Abstract
Background
There are currently two methods used to administer immunoglobulin: intravenous (IV) infusion, the conventional method, and subcutaneous (SC) infusion, a newer alternative. The aim of this assessment was to compare administration of SC immunoglobulin at home with IV immunoglobulin in hospital with respect to benefits, harm, and costs. We also investigated the lived experiences of patients, looking at their quality of life, satisfaction, opinions, and preferences.
Methods
We searched the literature for studies that compared home-based SC infusion with hospital- or clinic-based IV infusion of immunoglobulin in the treatment of primary and secondary immunodeficiency in adults and children. Two review authors reviewed the abstracts and full text of the relevant studies, and abstracted the data.
We also performed a review of the economic literature comparing SC infusion at home versus IV infusion of immunoglobulin in a hospital or outpatient clinic in patients with primary or secondary immunodeficiency disorders. We also performed a budget impact analysis to estimate the 5-year cost burden of funding home-based SC infusion programs. All costs were reported in 2017 Canadian dollars.
This health technology assessment followed a consultation plan for public engagement. We focused on interviews to examine the lived experience of patients with immunodeficiency, including those having experience of intravenous and/or subcutaneous immunoglobulin treatment.
Results
Sixteen studies met the inclusion criteria. The annual rate of serious bacterial infection per patient did not differ. The annual rate of all infections per patient was relatively lower with home-based SC infusion than with hospital-based IV infusion. Both methods provided an adequate blood (serum) level of immunoglobulin and the pooled mean difference in immunoglobulin level favoured home-based SC infusion. Severe adverse reactions were rare with either method. The risk of adverse events such as fever or headache were higher with IV, while SC infusion sometimes caused infusion site reactions such as swelling, redness, or pain. Where reported, incidence of hospitalization, antibiotic use, and missed days from work or school either did not differ or were lower for SC infusion. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) of evidence for these outcomes was determined to be low.
The scores for quality of life and treatment satisfaction either did not differ between the two methods or were significantly higher for some domains with home-based SC infusion. The three important concerns of patients in Ontario regarding home-based programs are loss of supervision, cost, and frequent injections.
We identified four economic studies with six analyses (five cost-minimization and one cost-utility). All six analyses suggested that home-based infusion has lower costs, with one also showing greater effectiveness. Results of the budget impact analysis suggest that funding home-based SC infusion program would yield savings of about $0.4 million in the first year, and about $1.6 million by year 5. The total savings from funding home-based SC infusion are approximately $5.0 million over 5 years. Greater savings are indicated when the analysis is conducted from the societal perspective.
In speaking directly with patients and their caregivers we found that immunodeficiency reduces quality of life. Intravenous treatment was said to be effective but consumed time and induced side-effects.
Conclusions
The best available evidence suggests that home-based SC infusion is safe and effective, with clinical outcomes that are comparable to the clinical outcomes of hospital IV infusion. The quality of evidence is low, however, meaning that we cannot be certain about these findings. The shift from hospital-based IV to home-based SC has the potential to reduce the health care costs due to savings in nursing time in Ontario. Patients and caregivers expressed preference for home-based SC treatment as it reduces treatment burden and improves overall quality of life.
OBJECTIVE
This health technology assessment compared the benefits, harms, and costs of home-based subcutaneous infusion of immunoglobulin in comparison with hospital- or clinic-based intravenous infusion of immunoglobulin in the treatment of primary and secondary immunodeficiencies in adults and children, and assessed the budget impact of developing a program in Ontario.
BACKGROUND
Health Condition
Immunodeficiency disorders are conditions caused by defects in the immune system that leave the body unable to produce sufficient antibodies to fight infection. Primary immunodeficiency disorders are inborn defects that a person has throughout life.1 Secondary immunodeficiency disorders can be acquired through exposure to an external agent such as infection, chemotherapy, malnutrition, or severe burns, and may be temporary.
Children and adults with immunodeficiency often suffer from recurrent bacterial infections which can sometimes be serious and life threatening. The United States Food and Drug Administration (FDA) has defined serious bacterial infection as the occurrence of any of the following infections: bacteremia/sepsis, bacterial meningitis, osteomyelitis/septic arthritis, bacterial pneumonia, and visceral abscess.2 Based on the FDA's examination of historical data, the rate of serious bacterial infection should be < 1.0 episode per person per year.2
Clinical Need and Target Population
Immunoglobulin replacement therapy is the mainstay treatment to prevent or reduce the severity of infections in patients with immunodeficiency. It can improve quality of life and lifespan. A successful immunoglobulin therapy will increase resistance to infection and give the patient strength and opportunity to participate in family and social activities.
Little is known about the prevalence of primary immunodeficiencies in Canada. However, the incidence rates for one form of primary immunodeficiency (severe combined immunodeficiency) among Canadians is 1.2 per 100,000 people.3 The prevalence of primary immunodeficiency in the United States is estimated to be between 1 in 4,000 and 1 in 10,000.4
Current Treatment Options
Immunoglobulin (or gammaglobulin) is a human-derived blood product used for a broad range of conditions, including primary and secondary immunodeficiency disorders and autoimmune diseases.5 Immunoglobulin is a sterile preparation derived from large pools of human plasma taken from healthy donors. There are several formulations available that differ in their characteristics. Because patients tolerate different products differently, the specific formulation used needs to be matched to patient characteristics.6
Immunoglobulin is currently administered through one of two methods: intravenous infusion of immunoglobulin (IVIG), which is the standard practice in Ontario, and subcutaneous infusion of immunoglobulin (SCIG), which is a relatively newer method. IVIG is usually performed in hospital every 3–4 weeks (13–17 outpatient visits per year). SCIG can be done at home using a pump or manual push (i.e., a syringe), usually once a week. Throughout this report, IVIG refers to hospital- or clinic-based administration and SCIG refers to home-based administration of immunoglobulin.
The total dosage for each method is usually the same, with the more frequent doses by SCIG administered in smaller amounts. Generally, a weekly dose of 100 mg/kg body weight immunoglobulin is known to raise serum immunoglobulin level to what is considered a normal range7; however, the physician may adjust the dosage according to the individual's condition.
Health Technology Under Review
The introduction of SCIG therapy may help improve patients’ psychological well-being by giving them some independence from the need for frequent hospital visits. SCIG allows patients to self-treat (or be treated) at home. Another advantage of SCIG is that it can provide a more stable immunoglobulin level by more frequent infusions at smaller dosages.8 It is known that with monthly IVIG therapy, blood levels of immunoglobulin may subside. Patients may experience low levels of immunoglobulin and complain of tiredness and not feeling well in the week before the next infusion.9 In addition, administration of IVIG can be difficult in patients with poor venous access (experience difficulty with needle insertion).10
SCIG may offer advantages from both patient and family perspectives and from the health care system perspective. It has the potential to reduce the incidence of systemic adverse reactions6 and improve quality of life for patients. It may also reduce reliance on hospital resources and result in lower costs to hospitals and patients.11
Ontario Context
In Ontario, immunoglobulin products are mainly administered intravenously by health professionals in health care settings such as hospitals or clinics. Only 9% of immunoglobulin administration is in the home.11 Intravenous immunoglobulin administration is covered under hospital budgeting. One study shows that switching 50% to 75% of IVIG therapy over to SCIG therapy has the potential to save 9 million to 13.5 million dollars in Ontario over 3 years.11
One Ontario hospital has trained more than 100 patients with immunodeficiency to self-administer immunoglobulin at home. The program involves the following:
Initial step:
Initial appointment with physician and nurse
Baseline patient evaluation
Explain IVIG and SCIG and demonstrate SCIG infusion set up
Organize training sessions
Training:
Demonstrate safe injection and allow patient to practice under nurse supervision over three visits
Explain medication log sheets
Explain adverse events for both SCIG and IVIG
Explain follow-up plan for adherence, serum immunoglobulin level, and dose adjustment, if necessary
Nurse role:
Educate patients and provide resources to patients and family
Support patients for continued education and monitor immunoglobulin trough levels
Monitor patient learning to ensure appropriate injection technique and dose adjustment in consultation with physician
Coordinate and triage, arrange supply delivery with blood bank
Provide for trouble shooting and phone support
SCIG has been implemented in the Atlantic provinces of Canada (Nova Scotia, New Brunswick, Newfoundland and Labrador, and Prince Edward Island), British Columbia, and Alberta.12
CLINICAL EVIDENCE
Research Question
What are the benefits and risks associated with home-based subcutaneous infusion of immunoglobulin (SCIG) in comparison with hospital-based intravenous infusion (IVIG) in the treatment of patients with primary and secondary immunodeficiency?
Methods
Research questions are developed by Health Quality Ontario in consultation with patients, health care providers, clinical experts, and other health system stakeholders.
Literature Search
We performed a literature search on December 13, 2016 to retrieve studies published from inception to the search date. We used the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, Database of Abstracts of Reviews of Effects, and National Health Service Economic Evaluation Database (NHSEED); and we used the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL).
Search strategies were developed by medical librarians using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.13 Database auto-alerts were created in MEDLINE, Embase, and CINAHL, and monitored for the duration of the HTA review.
We performed targeted grey literature searching of HTA agency sites and clinical trial registries. See Appendix 1 for Literature Search Strategies, including all search terms.
Citation Screening
Two reviewers used DistillerSR management software to conduct an initial screening of titles and abstracts, and obtained the full text of studies that appeared eligible for the review, according to the inclusion criteria. They examined the full text articles and selected studies that were eligible for inclusion. The authors examined the reference lists of the selected studies for any additional relevant studies not identified through the search. The review authors contacted authors of the studies to provide clarification as needed.
Inclusion and Exclusion Criteria
Inclusion Criteria
English-language full-text publications
Studies published from inception to December 13, 2016
Randomized and non-randomized controlled clinical trials that compared SCIG with IVIG
Studies on pediatric and adult populations with primary or secondary immunodeficiencies
Exclusion Criteria
Non-English-language publications
Studies in which the setting for IVIG was in the home, or was unclear
Editorials, case reports, or commentaries
Studies that did not report any of the outcomes for this review
Types of Outcomes Measures
Effectiveness (serum trough level, rate of serious bacterial infections, rate of all infections, duration of antibiotic therapy, and rate and duration of hospitalization due to infection)
Safety (serious, systemic, and local adverse events)
Quality of life
Patient satisfaction
Patient preference (Canadian studies)
Number of days lost from work or school due to the immunoglobulin therapy
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form and independently abstracted the relevant laboratory and clinical outcomes of the studies. Disagreements regarding abstracted data were discussed and resolved by the authors with input from the project team.
Statistical Analysis
We used STATA 11, (StataCorp LLC, College Station, Texas) to carry out a meta-analysis on the trough levels to calculate the weighted mean difference between the two methods and to produce a graph. In the two studies14,15 where only the median trough level was reported, we used a conversion formula ([lower confidence interval [CI] + 2 × median + upper CI]/4) for obtaining the mean.16 In the two studies14,17 where standard deviation (SD) was not given, we used the mean of all other available SDs to replace the missing data. We used weighted mean difference and its 95% CI as the summary statistic and displayed the difference of mean trough levels between the two groups through a forest plot. We used a random effects model for pooling the data and the chi-square test to determine statistical heterogeneity among the studies. For categorical variables (e.g., frequency of infection or adverse events), we used rate as a measure of the frequency of the event in each group.
Quality of Evidence
We evaluated the quality of the body of evidence for each outcome according to the GRADE handbook.18 We started with the assumption that RCTs are high quality, whereas observational studies are low quality. We then rated the studies based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, publication bias, magnitude of effect, dose-response gradient, and any residual confounding factors. The overall quality was determined to be high, moderate, low, or very low using a step-wise, structural methodology. The quality determination reflects our certainty about the evidence.
Expert Consultation
We consulted specialists in the field of immunology and family medicine to provide advice on the methods of immunoglobulin administration at home and in hospital and the feasibility of developing a home-based program for infusion of immunoglobulin in Ontario. Feedback from the Ministry of Health and Long-Term Care with respect to the population of Ontario was taken into consideration in the overall assessment.
Results
Literature Search
The database search yielded 1,570 citations published from inception to December 13, 2016 (with duplicates removed). We excluded a total of 1,530 articles based on information in the title and abstract. We obtained the full text of the remaining 40 articles for further assessment. Sixteen studies met the inclusion criteria. Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 1: PRISMA Flow Diagram — Clinical Search Strategy.
Source: Adapted from Moher et al.19
Study and Patient Characteristics
Sixteen observational studies conducted in eight countries met our inclusion criteria. Nine studies were multicentre and seven were single centre. The patient population in these studies were mainly those with primary immunodeficiency disorders. Two studies20,21 included only patients with secondary immunodeficiency. One study17 included both primary and secondary immunodeficiency. Five studies included only adult patients, five studies included only children, and six studies included both adults and children. The duration of the SCIG therapy varied across the studies, ranging from 6 to 24 months. Table 1 shows study and patient characteristics.
Table 1.
Studies Comparing SCIG With IVIG Therapy: Study and Patient Characteristics
| Author, Year | Country | Centres(N) | Design | Patients Evaluated for Efficacy (n) | Completers (n) | Age, Years Mean ± SD (Range) | Observation Period for Efficacy (Month) | Reported Outcomes |
|---|---|---|---|---|---|---|---|---|
| Bienvenuet al, 201622 | France | 35 | Prospective/Retrospective | 116 adults with PID (46 on hospital IVIG, 57 on home SCIG, and 13 patients on home IVIG). 15 patients switched method (10 from IVIG to SCIG, 2 from SCIG to IVIG, 2 from hospital IVIG to home IVIG, and 1 from home IVIG to SCIG). |
All | 41.8 ± 17.5 (15–84) | SCIG: 12 IVIG: 12 |
Infection, SF-36, LQI |
| Vultaggioet al, 201523 | Italy | 11 | Prospective/Retrospective | 43 adults and 7 children with PID. 44 patients switched from IVIG to SCIG and 6 patients changed their SCIG preparation. Retrospective data were analyzed for 41 patients. |
39 (per protocol). 44 (ITT). | 31.7 ± 15.7 | SCIG: 24 IVIG: 12 |
Infection, trough level, hospitalization, missed school/work days, SF-36, CHQ-PF50, LQI, adverse events |
| Compagno et al, 201420 | Italy | 1 | Retrospective | 61 adults with SID. 33 patients had been previously treated with IVIG. |
43 | 67.7 | Mean SCIG: 19 IVIG: 42 |
Infection, trough level, antibiotic use, adverse events |
| Samaan et al, 201424 | Quebec, Canada | 1 | Retrospective | 143 children who had been given the choice of IVIG or SCIG. | N/A | Switch cohort: 10.7 New cohort: 6.0 |
NA | Preference |
| Reid and Pires, 201425 | Ontario, Canada | 1 | Survey | 91 adults & children on IVIG were sent a survey. | N/A | 23 ± 15 (2–75) | NA | Preference |
| Bezrodnik et al, 201326 | Argentina | 3 | Prospective/Retrospective | 15 children with PID previously on IVIG were switched to SCIG. | 13 | 10.6 ± 3.7 | SCIG: 8.5 IVIG: 8.5 |
Infection, trough level, hospitalization, adverse events |
| Sundin et al, 201221 | Sweden | 1 | Retrospective & survey | 58 children with SID due to stem cell transplantation were treated with IG (12 with SCIG and 46 with IVIG). | N/A | SCIG: 2.6 (0–9) IVIG: 7.2 (0–17) |
Median SCIG: 9 IVIG: 5 |
Infection, family attitudes, adverse events |
| Hoffmannet al, 201017 | Germany | 24 | Prospective | 19 adults & 11 children (25 PID & 5 SID) previously on IVIG were shifted to SCIG. | All | 30 (3–74) | SCIG: 9 IVIG: N/A |
Trough level, SF-36, CHQ-PF50, preference, adverse events |
| Bergeret al, 20108 | USA | Multia | Prospective | 42 adults and 9 children with PID previously on IVIG were treated with SCIG. | 45 | 40.4 ± 20.24 (3–66) | SCIG: 12 IVIG: NA |
Infection, trough level, missed school/work days, SF-36, CHQ-PF50, adverse events |
| Thepotet al, 201015 | France | 1 | Prospective | 65 adults with PID receiving IVIG were switched to SCIG. | 60 | 43.8 (15–73) | SCIG: 12 IVIG: 12 |
Trough level, hospitalization, adverse events |
| Quintiet al, 200827 | Germany | Multia | Prospective | 12 adults & 1 child, all with PID who were not receiving immunoglobulin for long period because of severe adverse reactions resumed their therapy using SCIG. | All | 41.2 (13–67) | SCIG: 12 IVIG: NA |
Trough level, adverse events |
| Fasth & Nystrom, 200814 & Fasth & Nystrom, 200728 | Sweden | 1 | Prospective | 12 children with PID were switched from IVIG to SCIG and were followed for 6 months. | All | 10.9 (1.7–17.1) | SCIG: 6 IVIG: 6 |
Trough level, antibiotic use, hospitalization, missed school/work days, CHQ-P50 and CHQ child form, preference, adverse events |
| Nicolay et al, 200629 | USA & Canada | Multia | Prospective | 28 adults with PID were switched to SCIG. | 21 | 36.1 ± 13.6 | 12 | SF-36, LQI, preference |
| Kittner et al, 200630 | Germany | Multia | Survey | 61 adults with PID filled a questionnaire deigned to gather opinions on switching to SCIG. | SCIG: 37± 9.1 IVIG: 51.2 ± 14.5 |
NA | Preference | |
| Gardulf et al, 2004,9 Nicolay et al, 2005,31 & Gardulf et al 20067 | 6 European countries |
12 | Prospective | 44 adults and 16 children with PID were switched from IVIG to SCIG. 10 patients were previously on SCIG. | 52 | Median Adults 33.5 (14–74) Children 7 (3–13) |
SCIG: 10 IVIG: ≥ 6 |
Infection, trough level, hospitalization, missed school/work days, SF-36, CHQ-PF50, LQI, preference, adverse events |
| Gaspar et al, 199832 | UK | 1 | Prospective/Retrospective | 26 children with PID were treated with SCIG. 15 had previously been treated with IVIG. | (1.5 months–15 years) | SCIG: 12 IVIG: 6–42 |
Parental satisfaction |
Abbreviations: CHQ-PF50, child health questionnaire-parental form 50; ID, immunodeficiency; IVIG, hospital-based intravenous immunoglobulin; LQI, life quality index; NA, not available/not applicable; PID, primary immunodeficiency; SCIG, home-based subcutaneous immunoglobulin; SID, secondary immunodeficiency; SF-36, short-form 36.
Precise number of centres not reported in study.
Intervention Characteristics
Studies used different brands of immunoglobulin for SCIG administration. In a majority of the studies, an infusion pump was used to deliver the immunoglobulin subcutaneously. Seven studies used equal doses of immunoglobulin for SCIG and IVIG by dividing the monthly dosage of IVIG into 4 weekly doses. One study investigated a dosage of 0.37 times more than the IVIG dosage,8 and one study used a dosage that was 0.28 times less than the IVIG dosage.15 Table 2 shows the specific brands of immunoglobulin products used in these studies and the dosage of immunoglobulin per body weight used for SCIG and IVIG.
Table 2.
Studies Comparing SCIG With IVIG Therapy: Intervention Characteristics
| Author, Year | SCIG Product | Company | Dosage SCIG Mean (Range) | Dosage IVIG Mean (Range) | Dose Equivalence |
|---|---|---|---|---|---|
| Bienvenu et al, 201622 | NA | NA | Median 428 mg/kg/mo | Median 571 mg/kg/mo | NA |
| Vultaggio et al, 201523 | 16% Vivaglobin | CSL Behring GmbH, Marburg, Germany | NA | NA | Equal |
| Compagno et al, 201420 | Subcuvia Vivaglobin Hizentra |
75 mg/kg/wk | 300 mg/kg/mo | Equal | |
| Samaan et al, 201424 | NA | NA | 100 mg/kg/wk | 400 mg/kg/mo | Equal |
| Reid and Pires, 201425 | NA | NA | NA | NA | NA |
| Bezrodnik et al, 201326 | 16% IgG Beriglobina P |
CSL Behring GmbH, Marburg, Germany | 139 mg/kg/wk (range 105–181) |
556 mg/kg/mo (range 420–870) |
Equal |
| Sundin et al, 201221 | NA | NA | 100–200 mg/kg/1–2 wk | 300–500 mg/kg/2–4 wk | NA |
| Hoffmann et al, 201017 | 16% Vivaglobin | CSL Behring GmbH, Marburg, Germany | 370 mg/kg/mo | 390 mg/kg/mo | Equal |
| Berger et al, 20108 | 16% Vivaglobin | CSL Behring GmbH, Marburg, Germany | 100–200 mg/kg/wk | NA | 1.37× |
| Thepot et al, 201015 | Subcuvia Gammanorm Vivaglobin |
108 mg/kg/wk (range 62–174) |
507 mg/kg/mo (range 308–1000) |
0.72× | |
| Quinti et al, 200827 | Vivaglobin Subcuvia |
CSL Behring GmbH, Marburg, Germany/Baxter |
100 mg/kg/wk | NA | NA |
| Fasth & Nystrom, 200814 Fasth & Nystrom, 200728 | 16% Subcuvia | Baxter Medical AB, Kista, Sweden | Median 113.5 mg/kg/wk (range 56–159) |
Median 448.5 mg/kg/mo (range 81–763) |
Equal |
| Nicolay et al, 200629 | 16% Vivaglobin | ZLB Behring GmbH, Marburg, Germany | Median 152 mg/kg/wk |
NA | NA |
| Kittner et al, 200630 | NA | NA | NA | NA | NA |
| Gardulf et al, 2004,9 Nicolay et al, 2005, & Gardulf et al, 20067 | 16% Vivaglobin | ZLB Behring GmbH, Marburg, Germany | 100 mg/kg/wk | 400 mg/kg/mo | Equal |
| Gaspar et al, 1998 | 16% Gammabulin | Immuno Ltd, Newbury, Berks, UK | Mean 160 mg/kg/wk (range 70–260) |
NA | NA |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; NA, not available/not applicable; SCIG, home-based subcutaneous immunoglobulin.
