Abstract
Background
South Africa has high burdens of HIV, TB and drug-resistant tuberculosis (DR-TB, rifampicin-resistance). Treatment outcome data for HIV-infected versus uninfected patients is limited. We assessed the impact of HIV and other factors on DR-TB treatment success, time to culture conversion, loss-from-treatment and overall mortality after second-line treatment initiation.
Methods
A retrospective cohort analysis was conducted for patients initiated on DR-TB treatment from 2008 to 2012, within a community-based, decentralised programme in Khayelitsha, South Africa.
Results
Among 853 confirmed DR-TB patients initiating second-line treatment, 605 (70.9%) were HIV infected. HIV status did not impact on time to sputum culture conversion nor did it impact treatment success; 48.1% (259/539) and 45.9% (100/218), respectively (p=0.59). In a multivariate model, HIV was not associated with treatment success. Death during treatment was higher among HIV-infected patients, but overall mortality was not significantly higher. HIV-infected patients with CD4 <=100 cells/ml were significantly more likely to die after starting treatment.
Conclusions
Response to DR-TB treatment did not differ with HIV infection in a programmatic setting with access to antiretroviral treatment (ART). Earlier ART initiation at a primary care level could reduce mortality among HIV-infected patients presenting with low CD4 counts.
Keywords: ART, DR-TB, HIV, Programmatic treatment outcomes
Introduction
The human immunodeficiency virus (HIV) epidemic has led to increased TB rates in many high HIV prevalence settings. In 2013, 9 million people were estimated to develop TB, among whom 1.1 million were estimated to be HIV infected, predominantly in Africa.1 Similarly, HIV infection has also driven increasing levels of drug-resistant tuberculosis (DR-TB), with an estimated 480 000 cases of multi-drug resistant TB (MDR-TB) emerging globally in 2013.1 While estimates of DR-TB (defined as rifampicin resistance)2 prevalence are limited in many African countries, South Africa has among the highest global burdens of HIV, TB and DR-TB.1,3
Access to second-line treatment for DR-TB is limited in many high-burden settings, particularly in the African region where only 14 418 cases were reported to have been enrolled on treatment in 2013, predominantly in South Africa.1 In addition to limited access, treatment outcomes associated with MDR-TB are poor; WHO reports that only a half of all those who initiate second-line treatment are successfully treated.1 Lengthy, poorly efficacious and poorly tolerated treatment regimens1,4 result in high levels of incomplete treatment, treatment failure and death. MDR-TB patients infected with HIV might be expected to have even poorer outcomes, with reported higher mortality,5–9 increased side effects of treatment leading to higher loss-from-treatment (LFT, also known as default from treatment or loss to follow-up)10 and potential overlapping toxicities with anti-retroviral treatment (ART).11,12 Despite this, while there is substantial programmatic data on treatment outcomes for MDR-TB from a range of settings,1,13,14 data on treatment outcomes among HIV-infected individuals are more limited. A review aiming to assess the impact of ART among HIV-infected MDR-TB patients identified only 10 studies between 1980 and 2009 and these studies included only 217 subjects.15
The number of diagnosed DR-TB cases in South Africa has increased substantially in recent years, with more than 26 000 cases notified in 2013. While the proportion of HIV infection among these cases is not reported, 62% of all notified TB cases in South Africa are HIV infected.1 Given early reports of high and rapid mortality among HIV-infected individuals with MDR-TB and extensively drug-resistant TB (XDR-TB) in South Africa,7,8,16 decreased treatment success among HIV-infected patients compared to HIV-uninfected might be expected. Indeed, HIV-infected MDR-TB patients diagnosed between 2000 and 2004, prior to widespread public sector ART access, from eight South African provinces had lower treatment success and higher mortality than HIV-uninfected patients in the same programmes.6
Recently, we reported treatment outcomes from a community-based decentralised DR-TB treatment programme in Khayelitsha, South Africa.17 Among patients diagnosed with DR-TB between 2008 and 2011, high rates of treatment initiation lead to overall reduced mortality compared to previous reports, with no significant difference in treatment success between HIV-infected and uninfected patients.17 Here, we aim to extend the previous analysis and assess the impact of HIV infection and other factors on treatment success, time to culture conversion, mortality and LFT under programmatic conditions with wide availability of ART. The impact of ART provision on treatment outcomes and mortality was not assessed here as this has been analysed separately in a smaller cohort where more detailed ART data was available.18
