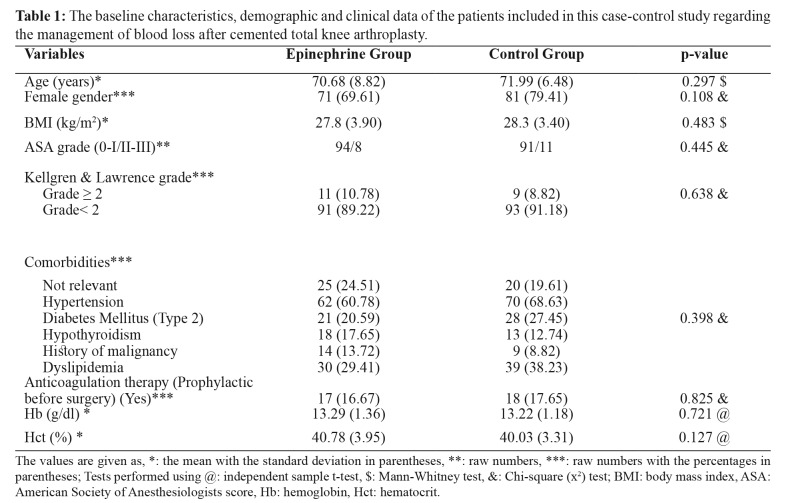
Two hundred and four patients were finally enrolled in the study. The two groups were comparable for age, sex, gender, body mass index, American Society of Anesthesiologists score, Kellgren and Lawrence grade of OA, history, anticoagulation therapy, and level of preoperative hemoglobin and hematocrit (Table 1).
Table 1. The baseline characteristics, demographic and clinical data of the patients included in this case-control study regarding the management of blood loss after cemented total knee arthroplasty.
The values are given as, *: the mean with the standard deviation in parentheses, **: raw numbers, ***: raw numbers with the percentages in parentheses; Tests performed using @: independent sample t-test, $: Mann-Whitney test, &: Chi-square (x2) test; BMI: body mass index, ASA: American Society of Anesthesiologists score, Hb: hemoglobin, Hct: hematocrit.
Interventions
All patients received general anesthesia. A senior arthroplasty surgeon (E.T.) performed all knee arthroplasties through a medial parapatellar approach. Cemented, posteriorly stabilized cobalt-chrome, metal-on-polyethylene prostheses were used in all cases. Thigh tourniquet pressure was applied before skin incision until skin closure. A deep drain was used routinely for the first postop day. All patients were mobilized during the first 24 hours postoperatively. They received anti-embolic stockings and were encouraged to ambulate with partial weight bearing for 20 days postoperatively. All patients received rivaroxaban 10 mg (Xarelto; Bayer, Germany), starting 6-8 hours postoperatively and administered once daily for a month. Transfusion was given when hemoglobin levels fell below 9 g/dl.
The patients of the EP group received a single dose of 1 gr of TXA intravenously diluted in a volume of 1,000 ml of solution at the induction of anesthesia. Besides, they received intraarticular injections with a mixture comprised of 45 ml of natural solution 0.9 %, 0.6 mg EP and 500 mg TXA. The patients of the control group received the same drug combination without EP. The injections were applied in equal doses in three phases. The initial dose was injected subcutaneously and to the capsular adhesion to the tibia at the beginning of surgery. The second dose was applied to the posterior synovial membrane and capsule during the femoral and tibial bone cuts. Before wound closure, the last injection was applied subcutaneously, to the pes anserinus and the tibial attachment of the iliotibial band.
Outcomes
The primary outcomes were the mean difference between pre- and postoperative hemoglobin and hematocrit level at the first postop day. All measurements were made in the morning by venous sampling, using the same blood analyzer. Secondary outcomes were the recorded complications (wound infection, wound hematoma, pulmonary embolism, infection, deep vein thrombosis), and the rate of transfusion during hospitalization between groups.
