ABSTRACT
PURPOSE OF REVIEW
Alzheimer disease (AD) is the most common cause of late-onset dementia. This article describes the epidemiology, genetic and environmental risk factors, clinical diagnosis, biomarkers, and treatment of late-onset AD, defined by age of onset of 65 years or older.
RECENT FINDINGS
An estimated 5.7 million Americans are living with AD dementia, with the number of affected individuals growing rapidly because of an aging population. Vascular risk factors, sleep disorders, and traumatic brain injury are associated with an increased risk of AD, while increased cognitive and physical activity throughout the lifespan reduce the risk of disease. The primary genetic risk factor for late-onset AD is the apolipoprotein E (APOE) ε4 allele. AD typically presents with early and prominent episodic memory loss, although this clinical syndrome is neither sensitive nor specific for underlying AD neuropathology. Emerging CSF and imaging biomarkers can now detect the key neuropathologic features of the disease (amyloid plaques, neurofibrillary tangles, and neurodegeneration) in living people, allowing for characterization of patients based on biological measures. A comprehensive treatment plan for AD includes use of symptomatic medications, optimal treatment of comorbid conditions and neuropsychiatric symptoms, counseling about safety and future planning, and referrals to community resources.
SUMMARY
AD is very common in older neurologic patients. Neurologists should set the standard for the diagnosis and care of patients with AD and should be familiar with emerging biomarkers that have transformed AD research and are primed to enter the clinical arena.
INTRODUCTION
Beginning with Alois Alzheimer’s seminal report “On an Unusual Illness of the Cerebral Cortex” in a 51-year-old woman1 and for most of the 20th century, Alzheimer disease (AD) was considered a rare cause of presenile dementia. In the 1970s, it became apparent that the “neurofibrils” (neurofibrillary tangles) and “miliary foci” (senile plaques) described by Alzheimer were present in the majority of people who developed dementia in late life. In subsequent decades, it has become clear that AD is very common, with a prevalence that rivals the most common age-related diseases and continues to grow in the setting of an aging population. This article focuses on late-onset AD, defined as symptom onset at age 65 or older, reviewing the epidemiology of the disease and known environmental and genetic risk factors. Current approaches to clinical diagnosis and treatment are discussed, and a growing armamentarium of biomarkers that are already transforming AD research and are likely to have an increasing future role in clinical care are introduced.
EPIDEMIOLOGY
An estimated 5.7 million Americans are living with AD dementia, and an additional 11.6 million Americans have mild cognitive impairment (MCI).2,3 The incidence and prevalence of AD increase dramatically with age. Approximately 80% of patients with AD are older than age 75, with disease incidence increasing from 2 per 1000 at ages 65 to 74 to 37 per 1000 at age 85 and older.2,4,5 The number of patients with AD in the United States is projected to nearly triple by 2050, with the majority of growth attributed to the 85 and older age group.5 Nearly two-thirds of patients with AD are women, likely reflecting both increased life duration and biological factors.5 Compared to non-Hispanic whites, the incidence of AD is higher in African Americans/blacks and Hispanics/Latinos and lower in Asian Americans.6 The overall lifetime risk for AD at age 65 is 21.1% for women and 11.6% for men.2,7 Average survival after diagnosis varies between 4 and 8 years across studies and is impacted by multiple factors, including age at diagnosis, sex, psychotic features, motor system involvement, and medical comorbidities.8 In otherwise healthy individuals, survival can extend to 15 to 20 years. The public health impact of AD and other forms of dementia cannot be overstated, with an estimated cost of $277 billion in the United States in 2018.2
ENVIRONMENTAL RISK FACTORS
Prospective population-based studies provide strong evidence that the risk of late-life cognitive impairment and dementia is modified by medical comorbidities, lifestyle choices, and other environmental factors.9 The risk of dementia is increased in patients with vascular risk factors, and growing evidence suggests that aggressive treatment of these risk factors as early as midlife can attenuate the risk of developing cognitive impairment in older age.10,11 Common sleep disturbances such as insomnia and obstructive sleep apnea are also associated with late-life cognitive decline.12,13 Traumatic brain injury is a potentially preventable risk factor for AD and other neurodegenerative disorders.14 Late-life depression is associated with increased risk of cognitive decline, although it is unclear whether this represents a risk factor or a consequence of early AD neuropathology in serotonergic and noradrenergic brainstem nuclei.15 Conversely, increased years of formal education, physical activity, and social engagement across the lifespan moderate the risk of late-life dementia.9 Links between many of these factors and the specific pathobiology of AD are less established, since few studies included autopsy or biomarker confirmation of AD in assessing outcomes. Nevertheless, aggressive treatment of dementia risk factors and encouragement of beneficial lifestyle choices should be considered universal recommendations for promoting healthy brain aging.
GENETIC RISK FACTORS
Late-onset AD is a complex genetic disorder, with an estimated heritability of 60% to 80%.16 The strongest genetic risk factor for late-onset AD is apolipoprotein E (APOE) genotype. APOE, which encodes the brain’s major cholesterol transporter, has three common alleles: ε2 (8.4% estimated allele frequency in the population), ε3 (77.9%), and ε4 (13.7%).17 APOE ε4 is associated with an increased risk of developing AD, with odds ratios of approximately 3 in heterozygotes and 8 to 12 in homozygotes compared to individuals with the ε3/ε3 genotype.17,18 Each APOE ε4 allele reduces the average age of symptom onset by about a decade. Female carriers of APOE ε4 are at increased risk compared to male carriers, particularly between the ages of 65 and 75.18 Conversely, the ε2/ε2 and ε2/ε3 genotypes are protective (odds ratio approximately 0.5 to 0.7 versus ε3/ε3). APOE ε4 contributes to AD risk via a multitude of mechanisms, including enhanced aggregation and decreased clearance of the amyloid-β (Aβ) polypeptide; increased tau phosphorylation; network hyperexcitability; reduced glucose metabolism, vascular, and mitochondrial function; and neurodevelopmental differences.17,19 APOE genotyping is currently not recommended in the clinical evaluation of patients with suspected AD, since the ε4 allele represents a risk factor rather than a deterministic gene.20
Genome-wide association studies have identified more than 20 additional common genetic variants that modify the risk of late-onset AD.16 These genes converge in biological pathways involving lipid metabolism, innate immunity, and endocytosis. The effects of each gene on AD risk is small (odds ratios of approximately 0.8 to 0.9 for protective alleles and 1.1 to 1.2 for risk alleles) and not clinically meaningful. Assessing the overall burden of AD risk alleles via polygenic hazard scores may enhance individual risk prediction.21 With the advent of next-generation sequencing, rare genetic variants with large effects on disease risk are coming to light. For example, rare variants in the triggering receptor expressed on myeloid cells 2 (TREM2) gene are associated with an odds ratio of approximately 3 to 4 for developing AD,22 highlighting the importance of immune and inflammatory pathways in disease pathogenesis. Mutations in amyloid-β precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) that lead to familial early-onset AD are rare in older patients, although pathogenic mutations in PSEN2 and rare variants in APP have been associated with late-onset disease.16
CLINICAL APPROACH TO PATIENTS WITH COGNITIVE SYMPTOMS
The clinical evaluation of patients with cognitive symptoms begins with a thorough clinical history and examination. It is important to obtain corroborative information from an additional source (eg, family member or close friend), since patient recall or insight may be limited. The history of present illness should query symptoms referable to specific cognitive domains and neuropsychiatric symptoms as well as motor and autonomic symptoms, sleep, dietary habits, emotional function, and social behavior (table 1-1). First and early symptoms are particularly salient, since they can help localize the earliest brain regions involved and thus inform the differential diagnosis. Determining the level of functional impairment (ie, the impact of cognitive symptoms on instrumental and basic activities of daily living) is critical for disease staging and appropriate counseling.
