The inner workings of the human body are largely invisible to the unaided eye, which is possibly why, in the era of the “quantified self,” the instinct to measure them is almost impossible to resist. Of course, millions of Americans have tried (and most have abandoned) using wearable devices that continuously monitor electrical signals generated by the body, such as sensors that measure heart rate and temperature and even provide crude versions of cardiac telemetry. Continuous measurement of internal biologic signals, as opposed to external electrical signals, is more difficult. Yet we do have a limited arsenal of monitoring devices, such as continuous glucose monitors. Although these devices have traditionally been reserved for patients with diabetes, they are increasingly being used by people without diabetes to interpret patterns of glucose regulation for informing health choices.
As of now, however, the repertoire of assays available for continuous internal monitoring is limited, and most assays — even those that measure a single molecule — take years, if not decades, to perfect. So how will we ever measure the hundreds to thousands of known molecules and proteins — and probably orders of magnitude more as-yet undiscovered molecules — that exist within us?
In a recent article, Mimee, Nadeau, and colleagues1 tackle this challenge by leveraging the flexibility of synthetic biology and engineering to create a modular, internal, continuous-measurement device that they call the ingestible micro-bio-electronic device (IMBED). They created and tested the device in three steps and showed the utility of their system for measuring heme released from blood within the gastrointestinal tract in a porcine model.
In the first step, the researchers created a flexible, sensitive, and specific biosensor. To do so, they co-opted genes from a variety of bacteria and combined them into a single biologic circuit that was genetically engineered into a strain of Escherichia coli: Nissle 1917. They generated a handful of circuits, each responsive to a different small molecule. In their research, they focused on a sensor that, on detection of heme, induces the transcription of a luminescent operon that produces light. They tested the strain in vitro and also fed the engineered heme-responsive strain to both control mice and mice that had been treated with indomethacin to induce gastroin-testinal bleeding. They detected bacteria in the stool of both sets of mice, but only the mice that were exposed to indomethacin had luminescent stool.
The second step involved an engineering challenge. The researchers needed to package the bacterial sensor so that it could be implanted or ingested, could detect light, could perform computations to convert that light into data, and finally, could transmit those data to an external device (Fig. 1). Moreover, because the device would need to be charged by battery, they had to develop it such that it would require only a very small amount of power (and thus permit a very small battery). They placed the sensor bacteria in a chamber in which one side was covered with a semipermeable membrane within the device. Fluids and metabolites (including heme) from the external environment could enter the device and activate it.
Figure 1. Schematic of the IMBED.
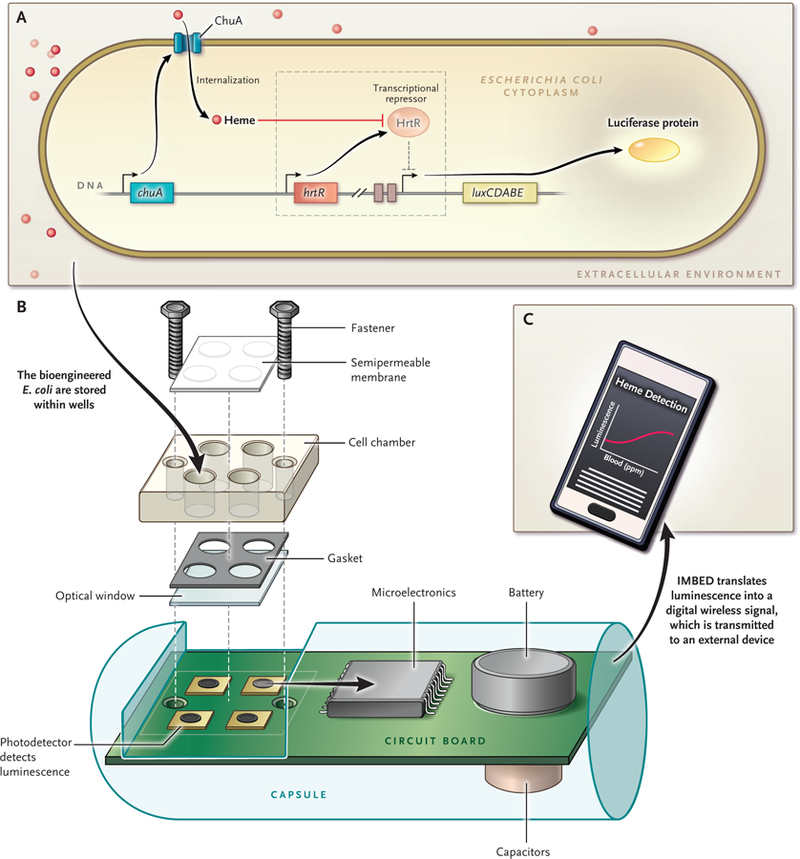
A genetically engineered bacterium is generated to create a biosensor (Panel A). The bacterium is capable of detecting a signal, in this case heme. The signal induces transcription of a genetic locus that encodes a luminescent protein. This portion of the system is essentially modular and can be engineered to sense a variety of individual signals and to produce a luminescent signal. Specifically, chuA encodes a membrane protein that allows heme to be trafficked into the bioengineered E. coli. Heme then inhibits the transcriptional repressor protein HrtR, which results in the derepression, or turning on, of the luxCDABE operon. The luxAB portion of the operon encodes the luciferase reporter gene, and the luxDEC portion of the operon encodes a fatty acid reductase complex. A simplified cross section of the ingestible micro-bio-electronic device (IMBED), which is approximately 3.5 cm in length, is shown (Panel B). The device consists of a chamber in which the bacteria are placed. The chamber has a semipermeable membrane that allows entry of small-molecule signals. When heme comes in contact with the bacteria, the bacteria generate light, which is detected by the photodetector. The light signals are converted into a digital code, which is then transmitted and detected wirelessly, in real time, by an external device, in this case an Android cell phone with a customized application for data analysis (Panel C).