Quality of Evidence
We used the grading system developed by the GRADE Collaboration18 to make judgements about the quality and strength of the evidence. We first used the Cochrane risk of bias tool for non-randomized studies and assessed issues such as appropriate eligibility criteria, measurement of exposure and the outcomes, prognostic imbalance, presence of co-intervention, and adequate follow-up in each individual study. We then rated the evidence for each outcome across the studies considering other elements of the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system. These are inconsistency, indirectness, imprecision, publication bias, magnitude of effect, dose-response gradient, and any residual confounding factors). Considering all key criteria, the quality of the body of evidence for each outcome was determined as low, meaning there is uncertainty in the results of the studies (Table A1).
Immunoglobulin Trough Level
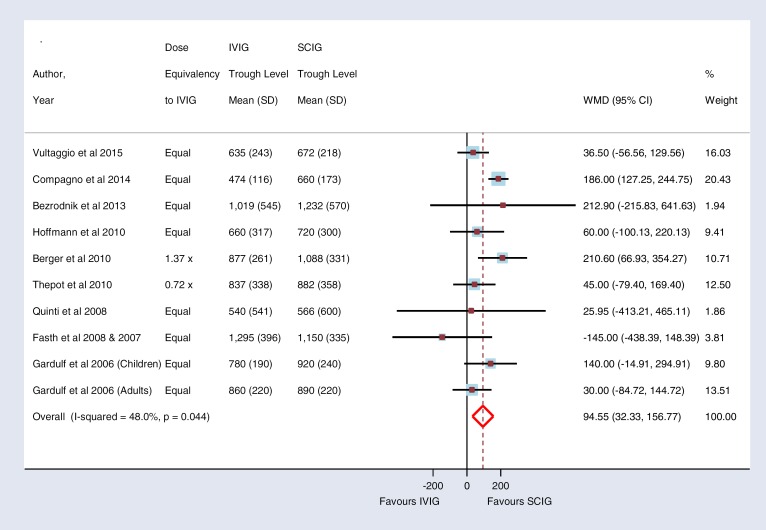
Nine studies compared serum immunoglobulin trough levels between SCIG and IVIG treatments. In one of these studies, SCIG was administered to patients who had previously experienced severe adverse reactions to IVIG, resulting in withdrawal of treatment.27 These patients received SCIG in hospital. The nine studies included 382 patients who received SCIG and 320 patients who received IVIG. The pooled weighted mean difference (WMD) in trough levels between the two methods indicated that SCIG provided a higher level of serum immunoglobulin than IVIG (WMD 94.55, 95% CI 32.33–156.77). There was a moderate degree of heterogeneity among the studies (I2 = 48%). Six of these studies7,14,17,20,23,26 used an equal dosage of immunoglobulin for SCIG and IVIG. One study8 used a higher dosage and another study15 used a lower dosage for SCIG. Five studies7,8,17,23,27 included data from both children and adults, but only one study7 presented the data separately. One study27 used SCIG dosage of 400 mg/kg but the previous dosage of IVIG was not available. The forest plot for immunoglobulin trough levels is shown in Figure 2.
Figure 2: Meta-analysis of Immunoglobulin Trough Levels: Comparison Between SCIG and IVIG.
Abbreviations: CI, confidence interval; IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin; SD, standard deviation.
Table 3 shows infection rates, antibiotic use, and hospitalization rates for SCIG and IVIG.
Table 3.
Results of Studies Comparing SCIG With IVIG Therapy: Infection Rates, Antibiotic Use, and Hospitalization
| Author, Year | Serious Infections | All Infections | Antibiotic Use | Hospitalization | ||
|---|---|---|---|---|---|---|
| Incidence | Annual Incidence per Patient | Incidence | Annual Incidence per Patient | |||
| Bienvenu et al, 201622 | 16 episodes in 10 patients in SCIG and IVIG, combined with no significant difference between the two methods | 0.19 (0.08–0.46) for SCIG and IVIG combined with no significant difference between the two methods | NA | NA | NA | NA |
| Vultaggioet al, 201523 |
ITT (n = 44) SCIG: 5 IVIG: NA |
ITT (n = 44) SCIG: 0.056 IVIG: NA |
Completers (n = 39) SCIG: 33 patients (84.6%) experienced at least one incidence of infection during 24-mo follow-up period IVIG: NA |
NA | NA |
Mean days in hospital SCIG: 0.64 ± 2.94 IVIG: 1.93 ± 4.08 (Reasons: NA) |
| Compagno et al, 201420 | SCIG: 11/61 IVIG: 12/33 |
SCIG: 0.11 IVIG: 0.10 |
SCIG: 170 IVIG: 260 |
SCIG: 1.76 IVIG: 2.29 |
Cycles of antibiotic per patient per year: SCIG: 1.43 IVIG: 1.82 |
NA |
| Bezrodnik et al, 201326 | SCIG: 0 IVIG: 0 |
SCIG: 0 IVIG: 0 |
SCIG: 4 IVIG: 14 |
SCIG: 0.4 IVIG: 1.4 |
NA | Incidence due to infection: SCIG: 0 IVIG: 1 |
| Sundin et al, 201221 | NA | NA | SCIG: 6.4 (3–13) IVIG: 5.5 (0–23) Not significant |
NA | NA | NA |
| Berger et al, 20108 | SCIG: 1 IVIG: NA |
SCIG: 0.03 IVIG: NA |
SCIG: 162 IVIG: NA |
SCIG: 3.42 (95%CI2.93–3.99) IVIG: NA |
NA | Incidence due to all causes: 5 in 51 patients Reasons: pneumonia, Crohn disease, MI, lithium toxicity, near syncope |
| Thepot et al, 201015 | NA | NA | NA | NA | NA | Incidence: SCIG: 56 in 15 patients (Incidence rate: 1.19/patient/yr) IVIG: 43 in 24 patients (Incidence rate: 0.84/patient/yr) |
| Fasth & Nystrom, 200814 & Fasth & Nystrom, 200728 | NA | NA | Rate of infections per month: SCIG: 2.0 IVIG: 2.4 Not significant |
NA | Days on antibiotic: SCIG: 3.5 (0–92) IVIG: 12.8 (0–92) Difference: −5.3 (–38.5–15.3; P = 0.373) |
Incidence due to infection: SCIG: 0 IVIG: 0 |
| Gardulfetal, 2004,9 Nicolay etal, 2005,31 & Gardulf et al, 20067 | SCIG: 1 IVIG: NA |
SCIG: 0.04 IVIG: NA |
SCIG: Adults: 174 Children: 72 IVIG: NA |
SCIG: URT: 3.6 LRT: 0.50 IVIG: NA |
NA | Incidence due to infection: SCIG: 2 (3%) IVIG: NA |
| Gaspar et al 199832 | SCIG: 0 IVIG: NA |
NA | NA | NA | NA | Incidence due to infection: SCIG: 0 IVIG: NA |
Abbreviations: ID, immunodeficiency; IG, immunoglobulin; ITT, intention to treat; IVIG, hospital-based intravenous immunoglobulin; LRT, lower respiratory tract; MI, myocardial infarction; NA, not available/not applicable; SCIG, home-based subcutaneous immunoglobulin; URT, upper respiratory tract.
Infection Rate
Serious Bacterial Infection
Six studies reported on the annual rate of serious bacterial infection per patient,7,8,20,22,23,26 but only three studies20,22,26 reported the rates for both methods. The annual rate of serious bacterial infection among patients who received SCIG ranged from 0.03 to 0.19 among the studies and was below the target of <1.0 per person per year that was set by the FDA.
All Infections
Seven studies reported on the incidence of all infections.7,8,20,21,23,26,28 Four compared SCIG with IVIG,20,21,26,28 Two of these studies also compared SCIG with IVIG for the annual rate of all infections per patient.20,26 In these studies, the annual rate for all infections per patient was lower in the SCIG group than in the IVIG group, but no P value was reported.
Antibiotic Use
Two studies reported on the use of antibiotics. Compagno et al20 reported 1.43 and 1.82 cycles per patient per year for SCIG and IVIG, respectively. Fasth and Nystrom14 reported 3.5 and 12.8 days of antibiotic use for SCIG and IVIG, respectively.
Hospitalization Rate
Seven studies reported on hospitalization.7,8,14,15,23,26,32 One study23 reported on the mean number of days in hospital and the remaining of the studies reported on the incidence of hospitalization. The incidence of hospitalization due to infection among studies that used equal or higher dosage of immunoglobulin was 0% to 3%. In one study that used 28% lower dosage of immunoglobulin for SCIG, the annual incidence of hospitalization was higher in SCIG than IVIG (1.19 and 0.84 per patient for SCIG and IVIG, respectively).
Missed Days of Work or School
Four studies7,8,14,23 reported on the number of missed days from work or school, and two of these studies compared IVIG to SCIG. Vultaggio et al23 reported that during home SCIG patients missed work or school for a mean of 2.26 ± 4.45 days during the 24-month observation period while they missed work or school for a mean of 15.27 ± 23.17 days during the 12-month period they were on hospital IVIG. Fasth and Nystrom14 reported that the median number of the days that children missed school during SCIG was 2 days (range, 0–10), while the median number of days they missed school during IVIG was 8.8 days (range, 0–45). Parents or caregivers of children on SCIG also missed a median of 1 day (range, 0–9), while they missed a median of 4.5 days (range, 0–10) when the child was on IVIG. Berger et al8 reported a mean number of missed days for SCIG of 4.5 days per patient per year. Table 4 shows missed days from work or school.
Table 4.
Results of Studies Comparing SCIG With IVIG Therapy: Missed Days of Work or School
| Author, Year | Patient | Parent/Caregiver |
|---|---|---|
| Vultaggio et al, 201523 | SCIG: 2.26 ± 4.45 IVIG: 15.27 ± 23.17 |
NA |
| Bergeretal, 20108 | SCIG: Mean of 4.5 d/patient/yr 26 patients (51%) had no missed day and 25 (49%) had an average of 9.2 missed days IVIG: NA |
NA |
| Fasth & Nystrom, 200814 & Fasth & Nystrom, 200728 & | Children school and work absence: SCIG: 2 (0–10) IVIG: 8.8 (0–45) Difference at 6 mo: median: −7.4 (–18.5, −3.0); P = 0.006 |
School/university/work absence: SCIG: 1.0 (0–9) IVIG: 4.5 (0–10) Difference at 6 mo: median: −3.5 (–5.5, −1.5) P = 0.008 |
| Gardulf et al, 2006,7 Gardulf et al, 2004,9 Nicolayetal, 2005,31 | SCIG: 10 adults and 6 children missed days from school or work; continuous absence from work for adults ranged from 1 to 36 days; continuous absence from school for children ranged from 1 to 9 days. IVIG: NA |
NA |
Abbreviations: NA, not available; IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Adverse Reactions
One anaphylactic reaction (hypersensitivity) was reported among the reviewed studies. It occurred in a patient receiving hospital-based IVIG. One vagal reaction was reported in a patient who received SCIG. No other severe reaction was reported in either method. Systemic reactions including fever, chills, headache, dizziness, nausea or vomiting, diarrhea, allergic reaction, and malaise were reported more frequently during IVIG therapy than during SCIG. On the other hand, infusion site reactions were frequently seen during SCIG (Table 5). One study reported that 2% of patients who received SCIG needed premedication, compared with 52% of patients on IVIG. Five studies that reported on withdrawal from SCIG gave a range of 1% to 7%.
Table 5.
Results of Studies Comparing SCIG With IVIG: Systemic and Local Adverse Event
| Adverse Reaction | During SCIG | During IVIG |
|---|---|---|
| Systemic Reactions | ||
| Severe or anaphylactic reaction | Vagal reaction in 1 patient7 | Anaphylactic reaction occurred in 1 patient20 |
| Fever | 4.7 to 13%7,20,23 | 34%20 |
| Headache | 3 to 8%14,20 | 13.7 to 54%8,21 |
| Dizziness | 2%7,8 | No study reported on this item. |
| Allergic reaction/diffuse skin reaction | 3%7 | 15% to 16%20,21 |
| Nausea/vomiting | 2% to 17%14,23 | 9%20 |
| Diarrhea | 4.7%23 | No study reported on this item. |
| Malaise | 2%7 | No study reported on this item. |
| Infusion Site Reactions | ||
| Reactions such as pain, rash, induration, redness, swelling, soreness, itching, bruising | 2% to 100%8,14,17,20,21,23,26,27 one-fourth of infusions7 | Not applicable |
Abbreviations: NA, not available; IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Quality of Life
Quality of life of adult patients was measured by short form-36 (SF-36) and quality of life of children was measured by the Child Health Questionnaire parental form (CHQ-PF50) and child form. We did not meta-analyze the data on quality of life scores because of the high degree of heterogeneity of reporting among the studies.
Quality of Life of Adult Patients
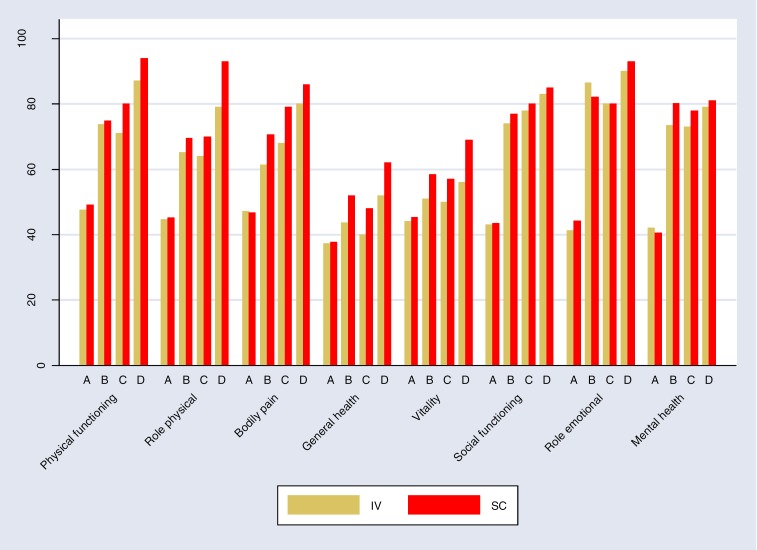
Overall, six studies reported on SF-36, but two8,21 reported only on some domains. Data from the other four studies8,17,22,29 were used to create a bar chart. Figure 3 shows that the scores for SF-36 for IVIG and SCIG were similar. Higher scores indicate better quality of life.
Figure 3: Quality of Life of Adult Patients Treated by SCIG Versus IVIG Therapy Measured by Short-Form-36 (SF-36) Instrumenta.
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
aA, Bienvenu et al 201622; B, Berger et al 20108; C, Hoffmann et al 201017; D, Nicolay et al 2006.29
Bienvenu et al22 reported no difference in any of the subscales between the two methods. Berger et al8 reported a significant difference at 6 months for general health, vitality, and mental health subscales in favour of SCIG. At 12 months, scores for all subscales were higher for SCIG except for role emotional, but only the general health subscale reached statistical significance, in favour of SCIG (P = 0.047). Hoffmann et al17 reported a significant difference in favour of SCIG in subscales of general health (P = 0.05), vitality (P = 0.05), and bodily pain (P = 0.02). Nicolay et al29 reported a significant difference (P < 0.05) favouring SCIG for subscales of role physical, general health, vitality, and health transition at 12 months. Vultaggio et al23 reported no improvement in quality of life of patients at 6, 12, and 24 months when switching from hospital IVIG to home SCIG. Gardulf et al9 reported significantly higher scores for vitality (P = 0.04), mental health (P = 0.05), and social functioning (P = 0.01) at 10 months, favouring SCIG.
Quality of Life of Children
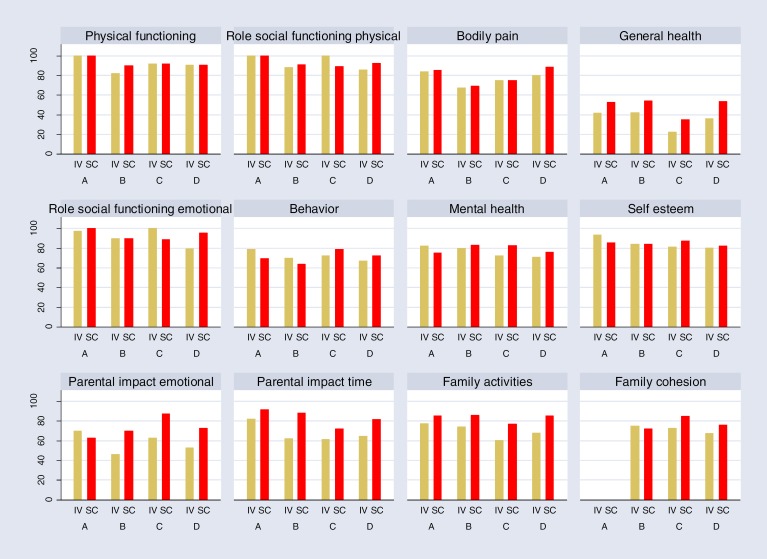
Five studies used CHQ to demonstrate changes in children's quality of life.8,9,14,17,23 Four of these studies provided data that could be used for creating a bar chart8,9,14,17 (Figure 4). Higher scores indicate a better quality of life.
Figure 4: Quality of Life of Children Treated by SCIG Versus IVIG Therapy Measured by Child Health Questionnaire Parental Form (CHQ-PF50) Instrumenta.
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
aA, Berger et al 20108; B, Hoffmann et al 201017; C, Fasth and Nystrom 200814; D, Gardulf et al 2004.9
Berger et al8 reported no difference between the two methods in quality of life of children. Hoffmann et al17 reported a statistically significant difference (P < 0.05) for subscales of general health, parental impact (emotional), parental impact (time), and family activities. Fasth and Nystrom14 reported statistically significant differences for subscales of mental health (P = 0.036), change in health (P = 0.041), and family activities (P = 0.037). Gardulf et al9 reported a significant difference in subscales of role social emotional/behavioral (P = 0.02), general health (P = 0.001), parental impact (emotional) (P = 0.02), parental impact (time) (P = 0.004), family activity (P = 0.002), and global health (P = 0.01). Vultaggio et al23 reported no significant improvement in quality of life of children who switched from IVIG to SCIG at 6, 12, or 24 months.
The CHQ child form was reported in only one study.14 Children scored most of the domains in favour of SCIG. The study reported a significant increase in CHQ scores 6 months after switching from hospital IVIG to home SCIG for subscales of global health (P = 0.042) and role social limitations-emotional (P = 0.041), which is a measure of whether school work or usual activities with friends were affected by problems like feeling sad or worried. There were no significant improvement for other subscales. Scores for bodily pain decreased from 90 to 80, but this difference did not reach statistical significance.