Methods
Setting and treatment programme
Khayelitsha, a township near Cape Town, South Africa, has approximately 450 000 inhabitants and has high rates of HIV, TB and DR-TB.19,20 Social factors such as informal, overcrowded housing, poor ventilation systems in homes, and high unemployment rates are determinants driving the HIV and TB epidemics.21 There are approximately 200 cases of DR-TB diagnosed in Khayelitsha each year, with a HIV infection rate of 72%.17 Eleven health facilities in Khayelitsha provide integrated HIV and TB services for patients, including DR-TB treatment.22
The decentralised DR-TB programme in Khayelitsha has been described previously in several reports.17,22 Through the decentralised programme, DR-TB patients are initiated on second-line treatment in their primary care clinic, and are hospitalised only if they were clinically unstable or unable to attend their clinic daily. HIV-infected patients, not already receiving ARTare initiated on ART as soon as possible after starting second-line TB treatment, according to current guidelines and the clinical status of the patient.23
Data collection
A retrospective analysis was conducted on routinely collected DR-TB data from the Khayelitsha cohort. DR-TB patients registered in Khayelitsha from January 2008 to 2012 were included and treatment outcomes were analysed to 2014. Data was initially collected from clinic registers and captured in a Microsoft Access (Microsoft Corp., Redmond, WA, USA) database. From late 2012 onward, data was imported directly from the decentralised national electronic DR-TB database in South Africa, with verification and additional data collection from clinic registers. The DR-TB database includes: information on clinical characteristics, DR-TB diagnostic and follow-up tests, HIV co-infection, DR-TB treatment regimens and treatment outcomes, including mortality. Outcomes were assigned at the sub-district primary health care (PHC) clinics in Khayelitsha by medical officers and were later reported on the national electronic database. Outcomes were reviewed to ensure they met the WHO recommended definitions2 or the South African National DR-TB Guidelines in the case of treatment failure.24
Only patients who initiated DR-TB treatment between 2008 and 2012 with known HIV status were included in the analysis. Patients with a previous history of DR-TB treatment, those transferred into a Khayelitsha sub-district health facility and those with bacteriologically unconfirmed DR-TB (predominantly children aged ≤5 years) were excluded from the analysis. Overall mortality was determined as any mortality experienced during or post treatment. As previously reported passive surveillance and DR-TB registers used in Khayelitsha ascertain 89% of mortality post treatment.17
Definitions
Case definitions for DR-TB and treatment outcomes, with the exception of treatment failure, were defined in line with WHO recommended definitions.2 Treatment failure, was defined in concordance with the South African National DR-TB Guidelines, which state patients whose cultures have not converted after 6 to 8 months of treatment are considered cases of treatment failure.24 Those patients who were cured or completed their treatment, without treatment failure, were considered to have treatment success, while patients who either died, were LFT, or for whom treatment failed were considered to have unsuccessful treatment outcomes. Culture conversion was defined as the first date of two consecutive negative cultures, taken at least 30 days apart, when previously the patient was culture positive.2 All cultures conducted in Khayelitsha for the duration of this study were done using a mycobacteria growth incubator tube (MGIT).
Data analysis
Data were analysed with STATA/IC version 12.1 (StataCorp, College Station, YX, USA). Clinical and demographic characteristics, culture conversion and treatment outcomes were stratified by HIV status and χ2 tests were performed to determine the level of significance between HIV status and the various factors investigated. Univariate logistic regression models were used to determine factors associated with treatment success. Significant factors on univariate analysis were entered into the multivariate logistic regression model.