TABLE 1-1.
Symptoms Associated With Neurodegenerative Dementia

The first goal of the clinical evaluation is to rule out potentially reversible causes of cognitive decline by reviewing medical comorbidities, medication and substance use, and environmental exposures. Neurodegenerative diseases typically have an insidious onset and are characterized by slow gradual progression. Thus, an acute or subacute change in mental status should raise concern for a nondegenerative process. Screening with brief cognitive tests such as the Mini-Mental State Examination (MMSE) or Montreal Cognitive Assessment (MoCA) represents a reasonable first step, but more detailed neuropsychological testing is often helpful in defining the pattern of cognitive deficits.
Per American Academy of Neurology (AAN) guidelines, the following laboratory tests should be ordered in the routine evaluation of patients with cognitive decline: complete blood cell count, serum electrolytes, liver and renal function tests, thyroid function tests, and serum vitamin B12.20 Additional laboratory testing may be appropriate depending on the clinical context. Brain imaging with CT or MRI without contrast is recommended to exclude structural lesions and can be helpful in identifying characteristic patterns of brain atrophy and white matter injury.
CLINICAL DIAGNOSIS OF ALZHEIMER DISEASE
Patients with acquired cognitive impairment that represents a decline from their previous level of performance and is objectively corroborated by history and examination, yet does not interfere with daily function, are considered to have MCI.3,23 When cognitive decline interferes with independent function, patients meet criteria for dementia.23 Equivalent categories of mild and major neurocognitive disorder are defined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).24 In reality, these distinct categories represent a continuum of cognitive decline that begins with subjective changes and culminates in dementia.23
A number of common neurodegenerative diseases can present with late-life cognitive decline, including the following:
Alzheimer disease
Vascular cognitive impairment
Dementia with Lewy bodies
Primary age-related tauopathy
Hippocampal sclerosis of aging (cerebral age-related transactive response DNA-binding protein 43 [TDP-43] and sclerosis)
Argyrophilic grain disease
Frontotemporal lobar degeneration
Late-onset AD manifests most commonly as a progressive amnestic disorder characterized by early and prominent deficits in episodic memory, with varying degrees of executive, language, and visuospatial impairment (case 1-1).25,26 Patients often show a gradient of memory impairment, with greatest difficulty recalling recent events and relative sparing of remote memory. On memory tests (eg, word list or story learning), patients show impaired learning, rapid forgetting, and poor delayed recall, characteristic of dysfunction of the hippocampal circuit.27 However, this clinical presentation is not specific for AD and may also occur in other conditions that affect the medial temporal lobes (case 1-2), such as vascular cognitive impairment, primary age-related tauopathy, hippocampal sclerosis of aging (cerebral age-related TDP-43 and sclerosis), and argyrophilic grain disease. For more information on primary age-related tauopathy, hippocampal sclerosis of aging, and argyrophilic grain disease, refer to the article “Hippocampal Sclerosis, Argyrophilic Grain Disease, and Primary Age-Related Tauopathy” by Gregory A. Jicha, MD, PhD, and Peter T. Nelson, MD, PhD,29 in this issue of Continuum. Nonamnestic syndromes associated with AD neuropathology (eg, logopenic variant primary progressive aphasia, posterior cortical atrophy) are less common in late-onset disease than in younger patients.30 For more information on posterior cortical atrophy, refer to the article “Posterior Cortical Atrophy” by Jonathan M. Schott, BSc, MD, FRCP, FEAN, SFHEA, and Sebastian J. Crutch, PhD, CPsych,31 in this issue of Continuum. Rarely, underlying dementia with Lewy bodies or frontotemporal lobar degeneration can mimic AD clinically, but these conditions are typically associated with differentiating clinical features.
CASE 1-1
A 73-year-old woman presented for evaluation of 3 years of progressive memory loss. Her husband reported that she frequently misplaced personal items, forgot passwords, and repeated the same questions. She had trouble locating her car in the parking lot and had been late paying bills. She had difficulty completing tasks and recently seemed overwhelmed when trying to plan travel for a vacation. She had shown less interest in previous hobbies but did not report low mood. She denied motor problems or disruption of sleep. Her husband had taken over managing finances and bill paying and had to remind her to take her medications. She was otherwise independent with day-to-day function.
Her past medical history was notable for long-standing hypertension, diabetes mellitus, and hyperlipidemia. She had an episode of delirium 5 years prior when hospitalized for a total hip replacement. Review of medications did not reveal any substances known to affect cognition. Family history was notable for late-onset dementia in her mother, brother, and maternal grandmother. She had 16 years of formal education, rarely drank alcohol, and did not smoke tobacco or use recreational drugs.
During the interview, she had difficulty describing her symptoms and often deferred to her husband to answer questions. Recall of recent personal and public events was impaired and lacked detail. Insight was limited. On cognitive testing, she scored 21/30 on the Mini Mental State Examination (MMSE), losing points for orientation, word recall, and serial 7s. She showed moderate impairment on tests of verbal and visual memory and mild deficits on tests of executive, language, and visuospatial functions. The rest of her neurologic examination was normal.
Laboratory evaluations for reversible causes of cognitive impairment were within normal limits. Brain MRI showed moderate atrophy of the hippocampus and medial temporal lobes bilaterally, mild atrophy of the lateral temporal and parietal lobes, and no significant vascular lesions. The patient was diagnosed with dementia due to probable Alzheimer disease (AD) and started on an acetylcholinesterase inhibitor.
A driving evaluation at the Department of Motor Vehicles was ordered. Regular exercise was recommended. The husband was provided information about attending a caregiver support group and working with a social worker.
COMMENT
This patient’s clinical presentation is highly suggestive of underlying AD, with early and prominent involvement of episodic memory and mild involvement of other cognitive domains. Her medical comorbidities and family history put her at elevated risk for AD. Her MRI showed an atrophy pattern that was consistent with the diagnosis and did not show evidence of significant vascular burden. Biomarker tests of amyloid and tau are not currently recommended for patients with “typical” age of onset and clinical symptoms. The care plan for this patient included a combination of drug treatment, lifestyle and safety recommendations, and referral to community resources.