Patient Satisfaction
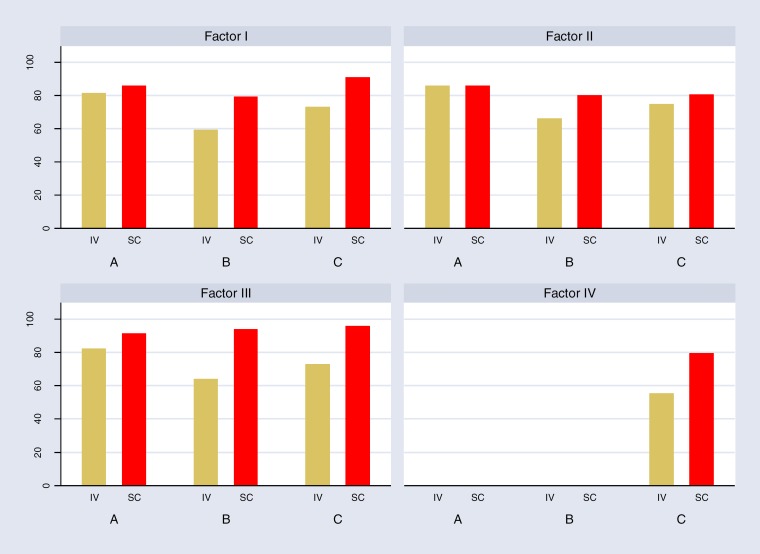
Patient satisfaction was addressed by three studies9,22,23 using the Life Quality Index (LQI) and its related factors. The LQI scale has been developed for primary immunodeficiency patients who receive immunoglobulin therapy. The LQI consists of 15 items.31 Each item is addressed on a seven-point Likert scale ranging from extremely good (7 points) to extremely bad (1 point). Investigators have made categories of treatment interference (Factor I), therapy-related problems (Factor II), and therapy setting (Factor III). The treatment cost (Factor IV) was reported by only one study.31 Scores for LQI are shown in Figure 5. Higher scores indicate a higher level of patient satisfaction.
Figure 5: Treatment Satisfaction of Children and Adult Patients Treated by Home SCIG Versus Hospital IVIG Therapy Measured by Life Quality Index Instrumenta,b.
aFactor I, treatment interference; Factor II, therapy-related problems; Factor III, therapy setting, Factor IV, treatment costs.
bA, Bienvenu et al 201622; B, Nicolay et al 200629; C, Nicolay et al 2005.
Bienvenu et al22 reported a significant difference on LQI factor III favouring SCIG (P = 0.005). Vultaggio et al23 reported the total mean LQI scores and showed a significant improvement in LQI scores 6 months after switching from SCIG to IVIG, which was sustained over time (IVIG: 76.88 ± 16.76; SCIG: 90.67 ± 11.64; P < 0.01). Gardulf et al9 reported a significant improvement in total mean summary LQI scores for adults (P = 0.001) and children (P = 0.0001) after switching from home SCIG to hospital IVIG.
Patient Preference
Canadian Studies
In Ontario, the willingness and preference of patients on IVIG to switch to SCIG was studied by Reid and Pires.25 A 25-question survey was mailed to patients receiving IVIG therapy in Ontario. The survey population included children and adults ranging in age from 2 to 75 years. Ninety one patients participated in the survey. Forty one questionnaires were completed by patients, and an additional 39 by parents or guardians of patients. Five questionnaires were completed by both patients and parents or guardians, and six were not specified. The mean age of the patients was 23 ± 8.5 years. The treatment locations were community hospitals (55 patients, 60%), teaching hospitals (27 patients, 30%), and clinics (1 patient, 1%). Location was not specified for eight patients (9%).
Patients were asked about the occurrence of the four most commonly reported adverse events associated with IVIG therapy (headaches, fever, hives, and chills). The majority of patients did not experience any of the common adverse effects. Patients were asked whether they would be willing to switch to home SCIG if this treatment were available and equally effective. Seventy eight percent of patients answered they would switch after consulting with their immunologist but none of the patients said they would switch to SCIG based on consultation with their family physician. Expenses associated with SCIG were less of a concern for patients under the age of 35, but it was an important issue for patients aged 35 and older, who were more likely to switch to SCIG only after inquiring about the costs.
Based on the qualitative analysis of the answers, researchers identified six concerns about switching to SCIG and ranked them in order of importance to patients. Loss of supervision was most important, followed by concerns about cost, frequent injections, lost time, self-injection, and, finally, safe and reliable storage of medication. Further analysis of the data comparing IVIG with SCIG showed that patients were significantly more likely to switch to home IVIG than home SCIG, but they had concerns regarding costs.
Patients were also asked to rank the five factors that make SCIG treatment more convenient. Patients ranked elimination of travel time as most important, followed by a preference to receive treatment in the home, safer treatments at home, better quality of life, and, finally, reduced travel costs.
Different subgroups had different views of the safety of SCIG. Patients under 35 years of age, parents, and patients receiving treatment at a teaching hospital ranked home SCIG as unsafe, while patients 35 years of age and older and those receiving community-based treatment ranked lack of safety among their least concerns. For detailed information, see Reid and Pires 2014.25
In Quebec, Samaan et al24 conducted a retrospective study to examine patient behaviour when given the choice of IVIG and SCIG. Patients were categorized into two groups: “switch cohort” and “new cohort.” Patients in the switch cohort were already on IVIG and were given the choice to stay on IVIG or to switch to SCIG. Patients in the new cohort were at the start of immunoglobulin therapy and were able to choose between the two methods. The physician and the nurse provided information about both methods to the patients, including technical information and side effects. Training was provided to patients who chose SCIG. Patients in the switch cohort received an equal dosage of immunoglobulin.
One hundred forty three patients with primary immunodeficiency were included in the study. Of the 51 patients in the switch cohort, 50 switched from IVIG to SCIG. Forty four (88%) remained on SCIG after a follow-up of (mean duration, 52 months; range, 30–72 months). Of 92 patients in the new cohort, about half (44 patients) initially decided to receive SCIG. Forty eight decided to receive IVIG. After a mean of 6.8 months, 35 of the IVIG patients (73%) switched to SCIG. Therefore, after a mean of 33.2 months (range, 7.9–66.3 months), 74 (80%) of the patients in the new cohort were on SCIG. During the course of the study, a total of 13 patients switched from SCIG to IVIG. Patients in the two groups had similar mean trough levels of immunoglobulin (IVIG, 920 mg/dl; SCIG, 900 mg/dl).
Discussion
Laboratory Results
Studies that compared SCIG with IVIG have shown that SCIG can provide an adequate serum trough level to prevent infection. The pooled data from nine studies shows a mean difference of 95 mg/dL in serum trough levels favouring SCIG between the two methods.
Clinical Outcomes
Overall, the quality of evidence for clinical outcomes was low because studies were prospective with retrospective data as control, or were retrospective in design. Comparisons between the two methods for clinical outcomes were not available in several of these studies.
The occurrence of severe adverse reactions was rare. Among patients who received IVIG, one case was reported (an anaphylactic reaction) and among patients who received SCIG, one case was reported (a vagal nerve reaction).
Subcutaneous IG was associated with a lower risk of adverse events such as fever, headache, and allergic reaction. But, SCIG caused local reactions such as pain, rash, induration, swelling, soreness, and itching at the site of infusion. In the studies we reviewed, up to 7% of patients withdrew from SCIG due to infusion site reactions.
Both methods had similar rates of serious bacterial infection, and the incidence was low (determined based on FDA recommendations that the rate of serious bacterial infection should be < 1.0 per patient per year).2 Two studies comparing the annual rate of all infections per patient between the two groups reported lower rates for SCIG than for IVIG.
Two studies comparing the use of antibiotics reported fewer cycles of antibiotic use, or reported fewer days on antibiotics for SCIG compared with IVIG.
One study using an equal dosage of immunoglobulin reported that fewer patients on SCIG required hospitalization than did those on IVIG. In another study using a 28% lower dosage of immunoglobulin, the hospitalization rate increased from 0.8 per patient per year during IVIG to 1.19 per patient per year during SCIG. The number of days in hospital was reported by one study and it showed fewer days in patients receiving SCIG.
Two studies comparing missed work and school days reported fewer missed days during SCIG than during IVIG. One of these studies also reported that parents or caregivers had fewer missed days when the child was on SCIG.
Quality of Life
Adult patients showed significant improvement for general health and vitality when they switched from IVIG to SCIG, but other subscales showed no significant improvement.
The parent-completed child health questionnaire showed that scores for quality of life of children were not different when comparing SCIG with IVIG for most subscales, but some studies reported significant improvement with SCIG in domains related to general health, mental health, parental impact, and family activities. The child-completed form showed significant improvement in subscales of global health and role social limitations-emotional.
Treatment Satisfaction
The Life Quality Index was measured for treatment satisfaction. Total mean Life Quality Index scores improved significantly after switching from hospital IVIG to home SCIG.
Patient Preference
Ontario patients were asked whether they are willing to switch to SCIG if this treatment were available and equally effective. Seventy eight percent of patients would switch after consulting with their immunologist, but none said they would switch to SCIG based on consultations with their family physician. Concerns form patients about switching to SCIG therapy, ranked from most to least important, were (1) loss of supervision, (2) cost, (3) frequency of injections, (4) lost time, (5) self-injections, and (6) safe and reliable storage of medication. Further analysis of the data comparing IVIG with SCIG showed that patients were significantly more likely to switch to home IVIG rather than home SCIG, but they had concerns regarding costs with home IVIG rather than home SCIG.
In Quebec, 88% of patients who switched from hospital IVIG to home SCIG remained on SCIG after follow-up (mean duration, 52 months). Patients at the start of immunoglobulin therapy were offered either treatment and about half chose IVIG, but 73% of these patients switched to SCIG after follow-up (mean duration, 6.8 months).
Conclusions
The best available evidence suggests that home-based subcutaneous infusion is safe and effective, with clinical outcomes that are comparable to the clinical outcomes of hospital-based IV infusion. However, the quality of evidence is low, meaning that we cannot be certain about these findings. Serum trough levels were higher after immunoglobulin replacement therapy with SCIG than was obtained by IVIG. The incidence of serious bacterial infection was similar between the two methods. The incidence of systemic adverse events with SCIG was low, and generally lower than with IVIG. However, infusion site reactions were reported with SCIG, with variable rates among the studies. The overall quality of life of adults and children did not differ between SCIG and IVIG, but there was improvement in specific domains with SCIG.
ECONOMIC EVIDENCE
Research Question
What is the published economic evidence for subcutaneous immunoglobulin delivered at home (SCIG) when compared to intravenous immunoglobulin administrated in hospital or outpatient clinic (IVIG) for children or adults with primary or secondary immunodeficiency disorders?
Methods
Economic Literature Search
We performed an economic literature search on December 23, 2016, for studies published from inception to the search date. To retrieve relevant studies, the search was developed using the clinical search strategy with an economic filter applied.
Database auto-alerts were created in MEDLINE, Embase, and CINAHL, and monitored for the duration of the HTA review. We performed targeted grey literature searching of HTA agency sites, clinical trial registries, and Tufts Cost-Effectiveness Analysis Registry. See Clinical Evidence, Literature Search, above, for further details on methods used, and Appendix 1 for Literature Search Strategies, including all search terms.
Literature Screening
A single reviewer reviewed titles and abstracts and, for those studies likely to meet the eligibility criteria, we obtained full-text articles and preformed further assessment for eligibility.
Inclusion Criteria
Studies comparing SCIG versus IVIG in patients with primary or secondary immunodeficiency disorders
English-language full-text publications
Studies published between January 1, 2007, and December 23, 2016
Cost-utility analyses, cost-effectiveness analysis, cost-benefit analyses, or cost minimization analyses
Exclusion Criteria
Reviews
Abstracts, letters, and editorials
Unpublished studies
Outcomes of Interest
Cost
Quality-adjusted life-years (QALYs)
Incremental cost and incremental effectiveness
Cost per QALY gained
Data Extraction
A single reviewer conducted the preliminary data extraction applying the inclusion criteria. For studies containing several comparators, we extracted only the results for the comparison of SCIG versus IVIG. We mainly extracted the following information:
Source (i.e., first author, country, year of publication)
Population, perspective, and time horizon
Interventions and comparators
Outcomes (e.g., health outcomes, costs, cost-effectiveness)
We contacted authors of the studies to provide clarification as needed.
Study Applicability
We determined the usefulness of each identified study for decision-making by applying a modified applicability checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of clinical guidelines by NICE.33 We retained questions from the NICE checklist related to study applicability and modified the wording of the questions to make it Ontario specific. A summary of the number of studies judged to be directly applicable, partially applicable, or not applicable to the research question is presented.
Results
Literature Search
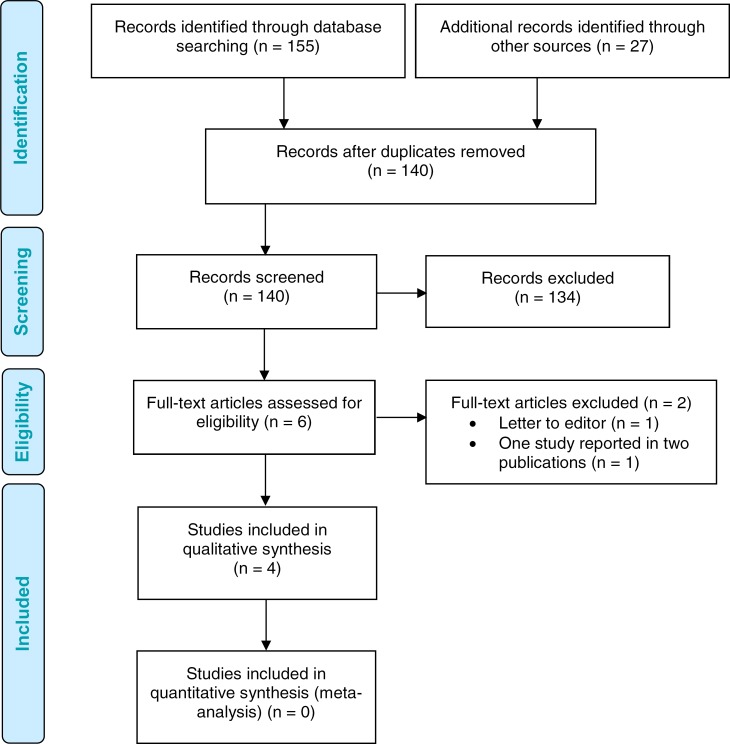
The literature search yielded 140 citations published before December 23, 2016 (with duplicates removed). We excluded a total of 134 articles based on information in the title, abstract, and publication date (i.e., before January 1, 2007). We then obtained the full texts of six potentially relevant articles for further assessment.11,12,34–37 Finally, we included four studies.11,34–36 Figure 6 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).19
Figure 6: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.19
Review of Included Economic Studies
We included four studies — three from Canada11,34,35 and one from France.36 The four studies include six analyses (five cost minimization analyses and one cost utility analysis) that compared SCIG with IVIG in patients with primary or secondary immunodeficiency.11,34–36 The results were consistent. All five cost minimization analyses indicated that SCIG creates cost savings when compared with IVIG. The cost-utility analysis (by CADTH) showed that SCIG had greater effectiveness and lower cost than IVIG.35 Table 6 provides a summary of the studies.
Table 6:
Results of Economic Literature Review—Summary
| Results | ||||||
|---|---|---|---|---|---|---|
| Name, Year, Location | Study Design and Perspective | Population | Interventions/Comparators | Health Outcomes | Costs | Cost-Effectiveness |
| Gerth et al, 2014,11 Canada |
Type of economic analysis: CMA Study design: CMA model Perspective:: Public payer Time horizon: 1 year |
Patients with primary and secondary immunodeficiency | SCIG IVIG |
NA |
First year SCIG: $691; IVIG: $3,292 Subsequent years SCIG: $345; IVIG: $3,292 Cost year: 2011 Note: Only nursing time was included. |
SCIG led to substantial cost-saving |
| Martin et al, 2013,34 Canada |
Type of economic analysis: CMA Study design: CMA model Perspective: : Public payer Time horizon: 3 year |
Adults with primary immunodeficiency | SCIG by manual rapid push IVIG | NA | SCIG: $1,978; IVIG: $7,714 Cost year: 2011 Note: 1) drug cost was not included and 2) discounting was not applied |
SCIG led to substantial cost-saving |
| Ho et al, 2008,35 Canada (first analysis)a |
Type of economic analysis: CMA Study design: CMA model Perspective: Public payer Time horizon: 1 year |
Adults and children with primary immunodeficiency | SCIG by pump Infusion administration IVIG | NA |
Adult (70 kg) SCIG: $20,417; IVIG: $21,777 Children (40 kg) SCIG: $12,101; IVIG: $13,460 Cost year: 2007 |
SCIG led to slight cost-saving |
| Ho et al, 2008,35 Canada (second analysis)b |
Type of economic analysis: CUA Study design: Decision-analytic model Perspective: : Public payer Time horizon: 1 year |
Adults with primary immunodeficiency | SCIG by pump Infusion Administration IVIG | SCIG: 0.675 QALY IVIG: 0.648 QALY |
Adult (70 kg) SCIG: $20,065; IVIG: $21,273 Cost year: 2007 |
SCIG dominated IVIG with lower cost and higher QALY |
| Beaute et al2010,36 France (first analysis)a |
Type of economic analysis: CMA Study design: CMA model Perspective: : French social insurance Time horizon: 1 year |
Patients with primary immunodeficiency | SCIG by pump Infusion Administration IVIG | NA |
Model (50 kg young adult) SCIG: €24,952; IVIG: €25,583 Cost year: not reported |
SCIG led to a slight cost-saving |
| Beaute et al2010,36 France (second analysis)c |
Type of economic analysis: CMA Study design: cohort study Perspective: French social insurance Time horizon: 1 year |
Patients with primary immunodeficiency | SCIG by pump Infusion Administration IVIG | NA |
Individual level data in the cohort (SCIG: 15.2 years old and 40 kg; IVIG: 15.6 years old and 41.8 kg) SCIG: €20,289 (IG cost: €12,935); IVIG: €26,428 (IG cost: €18,703) Cost year: Not reported |
SCIG showed substantial savings due to lower dose prescribed of IG |
Abbreviations: CUA, cost-utility analyses; CMA, cost minimization analysis; IG, immunoglobulin; IVIG, hospital-based intravenous immunoglobulin; NA, not applicable; QALY, quality-adjusted life year; SCIG, home-based subcutaneous immunoglobulin.
For the first analysis (cost minimization), authors assumed that SCIG and IVIG would yield identical clinical outcomes.
For the second analysis (cost utility), authors assumed that SCIG and IVIG would yield different clinical outcomes.
Authors include eight patients with SCIG therapy and 26 patients with IVIG therapy. The crude results are reported.
The report by CADTH includes two analyses, a cost-minimization analysis and a cost-utility analysis, with 1-year time horizon.35 The analyses compared SCIG by pump infusion with IVIG for patients with primary immunodeficiency. In the cost-minimization analysis, the authors assumed equal effectiveness of both treatments. The authors also assumed that the monthly dosage and price of SCIG and IVIG were same. SCIG resulted in lower cost than IVIG for both adults and children due to reduced nursing time (adult: $20,417 vs $21,777; children: $12,101 vs $13,460). The authors conducted a cost-utility study assuming favorable outcomes for SCIG and concluded that SCIG dominated IVIG with lower cost and greater QALY. Results should be interpreted with caution as the key parameters were based on experts’ assumptions (e.g., probability of remaining in a healthy state for both treatments, and the number and severity of infections for both treatments).
Martin et al (2013)34 conducted a cost-minimization analysis comparing SCIG by manual push with IVIG for adults with primary immunodeficiency. Authors included the cost of nursing time and infusion supplies but excluded the cost of IG. This study showed that SCIG resulted in substantial cost savings of $5,736 CAD per patient in three years.
Gerth et al (2014)11 conducted a cost-minimization analysis for both primary and secondary immunodeficiencies. They included only the cost of nursing time. Using the results from Martin et al,34 the authors estimated that, compared with IVIG, nursing time savings by SCIG was 45.2 hours in year 1, and 51.2 hours in each subsequent year. The hourly compensation rate was reported to be $57.58, including wages and benefits. The net economic benefits were $2,603 in year 1 and $2,948 in each subsequent year.
Beaute et al (2009)36 included two analyses (a cost-minimization model and a cohort study) that compared SCIG by pump with IVIG for young adults with primary immunodeficiency using the French social insurance perspective. The cost of renting a SCIG pump was reported to be €306.41 per month per unit, and the estimated total infusion pump/kit was €7,354 per year per patient. In the cost-minimization model, the authors assumed that both treatments had the same cost for the immunoglobulin drug, while SCIG had additional costs for pump rental and IVIG had additional costs for in-hospital treatment. The total yearly cost in the SCIG group was slightly lower than IVIG (€24,952 vs €25,583). In the cohort study, the authors collected individual level data from eight patients with SCIG treatment and 26 patients with IVIG treatment. This study showed substantial savings of approximately €6,000 per patient with SCIG compared with IVIG. However, the cost savings with SCIG were mainly driven by the lower immunoglobulin dose (23.4 g per month on average in the SCIG group vs 32.9 g per month in the IVIG group).
Applicability of the Included Studies
After reviewing the four studies using the quality appraisal checklist, we found the results of the Canadian analyses were partially applicable to the publicly funded health care system in Ontario, but the French analysis was not applicable. The complete results of the applicability checklist applied to all the included full-text articles can be found in Appendix 3.