Kaplan Meier analyses were used to determine time from DR-TB treatment initiation to events of culture conversion, loss-from-treatment and death. For culture conversion, data were censored at death or LFT. For LFT analyses, data were censored at death, while for mortality analyses, data were censored at LFT. Log rank tests were used to determine the significance of the relationships investigated in the Kaplan Meier survival analyses.
Results
Patient cohort
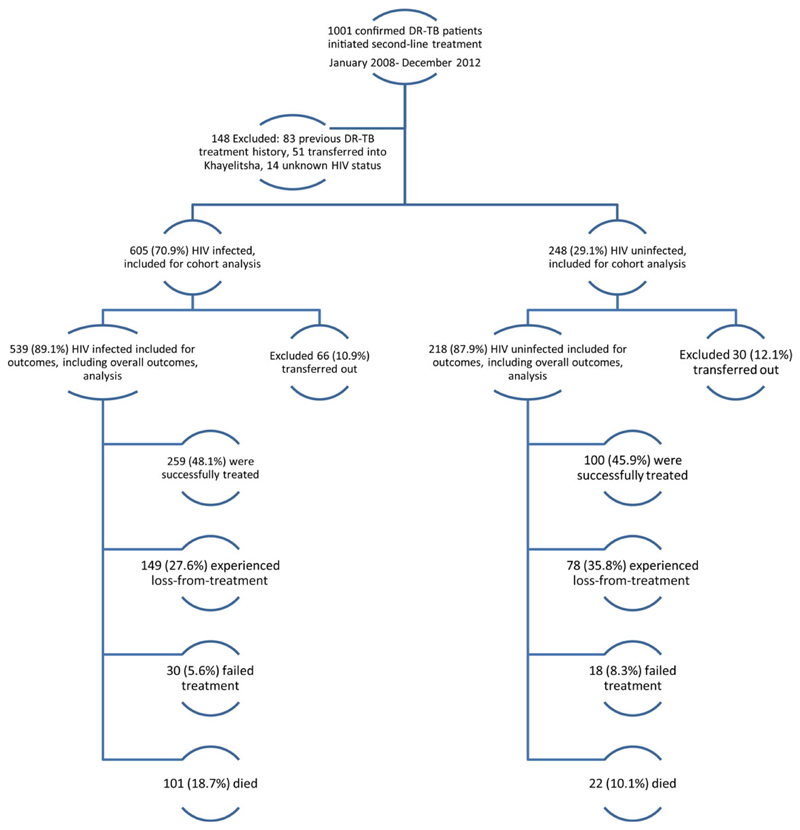
A total of 1001 bacteriologically confirmed DR-TB patients for second-line treatment initiation were recorded in Khayelitsha from January 2008 to December 2012. Overall, 148 patients were excluded; 14 with unknown HIV status, 51 who were transferred into Khayelitsha during their treatment course and 83 who had previously received second-line DR-TB treatment. This left 853 patients in the final analysis.
Demographic and clinical factors by HIV status are shown in Table 1. Overall, 70.9% (605/853) of patients were infected with HIV. Females were more likely to be HIV-infected (p<0.0001), while HIV-uninfected patients were more likely to be under the age of 25 years (p<0.0001). Median age for HIV-infected patients at diagnosis was 33 years (Interquartile Range [IQR] 28–39) while for HIV-uninfected patients it was 28 years (IQR 21–45). HIV-uninfected patients were more likely to have pulmonary TB (PTB) and less likely to have extra pulmonary TB (EPTB) (p<0.0001) and HIV-uninfected patients were more likely to be smear positive at baseline (p=0.011).