CASE 1-2
An 83-year-old man was evaluated for 2 years of progressive cognitive decline. He reported increasing problems remembering the names of distant acquaintances, thinking of words, and learning to use new devices. He started keeping a detailed to-do list and daily calendar because he had missed several medical appointments. His wife agreed that he had become more forgetful in the previous 2 years and commented that he now repeated himself in daily conversations. He remained active in local community organizations and was fully independent with all instrumental activities of daily living. His medical history was notable for well-controlled hypertension. He had been taking diphenhydramine at bedtime for many years for insomnia.
Examination was notable for a lack of detail when describing events in the news. His Mini-Mental State Examination (MMSE) score was 26/30, losing points for orientation and recall.
On formal cognitive testing, he scored 1.0 to 1.5 standard deviations below age-matched norms on tests of episodic memory and in the normal to above average range on tests of other cognitive domains. Laboratory evaluation was unremarkable, and brain MRI showed moderate to severe hippocampal atrophy, mild generalized cortical atrophy, and mild to moderate subcortical white matter changes.
Based on this evaluation, he was diagnosed with mild cognitive impairment (MCI) impacting primarily episodic memory. Diphenhydramine was discontinued, and sleep hygiene measures were instituted to help with insomnia. Amyloid positron emission tomography (PET), obtained as part of an observational research study, was negative for cortical tracer retention. He started exercising regularly under the supervision of a personal trainer at the local senior center and enrolled in adult classes offered at the local university. On follow-up 2 years later, he and his wife reported a mild decline in his memory, but cognitive testing was stable, and he remained highly functional and fully independent.
COMMENT
This patient presented with amnestic MCI, which can represent the prodromal stage of Alzheimer disease (AD) but is also associated with other age-related neuropathologies and nondegenerative causes. In this case, negative amyloid PET reduces the likelihood of underlying AD, while his MRI findings support the possibility of a non-AD degenerative process affecting the medial temporal lobes as well as a vascular contribution. The treatment plan involved discontinuation of anticholinergic medication and lifestyle recommendations. He remained clinically stable with follow-up, consistent with studies that suggest slower decline in patients with MCI who are negative on amyloid biomarkers.28
Common neuropsychiatric symptoms in AD include depression, anxiety, mild apathy, irritability, and sleep disturbances (eg, insomnia or disrupted circadian rhythm).32 Social and emotional function are generally preserved early in the disease course, and agitation and psychotic symptoms are uncommon. The general neurologic examination is either normal or shows minor signs (eg, restricted upgaze, paratonia, or mild rigidity).
BRAIN IMAGING
AAN guidelines support the use of brain imaging with CT or MRI in the initial assessment of dementia to exclude structural lesions such as neoplasms, subdural hematomas, and normal pressure hydrocephalus.20 CT and MRI can also inform the differential diagnosis of neurodegenerative diseases by identifying characteristic brain atrophy patterns and assessing for vascular injury. The hallmark MRI changes of late-onset AD include atrophy of the hippocampus and medial temporal lobes, cortical atrophy (primarily involving temporoparietal cortex), and ventricular enlargement (figures 1-1a, 1-1b, and 1-1c).33 Compared to early-onset AD, patients with late-onset disease show greater medial temporal atrophy and less cortical atrophy.34 While these changes are not entirely specific to AD versus normal aging or other causes of dementia, they can support the clinical impression of AD in the appropriate clinical context. Patients show varying degrees of white matter hyperintensities on T2-weighted/fluid-attenuated inversion recovery (FLAIR) sequences, which are nonspecific but most often associated with small vessel ischemic disease (figure 1-1d).35 Cerebral amyloid angiopathy (CAA), which can co-occur with AD, is suggested by the presence of cortical microbleeds or superficial siderosis on iron-sensitive sequences such as gradient recalled echo (GRE) or susceptibility-weighted imaging (SWI) and extensive white matter lesions on T2-weighted/FLAIR images (figures 1-1e and 1-1f).36
FIGURE 1-1.
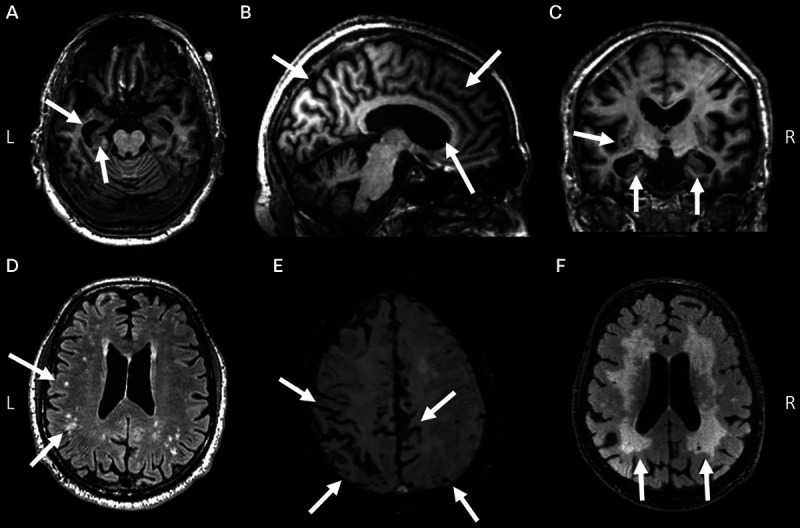
MRI findings in Alzheimer disease (AD). Axial (A), sagittal (B), and coronal (C) T1-weighted images demonstrate prominent hippocampal and medial temporal lobe atrophy, moderate diffuse cortical atrophy, and ventricular enlargement in an 81-year-old with AD dementia, subsequently confirmed at autopsy. D, Fluid-attenuated inversion recovery (FLAIR) sequence demonstrates subcortical and periventricular white matter hyperintensities in a 78-year-old with a clinical diagnosis of AD, likely representing comorbid small vessel ischemic disease. E, Hallmarks of cerebral amyloid angiopathy, including scattered microbleeds and superficial siderosis, are revealed on susceptibility-weighted imaging (SWI) in a 75-year-old with acute altered mental status superimposed on progressive memory and executive dysfunction. F, Confluent white matter hyperintensities on FLAIR in a 75-year-old with pathology-proven severe cerebral amyloid angiopathy and AD neuropathology. In all panels, key findings are highlighted by arrows.
L = left; R = right.