Discussion
There were three economic studies published in the last 10 years that compared SCIG with IVIG for the Canadian population.11,34,35 Although those studies provided elaborate analyses, the estimation of a key cost component—nursing time for IVIG—may be overestimated. The authors used the average duration of IVIG infusion time (4 hours) multiplied by the average number of infusion per year (14.3) to estimate the nursing time.34 However, it may be unrealistic to assume that one nurse would only treat the one IVIG patient during the entire infusion period. As a result, the cost savings by SCIG is likely overestimated. Thus, we judged that results in those studies were partially applicable to our setting.
Beaute et al36 (the French study) found that the cost of the infusion pump and kit was as high as €7,354 per year. This was much higher than the cost in Canada ($2,000 per year38). Also, the cost difference between the two administration methods in Beaute et al's second analysis suggested significant cost savings of SCIG due to lower dosage. However, the difference of immunoglobulin dose between two groups may be associated by patient characteristics (e.g., the severity of the disorder), rather than the administration method. Most clinical studies suggest that patients need the same or similar dose with SCIG as with IVIG.
Although the published studies11,34–36 provided valuable details and showed consistent results, their findings may be only partially applicable to our research question given the limitations discussed above.
Conclusions
The systematic review identified four economic studies on SCIG versus IVIG for patients with primary or secondary immunodeficiency. Those studies showed that, compared with IVIG in hospital, home-based SCIG lead to cost savings mainly due to the decrease in nursing time needed. Three studies were partially applicable to the Ontario context.
BUDGET IMPACT ANALYSIS
Our clinical evidence review found low-quality evidence comparing the clinical outcomes of SCIG and IVIG. Serious bacterial infections were rare and the risk was comparable between the two administration methods. Compared with IVIG, home-based SCIG carries less risk of systemic adverse effects, but higher risk of local reactions due to the more frequent infusions (weekly SCIG vs every 3 to 4 weeks with IVIG). See Tables 3 and 5, above, for details. Some studies suggest that the convenience of SCIG may enable better adherence, leading to improved health-related quality of life in the given domains. However, these studies had a high degree of heterogeneity and reported inconsistent results. Therefore, we could not quantify the difference in QALYs between the two administration methods. See Figures 3–5, above. Overall, the difference in outcomes for SCIG and IVIG is relatively small. For simplicity, we assumed that SCIG and IVIG have similar clinical outcomes and health-related quality of life, and focused only on the cost implications in this report.
We conducted a budget impact analysis to estimate the 5-year cost burden of funding the SCIG program for adults and children with primary or secondary immunodeficiency in Ontario. The analysis was conducted from the perspective of the Ontario Ministry of Health and Long-Term Care. All costs were reported in 2017 Canadian dollars.39
Research Question
From the perspective of the Ontario Ministry of Health and Long-Term Care, what is the potential budget impact of funding the home-based program of subcutaneous infusion of immunoglobulin (SCIG) for adults and children with primary and secondary immunodeficiency in Ontario?
Methods
Target Population
The target population was adults and children with primary or secondary immunodeficiency who are being treated with IVIG and are eligible for SCIG (e.g., they have had no allergic reactions to immunoglobulin products). Given the similarity of immunoglobulin therapies for primary and secondary immunodeficiency, we did not differentiate these two types of immunodeficiency in the present report.
In the base case, we estimated the size of the target population based on expert consultation estimating the potential impact of funding SCIG in Ontario (content expert, personal communication, February 20, 2017). Presently, there are 895 patients treated with IVIG and 248 patients treated with home-based SCIG through six hospitals in Toronto, Ottawa, Hamilton, London, and Sudbury. The target populations for IVIG and SCIG are expected to increase as a result of aging and increased cancer survival. According to Canadian Blood Services, which manages the blood supply in all provinces and territories except Quebec, in the past 3 years, total immunoglobulin use in Canada has increased 8%, 10%, and 6%.40 Therefore, we assumed an average 8% yearly increase of the target population (IVIG and SCIG users) in Ontario over the next few years. Based on a recent survey by the Ontario Regional Blood Coordinating Network, about 12% patients receiving IVIG treatment are in the neonatal and pediatric population.17 We assumed that this rate would remain stable over the next 5 years and that the percentage of patients receiving SCIG treatment is the same for children and adults. The expected number of patients receiving IVIG and SCIG therapy over the next 5 years in those six hospitals is presented in Table 7.
Table 7:
Expected Number in Target Population in the Next 5 Years in Ontario
| Expected Number of Patients | |||||
|---|---|---|---|---|---|
| Type of Treatment Method | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
| Total immunodeficiency patients treated with IVIG or SCIG | 1,143 | 1,234 | 1,333 | 1,440 | 1,555 |
| Reference scenario: current uptake rate of SCIG | 22% | 22% | 22% | 22% | 22% |
| SCIG (adults and children) | 248 | 268 | 289 | 312 | 337 |
| Adult patients (88%) | 218 | 236 | 254 | 275 | 297 |
| Pediatric patients (12%) | 30 | 32 | 35 | 37 | 40 |
| IVIG (adults and children) | 895 | 966 | 1,044 | 1,128 | 1,218 |
| Adult patients (88%) | 788 | 850 | 919 | 993 | 1,072 |
| Pediatric patients (12%) | 107 | 116 | 125 | 135 | 146 |
| New Scenario: increased uptake rate of SCIG | 43% | 50% | 57% | 64% | 70% |
| SCIG (adults and children) | 491 | 617 | 760 | 922 | 1,089 |
| Adult patients (88%) | 432 | 543 | 669 | 811 | 958 |
| Pediatric patients (12%) | 59 | 74 | 91 | 111 | 131 |
| IVIG (adults and children) | 652 | 617 | 573 | 518 | 466 |
| Adult patients (88%) | 574 | 543 | 504 | 456 | 410 |
| Pediatric patients (12%) | 78 | 74 | 69 | 62 | 56 |
Abbreviations: IVIG, intravenous immunoglobulin; SCIG, subcutaneous immunoglobulin.
There were few studies investigating the incidence and prevalence of primary or secondary immunodeficiency in Canada. Gerth et al estimated that there were 2,125 patients with primary and secondary immunodeficiency in Ontario in 2012, including 1,381 primary and 744 secondary.11 Hospitals need a certain volume to maintain competency on training patients, tracking product, and reporting adverse events. In our scenario analyses, we estimated that 60% of all immunodeficiency patients in Ontario could gain access to SCIG. Assuming an 8% annual increase, the target population in Ontario would be 1,873 in year 1 (2017), 2,023 in year 2 (2018), 2,185 in year 3 (2019), 2,360 in year 4 (2020) and 2,549 in year 5 (2021).
Current Use and Future Uptake of SCIG
Based on the expert consultation (content expert, personal communication, February 20, 2017), 22% of patients are currently receiving SCIG. We assumed that 43% of patients (i.e., 21% increase) would receive SCIG at year 1 if this program were publically funded. It is expected that the delivery system for SCIG would continue improving (e.g., moving to pre-fill syringes). Assuming greater accessibility to SCIG in the future, we estimated that the adoption rate would gradually increase to 70% in year 5. The expected number of patients with IVIG and SCIG treatments are presented in Table 7.
Resources and Canadian Costs
We used the unit price to estimate the costs of the two administration methods. Based on published economic studies of SCIG in Canada,11,12,34,35 we identified the resource uses related to the two administration methods. We included the cost of immunoglobulin product, nursing time, hospital charges for IVIG, infusion supplies (pump or manual push) for SCIG, and transportation for home visits by nurses for SCIG in the base case. The main advantage of SCIG treatment is the reduction of hospital visits. We conducted a scenario analysis using societal perspective to capture this advantage. We included the costs of transportation and time loss for patients and caregivers. Since both administration have similar risk and severity of infection, we excluded that cost. We also excluded physician cost since treatments are generally provided by nurses. The unit prices and resource use estimates were obtained from the literature or clinical experts. The clinical experts also verified all parameter inputs. Tables 8 and 9 show the cost inputs of IVIG and SCIG, respectively. More details of the cost components included in our analysis are presented below.
Table 8:
Cost Items for Hospital-Based Intravenous Immunoglobulin
| Cost Items | Unit Price | Resource Use Per Year | Cost Per Year |
|---|---|---|---|
| 1. Medication (immunoglobulin) | |||
| Adult patients35,41 | $50.69/g | 336 g (0.4g/kg × 70kg ×12 mo) | $17,032.72 |
| Pediatric patients35,41 | $50.69/g | 192 g (0.4g/kg × 40kg ×12 mo) | $9,733.26 |
| 2. Nursing hours per year34,35,35 | $58.88/hr | 22.93 hours [(4 ÷ 2.5) × 14.33] | $1,350.23 |
| 3. Hospital charge35 | $127.33/mo | 12 mo | $1,528.00 |
| 4. Transportation of patient visit to hospital in the first year | |||
| Adult patients35 | $11.93/visit | 14.33 visits | $170.93 |
| Pediatric patients (with parents)35 | $14.91/visit | 14.33 visits | $213.67 |
| 5. Productivity costs | |||
| Paid time42 | $36.91/hr | — | — |
| Unpaid time43 | $14.82/hr | — | — |
| Adult patients35,42–44 | — | 60.20 paid hours; 25.80 unpaid hours | $2,604.31 [(36.91 × 60.20) + (14.82 × 25.80)] |
| Caregivers for pediatric patient35,42–44 | — | 80.84 paid hours; 5.16 unpaid hours | $3,060.24 [(36.91 × 80.84) + (14.82 × 5.16)] |
Note: Numbers may be inexact due to rounding.
Table 9:
Cost Items of Home-Based Subcutaneous Immunoglobulin
| Cost Items | Unit Price | Resource Use Per Year | Cost |
|---|---|---|---|
| 1. Medication (immunoglobulin) | |||
| Adult patients35,41 | $50.69/g | 336g(0.4 g/kg × 70 kg × 12 mo) | $17,032.72 |
| Pediatric patients35,41 | $50.69/g | 192g(0.4g/kg × 40kg × 12 mo) | $9,733.26 |
| 2. Nursing hours per year | |||
| First year | $58.88/hr | 13 hr | $765.40 |
| Subsequent years | $58.88/hr | 7 hr | $412.14 |
| 3. Infusion supply | |||
| Infusion pump and materials38 | $2,000/yr | — | — |
| Manual push38 | $200/yr | — | — |
| Adult patients38,45 | — | Pump: 70%; Manual: 30% | $1,460 [(2,000 × 0.7) + (200 × 0.3)] |
| Pediatric patients38,45 | — | Pump: 90%; Manual: 10% | $1,820 [(2,000 × 0.9) +(200 ×0.1)] |
| Covered by hospital (estimated) | — | — | 20% of total supply cost |
| 4. Transportation of nurse's visit to patient's home (estimated) | $15/visit | 2 visits | $30 |
| 5. Transportation of patient's visit to hospital in the first year | |||
| Adult patients35 | $11.93/visit | 2 visits | $23.85 |
| Pediatric patients (with parents)35 | $14.91/visit | 2 visits | $29.81 |
| 6. Productivity costs (unpaid time) | |||
| Infusion pump (per year)43 | $14.82/hour | 78 hr (1.5 hr × 52 wk) |
$1,155.96 |
| Manual push (per year)43 | $14.82/hour | 26 hr (0.5 hr × 52 wk) |
$385.32 |
| Adult patients43 | — | — | $924.77 [(1,155.96 × 0.7) + (385.32× 0.3)] |
| Caregivers for pediatric patient43 | — | — | $1,078.90 [(1,155.96 × 0.9) + (385.32× 0.1)] |
Note: Numbers may be inexact due to rounding.
Medication
The Canadian Blood Services supply immunoglobulin products. Gammaguard S/D, Gamunex, Octagam (soon to be replaced by Panzyga), and Privigen are used for IVIG in Ontario. Hizentra is used for SCIG. Although prices are confidential, experts suggest that all immunoglobulin products are priced comparably. Based on the cost from the Atlantic Provinces, we estimate that the price for all immunoglobulin drugs is $50.69 per gram.41
Most publications identified in our clinical evidence review used the same monthly dosage for SCIG and IVIG. See Table 2, above. Also, the SCIG dosage recommended in Canada and most of Europe is equivalent to the IVIG dosage based on trough levels. Therefore, we assumed both administration methods have the same dose in the base case. We used estimates from Ho et al,35 and assumed that the average dose was 0.4 g/kg of body weight per month and the average weights were 70 kg and 40 kg for adults and children, respectively. The annual costs of immunoglobulin products are $9,732 (192 g) and $17,032 (336 g) for pediatric and adult patients, respectively.
Some U.S. publications suggested that SCIG requires a higher dose (e.g., 37% or 53% dose increase) to achieve the same area under the curve (pharmacokinetics) of plasma drug concentration and time,46 so we included one scenario analysis using a 37% higher dosage for SCIG than for IVIG.
Nursing Hours
Administration of IVIG requires 13 hospital visits per year (one every 4 weeks) for two thirds of the patients and 17 visits (one every 3 weeks) for the remaining one third,34 for an average of 14.33 visits per patient per year. Each IVIG infusion lasts about 4 hours. We estimated that one nurse can manage 2.5 patients at a time, for an average of about 1.6 hours (4 hours ÷ 2.5 patients) per IVIG infusion, or 22.93 hours per year. The cost of nursing time is $58.88 per hour, including salary and benefits (i.e., $97,500 for 1 full-time nurse at 1,656 hours per year [content expert, personal communication, February 20, 2017]). The cost of nursing time for each IVIG patient was $1,350 per year.
SCIG is usually administrated by patients or caregivers at home. On average, a nurse spends 6 hours training each patient in self-administration. We estimated that the nursing time for routine follow up and monitoring is about 7 hours per year, including 1 hour of travel time to a patient's home. The nurses monitor the effectiveness and safety of the SCIG therapy and keep track of the immunoglobulin products in the patient's home to ensure that supplies are available and properly stored.35 Nurses perform home visits for each patient every 6 months,34 and respond to questions related to SCIG therapy regularly through phone and email. Nursing time is estimated to be 13 hours ($765) in the first year of starting SCIG, and 7 hours ($412) in each subsequent year. We assumed that all patients with SCIG treatment are new patients in the first year. We assumed that in each subsequent year, 20% of SCIG patients (e.g., 123 of 617 in year 2 in the increasing uptake group) would be new patients needing 6 hours of training.
Average travel distance for nurses visiting patient homes was estimated to be about 30 kilometres (round trip) per visit. According to the current reimbursement policy, the rate is about $0.5 per kilometer. Thus, we estimated transportation costs of $15 per visit and $30 per year.
Hospital Cost for IVIG
The total hospital costs for IVIG, including administrative support, data management, and infusion materials, was approximately $1,528 per patient per year.35,47
Supply of SCIG Infusion at Home
SCIG could be administrated via two different methods. According to the Hizentra CARE Program,45 about 70% of adults and 90% of pediatric patients in Ontario with SCIG therapy use the infusion pump. The remaining patients use manual push infusion. The materials for the manual push (e.g., syringe, tubing, and sterility supplies) cost about $200 per patient per year.38 According to one Ontario hospital,38 the cost of supplies for the infusion pump was about $2,000 per year.
Currently, supplies of SCIG are paid by various sources, including hospitals, private insurance, compassionate support programs, and patient themselves. In the base case, we assumed that 20% of the costs of supplies of SCIG are paid by the hospital.
Patients’ Time and Transportation
One major advantage of SCIG is the reduced need for transportation and hospital visits. We captured these costs in a scenario analysis and adopted the societal perspective. Earlier publications suggest that the average round-trip travel cost for IVIG infusion is about $11.93 for adults.35 For the pediatric patients, we assumed an additional 25% for the caregiver (total: $14.91 per hospital visit). Patients new to SCIG require two hospital visits for self-administration training.
We used the human capital approach to estimate productivity costs. The human capital approach estimates any hour of absence of work due to illness and values it at the market salary rate. For salaried employees, the value of their time equals their gross earnings, which includes the salary, benefits, and employment overhead. We assumed that the benefits and employment overhead is 30% of salary.48 We used an average wage of $28.39 per hour for full-time employees,42 giving a cost for productivity loss of $36.91 per hour. There are many debates on how to value leisure time and unpaid work for the salaried employee, as well as time for unemployed people. We used the Ontario minimum wage rate of $11.40 per hour,43 giving a cost for productivity loss of $14.82 per hour including the benefits and overhead.
We considered the costs due to time loss for adult patients and caregivers for pediatric patients. We assumed that the caregivers are the parents, and therefore not compensated for their time. For patients receiving IVIG, we estimated a time loss is 6 hours per visit, including the infusion, traveling, and hospital administration. We estimated that 70% of adult patients and 94% of caregivers are in the labor force (we assume that caregivers are employed at the same rate as the general population).44 Thus, on average, the time loss is about 60 hours ([6 hr × 14.33 visits] × 70%) and 26 hours ([6 hr × 14.33 visits] × 30%) for the paid and unpaid time per year, respectively, for adult patients. The time loss, on average, is about 81 hours ([6 hr × 14.33 visits] × 94%) and 5 hours ([6 hr × 14.33 visits] × 6%) per year, respectively, for caregivers. Thus, the cost for productivity loss is $2,604 for per adult patient per year, and $3,060 per pediatric patient per year.
We assumed that all patients and caregivers use their unpaid time for SCIG due to the flexibility of schedule for SCIG administration. We estimated that it takes 1.5 hours for the infusion pump and 0.5 hours for manual infusion. About 70% of adults and 90% of children use the infusion pump for SCIG. Thus, the estimated total treatment times were 62.4 and 72.8 hours per year for adults and pediatric patients, respectively. The corresponding productivity cost per patient was about $925 for adults and $1,079 for children.
Analysis
The budget impact is calculated as the cost difference between the New Scenario (increased uptake of SCIG from 43% in year 1 to 70% in year 5) and the Reference Scenario (current uptake of SCIG, 22%). The total cost in each scenario is calculated using the average cost per patient multiplied by the number of patients per year.
In addition to the base case, we also conducted analyses in five scenarios:
In scenario one, we conduct the analysis from a societal perspective, including the cost of transportation and the productivity cost of treatment for the patients
In scenario two, we use the increased dose of SCIG to achieve an equivalent area under the curve of plasma drug concentration and time as that from IVIG treatment
In scenario three, we include only the cost of nursing time
In scenario four, we assume that the Ontario Ministry of Health and Long-Term Care will pay for the full amount of SCIG infusion supplies
In scenario five, we estimate the net budget impact for the greater target population, under the assumption that SCIG is accessible to 60% immunodeficiency patients in Ontario
We calculated the total net budget impact from SCIG over 5 years at base case and estimated the present value of the net budget impact over 5 years at the annual discounting rate of 1.5%, as recommended by the Canadian Agency for Drugs and Technologies in Health.49
The budget impact analysis was conducted using Excel 2013 (Microsoft Corp.).
Expert Consultation
We consulted specialist(s) in the field of immunology and primary care to provide advice on the methods of immunoglobulin administration at home and in hospital and the feasibility of developing a home-based program for infusion of immunoglobulin in Ontario. Feedback from the Ministry of Health and Long-Term Care with respect to the population of Ontario was taken into consideration in the overall assessment.
Results
Average Cost per Patient
Table 10 presents the results of the average yearly cost per patient in the base case. Compared with IVIG, SCIG was associated with lower costs, saving on average $1,790.83 per adult and $1,718.83 per child in the first year, and $2,144.09 per adult and $2,072.09 per child in each subsequent year.
Table 10:
Total Average Cost per Patient per Year of Intravenous and Subcutaneous Immunoglobulin, Base Case
| Treatment | Total Average Cost | Cost Items Included | Incremental Cost |
|---|---|---|---|
| Adult patients | |||
| IVIG | $19,910.48 | Items 1–3, Table 8 | – |
| SCIG | |||
| First year | $18,119.66 | Items 1–3 (20% cost for the infusion supply) and 4, Table 9 | −$1,790.83 |
| Subsequent years | $17,766.40 | Items 1–3 (20% cost for the infusion supply) and 4, Table 9 | −$2,144.09 |
| Pediatric patients | |||
| IVIG | $12,610.95 | Items 1–3, Table8 | – |
| SCIG | |||
| First year | $10,892.12 | Items 1–3 (20% cost for the infusion supply) and 4, Table 9 | −$1,718.83 |
| Subsequent years | $10,538.86 | Items 1–3 (20% cost for the infusion supply) and 4, Table 9 | −$2,072.09 |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Note: Numbers may be inexact due to rounding.
Table 11 presents the average yearly cost per patient in the scenario analyses. When considering the societal perspective, the cost savings of SCIG is greater. If SCIG therapy is associated with an increased dosage of immunoglobulin product (37% greater than IVIG), SCIG has a higher cost than IVIG. SCIG shows a savings of about 10 hours of nursing time ($584.83) per patient in the first year, and the yearly savings increased to about 16 hours ($938.09) per patient in the subsequent years. If the Ontario Ministry of Health and Long-Term Care pays the full cost of supplies for SCIG, the system savings substantially decrease.