In the final step, the authors carried out a proof-of-concept test of their device by implanting the device within the gastric cavity of pigs. The location of the device was confirmed by endoscopic visualization during placement and by radiography at the conclusion of the 2-hour experiment. One caveat of this beta-system model is the acidity of the gastric cavity; the bio-sensor does not function well in acidic environments. The animals were therefore treated with a neutralization solution to ensure that the gastric cavity was not too acidic; this limitation would need to be overcome if this sensor were to be widely used in clinical practice, although some uses for monitoring patients being treated for gastrointestinal bleeding could potentially be of value. When exogenous blood was administered orally, the IMBED was able to detect heme and transmit that signal to an external laptop computer and an Android cell phone with a custom application, which enabled real-time data acquisition.
This work opens up an exciting area of investigation. With its modular architecture, the bacterial component of the IMBED can be engineered to respond to a variety of signals, which suggests that the device could be tailored to measure any number of molecules of interest. In addition, engineered bacteria are actively being investigated as biosensors on their own; for example, Riglar et al. recently reported on the development and validation of an engineered organism that can detect and “record” exposure to the inflammatory molecule tetrathionate in the intestine.2 As with most of the other research published to date on the topic of engineered bacteria, their approach requires the collection of the biosensor (from the stool, for example) and an assay to quantify the signal. The IMBED, with its capacity to measure and report in real time, represents a conceptual advance, although only time will tell whether it is sufficiently sensitive, robust, and safe for human applications.
Given the strong likelihood that a new wave of biosensors that would enable the detection and assay of many types of molecules (perhaps continuously) is gathering speed, we must ask ourselves how all this monitoring will really affect health. Wearable devices have been on the market for more than a decade, yet their health benefits are unclear, with meta-analyses of early studies showing only very modest effects at best.3,4 Will we truly enjoy “better living through biotechnology”? And how will we be able to interpret the deluge of data that we are gearing up to collect? Given the volume and pace of innovation in this field, it seems unlikely that we will develop explanatory models grounded in physiology to make sense of the large amounts of data as they are generated. Rather, we are accelerating into an era of advanced computing and artificial intelligence with our ability to surveil anatomical sites that were previously inaccessible. Perhaps we will “machine-learn” our way into informed and effective decision making and, ultimately, better health.
Footnotes
Disclosure forms provided by the author are available at NEJM.org.
References
- 1.Mimee M, Nadeau P, Hayward A, et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 2018;360:915–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riglar DT, Giessen TW, Baym M, et al. Engineered bacteria can function in the mammalian gut long-term as live diagnostics of inflammation. Nat Biotechnol 2017;35:653–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Dunn J, Salins D, et al. Digital Health: Tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol 2017;15(1):e2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noah B, Keller MS, Mosadeghi S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. npj Digital Medicine January 15, 2018. (https://www.nature.com/articles/s41746-017-0002-4). [DOI] [PMC free article] [PubMed]