Functional brain imaging with fludeoxyglucose positron emission tomography (FDG-PET) or single-photon emission computed tomography (SPECT) perfusion scans can be helpful in differentiating AD from normal aging and other disorders,37,38 although routine use is not currently recommended. The classic pattern of hypometabolism (FDG-PET) or hypoperfusion (SPECT) associated with AD involves reduced activity in the temporoparietal cortex and posterior cingulate/precuneus. As with MRI, cortical changes are more prominent in early-onset than late-onset AD.39 In the United States, FDG-PET is reimbursed by the Centers for Medicare & Medicaid Services for differentiating between AD and frontotemporal dementia, the latter being associated with predominant hypometabolism in frontal and anterior temporal regions.40,41
NEUROPATHOLOGY
Gross examination of the postmortem AD brain typically reveals atrophy of the medial temporal lobes and cerebral cortex (figure 1-2a). On microscopic examination, evidence of synaptic and (to a lesser degree) neuronal loss is seen.42 The neuropathologic diagnosis of AD is established by the presence of two pathognomonic lesions, senile plaques and neurofibrillary tangles (figures 1-2b, 1-2c, 1-2d, and 1-2e).43 Senile plaques are extracellular deposits composed primarily of aggregated Aβ1-42 polypeptides. These appear as diffuse structures or as neuritic plaques when associated with dystrophic neurites. Aβ plaques first appear in the neocortex, then spread successively into the hippocampus and limbic structures, striatum and diencephalon, brainstem, and cerebellum.44 CAA is defined by vascular deposits consisting of aggregated Aβ1-40 polypeptides (figure 1-2f). Neurofibrillary tangles are composed of aggregated, hyperphosphorylated forms of the microtubule binding protein tau. The distribution of tangles typically follows a hierarchical pattern described by Braak stages, appearing first in the transentorhinal and entorhinal cortex (Braak stages I and II), followed by limbic (Braak stages III and IV) and isocortical (Braak stages V and VI) regions.45 The neuropathologic criteria for AD integrate the distribution and density of Aβ plaques with Braak staging of tau pathology in describing the overall burden of AD neuropathologic changes.43
FIGURE 1-2.
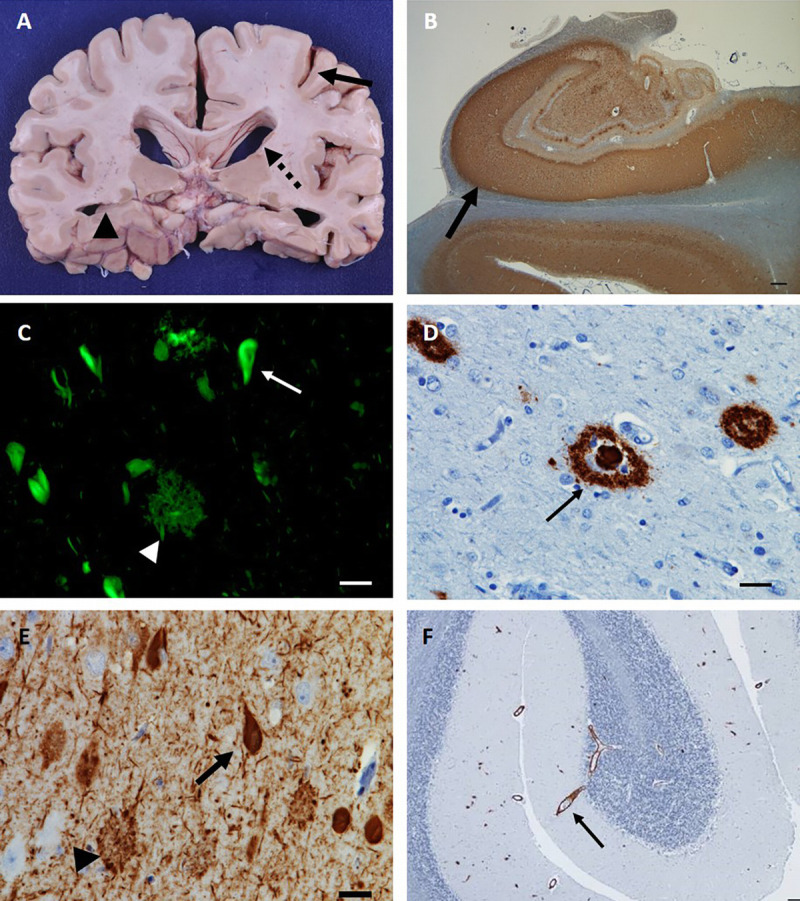
Neuropathologic features of Alzheimer disease (AD). A, Gross neuropathologic features of AD include cortical atrophy (arrow), ventricular enlargement (dashed arrow) and hippocampal atrophy (arrowhead). B, Low-power photomicrograph (1x) of hippocampus stained with anti–phosphorylated tau (p-tau) antibody (CP13), demonstrating an abundance of p-tau deposition, most prominent in CA1 (arrow). C, Thioflavin S staining identifies senile amyloid plaques (arrowhead) and tau neurofibrillary tangles (arrow), the pathologic hallmarks of AD. D, High-power photomicrographs (40x) of senile plaques (arrow) identified via anti–amyloid-β immunohistochemistry (1-16, clone DE2, mouse, 1:500). E, High-power photomicrograph (40x) of anti–p-tau (CP13) immunohistochemistry illustrates neurofibrillary tangles (arrow) and dystrophic neurites (arrowhead). F, Low-power photomicrograph (4x) of anti–amyloid-β immunostaining of cerebellum reveals amyloid deposition in blood vessel walls (arrow), the pathologic hallmark of cerebral amyloid angiopathy. Bars represent 25 microns.