Table 11:
Total Average Cost per Patient per Year of Intravenous and Subcutaneous Immunoglobulin, Scenario Analyses
| Total Average Cost ($) | Cost Items Included | Incremental Cost ($) | |
|---|---|---|---|
| Scenario 1: societal perspective | |||
|
Adult patients IVIG SCIG |
22,685.73 | Items 1–5, Table 8 | — |
| First year | 20,236.28 | Items 1–3 (full amount of the infusion supply) and 4–6, Table 9 | –2,449.46 |
| Subsequent years | 19,883.01 | Items 1–3 (full amount of the infusion supply) and 4–6, Table 9 | –2,802.72 |
|
Pediatric patients IVIG SCIG |
15,884.86 | Items 1–5, Table 8 | — |
| First year | 13,456.83 | Items 1–3 (full amount of the infusion supply) and 4–6, Table 9 | –2,428.03 |
| Subsequent years | 13,103.57 | Items 1–3 (full amount of the infusion supply) and 4–6, Table 9 | –2,781.29 |
| Scenario 2: higher dose for SCIG at home (IVIG is same as base case) | |||
|
Adult patients SCIG |
|||
| First year | 24,421.59 | Items 1 (37% higher), 2 & 3 (20% cost for the infusion supply), and 4, Table 9 | 4,511.11 |
| Subsequent years | 24,068.33 | Items 1 (37% higher), 2 & 3 (20% cost for the infusion supply), and 4, Table 9 | 4,157.85 |
|
Pediatric patients SCIG |
|||
| First year | 14,493.22 | Items 1 (37% higher), 2 & 3 (20% cost for the infusion supply), and 4, Table 9 | 1,882.28 |
| Subsequent years | 14,139.96 | Items 1 (37% higher), 2 & 3 (20% cost for the infusion supply), and 4, Table 9 | 1,529.02 |
| Scenario 3: nursing time only | |||
|
Adult or pediatric patients IVIG |
1,350.23 | Item 2 (22.93 hours of nursing time), Table 8 | — |
| SCIG | |||
| First year | 765.40 | Item 2 (13 hours of nursing time), Table 9 | –584.83 |
| Subsequent years | 412.14 | Item 2 (7 hours of nursing time), Table 9 | –938.09 |
| Scenario 4: SCIG infusion supplies fully paid by Ministry (IVIG is same as base case) | |||
|
Adult patients SCIG |
|||
| First year | 19,287.66 | Items 1–3 (full amount of the infusion supply), and 4, Table 9 | –622.83 |
| Subsequent years | 18,934.40 | Items 1–3 (full amount of the infusion supply), and 4, Table 9 | –976.09 |
|
Pediatric patients SCIG |
|||
| First year | 12,348.12 | Items 1–3 (full amount of the infusion supply), and 4, Table 9 | –262.83 |
| Subsequent years | 11,994.86 | Items 1–3 (full amount of the infusion supply), and 4, Table 9 | –616.09 |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Note: Numbers may be inexact due to rounding.
Base Case of Budget Impact Analysis
Table 12 presents the projected total costs of SCIG and IVIG at the current uptake rate and with an increased uptake rate. It also shows the expected net budget impact in the next 5 years. An increasing uptake rate of SCIG would lead to savings of about $0.4 million in the first year. With a continued trend of increasing uptake and an increasing target population, the net budget savings would reach about $1.6 million by year 5. The increased uptake of SCIG and the deferred savings in nursing time due to training needed by patients at the start of self-administration contribute to larger budget savings in later years. The total savings from funding the SCIG program is about $5.0 million over 5 years. The present value of the savings is $4.8 million at an annual discounting rate of 1.5%.
Table 12:
Total Costs and Net Budget Impact for an Increasing Uptake Rate for SCIG Versus Continued Current Uptake Rate in Ontario, Base Case
| Results ($) | |||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Total cost of reference scenario: current uptake rate of SCIG (22%) | 21,315,682 | 22,935,829 | 24,776,046 | 26,771,329 | 28,907,221 |
| SCIG (adults and children) | |||||
| Adult patients | 3,950,085 | 4,209,543 | 4,530,610 | 4,905,188 | 5,297,603 |
| Pediatric patients | 326,764 | 339,504 | 371,333 | 392,552 | 424,380 |
| IVIG (adults and children) | |||||
| Adult patients | 15,689,462 | 16,923,912 | 18,297,735 | 19,771,111 | 21,344,039 |
| Pediatric patients | 1,349,371 | 1,462,870 | 1,576,368 | 1,702,478 | 1,841,198 |
| Total cost of new scenario: increased uptake rate of SCIG | 20,882,598 | 22,215,224 | 23,803,490 | 25,504,561 | 27,347,249 |
| SCIG (adults and children) | |||||
| Adult patients | 7,827,691 | 9,685,517 | 11,932,985 | 14,465,846 | 17,087,892 |
| Pediatric patients | 642,635 | 785,104 | 965,465 | 1,177,655 | 1,389,846 |
| IVIG (adults and children) | |||||
| Adult patients | 11,428,618 | 10,811,393 | 10,034,884 | 9,079,181 | 8,163,299 |
| Pediatric patients | 983,654 | 933,210 | 870,155 | 781,879 | 706,213 |
| Net budget impact | –433,083 | –720,606 | –972,557 | –1,266,768 | –1,559,972 |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Note: Numbers may be inexact due to rounding.
There is likely to be no direct budget impact from SCIG since most of the theoretical savings is in nursing time and hospital administrative support. However, it may help improve the efficiency of hospitals, for example, by allowing a hospital to reallocate the saved nursing time to other patient care needs, increasing the volume of health care services and reducing wait times.
Scenario Analyses of Budget Impact Analysis
Table 13 presents the results of the scenario analyses. Compared with the base case, SCIG led to greater savings from the societal perspective. If SCIG is associated with 37% higher dose of immunoglobulin, it would increase the annual budget from $1 million to $3 million in next 5 years. When considering only nursing time, SCIG resulted in a savings of approximately 1.5 FTE (2,414 hours) in the first year and 7 FTEs (11,079 hours) in the fifth year after funding SCIG. If the ministry pays the full cost of the supplies for SCIG infusion, the budget savings will be much smaller. If the target population is large (e.g., 60% of all patients with primary and secondary immunodeficiency), the potential savings will be greater. However, because the bulk of the savings to the health care system is in nursing time and hospital administrative support, it may not lead to direct cost savings, but instead free up resources for other priorities.
Table 13:
Total Costs and Net Budget Impact for an Increasing Uptake Rate for SCIG Versus a Continued Current Uptake Rate in Ontario, Scenario Analyses
| Uptake Rate of SCIG | Results ($) | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
| Scenario analysis 1: societal perspective | ||||||
| Total cost of SCIG and IVIG | Current: 22% | 24,391,250 | 26,256,156 | 28,363,124 | 30,646,092 | 33,091,502 |
| Increasing | 23,796,653 | 25,303,565 | 27,077,521 | 28,974,317 | 31,032,138 | |
| Net budget impact | –594,597 | –952,591 | –1,285,603 | –1,671,775 | –2,059,364 | |
| Scenario analysis 2: higher dose for SCIG (IVIG is the same as the base case) | ||||||
| Total cost of SCIG and IVIG | Current: 22% | 22,797,537 | 24,538,321 | 26,502,777 | 28,637,602 | 30,922,940 |
| Increasing | 23,817,500 | 25,903,656 | 28,347,185 | 31,015,153 | 33,856,248 | |
| Net budget impact | 1,019,963 | 1,365,335 | 1,844,408 | 2,377,551 | 2,933,308 | |
| Scenario analysis 3: nursing time only | ||||||
| Total nursing hours of RARP and ORP | Current: 22% | 23,749 | 24,351 | 26,312 | 28,427 | 30,696 |
| Increasing | 21,335 | 19,209 | 19,373 | 19,440 | 19,617 | |
| Difference in nursing hours | –2,414 | –5,142 | –6,939 | –8,987 | –11,079 | |
| Total cost of SCIG and IVIG | Current: 22% | 1,398,274 | 1,433,709 | 1,549,165 | 1,673,689 | 1,807,279 |
| Increasing | 1,256,160 | 1,130,973 | 1,140,601 | 1,144,551 | 1,154,965 | |
| Net budget impact | –142,114 | –302,736 | –408,564 | –529,138 | –652,314 | |
| Scenario analysis 4: SCIG infusion supplies fully paid by Ministry (IVIG is the same as the base case) | ||||||
| Total cost of SCIG and IVIG | Current: 22% | 21,613,986 | 23,258,069 | 25,123,678 | 27,146,401 | 29,312,357 |
| Increasing | 21,473,078 | 22,957,192 | 24,717,378 | 26,613,425 | 28,656,929 | |
| Net budget impact | –140,907 | –300,878 | –406,301 | –532,976 | –655,428 | |
| Scenario analysis 5: increased number of target population, 60% immunodeficiency patients in Ontario | ||||||
| Total cost of SCIG and IVIG | Current: 22% | 34,922,948 | 37,593,558 | 40,598,782 | 43,851,324 | 47,359,792 |
| Increasing | 34,215,309 | 36,422,816 | 39,021,229 | 41,805,116 | 44,834,563 | |
| Net budget impact | –707,639 | –1,170,743 | –1,577,554 | –2,046,208 | –2,525,230 | |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
Note: Numbers may appear inexact because of rounding.
Discussion
Our analysis shows that funding SCIG could lead to cost savings, but the savings are likely to lead to improved efficiency in hospitals, rather than direct budget savings. But there is uncertainty in the findings. For example, 86% of the total cost for IVIG and 94% of the total cost for SCIG in adults were for the immunoglobulin products, but the exact prices and dosages of immunoglobulin products in Ontario are unclear. Different costs for immunoglobulin products would change the analysis. Canada and most European countries determine the dosage based on trough levels, but if the dosage is determined by the area under curve of plasma drug concentration and time, then more immunoglobulin would be used for SCIG than for IVIG. Consequently, SCIG could be more costly than IVIG.
If the ministry pays the full cost of supplies for SCIG infusion, the theoretical savings would be much lower. If the ministry provides extra funding for supplies, SCIG may lead to a budget increase. However, the most straightforward analysis shows that SCIG will reduce nursing time compared with IVIG, especially after the initial training period. With improvements to the delivery system, such as the use of pre-filled syringes, the training time for SCIG might be shortened, from present 6 hours to as little as 4 hours.
Our findings are consistent with earlier Canadian studies,11,34,35 as well as with a more recent prospective study in Ontario.50 However, the savings in nursing hours in our study is much lower than was found in the earlier studies. We estimate that, on average, one nurse can manage 2.5 patients on IVIG, while earlier studies assumed that one nurse would serve a single patient on IVIG during the entire infusion period.
Our analysis shows that SCIG is associated with lower costs than IVIG. Theoretically, hospitals could move nursing time from IVIG to SCIG to support a SCIG program, in which case there would be no need for new FTEs. However, in practice, reallocation of health care resources from an existing program to a specific new program is a complicated undertaking. For example, nurses in charge of IVIG infusion often have multiple responsibilities across a broad range of diseases and may not be available to switch from IVIG to SCIG.
We expect target populations for IVIG and SCIG infusion to increase. The more realistic approach to creating a SCIG program is through new FTEs. Nurses dedicated to SCIG are able to provide the best quality of care. Based on the experience of on Ontario hospital, we estimate the optimal ratio is around 150 patients per nurse, with a 180-patient maximum. The potential budget increase for each new nurse is $97,500 (content expert, personal communication, February 20, 2017). Our health system would gain productivity from savings in nursing time, and our society may also gain productivity from the reduced loss of working days by patients.
Study Strengths
Our study has the following strengths:
We estimated the budget impact from two perspectives and provided budget estimates for multiple scenarios
Our experts verified the main assumptions and cost parameters
We presented the cost differences of two immunoglobulin administration methods for subgroups of the adults and children, in the first year and in subsequent years
Study Limitations
The following limitations should be noted when interpreting the findings of this analysis:
There were no high quality, clinical trials, to compare the effects, adverse events, or health related quality of life for the two administration methods
The exact price of immunoglobulin products in Ontario is unknown
Published data suggest that the variability of the dosage of SCIG and IVIG is large, but local data directly comparing dosages of SCIG versus IVIG is not available
Conclusions
Our analysis shows that funding SCIG may lead to net budget savings due to savings in nursing time, but the cost savings may be not translated into monetary benefit. It is more likely to create opportunities to improve the efficiency of hospitals and/or the health care system.
PATIENT, CAREGIVER, AND PUBLIC ENGAGEMENT
Background
Public and patient engagement explores the lived experience of people with a health condition, including the impact that the condition and its treatment has on the patient, the patient's family, or other caregivers, and on the patient's personal environment. Public and patient engagement increases awareness and builds appreciation for the needs, priorities, and preferences of the person at the centre of a treatment program. The insights gained through public and patient engagement provide an in-depth picture of lived experience, through an intimate look at the values that underpin the experience.
Lived experience is a unique source of evidence about the personal impact of a health condition and how that condition is managed, including what it is like to navigate the health care system, and how technologies may or may not make a difference in people's lives. Information shared from lived experience can also identify gaps or limitations in published research (for example, outcome measures that do not reflect what is important to those with lived experience).51–53 Additionally, lived experience can provide information or perspectives on the ethical and social values implications of technologies and treatments. Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored by published literature, Health Quality Ontario reaches out to and directly speaks with people who live with the health condition, including those who may have experience with the intervention in question.
For this study, eight individuals were engaged to discuss their lived experience with immunoglobulin treatment. We spoke to individuals who had experience with both hospital-based intravenous immunoglobulin therapy (IVIG) and home-based subcutaneous immunoglobulin therapy (SCIG) and individuals who had experience with SCIG alone. Understanding and appreciating their day-to-day functioning and experience of different treatments helped to contextualize the potential value of the interventions from a lived experience perspective.
Methods
Engagement Plan
Engagement as a concept captures a range of efforts used to involve the public and patients in various domains and stages of health technology assessment decision-making.54 Rowe and Frewer outline three types of engagement: communication, consultation, and participation.55 Communication constitutes a one-way transfer of information from the sponsor to the patient, while participation involves the sponsor and patient collaborating through real-time dialogue. Consultation, on the other hand, refers to the sponsor's seeking out and soliciting information (e.g., experiential input) from the public, patients, and caregivers affected by the health technology or intervention in question.
The engagement plan for this health technology assessment was consultation. Within this typology, the engagement design focused on interviews to examine the lived experience of patients with immunodeficiency, including those having experience of intravenous and/or subcutaneous immunoglobulin treatment.56
The interview format was selected as an appropriate investigative method because it allows Health Quality Ontario staff to deeply explore the central themes in the lived experience of the participants. The main purpose of interviewing is to understand the meaning of what participants say.57 Interviews are particularly useful for getting the story and context behind a participant's experiences, which was the objective in this portion of the report. The sensitive nature of quality-of-life issues is another reason for using confidential one-on-one interviews for this project.
Participant Recruitment
For this project, we used a recruitment strategy called purposive sampling to actively recruit individuals with direct lived experience with the condition we are investigating. Patient, Caregiver, and Public Engagement staff contacted patients through a variety of health care organizations, as well as advocacy and patient groups and other social media groups.
Inclusion Criteria
We sought participants who had lived experience with immunoglobulin treatment, including patients and their caregivers. To capture equity issues and different decision making priorities across the province, we sought patients varying in age, gender, socio-economic background, and geographic location.
Exclusion Criteria
We set no specific exclusion criteria.
Participants
Patient, Caregiver, and Public Engagement staff spoke to eight individuals, seven patients and one caregiver, with lived experience of immunodeficiency across Ontario. All eight people were familiar with the standard treatment for immunodeficiency and three had experience with both standard and subcutaneous immunoglobulin treatment.
Interview Approach
At the outset of the interview, we explained the mandate of Health Quality Ontario, the role of the Ontario Health Technology Advisory Committee, and the purpose of the health technology assessment process. The risks of participation and protection of personal health information were outlined through a letter of information (see Appendix 4). Verbal consent was obtained before the start of the interview. Interviews were recorded and transcribed.
The interviews consisted of a series of open-ended questions, and lasted for approximately 30 to 45 minutes. Interview questions were based on a list of questions developed by Health Technology Assessment International's Patient and Citizen Involvement Group and were designed to elicit information specific to how a health technology or intervention affects lived experience and quality of life.58
Interview questions focussed on the impact of immunodeficiency on the patients’ and families’ quality of life, experiences with other treatment options, and perceived benefits and limitations of subcutaneous immunoglobulin therapy. The interview guide is attached as Appendix 5.
Data Extraction and Analysis
To capture themes and compare elements of lived experience among participants, we selected a modified version of a grounded theory methodology to analyze interview transcripts. The inductive nature of grounded theory follows an iterative process of eliciting, documenting, and analyzing responses, while simultaneously collecting and analyzing data using a constant comparative approach.59,60 Staff coded transcripts and compared themes using NVivo, a qualitative software program that enables the identification and interpretation of patterns in the interview data about the meaning and implications of the lived condition (QSR International, Doncaster, Victoria, Australia).
Results
Physical, Psychological, and Social Impact of Immunodeficiency
Patients and caregivers described several health conditions that co-exist with immunodeficiency, such as lymphoma, fibromyalgia, allergies, diabetes, etc. They also attributed a spectrum of challenges to immunodeficiency itself. Specific challenges depend on the severity of the condition, other medical conditions, and the length of time their condition went undiagnosed.
Patients’ perceptions were that immunodeficiency reduced their quality of life and impeded daily life activities. They reported physical challenges such as frequent and severe infections, and psychological challenges such as health anxiety around developing new illnesses.
“I was sick all the time. I would get pneumonia three or four times a year, I would get bronchitis, I coughed all the time.”
“[Now] if I have a symptom of something …my mind always goes to the worst case scenario because I know that people with my disorder are more likely to have lymphoma or certain kinds of cancers.”
Patients reported that knowledge and awareness regarding immunodeficiency in the general public was minimal. This lack of awareness about the condition was associated with isolation, stressful relationships, and reduced social functioning.
“Because he's anaphylactic to the milk, everyone just doesn't invite him because the parents will say to me, ‘Oh I feel like I can't get my house safe for him.’ So they just won't invite him.”
“My husband's best friend was getting married in Jamaica and I couldn't go because there's no insurance company that'll cover me. But trying to get people to understand that it's not my choice that I'm not going…I found more difficult than not going.”
Patients living in remote areas spoke of facing greater stigma due to lack of public knowledge and awareness of immunodeficiency. As one patient said, “because they worry that I'm infectious…which is ridiculous, but…you know, people aren't educated enough out there…”
Lack of knowledge and awareness leads to a negative impact on the professional lives of both patients and their caregivers. Frequent medical appointments means that patients and caregivers often have to miss work, with serious psychological and financial implications.
“Well, other than being threatened at work…I mean people just don't like that you're sick all the time. They just don't get it…. When I was working, it really became a very big stressor. Because you need to work financially, but you're sick. It's not your fault you're sick.”
“If you're used to working and…having the income of a senior executive and suddenly you can't work anymore, yeah, major impact. No income.”
Patients also noted a lack of awareness of the immunodeficiency condition within the primary care setting. Physicians often treated the infections without understanding the underlying problem. According to one patient, “I was never given a unifying diagnosis. There were lots of things that were wrong that were dealt with individually, but never was the whole picture understood.” This lack of understanding led to delays in diagnoses and sometimes irreversible damage to patient health. Another patient noted, “I have had [many] chronic ear problems to the point where now, as an adult, I have to wear a hearing aid.”
Despite the challenges, patients noted the positive impact of supportive caregivers in their life. However, this support wavered over time, with some family members displaying burn-out and distancing from them. Patients said that support systems for their families and caregivers was essential but lacking. Caregivers also acknowledged feeling chronic mental fatigue from taking care of their loved ones. They confessed feeling guilt and anxiety over the competing priorities in their life.
“My husband is not super supportive anymore. I think his patience has gone …”
“I feel like I'm watching him and noting everything that is going on. Sometimes I feel like I pass over my daughter if she's not feeling good because she doesn't have the immune deficiency. It just seems like I can never balance it.”
Some caregivers spoke of struggling with life-choice questions, including whether the condition should affect their family planning.
“Am I selfish for wanting to have another child? But then what if this child has what (he) has so now this child's going to have to go through everything too.”
Currently Available Intravenous Immunoglobulin Treatment for Immunodeficiency
Patients and caregivers said that antibiotics and intravenous immunoglobulin were widely available treatment options for individuals with immunodeficiency. They reported encountering these options at their doctor's clinics and in the hospitals. Patients also noted that intravenous immunoglobulin treatment effectively controls infections and improves overall health.
“I think almost right away I was feeling better. The gastro problems stopped, the ulcers went away, my sinus infections stopped. I gained a little bit of weight, like healthy weight…I looked better, I had more energy, I played all sorts of sports and I did well. It just totally changed my life.”