Demographics in the form of gender, age, body mass index, and American Society of Anaesthesiologists score were collected. Furthermore, the Kellgren and Lawrence grade of OA, diagnosis, prophylactic anticoagulation therapy for other reasons than surgery, comorbidities, levels of preoperative and postoperative hemoglobin and hematocrit at 24 hours, number of blood transfusions and complications were also recorded.
Statistical analysis
The determination of the samples’ necessary size was made according to the reported postoperative drop of hemoglobin following TKA in the previous studies1,2. The fact that the postoperative drop of hemoglobin has been reported to range between 2.8 and 4.8 g/dl1,2 was taken into consideration. Besides, no Minimal Clinical Important Difference concerning the reduction of the hemoglobin postoperatively had previously been published. The statistical analysis showed that with sufficient power of 0.8 and α value of 0.05, in order to see a hemoglobin difference of 0.5 g/dl between the groups, at least 64 patients had to be enrolled in each group.
The normality of the data distribution was tested according to the Kolmogorov-Smirnov and Shapiro-Wilk tests. All statistical tests were two-tailed. The alpha level was set at 0.05. Standard statistical methods were used for descriptive statistics. Continuous variables normally distributed were compared using a two-sided independent sample t-test. Variables not normally distributed were evaluated with the Mann-Whitney U-test and categorical data using Chi-squared or Fisher’s test. A multivariate linear regression analysis was performed to investigate variables affecting the mean difference between preoperative and postoperative hemoglobin. Statistical analysis was performed using R studio (version 1.0.136; 2009-2016 R Studio, Inc.) and R (version 3.3.2).
Results
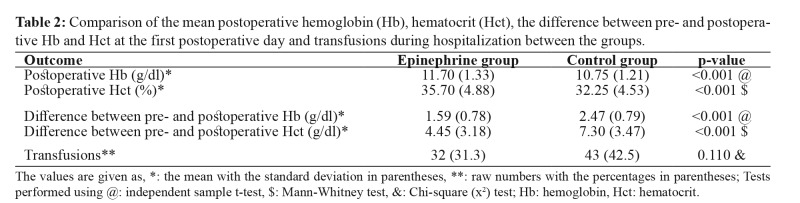
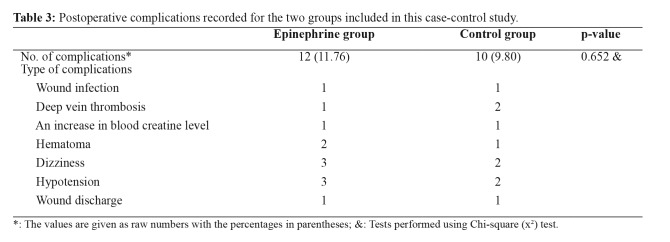
The EP group demonstrated statistically significant higher postoperative hemoglobin (11.70 vs 10.75, p <0.001) and hematocrit (35.70 vs 32.25, p <0.001) compared to the control group at the first postop day. The difference between the preoperative and postoperative hemoglobin (1.59 vs 2.47, p <0.001) and hematocrit (4.45 vs 7.30, p <0.001) was also significantly smaller, favoring the EP group. The EP group received fewer transfusions, but not reaching statistical significance (p =0.110) (Table 2). There were 12 complications recorded in the EP and ten in the control group. The complication rate did not differ significantly between groups (11.7 % vs 9.8 %, p =0.652) (Table 3).
Table 2. Comparison of the mean postoperative hemoglobin (Hb), hematocrit (Hct), the difference between pre- and postoperative Hb and Hct at the first postoperative day and transfusions during hospitalization between the groups.
The values are given as, *: the mean with the standard deviation in parentheses, **: raw numbers with the percentages in parentheses; Tests performed using @: independent sample t-test, $: Mann-Whitney test, &: Chi-square (x2) test; Hb: hemoglobin, Hct: hematocrit.
Table 3. Postoperative complications recorded for the two groups included in this case-control study.