Cognitive symptoms correlate more strongly with the burden and distribution of neurofibrillary pathology than with Aβ pathology.46 Furthermore, AD neuropathology is rarely found in isolation in older patients. The most common pathologies found in tandem with AD include vascular brain injury, Lewy body pathology, TDP-43 inclusions, and hippocampal sclerosis.43 Each of these copathologies contributes synergistically to cognitive decline.47,48 At autopsy, late-onset dementia and even MCI are nearly always associated with multiple pathologies,48 and this is especially true in the very old (age 90 plus).49
AMYLOID-β AND TAU BIOMARKERS
In recent years, major progress has been made in developing and validating CSF and imaging biomarkers of Aβ and tau, the core molecular features that define AD neuropathology.50 The CSF fingerprint of AD includes reductions in levels of Aβ1-42 and increases in total tau (t-tau) and phosphorylated tau (p-tau).51 Changes in Aβ1-42 and p-tau are associated with amyloid plaques and neurofibrillary pathology, respectively, while increases in t-tau are a nonspecific marker of neurodegeneration and can be seen in other conditions associated with rapid neuronal death (eg, Creutzfeldt-Jakob disease, acute ischemia, and traumatic brain injury). When performed in expert laboratories, combined CSF measures of Aβ and tau (eg, ratios of t-tau to Aβ1-42 or p-tau to Aβ1-42) show 80% to 90% sensitivity and specificity in discriminating between AD, healthy controls, and non-AD dementias.51,52 CSF biomarkers are also helpful in predicting clinical progression in MCI, presumably by identifying those individuals with underlying AD pathology. However, current assays are sensitive to a number of preanalytic and analytic factors, resulting in high variability across laboratories, particularly for Aβ1-42 assays.53 Major efforts around standardization combined with technical advances in the new generation of assays are likely to improve the reliability of these markers and increase their clinical use.53
Amyloid plaques can be directly detected in living people using PET ligands that bind to neuritic plaques.54 Three Aβ PET tracers, [18F]florbetapir, [18F]flutemetamol, and [18F]florbetaben, are approved for clinical use in the United States and other countries, and additional ligands are used in the research setting. For all tracers, a scan is considered “negative” when ligand retention is limited to white matter, while a “positive” scan is defined by extension of tracer binding into cortical gray matter (figure 1-3). PET-to-autopsy studies have shown that scans acquired during life reliably predict the burden of neuritic plaques found at autopsy.54 However, amyloid deposition is not specific to AD clinical phenotypes and can be also seen in dementia with Lewy bodies, CAA, and in a proportion of cognitively healthy older individuals.55 More recently, PET ligands that bind selectively to paired helical filaments of tau have been developed, allowing for in vivo visualization of neurofibrillary tangles.54 Early in vivo tau PET studies largely conform to expectations from neuropathology, with tracer binding following the topography of Braak staging and showing strong correlations with disease stage and cognitive performance.56–58 Tau PET is not yet approved for clinical use and is currently available only through research studies.
FIGURE 1-3.
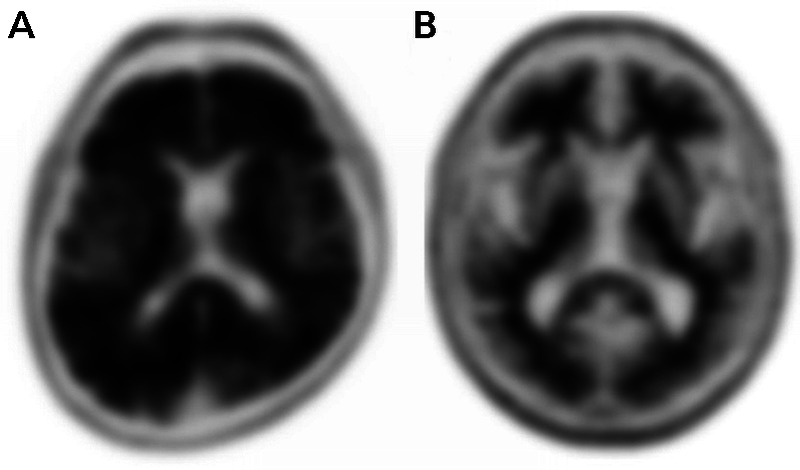
Amyloid positron emission tomography (PET) with [18F]florbetapir. A, Example of a positive scan in a 72-year-old with amnestic mild cognitive impairment, as defined by absent gray-white matter contrast and intense gray matter binding that exceeds the binding in adjacent white matter. B, Example of a negative scan in an 80-year-old with amnestic mild cognitive impairment, as defined by greater tracer binding in white matter compared with gray matter, creating clear gray-white contrast.
Implications of Alzheimer Disease Biomarkers for Research and Clinical Care
AD biomarkers allow us to capture in vivo the key components that define the neuropathology of AD and to prospectively follow these processes across the disease continuum (figure 1-4). Biomarkers have helped establish the existence of a prolonged preclinical phase of AD, demonstrating that plaques and tangles deposit in the brain a decade or more before the onset of clinical symptoms.59,60 Two sets of diagnostic criteria have suggested augmenting clinical diagnosis with biomarkers to increase confidence in underlying AD pathophysiology.25,61 More recently, a proposed research framework defines AD purely on biological grounds, based on abnormalities in biomarkers of amyloid, tau, and neurodegeneration, irrespective of clinical state (table 1-2).23
FIGURE 1-4.
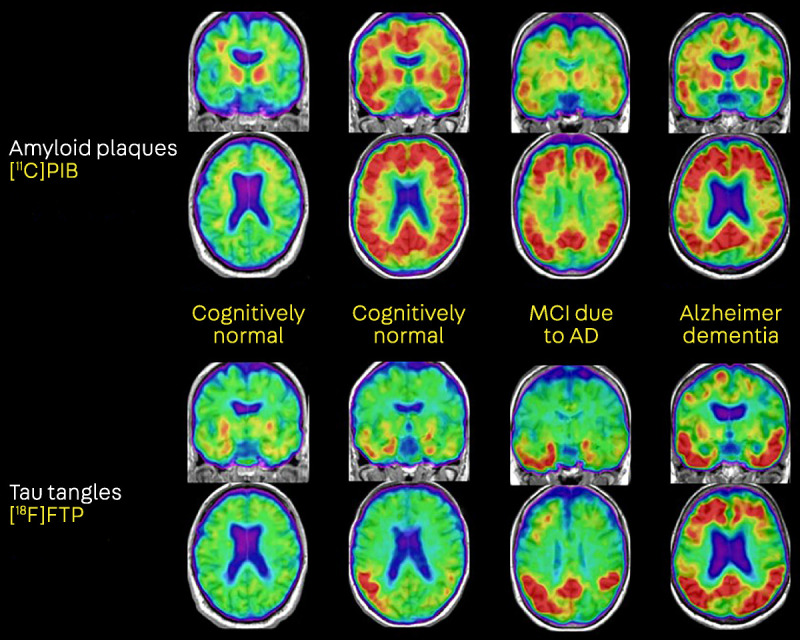
Amyloid and tau positron emission tomography (PET) of the Alzheimer disease (AD) continuum. The first column shows a cognitively normal individual with negative amyloid PET (no cortical tracer retention) and tau PET uptake restricted to the medial temporal lobes. The second column shows a cognitively normal individual with evidence of both cortical amyloid and spread of tau into temporal and parietal cortices. The third and fourth columns, representing patients with mild cognitive impairment (MCI) and dementia, show a plateau of amyloid PET signal but increased spread of tau with increasing clinical stages of AD.
[18F]FTP = [18F]flortaucipir, a radiotracer selective for paired helical filaments of tau; [11C]PIB = [11C]Pittsburgh Compound B, an amyloid-β–specific tracer.
TABLE 1-2.