Table 1. Clinical and demographic characteristics of patients initiated on drug resistant-TB (DR-TB) treatment from 2008 to 2012, stratified by HIV status.
| HIV-infected, n (%) n=605 | HIV-uninfected, n (%) n=248 | Total n=853 | |
|---|---|---|---|
| Sex | |||
| Female | 330 (54.6%) | 84 (33.9%) | 414 |
| Age (years) | |||
| 0–15 | 8 (1.3%) | 10 (4.0%) | 18 |
| 16–24 | 54 (8.9%) | 86 (34.7%) | 140 |
| 25–34 | 263 (43.5%) | 55 (22.2%) | 318 |
| 35–44 | 216 (35.7%) | 34 (13.7%) | 250 |
| >44 | 64 (10.6%) | 63 (25.4%) | 127 |
| Disease site | |||
| PTB | 535 (88.4%) | 242 (97.6%) | 777 |
| EPTB | 50 (8.3%) | 4 (1.6%) | 54 |
| Both | 20 (3.3%) | 2 (0.8%) | 22 |
| Baseline sputum smear status (if PTB) | |||
| Positive | 244 (44.0%) | 137 (56.2%) | 381 |
| Negative | 279 (50.3%) | 98 (40.2%) | 377 |
| Unknown | 32 (5.8%) | 9 (3.7%) | 41 |
| DR-TB classification | |||
| GeneXpert rifampicin resistance (no further DST) | 10 (1.7%) | 1 (0.4%) | 11 |
| Rifampicin-mono resistance | 136 (22.5%) | 43 (17.3%) | 179 |
| MDR-TBa | 379 (62.6%) | 166 (66.9%) | 545 |
| MDR-TB with second-line drug resistanceb | 80 (13.2%) | 38 (15.3%) | 118 |
| Year of treatment initiation | |||
| 2008 | 112 (71.82%) | 44 (28.0%) | 156 |
| 2009 | 138 (75.0%) | 46 (25.0%) | 184 |
| 2010 | 119 (71.3%) | 48 (28.7%) | 167 |
| 2011 | 116 (71.2%) | 47 (28.8%) | 163 |
| 2012 | 120 (65.6%) | 63 (34.4%) | 183 |
| ART status | |||
| On ART at DR-TB treatment initiation | 312 (51.6%) | ND | |
| On ART following DR-TB treatment initiation | 243 (40.2%) | ND | |
| Never on ART | 50 (8.3%) | ND | |
| CD4 count (cells/ml) | |||
| 0–100 | 257 (42.5%) | ND | |
| 101–200 | 141 (23.3%) | ND | |
| 201–300 | 84 (13.9%) | ND | |
| >300 | 120 (19.8%) | ND | |
| Missing | 3 (0.5%) | ND | |
ART: anti-retroviral treatment; DR-TB: drug resistant TB; DST: drug susceptibility testing; EPTB: extra-pulmonary TB; MDR-TB: multi-drug resistant TB; ND: not done; PTB: pulmonary TB.
MDR-TB with no confirmed second-line resistance.
Second-line drug resistance includes resistance to a fluoroquinolone (most commonly ofloxacin) or a second-line injectable agent (amikacin, kanamycin or capreomycin).
Overall, 51.6% (312/605) of HIV-infected patients were already receiving ART at DR-TB treatment initiation, with another 40.2% (243/605) initiating ART after DR-TB treatment initiation (Table 1). A small proportion of patients, 8.3% (50/605) never initiated ART. Of those who never initiated ART 21 (42.0%) died and 15 (30.0%) were LFT before ART could be initiated. Overall, 42.5% of HIV-infected patients started DR-TB treatment with CD4 levels below 100 cells/ml (Table 1).
Culture conversion
Among sputum culture positive patients with pulmonary TB, 71.2% (502/705) converted cultures to negative at a median of 2.1 months (IQR 1.1–3.8) after DR-TB treatment initiation. Time to culture conversion, with censoring for death and default from LFT, did not differ between HIV-infected and uninfected patients (p=0.44). By 6 months, close to 79.1% (489/618) of pateints remaining on treatment, regardless of HIV status, had converted positive sputum cultures to negative.