*: The values are given as raw numbers with the percentages in parentheses; &: Tests performed using Chi-square (x2) test.
Multivariate linear regression analysis revealed that application of intraarticular EP was negatively associated (adjusted βepin = -0.93, p <0.001), whereas anticoagulation therapy before the TKA was positively associated (adjusted βanticoag =0.43, p =0.0162), with the drop of hemoglobin and hematocrit at the first postoperative day.
Discussion
The present study demonstrated that the application of intravenous and intraarticular TXA combined with intraarticular EP was efficient to reduce the acute postoperative hematocrit and hemoglobin drop at the first postop day in patients undergoing cemented TKA. This drug combination was also found safe with a low rate of complications.
Various pre-, intra- and postoperative techniques have been used to control blood loss following TKA; however, the best is still missing. The use of TXA is an established method to control blood loss in several procedures, based on its antifibrinolytic action5. Although there were many concerns, especially for thromboembolic side effects, the application of TXA was proved safe and effective. Newer published data demonstrated that TXA is safe even for high-risk patients5. Although different modes of application of TXA have been proposed, there is no universally accepted method5. Both intravenous and intraarticular use of TXA is well-established routes of administration4,12,20-22. A recent meta-analysis demonstrated that the combined intraarticular and intravenous administration of TXA were more efficient than intravenous TXA and control group to reduce blood loss, need for transfusion, and drop of hemoglobin in primary TKA23. The combined TXA administration was associated with less drop of hemoglobin compared to the local application of TXA; however, the combined TXA and local TXA groups were proven equally effective concerning the blood loss and need for transfusion23. There was no significant difference between the groups as far as complications, namely deep vein thrombosis, pulmonary embolism, and infection were concerned23.
EP is a potent peripheral vasoconstrictor causing aggregation of platelets and release of coagulation factors16. The effectiveness of the EP to reduce blood loss following TKA is not fully elucidated19. A recent meta-analysis showed that EP was efficient to reduce the postoperative bleeding volume but not the postoperative hemoglobin drop and transfusion rate19. EP should be applied intraoperatively before the tourniquet is released to maximize its efficacy10. The intraarticular EP could also reduce hemorrhage by delaying the absorption and prolonging the action of TXA and reducing the risk of toxicity through several mechanisms10. A current meta-analysis16 showed that the combined intraarticular TXA plus EP in patients undergoing total joint arthroplasty was significantly more effective than TXA alone in reducing blood loss, transfusion rates, and hemoglobin drift. The incidence of postoperative hematoma and deep vein thrombosis was also similar between the groups16.
No previous studies have evaluated the efficacy of combined intravenous and intraarticular administration of TXA and intraarticular EP in TKA patients. Based on the previous favorable results of studies with combined intraarticular and intravenous TXA21 or combined intraarticular TXA and EP16 we evaluated the effectiveness and safety of combined intravenous and intraarticular TXA with intraarticular EP. This drug combination significantly reduced the postoperative hematocrit and hemoglobin at the first postop day compared to the control group. Although the EP group received fewer transfusions compared to the control group, this did not reach statistical significance. The optimal dosage and timing for TXA and EP administration are not determined in the literature16; the volume of locally injected TXA and EP ranges from 1-3 g and 0.25-0.33 mg, respectively. We administrated 1 g of intravenous TXA just before the incision and 500 mg TXA and 0.6mg of EP locally intraoperatively. Another drug combination might have different results.
Besides, no difference was found in the complication rate between the two groups. EP has been associated with complications such as pulmonary edema, delayed wound healing, hematoma, and edge necrosis10. The use of TXA has been related to deep vein thrombosis or pulmonary embolism24. In the current study, the additional use of EP did not increase the rate of complications. There was an equal number of wound infections, slightly higher cases of deep vein thromboses for the control group, and hematomas for the EP group (Table 3). Gao et al did not report any serious complications using intraarticular EP and TXA in TKA patents17. A recent meta-analysis also showed that EP was efficient to reduce the postoperative bleeding volume in total joint arthroplasties without increasing the risk of deep vein thrombosis19.