Biomarkers of Amyloid, Tau, and Neurodegeneration Proposed in the National Institute of Aging-Alzheimer’s Association Research Framework for Alzheimer Diseasea

While biomarkers are used extensively in research and drug development, their clinical impact has been modest because of several factors, including limited patient access, cost, and lack of reimbursement by third-party payers. Ongoing studies are assessing the clinical impact of biomarkers on patient management and outcomes, which may pave the way for reimbursement in the future.62–64 Appropriate use criteria for amyloid PET state that clinical scans should be restricted to diagnostically challenging cases in which the cause of cognitive impairment remains uncertain after a comprehensive evaluation by a specialist and in which knowledge of amyloid status is likely to change diagnosis and management.65 Broader clinical use can be anticipated in future years, and neurologists should familiarize themselves with the AD biomarker armamentarium and best practices around pretest counseling and disclosure of results to patients and families.66
TREATMENT
A comprehensive care plan includes treatment with AD-specific medications; treatment of vascular risk factors, sleep and mood disorders, and other relevant comorbid conditions; counseling about safety and future planning; and referrals to community resources (case 1-1).
First approved in 1993, acetylcholinesterase inhibitors (AChEIs) remain the mainstay of pharmacologic therapy for AD.67 AD is associated with loss of cholinergic neurons in the basal forebrain. AChEIs enhance cholinergic transmission by inhibiting the hydrolysis of acetylcholine in the synaptic cleft. Three AChEIs are used in clinical practice: donepezil, galantamine, and rivastigmine (table 1-3). Double-blinded randomized controlled trials demonstrate a symptomatic benefit in cognitive and functional outcomes across the spectrum from mild to severe AD dementia67 but do not show a benefit in MCI.3 Although subtle differences exist in the biological effects of different AChEIs, efficacy is similar across agents. While deemed clinically meaningful, the effects of AChEIs are modest and, in practice, difficult to appreciate, since patients rarely show improvement when starting treatment and over time inevitably decline. Common adverse effects include gastrointestinal upset and loss of appetite (potentially reduced by using the rivastigmine patch instead of the oral formulations), urinary frequency, muscle cramps, and vivid dreams. Slowing of cardiac conduction can occur, and AChEIs should be used with caution in patients at risk for bradycardia. Additional rare, but potentially serious, adverse effects reported for rivastigmine include increased pulmonary and gastric secretions, warranting caution in patients with uncontrolled obstructive airway disease, gastrointestinal bleeding, and gastric ulcer disease. A single study supports the use of very-high-dose (23 mg/d) donepezil in moderate to severe AD,68 but the added benefit may be counterbalanced by an increased risk of dose-dependent side effects, particularly in older patients, and slow titration is recommended for all patients.
TABLE 1-3.
Medications Approved for the Symptomatic Treatment of Alzheimer Disease Dementiaa
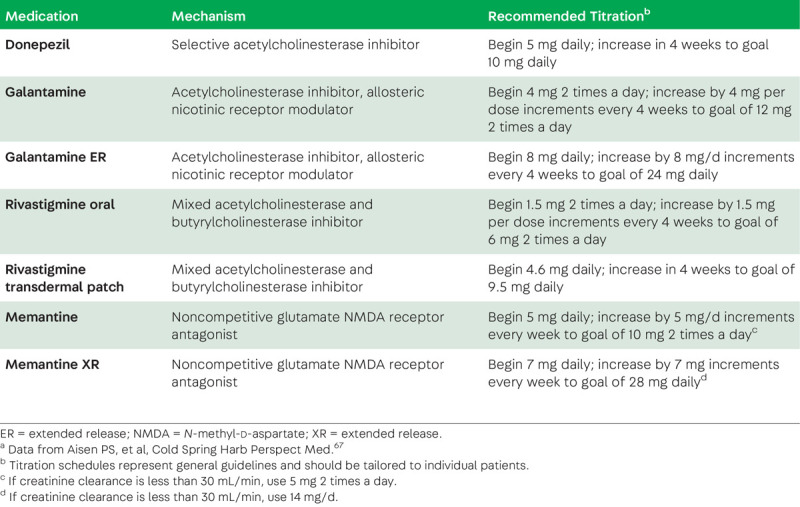
Memantine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, is approved for the treatment of moderate to severe AD dementia but does not show a benefit in mild dementia or MCI.67 Similar to AChEIs, the benefit is purely symptomatic and manifests as less decline compared to placebo on cognitive and functional scales. Potential adverse effects include constipation, dizziness, headache, and somnolence. Trials assessing the benefit of combination therapy with AChEIs and memantine versus monotherapy with each have yielded mixed results.69 A rational approach to AD drug treatment includes the initiation of AChEIs in mild AD dementia and the addition of memantine when patients enter the moderate stage of disease. In the absence of adverse effects, symptomatic treatment can be continued as long as enhanced cognition is associated with meaningful quality of life.
Treatment of behavioral symptoms in AD represents a clinical challenge, with little evidence to support any specific intervention and significant morbidity and mortality associated with many pharmacologic options.70 Nonpharmacologic interventions should always be attempted first. A careful history can often identify provoking factors that can be addressed by modifications to the environment and caregiver education (case 1-3). AChEIs can improve behavioral symptoms, particularly depression, apathy, and aberrant motor behavior, while memantine has been shown to improve agitation, lability, and irritability.70 Antidepressants (eg, selective serotonin reuptake inhibitors [SSRIs] or serotonin norepinephrine reuptake inhibitors [SNRIs]) should be used as first-line therapy for depression and anxiety and can also reduce agitation. Use of tricyclic antidepressants should be avoided because of their anticholinergic properties. Use of conventional and atypical neuroleptics should be avoided, if possible, because of evidence for increased morbidity and mortality in patients with dementia,71,72 leading to a class-wide US Food and Drug Administration (FDA) boxed warning. For more information on the boxed warning on antipsychotic medication use in dementia, refer to the article “Prescribing Antipsychotic Medications to Patients With Dementia: Boxed Warnings and Mitigation of Legal Liability” by Rachel V. Rose, JD, MBA, and Joseph S. Kass, MD, JD, FAAN,73 in this issue of Continuum. Benzodiazepines should be avoided because of their sedating effects and the potential for further worsening cognition.
CASE 1-3
A 93-year-old man was referred from a skilled nursing facility for assistance with managing difficult behaviors. He had been diagnosed with Alzheimer disease 8 years earlier and was now fully dependent on others to assist with activities of daily living. He became angry and aggressive when staff tried to get him out of bed or assist him with daily bathing. He had previously been taking donepezil and memantine, but these were stopped when he moved to the skilled nursing facility. He was prescribed lorazepam at bedtime to help with sleep and was reported to be drowsy and difficult to arouse in the morning. Examination was notable for diffuse osteoarthritic changes and tenderness to palpation of the low back and both knees. He otherwise appeared calm and cooperative during the examination. Laboratory assessment, including urinalysis, was normal.
The treating neurologist called the supervisor at the nursing facility, and together they formulated a multipronged care plan. An experienced staff member at the facility provided staff with training on how to interact with patients with severe cognitive impairment. His bathing schedule was reduced to twice a week. Low-dose acetaminophen was started to empirically treat presumed pain from degenerative joint disease. Memantine was restarted, while lorazepam was tapered off. On follow-up 8 weeks later, the frequency and severity of his difficult behaviors had greatly diminished, and staff felt better able to manage these events when they did occur.