Treatment outcomes
Of the 853 DR-TB patients who initiated treatment 757 (87.0%) were included in the assessment of treatment outcomes; 96 DR-TB patients were transferred out during treatment and therefore excluded from the analysis of treatment outcome (Figure 1). Overall, 47.4% (359/757) of patients were successfully treated, with no difference between HIV-infected and uninfected DR-TB patients (p=0.59). LFT was high at 30.0% (227/757) and was significantly higher among HIV-uninfected patients compared to HIV-infected; 27.6% (149/539) versus 35.8% (78/218), respectively (p=0.027). In contrast, mortality was significantly lower among HIV-uninfected patients, compared to the HIV-infected; 10.1% (22/218) and 18.7% (101/539), respectively (p=0.003). There was no significant difference in treatment failure (p=0.17).
Figure 1.
Drug resistant-TB (DR-TB) enrollment and treament outcomes among patient initiated on DR-TB treatment from 2008 to 2012, by HIV status. This figure is available in black and white in print and in color at Transactions online.
On univariate analysis using logistic regression, HIV status was not significantly associated with treatment success. Significant factors associated with increased treatment success were being female, aged 15 years or less and having EPTB. Factors associated with decreased treatment success were: age 16 to 24 years, having MDR-TB with confirmed second-line resistance and having a previous history of treatment with first-line anti-TB drugs. In a multivariate logistic regression model the factors significantly associated with increased treatment success were being female and 35 to 44 years of age. Factors associated with decreased treatment success were age 16 to 24 years, having MDR-TB with confirmed second-line resistance and having a previous treatment history with first-line anti-TB drugs (Table 2). HIV infection was not a significant predictor of poor treatment success in either the univariate or multivariate analyses.
Table 2. Logistic regression univariate and multivariate analysis of factors associated with treatment success in patients starting drug resistant-TB treatment (DR-TB) from 2008 to 2012.
| Factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Adjusted odds ratio (95% CI) | p-value | |
| HIV status | ||||
| Uninfected | 1.0 (reference) | 1.0 (reference) | ||
| Infected | 1.1 (0.80–1.5) | 0.59 | 0.78 (0.54–1.1) | 0.21 |
| Gender | ||||
| Male | 1.0 (reference) | 1.0 (reference) | ||
| Female | 1.4 (1.0–1.8) | 0.035 | 1.6 (1.1–2.2) | 0.005 |
| Age category (years) | ||||
| <=15 | 3.0 (1.1–8.8) | 0.039 | 2.1 (0.71–6.4) | 0.17 |
| 16–24 | 0.62 (0.40–0.96) | 0.032 | 0.57 (0.35–0.93) | 0.024 |
| 25–34 | 1.0 (reference) | 1.0 (reference) | ||
| 35–44 | 1.3 (0.94–1.9) | 0.10 | 1.5 (1.1–2.2) | 0.024 |
| >44 | 1.2 (0.74–1.8) | 0.54 | 1.4 (0.86–2.2) | 0.17 |
| Disease site | ||||
| PTB | 1.0 (reference) | 1.0 (reference) | ||
| EPTB | 2.7 (1.5–5.0) | 0.001 | 2.6 (1.4–5.1) | 0.004 |
| Both | 1.9 (0.72–4.9) | 0.19 | 2.0 (0.73–5.3) | 0.18 |
| Baseline smear status (if PTB) | ||||
| Negative | 1.0 (reference) | 1.0 (reference) | ||
| Positive | 0.77 (0.57–1.1) | 0.10 | 0.86 (0.63–1.2) | 0.36 |
| Unknown | 1.4 (0.65–3.1) | 0.39 | 1.3 (0.61–3.0) | 0.46 |
| EPTB- no smear | 2.4 (1.3–4.4) | 0.007 | NA | NA |
| DR-TB classification | ||||
| Rifampicin-mono TBa | 1.0 (reference) | 1.0 (reference) | ||
| MDR-TBb | 0.95 (0.67–1.3) | 0.76 | 0.99 (0.68–1.4) | 0.95 |
| MDR-TB with second-line drug resistance | 0.55 (0.33–0.90) | 0.018 | 0.54 (0.32–0.91) | 0.021 |
| Previous TB treatment | ||||
| No previous TB treatment | 1.0 (reference) | NA | 1.0 (reference) | NA |
| Previous first-line TB treatment | 0.68 (0.50–0.92) | 0.014 | 0.66 (0.47–0.91) | 0.012 |
DR-TB: drug resistant TB; EPTB: extra-pulmonary TB; MDR-TB: multi-drug resistant TB; NA: not applicable; PTB: pulmonary TB.