The present study has some limitations. First of all, the control group was retrospectively formed. The determination of the necessary sample size, however, strengthens reported results. Also, the postoperative blood test was performed in less than 24 postoperative hours. This may not be accurate as the “true” postop hemoglobin is usually checked at 48 hrs. The main reason for this choice was the belief that the drugs’ effectiveness could not be prolonged after 24 hours. TXA inhibits fibrinolysis for up to 17 hours by blocking the lysine binding site of plasminogen and plasminogen binding to fibrin4,10,19. As a result, no relation to the change in hemoglobin level could be further validated. Another concern is that the used threshold for transfusion was quite low (<9 mg/dl), and this might have skewed the results. Current guidelines in most European countries suggest that transfusions are indicated when hemoglobin is below 8 g/dl; however, this was the existing policy of our department during the study period.
Conclusion
This study is presenting the efficacy of the combined use of intravenous and intraarticular TXA plus EP to diminish the drop in the postoperative hemoglobin and hematocrit at the first postoperative day in patients undergoing TKA. Transfusions were fewer for the EP group, without reaching significance between the groups. This drug combination was found safe, with a low rate of complications. Results are promising; however, they are not considered as definite to recommend the intraarticular use of EP. Future high-level of evidence studies are certainly needed to validate reported results.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Rosencher N, Kerkkamp HE, Macheras G, Munuera LM, Menichella G, Barton DM, et al. Orthopedic Surgery Transfusion Hemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 2.Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee arthroplasty?. Correct blood loss management should take hidden loss into account. Knee. 2000;7:151–155. doi: 10.1016/s0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Lv YM, Ding PJ, Li J, Ying-Ze Z. The reduction in blood loss with intra-articular injection of tranexamic acid in unilateral total knee arthroplasty without operative drains: a randomized controlled trial. Eur J Orthop Surg Traumatol. 2015;25:135–139. doi: 10.1007/s00590-014-1461-9. [DOI] [PubMed] [Google Scholar]
- 4.Hourlier H, Fennema P. Chemoprophylaxis without intra-articular wound drainage can replace autotransfusion in primary TKA. Orthopedics. 2011;34:154. doi: 10.3928/01477447-20110427-11. [DOI] [PubMed] [Google Scholar]
- 5.White CC 4th, Eichinger JK, Friedman RJ. Minimizing Blood Loss and Transfusions in Total Knee Arthroplasty. J Knee Surg. 2018;31:594–599. doi: 10.1055/s-0038-1648223. [DOI] [PubMed] [Google Scholar]
- 6.Lu Q, Peng H, Zhou GJ, Yin D. Perioperative Blood Management Strategies for Total Knee Arthroplasty. Orthop Surg. 2018;10:8–16. doi: 10.1111/os.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee S, Issa K, Pivec R, McElroy MJ, Khanuja HS, Harwin SF, et al. Intraoperative pharmacotherapeutic blood management strategies in total knee arthroplasty. J Knee Surg. 2013;26:379–385. doi: 10.1055/s-0033-1353992. [DOI] [PubMed] [Google Scholar]
- 8.Shemshaki H, Nourian SM, Nourian N, Dehghani M, Mokhtari M, Mazoochian F. One step closer to sparing total blood loss and transfusion rate in total knee arthroplasty: a meta-analysis of different methods of tranexamic acid administration. Arch Orthop Trauma Surg. 2015;135:573–588. doi: 10.1007/s00402-015-2189-7. [DOI] [PubMed] [Google Scholar]
- 9.Ker K, Roberts I. Tranexamic acid for surgical bleeding. BMJ. 2014;349:g4934. doi: 10.1136/bmj.g4934. [DOI] [PubMed] [Google Scholar]