Although the treatment improved health, patients reported more side-effects immediately after the treatment and reported higher chance of contracting infections in the last week before the treatment.
“When I had IVIG, the first day I would be in bed that night and into the following day with kind of flu like symptoms and pain and I would be out for that day and the next day. So and then it would be great for a couple of weeks and then that last week before my next infusion–my immune system bottomed out and I would be at risk for getting sick again.”
Furthermore, the time required to obtain treatment at the hospital and to recuperate from side effects added stress on their work, family, and daily life activities. Patients reported facing stigma at work and to rely on their caregivers.
“I would have to miss 13 days a year to go to the hospital to have my treatment and just that anxiety was terrible…and HR put me on an attendance enhancement plan”
“It was an hour drive to the hospital, four hours for the infusion, an hour drive back, and then for 24 hours I was out so he had to take care of the kids. You know, it was…So I mean it was all on him and none of it on me. I just had to rest.”
Patients receiving care in large urban hospitals noted frustration related to hospital protocols, the cost of parking, and difficulty rescheduling missed appointments.
“They [health care professionals] just don't seem to look at the patient. They look at the paperwork…and the patient gets left behind.”
“And also with IVIG there were times if I couldn't make that date I'd be rescheduled for like three weeks later. So I'd actually have to miss an infusion. So that was really not great either.”
Patients and caregivers discussed several unmet needs with IVIG treatment. First, they found that the intravenous treatment interfered with their daily life activities. Additionally, patients and caregivers were also seeking reduction in side effects and decreased visits to the hospital. With this mindset, patients with intravenous treatment experience were open to seeking effective but less burdensome treatment.
“I take the car, I go to the hospital, I pay for parking, I go to the unit, and wait in line, I get a needle, if the needle doesn't work I get another needle, if it doesn't work I get another needle. And I have to start with the saline flush—then they start my actual treatment. But they start me very, very slow to begin with, then they're constantly taking my blood pressure and my temperature and my heart rate…I would like that time to just sleep, but if they're constantly doing your heart rate and your blood pressure and the pump is beeping beside you…it's so much that you don't need.”
“The biggest part is the side effects of IVIG; they were terrible. For the rest of the day after having my treatment, I would be very sluggish and I would have a headache and then for a couple of days after I would have severe pain in my spine and in my head. Walking was painful.”
“When you have an immune disorder, the last place you should be is at…a hospital…or public places [which] are not always clean and safe.”
“I avoid hospitals like the plague. So you have to go to the hospital.”
Perceived Impact of Home-Based Subcutaneous Immunoglobulin Treatment
The perceived impact of SCIG treatment was explored through questions related to the treatment process, effectiveness, efficiency, cost, and access.
Treatment Process
Patients and caregivers received training from health care professionals—nurses or doctors—in the process and method of subcutaneous immunoglobulin treatment. Patients and caregivers reported that health care professionals were willing to explain the benefits, risks, and alternatives to their satisfaction.
“I received training at the doctor's office and I was okay. People watched me do it and I was very confident doing it.”
“It's fantastic, I was trained in how to do the injections. But that was easy. I get the product every three months from the hospital, I do all the injections at home myself, and I've been doing that now for about a year and a half.”
Depending on their level of health knowledge and other co-existing medical conditions, patients reported varying levels of ease with treatment. They noted that the treatment could be personalized to an individual's life and preferences. They had the choice of manual injection through syringes or infusion through a pump. Patients said that pushing a large amount of fluid into the body using syringes requires strength in hands and fingers. They preferred the infusion pump as it offers better control of infusion rate and allows them to continue their daily life activities unhindered.
“I would push these syringes so that the fluid would go into my stomach. After a while my thumbs would get really sore…it hurts if you go too fast, so you would go pretty slowly.”
“I put the syringe in a pump and I dial the pump and it pushes itself and there's like a speed, you can slow it down if it's hurting or speed it up if you can tolerate it.”
“I hook myself up to a little pump and then the pump goes in this little, like almost like a backpack…And you cover up the tubing and you can do whatever you want. I've actually run out to the store to get something with the needles in my stomach and nobody would notice. I can do anything.”
Patients noted pain at the injection site that was dependent on the frequency of treatment and co-existing conditions such as diabetes or pain disorders. However, patients appreciated the ability to control the pain by infusing slower and by distributing the fluid over four sites rather than two. They also appreciated the choice of injecting in their abdomen, thighs, or buttocks.
“I have four needles right now, but if I want I could get tubing for two needles. However, I choose to have four needles as it balances out the fluid per site better and I can go faster if I have four needles.”
“I am supposed to change location, but my treatment is every two weeks so I stick with my stomach. I have the option to change site to thighs or buttocks.”
Subcutaneous treatment involves extensive supplies and some patients and their families found it difficult to store and dispose of them.
“It's certainly not fun for him and then he gets really frustrated with all my equipment around because I have lots of sharp containers and the equipment – because I have to keep a few months’ supply, it's kind of a hassle to store. But my kids aren't young anymore, so the sharps container isn't an issue. Just clutter.”
“At Brighton…the biggest problem is, ‘how do I dispose of the…syringes and vials?’”
Caregivers noted that children displayed anxiety and mood changes when anticipating treatment. However, the pain from subcutaneous needles was less traumatic than the IV needles.
“The first year, I couldn't do it [subcutaneous treatment] without my husband…because somebody would have to hold him while the other put the needles in because he would just fight and scream…but the subQ is much better…comparing anxiety from the IVIG to the subQ, it's a world of difference.”
“He would be screaming for an hour as they tried to put the IV in.”
Perception of Treatment Effectiveness
Patients and caregivers reported a high degree of satisfaction with the SCIG treatment. Patients who had experience with standard intravenous treatment were able to compare and contrast the number of infections and side-effects experienced with the two methods. Quality of life was rated much better with the SCIG treatment, which some patients referred to as “subQ”.
“The pushing factor towards subQ—I really didn't want to miss work anymore.”
“I started eating a little better. I started exercising a little more. Maybe I started doing that because I was feeling better and I was feeling a little more in the mood to do that because I was feeling a little healthier…it made a huge difference to my life.”
The SCIG treatment empowered patients to choose the frequency and speed of treatment. It allowed them to pursue daily activities of their choice and improved their overall quality of life.
“Travelling is amazing—I have the choice if I want to bring the supplies with me or I can just manoeuvre my treatment days so I don't have to bring any medical supplies with me …”
“I was on disability initially and since I started subQ, I've been able to go back to work, which is nice, but I do work from home so I'm not in that open concept kind of workplace.”
The SCIG treatment had a positive impact on patients’ personal resources and reduced their treatment burden.
“I'm not buying as many antibiotics. I'm not seeing as many specialists. I'm not having as many x-rays, ultrasounds, or other tests run. My number of tests and my stays in the hospital are down. The only tests I'm having done at this point are routine every—you know routine once a year.”
Perceived Efficiency
Patients reported that as a result of treatment, they suffered from chronic fatigue, infections, hospitalizations, and side-effects. They generally reported that it took a few months to see a considerable reduction in side-effects, fatigue, and rate of infections after switching to subcutaneous treatment.
“The first couple months…I felt the same really. I didn't have that bottom out like that last week ever—which was really nice not to have that kind of feeling, that you just had nothing left in your tank. I didn't miss that feeling ever, but as time went on, I felt the healthiest I've ever felt in my life, which I've always been a really sickly person, even when I was a kid.”
“Within a few months I noticed the difference and I wasn't hospitalized…I don't think I've been hospitalized since I've been on it.”
“My side effects were fatigue and headaches, when I first started…but at least I was at home.”
Cost and Access
The financial burden of subcutaneous immunoglobulin treatment on the patients was said to be minimal. The equipment, product, and supplies were covered by workplace insurance or the pharmaceutical company. One patient noted that they were unable to access infusion pumps initially due to lack of insurance coverage: “I had to use syringes three times a week. The insurance covered injections and supplies, if I self-injected.” However, in this patient's case, the pharmaceutical company stepped up to cover the cost for the pump.
Location and Access
Geographical location impacted patient access to subcutaneous immunoglobulin treatment. Patients in remote areas reported facing difficulty finding supplies and faxing log sheets to the urban immunology clinic. When hospitalized, they had to educate their health care professionals on their condition and treatment.
“Because it's a small community, they don't know about it [subcutaneous treatment]. They don't have a protocol to provide supplies and treatment.”
“It is a challenge because I don't have a fax machine. To mail it is expensive.”
Conclusions
In our interviews with a range of patients and caregivers impacted by immunodeficiency, a consistent theme emerged that it reduced their quality of life. Intravenous treatment was said to be effective but consumed time and induced side-effects. Patients and caregivers expressed preference for SCIG as it reduced treatment burden and improved their overall quality of life.
SUMMARY OF THE HEALTH TECHNOLOGY ASSESSMENT
The best available evidence suggests that home-based subcutaneous infusion of immunoglobulin (SCIG) for primary and secondary immunodeficiencies is safe and effective, with clinical outcomes that are comparable to the clinical outcomes of hospital-based intravenous infusion (IVIG). Studies that compared SCIG with IVIG showed that SCIG can provide an adequate serum trough level to prevent infection. The occurrence of severe adverse reactions with SCIG was rare. Subcutaneous IG was associated with a lower risk of adverse events such as fever, headache, and allergic reaction. But SCIG caused local reactions such as pain, rash, induration, swelling, soreness, and itching at the site of infusion. The number of days in hospital was reported by one study and it showed fewer days in patients receiving SCIG. However, the quality of evidence is low, meaning that we cannot be certain about these findings.
Quality of life questionnaires showed no significant difference between IVIG and SCIG for most subscales. The parent-completed child health questionnaire showed significant improvement with SCIG in domains related to general health, mental health, parental impact, and family activities. The child-completed form showed significant improvement in subscales of global health and role emotional.
In interviews, patients and caregivers expressed preference for SCIG as it reduced treatment burden and improved their overall quality of life. In Canadian studies, concerns from patients about switching from IVIG to SCIG, ranked from most to least important, were (1) loss of supervision, (2) cost, (3) frequency of injections, (4) lost time, (5) administration of doses (self-injections), and (6) safe and reliable storage of medication.
Funding home-based SCIG should lead to net budget savings, as well as savings in nursing time, but the cost savings may be not translated into monetary benefit. It is more likely to create opportunities to improve the efficiency of hospitals and/or the health care system.
Acknowledgments
The medical editor was Tim Maguire; others involved in the development and production of this report were Merissa Mohamed, Ana Laing, Claude Soulodre, Kellee Kaulback, Andrée Mitchell, Vivian Ng, Anil Thota, Sarah McDowell, Amy Lang, Nancy Sikich, and Irfan Dhalla.
We are grateful to David Tannenbaum, associate professor and physician-in-chief, Department of Family and Community Medicine, Mount Sinai Hospital, Stephen Betschel, assistant professor of medicine, program director clinical immunology and allergy, Department of Internal Medicine, University of Toronto, and Division of Clinical Immunology and Allergy, St. Michael's Hospital, Antonio Giulivi: associate professor, Department of Pathology and Laboratory Medicine, Faculty of Medicine, The Ottawa Hospital, and Juthaporn Cowan: assistant professor, Biochemistry, Microbiology and Immunology, Faculty of Medicine, University of Ottawa, and associate scientist, Clinical Epidemiology Program, The Ottawa Hospital Research Institute, for their expert contributions to this report.
ABBREVIATIONS
- CI
Confidence interval
- FTE
Full-time employee
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- IVIG
Hospital-based intravenous immunoglobulin therapy
- QALY
Quality-adjusted life-year
- SCIG
Home-based subcutaneous immunoglobulin therapy
- SD
Standard deviation
GLOSSARY
- Human capital approach
Calculates productivity cost based on missed work due to illness or injury.
- Plasma
The liquid portion of the blood in which the other blood components (red cells, white cells, platelets, etc.) are suspended.
- Random-effects model
A statistical approach used in meta-analysis to combine the results of several studies where the true effect size varies among the studies.
- SF-36
A tool to measure patient quality of life. A questionnaire to be filled out by the patient including a series of 36 health-related questions.
- Societal perspective
Considers the full effect on society of a condition, including all costs, regardless of who pays, and all benefits, regardless of who receives the benefits.
- Trough level
The concentration of drug in the blood just before the next administration.
- Quality-adjusted life-year (QALY)
A measurement that takes into account both the number of years gained by a patient from a procedure and the quality of those extra years (ability to function, freedom from pain, etc.). The QALY is commonly used as an outcome measure in cost–utility analyses.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: Dec 13, 2016
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database; EBSCO CINAHL
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <November 2016>, EBM Reviews –Cochrane Database of Systematic Reviews <2005 to December 07, 2016>, EBM Reviews – Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2016 Week 50>, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Immunologic Deficiency Syndromes/ (619910)
-
2
exp HIV/ (347682)
-
3
(immunodeficien∗ or PIDD or PIDDs or PID or PIDs or SID or SIDS).ti,ab,kf. (308909)
-
4
(HIV or AIDS or XLA or CVID).ti,ab,kf. (803163)
-
5
(agammaglobulin?emi∗ or agamma globulin?emi∗ or Hypogammaglobulin?emi∗ or Hypogamma globulin?emi∗ or hypo gammaglobulin?emi∗ or hypo gamma globulin?emi∗ or hypo IgG or hypergammaglobulin?emi∗ or hypergamma globulin?emi∗ or hyper gammaglobulin?emi∗ or hyper gamma globulin?emi∗ or Hyperimmunoglobulin∗ or hyper immunoglobulin∗ or hyper IgM or HIGM or hyper IgE or HIES or hyper IgD or HIDS or hypoimmunoglobulin?emi∗ or hypo immunoglobulin?emi∗ or Dysgammaglobulin?emi∗).ti,ab,kf. (24926)
-
6
((Tcell or T cell or Bcell or B cell or TB cell or TB cell or Adenosine deaminase or ZAP70 or immune or immuno or immunity or immunologic∗ or antibody or immunoglobulin∗ or immune globulin∗ or gammaglobulin or gamma globulin or gammaglobulins or gamma globulins or IgG or IgA or Leukocyte-Adhesion) adj2 (deficien∗ or defect∗ or disorder∗)).ti,ab,kf. (89128)
-
7
((immun∗ or idiopathic or purpura) adj2 thrombocytopeni∗).ti,ab,kf. (35042)
-
8
or/1–7 (1241602)
-
9
immunoglobulins/ (172214)
-
10
Immunoglobulin G/ (282346)
-
11
Immunoglobulins, Intravenous/ (139485)
-
12
immunotherapy/ (118344)
-
13
Immunization, Passive/ (32058)
-
14
home infusion therapy/ (686)
-
15
gamma globulins/ (147310)
-
16
or/9–15 (587320)
-
17
infusions, subcutaneous/ (100254)
-
18
injections, subcutaneous/ (134130)
-
19
subcutaneous absorption/ (7161)
-
20
(subcutaneous∗ or “sub cutaneous” or “sub cutaneously”).ti,ab,kf. (351077)
-
21
or/17–20 (442687)
-
22
16 and 21 (14282)
-
23
((subcutaneous∗ or “sub cutaneous” or “sub cutaneously”) adj6 (immunoglobulin∗ or Ig or IgG or immune globulin∗ or gammaglobulin or gammaglobulins or gamma globulin or gamma globulins or immunotherap∗ or immune therap∗ or Gamunex∗ or Gammagard∗ or Subcuvia∗ or Endobulin∗ or Privigen∗ or Nordimmun∗ or Gammaplex∗ or Octagam∗ or Gammanorm∗ or KIOVIG∗)).ti,ab,kf. (5013)
-
24
(SCIG or SCIGG or SC IG or SC IGG or IGSC).ti,ab,kf. (870)
-
25
(Hizentra∗ or IgPro20∗ or Vivaglobin∗ or Beriglobin∗ or Evogam∗ or Gammabulin∗ or HYQVIA∗ or IGHy or Subgam∗).ti,ab,kf. (315)
-
26
or/22–25 (16331)
-
27
8 and 26 (2027)
-
28
exp Animals/ not Humans/ (15835607)
-
29
27 not 28 (1065)
-
30
limit 29 to english language [Limit not valid in CDSR,DARE; records were retained] (944)
-
31
30 use ppez,coch,cctr,clhta,cleed,dare (446)
-
32
immune deficiency/ (69149)
-
33
exp cellular immunodeficiency/ (5027)
-
34
exp combined immunodeficiency/ (167760)
-
35
griscelli syndrome/ (414)
-
36
exp humoral immune deficiency/ (16827)