COMMENT
Managing difficult behaviors in Alzheimer disease involves identifying potential triggers, modifying the environment, and training family members and caregivers. Underlying medical causes such as pain and infection should be excluded. Nonpharmacologic approaches tend to be more effective than drug treatment, although acetylcholinesterase inhibitors and memantine can also be helpful.
Many over-the-counter vitamins, supplements, and medical foods claim to “improve brain function,” and anecdotal reports exist describing benefits for patients with AD. Some of these treatments are founded on a reasonable biological premise, and a number have shown efficacy in AD animal models. However, to date, no nutraceutical intervention has proven beneficial when subjected to rigorous clinical trials.67
Despite recent setbacks and high-profile failures, a myriad of biologically specific AD treatments are currently being tested in clinical trials and in the drug development pipeline.74 Many experimental therapies target the amyloid and tau pathways, while others address changes in brain metabolism, network activity, innate immunity, and modifiable lifestyle factors. Increasingly, clinical trials in AD are focused on preclinical or early symptomatic stages of the disease, employing biomarkers for subject selection and to assess target engagement. Ultimately, a “cocktail” of drugs synergistically targeting different elements of the disease’s complex pathophysiology may be required to truly modify the course of AD.
CONCLUSION
More than a century following Alzheimer’s initial description of the disease, AD is recognized as the most common cause of late-life dementia and a major public health problem. While the current approach to diagnosis and treatment remains grounded in clinical symptoms, emerging biomarkers for amyloid, tau, and neurodegeneration enable the detection of the core neuropathologic features of the disease in living people a decade or more before the onset of cognitive decline. These advances in diagnostic tools are already having a major impact on AD research and drug development and raise hope that breakthroughs in therapies will soon follow.
KEY POINTS
Alzheimer disease is the most common cause of dementia, affecting an estimated 5.7 million Americans. The number of affected individuals is expected to triple by 2050 because of an aging population.
Vascular risk factors, sleep disturbances, and traumatic brain injury increase the risk of Alzheimer disease. Increased years of education and greater cognitive and physical activity throughout the lifespan decrease the risk of Alzheimer disease.
The estimated heritability of late-onset Alzheimer disease is approximately 60% to 80%. The primary genetic risk factor for sporadic late-onset Alzheimer disease is the apolipoprotein E (APOE) ε4 allele.
The clinical evaluation of patients with cognitive symptoms should first and foremost exclude reversible causes based on history, examination, and laboratory testing.
Mild cognitive impairment is defined as objectively confirmed cognitive decline that does not interfere with independent function. When cognitive decline interferes with independent function, patients meet criteria for dementia.
Late-onset Alzheimer disease typically presents with progressive decline in episodic memory, with variable involvement of other cognitive domains. Progressive memory impairment can also be caused by other neurodegenerative processes affecting the medial temporal lobes.
Common neuropsychiatric symptoms in Alzheimer disease include depression, anxiety, mild apathy, irritability, and sleep disturbances.
MRI findings in Alzheimer disease include medial temporal lobe and posterior-predominant cortical atrophy. Iron-sensitive sequences should be performed to assess for hemorrhages associated with cerebral amyloid angiopathy.
The neuropathology of Alzheimer disease is defined by the presence of senile amyloid plaques and tau neurofibrillary tangles. The burden and distribution of these two lesions define the degree of Alzheimer disease neuropathologic changes.
Late-life dementia is often associated with multiple brain pathologies. The most common are Alzheimer disease, Lewy bodies, vascular brain injury, hippocampal sclerosis, and TDP-43 inclusions.
Amyloid plaques and tau tangles can be detected in living people based on changes in CSF levels of amyloid-β and phosphorylated tau or by using positron emission tomography radiotracers that selectively bind amyloid-β or tau aggregates.
A comprehensive care plan for patients with Alzheimer disease includes treatment with Alzheimer disease–specific medications, treatment of relevant comorbid conditions, counseling about safety and future planning, and referrals to community resources.
Acetylcholinesterase inhibitors are approved for the treatment of mild to severe Alzheimer disease dementia. Memantine is approved for the treatment of moderate to severe Alzheimer disease dementia.
Difficult behaviors in Alzheimer disease should be addressed primarily with nonpharmacologic approaches, use of Alzheimer disease symptomatic drugs, and judicious use of antidepressants. Use of neuroleptics should be avoided, if possible, because of increased morbidity and mortality.
ACKNOWLEDGMENTS
This work was supported by grants (R01-AG045611, P50-AG23501) from the National Institutes of Health. The author would like to thank Orit Lesman-Segev, MD; Lea Grinberg, MD, PhD; Salvatore Spina, MD, PhD; and Adrienne Visani, BA for assistance in preparing figures.
REFERENCES
- 1.Alzheimer A. Uber eine eigenaritage, schweren Erkrankung der Hirnrinde. Neurol Zbl 1907;25:1134. [Google Scholar]
- 2.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement 2018;14(3):367–429. doi:10.1016/j.jalz.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018;90(3):126–135. doi:10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord 2001;15(4):169–173. [DOI] [PubMed] [Google Scholar]
- 5.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013;80(19):1778–1783. doi:10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement 2016;12(3):216–224. doi:10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement 2015;11(3):310–320. doi:10.1016/j.jalz.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodaty H, Seeher K, Gibson L. Dementia time to death: a systematic literature review on survival time and years of life lost in people with dementia. Int Psychogeriatr 2012;24(7):1034–1045. doi:10.1017/S1041610211002924. [DOI] [PubMed] [Google Scholar]
- 9.Baumgart M, Snyder HM, Carrillo MC, et al. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement 2015;11(6):718–726. doi:10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Ritchie K, Ritchie CW, Yaffe K, et al. Is late-onset Alzheimer’s disease really a disease of midlife? Alzheimers Dement (N Y) 2015;1(2):122–130. doi:10.1016/j.trci.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011;10(9):819–828. doi:10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nat Rev Neurol 2014;10(2):115–119. doi:10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306(6):613–619. doi:10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg 2016;124(2):511–526. doi:10.3171/2015.2.JNS14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehrenberg AJ, Nguy AK, Theofilas P, et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 2017;43(5):393–408. doi:10.1111/nan.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona S, Hardy J, Guerreiro R. The genetic landscape of Alzheimer disease. Handb Clin Neurol 2018;148:395–408. doi:10.1016/B978-0-444-64076-5.00026-0. [DOI] [PubMed] [Google Scholar]