- 10.Gasparini G, Papaleo P, Pola P, Cerciello S, Pola E, Fabbriciani C. Local infusion of norepinephrine reduces blood losses and need of transfusion in total knee arthroplasty. Int Orthop. 2006;30:253–256. doi: 10.1007/s00264-005-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasanuma H, Sekiya H, Takatoku K, Takada H, Sugimoto N, Hoshino Y. Efficient strategy for controlling postoperative hemorrhage in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19:921–925. doi: 10.1007/s00167-010-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen CS, Jans Ø, Ørsnes T, Foss NB, Troelsen A, Husted H. Combined Intra-Articular and Intravenous Tranexamic Acid Reduces Blood Loss in Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial. J Bone Joint Surg Am. 2016;98:835–841. doi: 10.2106/JBJS.15.00810. [DOI] [PubMed] [Google Scholar]
- 13.Shang J, Wang H, Zheng B, Rui M, Wang Y. Combined intravenous and topical tranexamic acid versus intravenous use alone in primary total knee and hip arthroplasty: A meta-analysis of randomized controlled trials. Int J Surg. 2016;36:324–329. doi: 10.1016/j.ijsu.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 14.Lin C, Qi Y, Jie L, Li HB, Zhao XC, Qin L, et al. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty: A meta-analysis. Medicine (Baltimore) 2016;95:e5344. doi: 10.1097/MD.0000000000005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TP, Chen YM, Jiao JB, Wang YF, Qian LG, Guo Z, et al. Comparison of the effectiveness and safety of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:11. doi: 10.1186/s13018-017-0512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Z, Yao L, Yang Q. Tranexamic acid plus diluted-epinephrine versus tranexamic acid alone for blood loss in total joint arthroplasty: A meta-analysis. Medicine (Baltimore) 2017;96:e7095. doi: 10.1097/MD.0000000000007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, Sun W, Guo W, Li Z, Wang W, Cheng L. Topical Application of Tranexamic Acid Plus Diluted Epinephrine Reduces Postoperative Hidden Blood Loss in Total Hip Arthroplasty. J Arthroplasty. 2015;30:2196–2200. doi: 10.1016/j.arth.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Jans Ø, Grevstad U, Mandøe H, Kehlet H, Johansson PI. A randomized trial of the effect of low dose epinephrine infusion in addition to tranexamic acid on blood loss during total hip arthroplasty. Br J Anaesth. 2016;116:357–362. doi: 10.1093/bja/aev408. [DOI] [PubMed] [Google Scholar]
- 19.Teng Y, Ma J, Ma X, Wang Y, Lu B, Guo C. The efficacy and safety of epinephrine for postoperative bleeding in total joint arthroplasty: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2017;96:e6763. doi: 10.1097/MD.0000000000006763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JY, Chin PL, Moo IH, Pang HN, Tay DK, Chia SL, et al. Intravenous versus intra-articular tranexamic acid in total knee arthroplasty: A double-blinded randomized controlled noninferiority trial. Knee. 2016;23:152–156. doi: 10.1016/j.knee.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Digas G, Koutsogiannis I, Meletiadis G, Antonopoulou E, Karamoulas V, Bikos Ch. Intra-articular injection of tranexamic acid reduce blood loss in cemented total knee arthroplasty. Eur J Orthop Surg Traumatol. 2015;25:1181–1188. doi: 10.1007/s00590-015-1664-8. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Chong S, Balasubramanian D, Na YG, Kim TK. What is the Ideal Route of Administration of Tranexamic Acid in TKA? A Randomized Controlled Trial. Clin Orthop Relat Res. 2017;475:1987–1996. doi: 10.1007/s11999-017-5311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Shen X. The efficacy of combined intra-articular and intravenous tranexamic acid for blood loss in primary total knee arthroplasty: A meta-analysis. Medicine (Baltimore) 2017;96:e8123. doi: 10.1097/MD.0000000000008123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–1585. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]