-
37
exp phagocyte dysfunction/ (12202)
-
38
immunoglobulin deficiency/ (5922)
-
39
immunoglobulin G deficiency/ (893)
-
40
immunoglobulin G4 related disease/ (1368)
-
41
(immunodeficien∗ or PIDD or PIDDs or PID or PIDs or SID or SIDs).tw,kw. (313420)
-
42
(HIV or AIDS or XLA or CVID).tw,kw. (809813)
-
43
(agammaglobulin?emi∗ or agamma globulin?emi∗ or Hypogammaglobulin?emi∗ or Hypogamma globulin?emi∗ or hypo gammaglobulin?emi∗ or hypo gamma globulin?emi∗ or hypo IgG or hypergammaglobulin?emi∗ or hypergamma globulin?emi∗ or hyper gammaglobulin?emi∗ or hyper gamma globulin?emi∗ or Hyperimmunoglobulin∗ or hyper immunoglobulin∗ or hyper IgM or HIGM or hyper IgE or HIES or hyper IgD or HIDS or hypoimmunoglobulin?emi∗ or hypo immunoglobulin?emi∗ or Dysgammaglobulin?emi∗).tw,kw. (25304)
-
44
((Tcell or T cell or Bcell or B cell or TBcell or TB cell or Adenosine deaminase or ZAP70 or immune or immuno or immunity or immunologic∗ or antibody or immunoglobulin∗ or immune globulin∗ or gammaglobulin or gamma globulin or gammaglobulins or gamma globulins or IgG or IgA or Leukocyte-Adhesion) adj2 (deficien∗ or defect∗ or disorder∗)).tw,kw. (90752)
-
45
((immun∗ or idiopathic or purpura) adj2 thrombocytopeni∗).tw,kw. (35446)
-
46
or/32–45 (1144839)
-
47
immunoglobulin/ (172214)
-
48
immunoglobulin G/ (282346)
-
49
immunoglobulin G1/ (12458)
-
50
immunoglobulin G2/ (4247)
-
51
immunoglobulin G2a/ (4671)
-
52
immunoglobulin G2b/ (1921)
-
53
immunoglobulin G3/ (3558)
-
54
immunoglobulin G4/ (7042)
-
55
human immunoglobulin/ (11027)
-
56
immunoglobulin G antibody/ (32643)
-
57
immunoglobulin G1 antibody/ (2674)
-
58
immunoglobulin G2a antibody/ (1243)
-
59
immunoglobulin G3 antibody/ (408)
-
60
immunotherapy/ (118344)
-
61
or/47–60 (575192)
-
62
subcutaneous drug administration/ (99290)
-
63
(subcutaneous∗ or “sub cutaneous” or “sub cutaneously”).tw,kw,dv. (352951)
-
64
62 or 63 (421185)
-
65
61 and 64 (13966)
-
66
subcutaneous immunotherapy/ (1252)
-
67
immunoglobulin/sc (559)
-
68
((subcutaneous∗ or “sub cutaneous” or “sub cutaneously”) adj6 (immunoglobulin∗ or Ig or IgG or immune globulin∗ or gammaglobulin or gammaglobulins or gamma globulin or gamma globulins or immunotherap∗ or immune therap∗ or Gamunex∗ or Gammagard∗ or Subcuvia∗ or Endobulin∗ or Privigen∗ or Nordimmun∗ or Gammaplex∗ or Octagam∗ or Gammanorm∗ or KIOVIG∗)).tw,kw,dv. (5119)
-
69
(SCIG or SCIGG or SC IG or SC IGG or IGSC).tw,kw,dv. (901)
-
70
(Hizentra∗ or IgPro20∗ or Vivaglobin∗ or Beriglobin∗ or Evogam∗ or Gammabulin∗ or HYQVIA∗ or IGHy or Subgam∗).tw,kw,dv. (508)
-
71
or/65–70 (16870)
-
72
46 and 71 (2187)
-
73
(exp animal/ or nonhuman/) not exp human/ (10362903)
-
74
72 not 73 (2013)
-
75
limit 74 to english language [Limit not valid in CDSR,DARE; records were retained] (1870)
-
76
75 use emez (1478)
-
77
31 or 76 (1924)
-
78
77 use ppez (417)
-
79
77 use coch (0)
-
80
77 use cctr (24)
-
81
77 use dare (2)
-
82
77 use clhta (1)
-
83
77 use cleed (2)
-
84
77 use emez (1478)
-
85
remove duplicates from 77 (1547)
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Immunologic Deficiency Syndromes+”) | 68,894 |
| S2 | (MH “Human Immunodeficiency Virus+”) | 6,442 |
| S3 | (immunodeficien∗ OR PIDD OR PIDDs OR PID OR PIDs OR SID OR SIDs) | 27,703 |
| S4 | (HIV OR AIDS OR XLA OR CVID) | 104,696 |
| S5 | (agammaglobulin#emi∗ OR agamma globulin#emi∗ OR Hypogammaglobulin#emi∗ OR Hypogamma globulin#emi∗ OR hypo gammaglobulin#emi∗ OR hypo gamma globulin#emi∗ OR hypo IgG OR hypergammaglobulin#emi∗ OR hypergamma globulin#emi∗ OR hyper gammaglobulin#emi∗ OR hyper gamma globulin#emi∗ OR Hyperimmunoglobulin∗ OR hyper immunoglobulin∗ OR hyper IgM OR HIGM OR hyper IgE OR HIES OR hyper IgD OR HIDS OR hypoimmunoglobulin#emi∗ OR hypo immunoglobulin#emi∗ OR Dysgammaglobulin#emi∗) | 866 |
| S6 | ((Tcell OR T cell OR Bcell OR B cell OR TBcell OR TB cell OR Adenosine deaminase OR ZAP70 OR immune OR immuno OR immunity OR immunologic∗ OR antibody OR immunoglobulin∗ OR immune globulin∗ OR gammaglobulin OR gamma globulin OR IgG OR IgA OR Leukocyte-Adhesion) N2 (deficien∗ OR defect∗ OR disorder∗)) | 3,466 |
| S7 | ((immun∗ OR idiopathic OR purpura) N2 thrombocytopeni∗) | 1,891 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 114,606 |
| S9 | (MH “Immunoglobulins”) | 11,087 |
| S10 | (MH “Immunoglobulins, Intravenous”) | 1,660 |
| S11 | (MH “Immunotherapy”) | 4,341 |
| S12 | (MH “Gamma Globulins”) | 133 |
| S13 | S9 OR S10 OR S11 OR S12 | 16,842 |
| S14 | (MH “Injections, Subcutaneous+”) | 3,077 |
| S15 | (MH “Infusions, Subcutaneous”) | 627 |
| S16 | (subcutaneous∗ OR “sub cutaneous” OR “sub cutaneously”) | 13,085 |
| S17 | S14 OR S15 OR S16 | 13,170 |
| S18 | S13 AND S17 | 343 |
| S19 | ((subcutaneous∗ OR “sub cutaneous” OR “sub cutaneously”) N6 (immunoglobulin∗ OR Ig OR IgG OR immune globulin∗ OR gammaglobulin OR gammaglobulins OR gamma globulin OR gamma globulins OR immunotherap∗ OR immune therap∗ OR Gamunex∗ OR Gammagard∗ OR Subcuvia∗ OR Endobulin∗ OR Privigen∗ OR Nordimmun∗ OR Gammaplex∗ OR Octagam∗ OR Gammanorm∗ OR KIOVIG∗)) | 270 |
| S20 | (SCIG OR SCIGG OR SC IG OR SC IGG OR IGSC) | 28 |
| S21 | (Hizentra∗ OR IgPro20∗ OR Vivaglobin∗ OR Beriglobin∗ OR Evogam∗ OR Gammabulin∗ OR HYQVIA∗ OR IGHy OR Subgam∗) | 16 |
| S22 | S18 OR S19 OR S20 OR S21 | 478 |
| S23 | S8 AND S22 S23 | 70 |
| S24 | Limiters – English Language | 70 |
Grey Literature
Performed on: Nov 10–11, 2016
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, Tufts Cost-Effectiveness Analysis Registry
Keywords used:
Immunoglobulin, immunoglobulins, immune globulin, immune globulins, subcutaneous, immunodeficiency, immunodeficiencies, immune deficiency, immune deficiencies, immunoglobuline, immunodeficience, IG, SCIG
Results: 27
Economic Evidence Search
Databases searched: All Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, and NHS Economic Evaluation Database; EBSCO CINAHL
Database: EBM Reviews – Cochrane Central Register of Controlled Trials <November 2016>, EBM Reviews – Cochrane Database of Systematic Reviews <2005 to December 21, 2016>, EBM Reviews – Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews – Health Technology Assessment <4th Quarter 2016>, EBM Reviews – NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2016 Week 51>, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Immunologic Deficiency Syndromes/ (628828)
-
2
exp HIV/ (350584)
-
3
(immunodeficien∗ or PIDD or PIDDs or PID or PIDs or SID or SIDS).ti,ab,kf. (312313)
-
4
(HIV or AIDS or XLA or CVID).ti,ab,kf. (812726)
-
5
(agammaglobulin?emi∗ or agamma globulin?emi∗ or Hypogammaglobulin?emi∗ or Hypogamma globulin?emi∗ or hypo gammaglobulin?emi∗ or hypo gamma globulin?emi∗ or hypo IgG or hypergammaglobulin?emi∗ or hypergamma globulin?emi∗ or hyper gammaglobulin?emi∗ or hyper gamma globulin?emi∗ or Hyperimmunoglobulin∗ or hyper immunoglobulin∗ or hyper IgM or HIGM or hyper IgE or HIES or hyper IgD or HIDS or hypoimmunoglobulin?emi∗ or hypo immunoglobulin?emi∗ or Dysgammaglobulin?emi∗).ti,ab,kf. (25211)
-
6
((Tcell or T cell or Bcell or B cell or TB cell or TB cell or Adenosine deaminase or ZAP70 or immune or immuno or immunity or immunologic∗ or antibody or immunoglobulin∗ or immune globulin∗ or gammaglobulin or gamma globulin or gammaglobulins or gamma globulins or IgG or IgA or Leukocyte-Adhesion) adj2 (deficien∗ or defect∗ or disorder∗)).ti,ab,kf. (90211)
-
7
((immun∗ or idiopathic or purpura) adj2 thrombocytopeni∗).ti,ab,kf. (35448)
-
8
or/1–7 (1255414)
-
9
immunoglobulins/ (173459)
-
10
Immunoglobulin G/ (285651)
-
11
Immunoglobulins, Intravenous/ (139940)
-
12
immunotherapy/ (120322)
-
13
Immunization, Passive/ (32523)
-
14
home infusion therapy/ (698)
-
15
gamma globulins/ (148190)
-
16
or/9–15 (594801)
-
17
infusions, subcutaneous/ (100334)
-
18
injections, subcutaneous/ (135398)
-
19
subcutaneous absorption/ (7172)
-
20
(subcutaneous∗ or “sub cutaneous” or “sub cutaneously”).ti,ab,kf. (356578)
-
21
or/17–20 (448726)
-
22
16 and 21 (14442)
-
23
((subcutaneous∗ or “sub cutaneous” or “sub cutaneously”) adj6 (immunoglobulin∗ or Ig or IgG or immune globulin∗ or gammaglobulin or gammaglobulins or gamma globulin or gamma globulins or immunotherap∗ or immune therap∗ or Gamunex∗ or Gammagard∗ or Subcuvia∗ or Endobulin∗ or Privigen∗ or Nordimmun∗ or Gammaplex∗ or Octagam∗ or Gammanorm∗ or KIOVIG∗)).ti,ab,kf. (5060)
-
24
(SCIG or SCIGG or SC IG or SC IGG or IGSC).ti,ab,kf. (886)
-
25
(Hizentra∗ or IgPro20∗ or Vivaglobin∗ or Beriglobin∗ or Evogam∗ or Gammabulin∗ or HYQVIA∗ or IGHy or Subgam∗).ti,ab,kf. (323)
-
26
or/22–25 (16508)
-
27
8 and 26 (2050)
-
28
Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. or Congresses.pt. (5010005)
-
29
27 not 28 (1932)
-
30
economics/ (255211)
-
31
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (781869)
-
32
economics.fs. (422737)
-
33
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw. (754033)
-
34
exp “costs and cost analysis”/ (551094)
-
35
cost∗.ti. (253484)
-
36
cost effective∗.tw. (273962)
-
37
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab. (172517)
-
38
models, economic/ (163915)
-
39
markov chains/ or monte carlo method/ (71535)
-
40
(decision adj1 (tree∗ or analy∗ or model∗)).tw. (37220)
-
41
(markov or markow or monte carlo).tw. (111708)
-
42
quality-adjusted life years/ (33764)
-
43
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (57867)
-
44
((adjusted adj (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw. (109671)
-
45
or/30–44 (2439472)
-
46
29 and 45 (169)
-
47
limit 46 to english language [Limit not valid in CDSR,DARE; records were retained] (150)
-
48
limit 29 to english language [Limit not valid in CDSR,DARE; records were retained] (1796)
-
49
47 use ppez,coch,cctr,clhta,dare (38)
-
50
48 use cleed (2)
-
51
immune deficiency/ (69221)
-
52
exp cellular immunodeficiency/ (5036)
-
53
exp combined immunodeficiency/ (167880)
-
54
griscelli syndrome/ (415)
-
55
exp humoral immune deficiency/ (16863)
-
56
exp phagocyte dysfunction/ (12211)
-
57
immunoglobulin deficiency/ (5971)
-
58
immunoglobulin G deficiency/ (894)
-
59
immunoglobulin G4 related disease/ (1376)
-
60
(immunodeficien∗ or PIDD or PIDDs or PID or PIDs or SID or SIDs).tw,kw. (316746)
-
61
(HIV or AIDS or XLA or CVID).tw,kw. (819355)
-
62
(agammaglobulin?emi∗ or agamma globulin?emi∗ or Hypogammaglobulin?emi∗ or Hypogamma globulin?emi∗ or hypo gammaglobulin?emi∗ or hypo gamma globulin?emi∗ or hypo IgG or hypergammaglobulin?emi∗ or hypergamma globulin?emi∗ or hyper gammaglobulin?emi∗ or hyper gamma globulin?emi∗ or Hyperimmunoglobulin∗ or hyper immunoglobulin∗ or hyper IgM or HIGM or hyper IgE or HIES or hyper IgD or HIDS or hypoimmunoglobulin?emi∗ or hypo immunoglobulin?emi∗ or Dysgammaglobulin?emi∗).tw,kw. (25591)
-
63
((Tcell or T cell or Bcell or B cell or TBcell or TB cell or Adenosine deaminase or ZAP70 or immune or immuno or immunity or immunologic∗ or antibody or immunoglobulin∗ or immune globulin∗ or gammaglobulin or gamma globulin or gammaglobulins or gamma globulins or IgG or IgA or Leukocyte-Adhesion) adj2 (deficien∗ or defect∗ or disorder∗)).tw,kw. (91829)
-
64
((immun∗ or idiopathic or purpura) adj2 thrombocytopeni∗).tw,kw. (35851)
-
65
or/51–64 (1157259)
-
66
immunoglobulin/ (173459)
-
67
immunoglobulin G/ (285651)
-
68
immunoglobulin G1/ (12485)
-
69
immunoglobulin G2/ (4261)
-
70
immunoglobulin G2a/ (4675)
-
71
immunoglobulin G2b/ (1924)
-
72
immunoglobulin G3/ (3568)
-
73
immunoglobulin G4/ (7066)
-
74
human immunoglobulin/ (11058)
-
75
immunoglobulin G antibody/ (32697)
-
76
immunoglobulin G1 antibody/ (2683)
-
77
immunoglobulin G2a antibody/ (1245)
-
78
immunoglobulin G3 antibody/ (411)
-
79
immunotherapy/ (120322)
-
80
or/66–79 (581591)
-
81
subcutaneous drug administration/ (99307)
-
82
(subcutaneous∗ or “sub cutaneous” or “sub cutaneously”).tw,kw,dv. (358434)
-
83
81 or 82 (426670)
-
84
80 and 83 (14096)
-
85
subcutaneous immunotherapy/ (1257)
-
86
immunoglobulin/sc (560)
-
87
((subcutaneous∗ or “sub cutaneous” or “sub cutaneously”) adj6 (immunoglobulin∗ or Ig or IgG or immune globulin∗ or gammaglobulin or gammaglobulins or gamma globulin or gamma globulins or immunotherap∗ or immune therap∗ or Gamunex∗ or Gammagard∗ or Subcuvia∗ or Endobulin∗ or Privigen∗ or Nordimmun∗ or Gammaplex∗ or Octagam∗ or Gammanorm∗ or KIOVIG∗)).tw,kw,dv. (5160)
-
88
(SCIG or SCIGG or SC IG or SC IGG or IGSC).tw,kw,dv. (917)
-
89
(Hizentra∗ or IgPro20∗ or Vivaglobin∗ or Beriglobin∗ or Evogam∗ or Gammabulin∗ or HYQVIA∗ or IGHy or Subgam∗).tw,kw,dv. (518)
-
90
or/84–89 (17023)
-
91
65 and 90 (2209)
-
92
Economics/ (255211)
-
93
Health Economics/ or exp Pharmacoeconomics/ (222179)
-
94
Economic Aspect/ or exp Economic Evaluation/ (432951)
-
95
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw. (754033)
-
96
exp “Cost”/ (551094)
-
97
cost∗.ti. (253484)
-
98
cost effective∗.tw. (273962)
-
99
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab. (172517)
-
100
Monte Carlo Method/ (57810)
-
101
(decision adj1 (tree∗ or analy∗ or model∗)).tw. (37220)
-
102
(markov or markow or monte carlo).tw. (111708)
-
103
Quality-Adjusted Life Years/ (33764)
-
104
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (57867)
-
105
((adjusted adj (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw. (109671)
-
106
or/92–105 (2020919)
-
107
91 and 106 (201)
-
108
Case Report.pt. or Comment/ or Editorial/ or Letter/ or conference abstract.pt. (5522939)
-
109
107 not 108 (155)
-
110
limit 109 to english language [Limit not valid in CDSR,DARE; records were retained] (136)
-
111
110 use emez (100)
-
112
49 or 50 or 111 (140)
-
113
112 use ppez (36)
-
114
112 use coch (0)
-
115
112 use cctr (0)
-
116
112 use clhta (1)
-
117
112 use dare (1)
-
118
112 use cleed (2)
-
119
112 use emez (100)
-
120
remove duplicates from 112 (111)
CINAHL
| # | Query | Results |
|---|---|---|
| S1 | (MH “Immunologic Deficiency Syndromes+”) | 71,189 |
| S2 | (MH “Human Immunodeficiency Virus+”) | 6,682 |
| S3 | (immunodeficien∗ OR PIDD OR PIDDs OR PID OR PIDs OR SID OR SIDs) | 29,272 |
| S4 | (HIV OR AIDS OR XLA OR CVID) | 110,135 |
| S5 | (agammaglobulin#emi∗ OR agamma globulin#emi∗ OR Hypogammaglobulin#emi∗ OR Hypogamma globulin#emi∗ OR hypo gammaglobulin#emi∗ OR hypo gamma globulin#emi∗ OR hypo IgG OR hypergammaglobulin#emi∗ OR hypergamma globulin#emi∗ OR hyper gammaglobulin#emi∗ OR hyper gamma globulin#emi∗ OR Hyperimmunoglobulin∗ OR hyper immunoglobulin∗ OR hyper IgM OR HIGM OR hyper IgE OR HIES OR hyper IgD OR HIDS OR hypoimmunoglobulin#emi∗ OR hypo immunoglobulin#emi∗ OR Dysgammaglobulin#emi∗) | 967 |
| S6 | ((Tcell OR T cell OR Bcell OR B cell OR TBcell OR TB cell OR Adenosine deaminase OR ZAP70 OR immune OR immuno OR immunity OR immunologic∗ OR antibody OR immunoglobulin∗ OR immune globulin∗ OR gammaglobulin OR gamma globulin OR IgG OR IgA OR Leukocyte-Adhesion) N2 (deficien∗ OR defect∗ OR disorder∗)) | 3,887 |
| S7 | ((immun∗ OR idiopathic OR purpura) N2 thrombocytopeni∗) | 2,008 |
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | 120,895 |
| S9 | (MH “Immunoglobulins”) | 11,680 |
| S10 | (MH “Immunoglobulins, Intravenous”) | 1,775 |
| S11 | (MH “Immunotherapy”) | 4,714 |
| S12 | (MH “Gamma Globulins”) | 139 |
| S13 | S9 OR S10 OR S11 OR S12 | 17,916 |
| S14 | (MH “Injections, Subcutaneous+”) | 3,195 |
| S15 | (MH “Infusions, Subcutaneous”) | 658 |
| S16 | (subcutaneous∗ OR “sub cutaneous” OR “sub cutaneously”) | 14,850 |
| S17 | S14 OR S15 OR S16 | 14,938 |
| S18 | S13 AND S17 | 373 |
| S19 | ((subcutaneous∗ OR “sub cutaneous” OR “sub cutaneously”) N6 (immunoglobulin∗ OR Ig OR IgG OR immune globulin∗ OR gammaglobulin OR gammaglobulins OR gamma globulin OR gamma globulins OR immunotherap∗ OR immune therap∗ OR Gamunex∗ OR Gammagard∗ OR Subcuvia∗ OR Endobulin∗ OR Privigen∗ OR Nordimmun∗ OR Gammaplex∗ OR Octagam∗ OR Gammanorm∗ OR KIOVIG∗)) | 297 |
| S20 | (SCIG OR SCIGG OR SC IG OR SC IGG OR IGSC) | 33 |
| S21 | (Hizentra∗ OR IgPro20∗ OR Vivaglobin∗ OR Beriglobin∗ OR Evogam∗ OR Gammabulin∗ OR HYQVIA∗ OR IGHy OR Subgam∗) | 19 |
| S22 | S18 OR S19 OR S20 OR S21 | 520 |
| S23 | S8 AND S22 | 77 |
| S24 | S23 | 76 |
| S25 | (MH “Economics”) | 11,303 |
| S26 | (MH “Economic Aspects of Illness”) | 6,876 |
| S27 | (MH “Economic Value of Life”) | 521 |
| S28 | MH “Economics, Dental” | 109 |
| S29 | MH “Economics, Pharmaceutical” | 1,795 |
| S30 | MW “ec” | 144,148 |
| S31 | (econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗) | 221,952 |
| S32 | (MH “Costs and Cost Analysis+”) | 86,622 |
| S33 | TI cost∗ | 40,864 |
| S34 | (cost effective∗) | 29,781 |
| S35 | AB (cost∗ N2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)) | 20,585 |
| S36 | (decision N1 (tree∗ or analy∗ or model∗)) | 5,381 |
| S37 | (markov or markow or monte carlo) | 3,537 |
| S38 | (MH “Quality-Adjusted Life Years”) | 2,782 |
| S39 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 6,703 |
| S40 | ((adjusted N1 (quality or life)) or (willing∗ N2 pay) or sensitivity analys?s) | 12,555 |
| S41 | S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 | 296,935 |
| S42 | S24 AND S41 | 16 |
| S43 | S24 AND Limiters – English Language | 16 |
Appendix 2: Clinical Evidence Quality Assessment
Table A1:
Quality of the Body of Evidence (GRADE System)
| Number of Studiesa | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Trough level | |||||||
| 9 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕Low |
| Serious bacterial infection | |||||||
| 3 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕Low |
| All infections | |||||||
| 2 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕Low |
| Antibiotic use | |||||||
| 2 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Days in hospital | |||||||
| 2 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕Low |
| Incidence of hospitalization | |||||||
| 2 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Missed days from work or school for patients | |||||||
| 2 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Missed days from work or school for parents or caregivers | |||||||
| 1 study | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Quality of life of adults (SF-36) | |||||||
| 4 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Quality of life of children (CHQ-PF50, parental form) | |||||||
| 4 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Quality of life of children (CHQ, child form) | |||||||
| 1 study | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Satisfaction (LQI) | |||||||
| 3 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Preference | |||||||
| 2 Canadian studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| Adverse events | |||||||
| 10 studies | No serious limitations | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
Abbreviations: CHQ, child health questionnaire; LQI, Life quality Index; SF-36, Short-form 36.
All studies had prospective and/or retrospective design.