- 17.Liu CC, Liu CC, Kanekiyo T, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 2013;9(2):106–118. doi:10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol 2017;74(10):1178–1189. doi:10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis 2014;72(pt A):3–12. doi:10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56(9):1143–1153. doi:10.1212/WNL.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 21.Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med 2017;14(3):e1002258 doi:10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol 2018;17(8):721–730. doi:10.1016/S1474-4422(18)30232-1. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR, Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14(4):535–562. doi:10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 25.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):263–269. doi:10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014;13(6):614–629. doi:10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 27.Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s disease: past, present, and future. J Int Neuropsychol Soc 2017;23(9–10):818–831. doi:10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landau SM, Horng A, Fero A, Jagust WJ; Alzheimer’s Disease Neuroimaging Initiative. Amyloid negativity in patients with clinically diagnosed Alzheimer disease and MCI. Neurology 2016;86(15):1377–1385. doi:10.1212/WNL.0000000000002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jicha GA, Nelson PT. Hippocampal sclerosis, argyrophilic grain disease, and primary age-related tauopathy. Continuum (Minneap Minn) 2019;25(1 Dementia):208–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2012;27(6):413–420. doi:10.1177/1533317512454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schott JM, Crutch SJ. Posterior cortical atrophy. Continuum (Minneap Minn) 2019;25(1 Dementia):52–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geda YE, Schneider LS, Gitlin LN, et al. Neuropsychiatric symptoms in Alzheimer’s disease: past progress and anticipation of the future. Alzheimers Dement 2013;9(5):602–608. doi:10.1016/j.jalz.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desikan RS, Rafii MS, Brewer JB, Hess CP. An expanded role for neuroimaging in the evaluation of memory impairment. AJNR Am J Neuroradiol 2013;34(11):2075–2082. doi:10.3174/ajnr.A3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frisoni GB, Pievani M, Testa C, et al. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain 2007;130(pt 3):720–730. doi:10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- 35.Lockhart SN, DeCarli C. Structural imaging measures of brain aging. Neuropsychol Rev 2014;24(3):271–289. doi:10.1007/s11065-014-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke 2018;49(2):491–497. doi:10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001;286(17):2120–2127. doi:10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 38.Jagust WJ, Johnson KA, Holman BL. SPECT perfusion imaging in the diagnosis of dementia. J Neuroimaging 1995;5(suppl 1):S45–S52. doi:10.1111/jon19955s1s45. [DOI] [PubMed] [Google Scholar]
- 39.Rabinovici GD, Furst AJ, Alkalay A, et al. Increased metabolic vulnerability in early-onset Alzheimer’s disease is not related to amyloid burden. Brain 2010;133(pt 2):512–528. doi:10.1093/brain/awp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007;130(pt 10):2616–2635. doi:10.1093/brain/awm177. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology 2011;77(23):2034–2042. doi:10.1212/WNL.0b013e31823b9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Calderon-Garcidueñas AL, Duyckaerts C. Alzheimer disease. Handb Clin Neurol 2017;145:325–337. doi:10.1016/B978-0-12-802395-2.00023-7. [DOI] [PubMed] [Google Scholar]
- 43.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 2012;8(1):1–13. doi:10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002;58(12):1791–1800. doi:10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 45.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82(4):239–259. [DOI] [PubMed] [Google Scholar]
- 46.Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 2012;71(5):362–381. doi:10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain 2016;139(11):2983–2993. doi:10.1093/brain/aww224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66(2):200–208. doi:10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawas CH, Kim RC, Sonnen JA, et al. Multiple pathologies are common and related to dementia in the oldest-old: the 90+ study. Neurology 2015;85(6):535–542. doi:10.1212/WNL.0000000000001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol 2017;16(8):661–676. doi:10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 51.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 2016;15(7):673–684. doi:10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 52.Mattsson N, Lönneborg A, Boccardi M, et al. ; Geneva Task Force for the Roadmap of Alzheimer’s Biomarkers. Clinical validity of cerebrospinal fluid Aβ1-42, tau, and phospho-tau as biomarkers for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging 2017;52:196–213. doi:10.1016/j.neurobiolaging.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 53.Teunissen CE, Otto M, Engelborghs S, et al. White paper by the Society for CSF Analysis and Clinical Neurochemistry: overcoming barriers in biomarker development and clinical translation. Alzheimers Res Ther 2018;10(1):30 doi:10.1186/s13195-018-0359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villemagne VL, Doré V, Burnham SC, et al. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol 2018;14(4):225–236. doi:10.1038/nrneurol.2018.9. [DOI] [PubMed] [Google Scholar]
- 55.Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA 2015;313(19):1939–1949. doi:10.1001/jama.2015.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maass A, Landau S, Baker SL, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage 2017;157:448–463. doi:10.1016/j.neuroimage.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 2016;139(pt 5):1551–1567. doi:10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79(1):110–119. doi:10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement 2016;12(3):292–323. doi:10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroup. Alzheimers Dement 2011;7(3):280–292. doi:10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7(3):270–279. doi:10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Wilde A, van der Flier WM, Pelkmans W, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol 2018;75(9):1062–1070. doi:10.1001/jamaneurol.2018.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabinovici GD, Gatsonis C, Apgar C, et al. Impact of amyloid PET on patient management: early results from the IDEAS study. Alzheimer’s Association International Conference, 2017; London, UK. [Google Scholar]
- 64.Duits FH, Prins ND, Lemstra AW, et al. Diagnostic impact of CSF biomarkers for Alzheimer’s disease in a tertiary memory clinic. Alzheimers Dement 2015;11(5):523–532. doi:10.1016/j.jalz.2014.05.1753. [DOI] [PubMed] [Google Scholar]
- 65.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 2013;9(1):e1–e16. doi:10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabinovici GD, Karlawish J, Knopman D, et al. Testing and disclosures related to amyloid imaging and Alzheimer’s disease: common questions and fact sheet summary. Alzheimers Dement 2016;12(4):510–515. doi:10.1016/j.jalz.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med 2012;2(3):a006395 doi:10.1101/cshperspect.a006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farlow MR, Salloway S, Tariot PN, et al. Effectiveness and tolerability of high-dose (23 mg/d) versus standard-dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: a 24-week, randomized, double-blind study. Clin Ther 2010;32(7):1234–1251. doi:10.1016/j.clinthera.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmidt R, Hofer E, Bouwman FH, et al. EFNS-ENS/EAN Guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur J Neurol 2015;22(6):889–898. doi:10.1111/ene.12707. [DOI] [PubMed] [Google Scholar]
- 70.Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr 2010;22(3):346–372. doi:10.1017/S1041610209991505. [DOI] [PubMed] [Google Scholar]
- 71.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146(11):775–786. doi:10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 72.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353(22):2335–2341. doi:10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 73.Rose RV, Kass JS. Prescribing antipsychotic medications to patients with dementia: boxed warnings and mitigation of legal liability. Continuum (Minneap Minn) 2019;25(1 Dementia):254–259. [DOI] [PubMed] [Google Scholar]
- 74.Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimers Dement (N Y) 2017;3(3):367–384. doi:10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