Appendix 3: Results of Applicability Checklist for Studies Included in the Economic Literature Review
Table A2:
Applicability of Included Studies in the Economic Literature Review
| Objective: To assess the cost-effectiveness of SCIG versus IVIG treatment | |||||
|---|---|---|---|---|---|
| Author, year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Was the perspective clearly stated and what was it? | Are estimates of relative treatment effect from the best available source? |
| Gerth et al,201411 | Yes | Yes | Yes (Canada) | Yes; Public payer | Not applicable |
| Martin et al,201334 | Yes | Yes | Yes (Canada) | Yes; Public payer | Not applicable |
| CADTH, 200835 (First analysis)a | Yes | Yes | Yes (Canada) | Yes; Public payer | Not applicable |
| CADTH, 2008 (Second analysis)b | Yes | Yes | Yes (Canada) | Yes; Public payer | Partly |
| Beauteet al200936 (First analysis)a | Yes | Yes | Yes (France) | Yes; Public payer | Not applicable |
| Beauteet al 200936 (Second analysis)c | Yes | Yes | Yes (France) | Yes; Public payer | Not applicable |
| Author, year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs from other sectors fully and appropriately measured and valued? | Overall judgement (directly applicable/partially applicable/not applicable) | |
|---|---|---|---|---|---|
| Gerth et al, 201411 | Not applicable | Not applicable | Partly | Partially Applicable | |
| Martin et al, 201334 | No | Not applicable | Partly | Partially Applicable | |
| CADTH, 200835 (First analysis)a | Not applicable | Not applicable | Partly | Partially Applicable | |
| CADTH, 2008 (Second analysis)b | Not applicable | Yes | Partly | Partially Applicable | |
| Beauteet al 200936 (First analysis)a | Not applicable | Not applicable | Partly | Not Applicable | |
| Beauteet al 200936 (Second analysis)c | Not applicable | Not applicable | No | Not Applicable | |
Abbreviations: IVIG, hospital-based intravenous immunoglobulin; SCIG, home-based subcutaneous immunoglobulin.
In the first analysis, authors assumed that SCIG and IVIG would yield identical clinical outcomes, and then conducted the cost minimization analysis.
In the second analysis, authors assumed that SCIG and IVIG would yield different clinical outcomes, and then conducted the cost utility analysis.
Authors included eight patients with SCIG therapy and 26 patients with IVIG therapy. The crude results were reported.
Appendix 4: Letter of Information
LETTER OF INFORMATION
SUMMARY:
Health Quality Ontario (HQO) is conducting a formal assessment of home-based subcutaneous immunoglobulin therapy, to better understand how this treatment option should be funded by the healthcare system. An important part of this assessment involves speaking to patients and families of those who suffer (or may have suffered) from immunodeficiency and may have used subcutaneous immunoglobulin treatment. Our goal is to ensure that recommendations about funding are informed by the lived-experience of patients and families who have been or are currently being treated with subcutaneous immunoglobulin.
WHAT DO YOU NEED FROM ME?
-
✓
Willingness to share your story
-
✓
40–60 minutes of your time for a phone or in-person interview
-
✓
Permission to audio- (not video-) record the interview
WHY DO YOU NEED THIS INFORMATION?
Health Quality Ontario (HQO) is conducting a Health Technology Assessment of the effectiveness and safety of subcutaneous immunoglobulin treatment for immunodeficiency. As part of HQO's core function to promote health care supported by the best evidence available, established scientific methods are used to analyze the evidence for a wide range of health interventions, including diagnostic tests, medical devices, interventional and surgical procedures, health care programs and models of care. These analyses may be informed and complemented by input from a range of individuals, including patients and clinical experts, and serve as the basis recommendations about whether health care interventions should be publicly funded or not.
The perspective that you share will be useful to help provide context to the day-to-day realities of patients with immunodeficiencies and the decisions they face in terms of therapies. The ultimate goal of the project is to provide recommendations to the Ontario Health Technology Assessment Committee who advises the Ontario Ministry of Health and Long-Term Care on the appropriateness of funding.
WHAT YOUR PARTICIPATION INVOLVES
If you agree to enroll, you will be asked to participate in an interview conducted by HQO staff. The interview will likely last 40–60 minutes. The session will be conducted in a private location and will be audio-taped. The interviewer will ask you questions about your lived experience with home-based subcutaneous immunoglobulin and your perspectives on immunoglobulin therapy in Ontario.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect care you receive.
CONFIDENTIALITY
All information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION:
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
HEALTH QUALITY ONTARIO STAFF:
Appendix 5: Interview Guide
| CANDIDATE: | INTERVIEWER: | DATE: |
|---|---|---|
|
Overview – What is Health Quality Ontario's mandate? What is Health Technology Assessment? Health Quality Ontario (HQO) is a provincial agency dedicated to ensuring our health care system delivers a better experience of care and better outcomes for Ontarians at better value for money. Part of this role includes evaluating the effectiveness of health care technologies and services through a process called Health Technology Assessment. Health Technology Assessment projects involve rigorous clinical and economic evidence review on the effective, safety, and cost of technologies while considering the perspectives of patients and caregivers who have experience with the particular condition or technology in question. We are currently reviewing home-based sub cutaneous immunoglobulin treatment in adult and children patients. I am calling you to hear about your experience with immunodeficiency and the treatment options available. | ||
|
QUESTION 1: What is it like to live with immunodeficiency? Is it primary or secondary? How does it impact your day to day routine? How would you describe your quality of life? (e.g. emotional/psychological effects, fatigue, stress, depression, physical challenges, financial, inability to work or go to school, etc.) If Caregiver, What is it like to care for someone with immunodeficiency? What is your day to day routine? How would you describe your quality of life? | ||
| CANDIDATE RESPONSE: | ||
|
QUESTION 2: What are the treatments that are accessible to you and which are the ones you have explored? How are currently available technologies meeting your needs? (What other technologies are they aware of? Which ones are accessible to them? How are these helpful to them in terms of addressing the challenges and what are the most important benefits? what are their unmet needs from these technologies? What is the positive and negative impact of currently available technologies, what are the side-effect of currently available technologies and are these tolerable?) | ||
| CANDIDATE RESPONSE: | ||
|
QUESTION 3: For those people who did not use this technology or who had tried both standard treatment and the technology under evaluation: What challenges would SCIG address? (Is there a long journey through many healthcare providers, Are there financial burden not supported by health insurance? Travel required for technology? Accessible? Repeat visits, uncomfortable/painful procedure, Embarrassing? Time off work/school, are there other choices available?) | ||
| CANDIDATE RESPONSE: | ||
|
QUESTION 4: For those people who did not use this technology or who had tried both standard treatment and the technology under evaluation: Are there any other benefits you see to this technology being available? What are the most important things you would like to gain from SCIG? (Does it offer accurate diagnosis/treatment? Better access or easier use? Is it more effective or safer? Control of condition or symptoms? Less intrusive or painful, future benefits, better quality of life, ability to go about your daily life, improve experience?) | ||
| CANDIDATE RESPONSE: | ||
|
QUESTION 5: For those people with experience of using SCIG, what difference did it make to their lives? What was the impact of having information from the technology: Treatment changes, Quality of Life change, empowerment and ownership of condition, improvement in adherence to treatment, lifestyle change, more tests after the first test, invasiveness, fewer tests, consequences of treatment, Financial burden, other healthcare services) What was the impact of having/taking the test: (Anxiety before/after, pain, side effects, embarrassment, time off work/school etc) If the test is handled directly by the patient: (new technology easy or more difficult, easy to understand, is daily life less impeded, what is the financial impact on family and patients) Have people actually gained what was important to them with the new test? Did the new diagnostic technology meet expectations? | ||
| CANDIDATE RESPONSE: | ||
The biggest challenges of living with immunodeficiency are…________________________
IVIG is adequate/inadequate because…________________________
Home-based SCIG being assessed will be/will not be beneficial because…________________________
Thank you for sharing your story and your insights on this condition and the available technologies. We will use these insights to draft report and recommendation for funding. The draft report will be posted on our public website for comments and would welcome you to review and share your thoughts on it. If you wish we could email you to alert you about this posting
If we do not have their email, request it and add to the stakeholder list ________________________
Author contributions
This report was developed by a multi-disciplinary team from Health Quality Ontario. The clinical epidemiologist was Shayan Sehatzadeh, the health economist was Xuanqian Xie, the public and patient engagement specialist was Arshia Ali, and the medical librarian was Melissa Walter.
Footnotes
What Is This Health Technology Assessment About?
Immunodeficiency disorders are conditions caused by defects in the immune system that leave the body unable to produce sufficient antibodies to fight infection. A person may be born with immunodificiency or acquire it through infection or as a side-effect of medical treatment. It may be temporary, resolving as the body heals form the damaging events, or it may be a life-long condition.
Standard therapy consists of regular injections of immunoglobulin, which is made from blood plasma taken from healthy donors. Treatment is usually done in hospital, but some provinces and hospitals are embracing an at-home therapy method that may help reduce the burden on patients and the system.
This health technology assessment compares the benefits, harms, safety, costs and patient experience of home-based subcutaneous infusion of immunoglobulin with hospital- or clinic-based intravenous infusion.
What Did This Health Technology Assessment Find?
Studies found that home-based subcutaneous immunoglobulin (SCIG) and hospital-based intravenous immunoglobulin therapies have comparable effectiveness, with a very low rate of serious adverse effects with either method. However, the quality of the evidence was low, meaning that we cannot be certain about these findings. People receiving SCIG treatment reported higher levels of satisfaction, largely due to the greater convenience and cost savings from not having to travel to the hospital for treatment. There are also system savings from SCIG treatment, mostly through reduced nursing time.
Contributor Information
Health Quality Ontario:
Shayan Sehatzadeh, Xuanqian Xie, Arshia Ali, and Melissa Walter
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are.
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do.
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters.
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Bonilla FA. Bernstein IL. Khan DA. Ballas ZK. Chinen J. Frank MM. et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(5 Suppl 1):S1–S63. [DOI] [PubMed] [Google Scholar]
- (2).Guidance for industry: safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency [Internet]. 2008. [cited 2017 May 10]. Available from: https://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/ucm072130.htm [Google Scholar]
- (3).O'Keefe AW. Halbrich M. Ben-Shoshan M. McCusker C. Primary immunodeficiency for the primary care provider. Paediatr Child Health. 2016;21(2):e10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Shehata N. Palda VA. Meyer RM. Blydt-Hansen TD. Campbell P. Cardella C. et al. The use of immunoglobulin therapy for patients undergoing solid organ transplantation: an evidence-based practice guideline. Transfus Med Rev. 2010;24 Suppl 1:S7–s27. [DOI] [PubMed] [Google Scholar]
- (5).Cherin P. Marie I. Michallet M. Pelus E. Dantal J. Crave JC. et al. Management of adverse events in the treatment of patients with immunoglobulin therapy: A review of evidence. Autoimmun Rev. 2016; 15(1):71–81. [DOI] [PubMed] [Google Scholar]
- (6).Jolles S. Orange JS. Gardulf A. Stein MR. Shapiro R. Borte M. et al. Current treatment options with immunoglobulin G for the individualization of care in patients with primary immunodeficiency disease. Clin Exp Immunol. 2015;179(2):146–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gardulf A. Nicolay U. Asensio O. Bernatowska E. Bock A. Carvalho BC. et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies – A prospective, multi-national study. J Clin Immunol. 2006;26(2):177–85. [DOI] [PubMed] [Google Scholar]
- (8).Berger M. Murphy E. Riley P. Bergman GE. Improved quality of life, immunoglobulin G levels, and infection rates in patients with primary immunodeficiency diseases during self-treatment with subcutaneous immunoglobulin G. South Med J. 2010;103(9):856–63. [DOI] [PubMed] [Google Scholar]
- (9).Gardulf A. Nicolay U. Math D. Asensio O. Bernatowska E. Bock A. et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42. [DOI] [PubMed] [Google Scholar]
- (10).Ochs HD. Gupta S. Kiessling P. Nicolay U. Berger M. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26(3):265–73. [DOI] [PubMed] [Google Scholar]
- (11).Gerth WC. Betschel SD. Zbrozek AS. Implications to payers of switch from hospital-based intravenous immunoglobulin to home-based subcutaneous immunoglobulin therapy in patients with primary and secondary immunodeficiencies in Canada. Allergy Asthma Clin Immunol. 2014;10(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ducruet T. Levasseur MC. Des Roches A. Kafal A. Dicaire R. Haddad E. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol. 2013;131(2):585–7.e1–3. [DOI] [PubMed] [Google Scholar]
- (13).McGowan J. Sampson M. Salzwedel D. Cogo E. Foerster V. Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (14).Fasth A. Nystrom J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28(4):370–8. [DOI] [PubMed] [Google Scholar]
- (15).Thepot S. Malphettes M. Gardeur A. Galicier L. Asli B. Karlin L. et al. Immunoglobulin dosage and switch from intravenous to subcutaneous immunoglobulin replacement therapy in patients with primary hypogammaglobulinemia: Decreasing dosage does not alter serum IgG levels. J Clin Immunol. 2010;30(4):602–6. [DOI] [PubMed] [Google Scholar]
- (16).Hozo SP. Djulbegovic B. Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hoffmann F. Grimbacher B. Thiel J. Peter HH. Belohradsky BH. Home-based subcutaneous immunoglobulin G replacement therapy under real-life conditions in children and adults with antibody deficiency. Eur J Med Res. 2010;15(6):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Guyatt GH. Oxman AD. Schunemann HJ. Tugwell P. Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (19).Moher D. Liberati A. Tetzlaff J. Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- (20).Compagno N. Cinetto F. Semenzato G. Agostini C. Subcutaneous immunoglobulin in lymphoproliferative disorders and rituximab-related secondary hypogammaglobulinemia: A single-center experience in 61 patients. Haematologica. 2014;99(6):1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sundin M. Nordin K. Jostemyr Y. Winiarski J. Subcutaneous IgG replacement after pediatric SCT. Pediatr Transplant. 2012;16(8):866–71. [DOI] [PubMed] [Google Scholar]
- (22).Bienvenu B. Cozon G. Hoarau C. Pasquet M. Cherin P. Clerson P. et al. Does the route of immunoglobin replacement therapy impact quality of life and satisfaction in patients with primary immunodeficiency? Insights from the French cohort “visages”. Orphanet J Rare Dis. 2016;11(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Vultaggio A. Azzari C. Milito C. Finocchi A. Toppino C. Spadaro G. et al. Subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency in routine clinical practice: The VISPO prospective multicenter study. Clin Drug Investig. 2015;35(3):179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Samaan K. Levasseur MC. Decaluwe H. St-Cyr C. Chapdelaine H. Des Roches A. et al. SCIg vs. IVIg: Let's give patients the choice! J Clin Immunol. 2014;34(6):611–4. [DOI] [PubMed] [Google Scholar]
- (25).Reid B. Pires L. Home gammaglobulin therapy: A patient survey of intravenous and subcutaneous options in Canada. LymphoSign Journal. 2014;1(1):27–37. [Google Scholar]
- (26).Bezrodnik L. Gomez Raccio A. Belardinelli G. Regairaz L. Diaz Ballve D. Seminario G. et al. Comparative study of subcutaneous versus intravenous IgG replacement therapy in pediatric patients with primary immunodeficiency diseases: A multicenter study in Argentina. J Clin Immunol. 2013;33(7):1216–22. [DOI] [PubMed] [Google Scholar]
- (27).Quinti I. Soresina A. Agostini C. Spadaro G. Matucci A. Sfika I. et al. Prospective study on CVID patients with adverse reactions to intravenous or subcutaneous IgG administration. J Clin Immunol. 2008;28(3):263–7. [DOI] [PubMed] [Google Scholar]
- (28).Fasth A. Nystrom J. Safety and efficacy of subcutaneous human immunoglobulin in children with primary immunodeficiency. Acta Paediatr. 2007;96(10):1474–8. [DOI] [PubMed] [Google Scholar]
- (29).Nicolay U. Kiessling P. Berger M. Gupta S. Yel L. Roifman CM. et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26(1):65–72. [DOI] [PubMed] [Google Scholar]
- (30).Kittner JM. Grimbacher B. Wulff W. Jager B. Schmidt RE. Patients’ attitude to subcutaneous immunoglobulin substitution as home therapy. J Clin Immunol. 2006;26(4):400–5. [DOI] [PubMed] [Google Scholar]
- (31).Nicolay U. Haag S. Eichmann F. Herget S. Spruck D. Gardulf A. Measuring treatment satisfaction in patients with primary immunodeficiency diseases receiving lifelong immunoglobulin replacement therapy. Qual Life Res. 2005;14(7):1683–91. [DOI] [PubMed] [Google Scholar]
- (32).Gaspar J. Gerritsen B. Jones A. Immunoglobulin replacement treatment by rapid subcutaneous infusion. Arch Dis Child. 1998;79(1):48–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).National Institute for Health and Care Excellence. Process and methods guides. The guidelines manual: Appendix G: methodology checklist [Internet]. London (UK): The Institute; 2012. [cited 2015 May 27]. Available from: http://publications.nice.org.uk/the-guidelines-manual-appendices-bi-pmg6b/appendix-g-methodology-checklist-economic-evaluations. [Google Scholar]
- (34).Martin A. Lavoie L. Goetghebeur M. Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Ho C. Membe S. Cimon K. Roifman C. Kanani A. Morrison A. Subcutaneous versus intravenous immunoglobulin for primary immunodeficiencies: Systematic review and economic evaluation [Technology report number 98]. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2008. [Google Scholar]
- (36).Beaute J. Levy P. Millet V. Debre M. Dudoit Y. Le Mignot L. et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160(2):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Membe SK. Ho C. Cimon K. Morrison A. Kanani A. Roifman CM. Economic assessment of different modalities of immunoglobulin replacement therapy. Immunol Allergy Clin North Am. 2008;28(4):861–74. [DOI] [PubMed] [Google Scholar]
- (38).Theoret L. SCIG Home Infusion at The Ottawa Hospital. [Internet]. 2015. [cited 2017 May 10]. Available from: http://transfusionontario.org/en/wp-content/uploads/sites/4/2015/05/SS2015_Theoret.pdf [Google Scholar]
- (39).Statistics Canada. Consumer Price Index, by province, health and personal care (Canada). [Internet]. Ottawa (ON): Statistics Canada; 2017. [updated 2017 Feb 24. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/cpis01a-eng.htm [Google Scholar]
- (40).Canadian Blood Services. Plasma Protein Products: Immune Globulin Information [Internet]. 2017. [cited 2017 May 10]. Available from: https://blood.ca/en/hospitals/plasma-products [Google Scholar]
- (41).IVIG and SCIG Utilization in the Atlantic Provinces in FY 2014/15. Nova Scotia Provincial Blood Coordinating Program; September 2015. [Google Scholar]
- (42).Government of Canada. Current and forthcoming minimum hourly wage rates for experienced adult workers in Canada. [Internet]. 2017. [cited 2017 May 10]. Available from: http://srv116.services.gc.ca/dimt-wid/sm-mw/rpt1.aspx [Google Scholar]
- (43).Ontario Ministry of Labour. Minimum Wage. [Internet]. 2017. [cited 2017 May 10]. Available from: https://www.labour.gov.on.ca/english/es/pubs/guide/minwage.php [Google Scholar]
- (44).Statistics Canada. Labour force characteristics, seasonally adjusted, by province. [Internet]. 2017. [cited 2017 May 10]. Available from: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/lfss01b-eng.htm [Google Scholar]
- (45).CSL Behring. Hizentra® Care Program. [Internet]. 2015. [cited 2017 May 10]. Available from: http://www.health.gov.nl.ca/health/bloodservices/pdf/HizentraCAREenrolmentform.pdf [Google Scholar]
- (46).Kobrynski L. Subcutaneous immunoglobulin therapy: a new option for patients with primary immunodeficiency diseases. Biologics. 2012;6:277–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Chapel HM. Spickett GP. Ericson D. Engl W. Eibl MM. Bjorkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20(2):94–100. [DOI] [PubMed] [Google Scholar]
- (48).Drummond MF SM. Claxton K. Stoddart GL. Torrance GW. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford Medical Publications; 2015. [Google Scholar]
- (49).Guidelines for the economic evaluation of health technologies: Canada. 4th ed. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2017. [Google Scholar]
- (50).Fu L. Song C. Betschel S. Prospective economic analysis of home-based subcutaneous immunoglobulin therapy vs hospital-based intravenous immunoglobulin therapy. Ann Allergy Asthma Immunol. 2016;117(5 (Suppl)):S96. [DOI] [PubMed] [Google Scholar]
- (51).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (52).Messina J. Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (53).Subcommittee OPE. Public Engagement for Health Technology Assessment at Health Quality Ontario—Final Report From the Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Toronto, ON: Queen's Printer for Ontario; 2015. [Google Scholar]
- (54).Tjornhoj-Thomsen T. Hansen HP. Knowledge in health technology assessment: Who, what, how? Int J Technol Assess Health Care. 2011;27(4):324–9. [DOI] [PubMed] [Google Scholar]
- (55).Rowe G. Frewer LJ. A typology of public engagement mechanisms. Sci Technol Hum Val. 2005;30(2):251–90. [Google Scholar]
- (56).Brundisini F. Giacomini M. DeJean D. Vanstone M. Winsor S. Smith A. Chronic disease patients’ experiences with accessing health care in rural and remote areas: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser. 2013;13(15):1–33. [PMC free article] [PubMed] [Google Scholar]
- (57).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (58).Health Technology Assessment international Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. 2015. [updated 2015]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA_KFacey_Jun13.pdf [Google Scholar]
- (59).Strauss AL. Corbin JM. Grounded theory research: Procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (60).Strauss AL. Corbin JM. Grounded theory methodology: an overview. In: Denzin NK. Lincoln YS. editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]