Includes unconfirmed GeneXpert rifampicin-resistant TB.
MDR-TB with no confirmed second-line resistance.
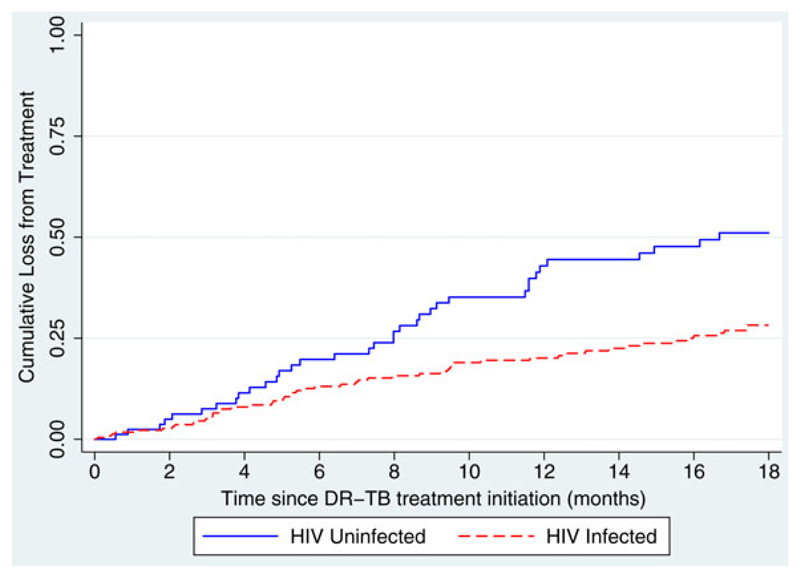
LFT was high across all programme years, and was signficantly higher among HIV-uninfected patients in the earlier years of 2008/09, before all the elements of the decentralised model of care were in place.25 There was no difference in LFT between HIV-infected and uninfected patients in subequent programme years (Table 3). Given this variation, and to assess whether timing of LFT was different between HIV-infected and uninfected pateints, Kaplan Meier curves were used to determine time to LFT, based on treatment initiation year. Among patients initiating treatment in 2008/09, HIV-uninfected patients had a higher risk of LFT during treatment compared to HIV-infected patients, with the difference more apparent during the continuation phase of treatment (Figure 2; p=0.001). For patients initiating treatment between 2010 and 2012, there was no difference between HIV-infected and uninfected patients, and the risk of LFT was consistent across 18 months of treatment (p=0.98).
Table 3. Loss-from-treatment (LFT, default of loss-to-follow-up) among HIV-infected and uninfected pateints by year of treatment initiation.
| Treatment initiation year | LFT/total (%) |
|||
|---|---|---|---|---|
| HIV-infected | HIV-uninfected | p-value | Total | |
| 2008–2009 | 57/229 (25%) | 36/83 (43.0%) | 0.002 | 93/312 (30%) |
| 2009–2012 | 92/310 (30%) | 42/135 (31%) | 0.76 | 134/445 (30%) |
| Total | 149/539 (28%) | 78/218 (36%) | 227/757 (30%) | |
Figure 2.
Time to cumulative loss-from-treatment (Kaplan Meier Curves) for HIV-infected and uninfected patients who started treatment 2008–2009 (p=0.001). This figure is available in black and white in print and in color at Transactions online.
Mortality
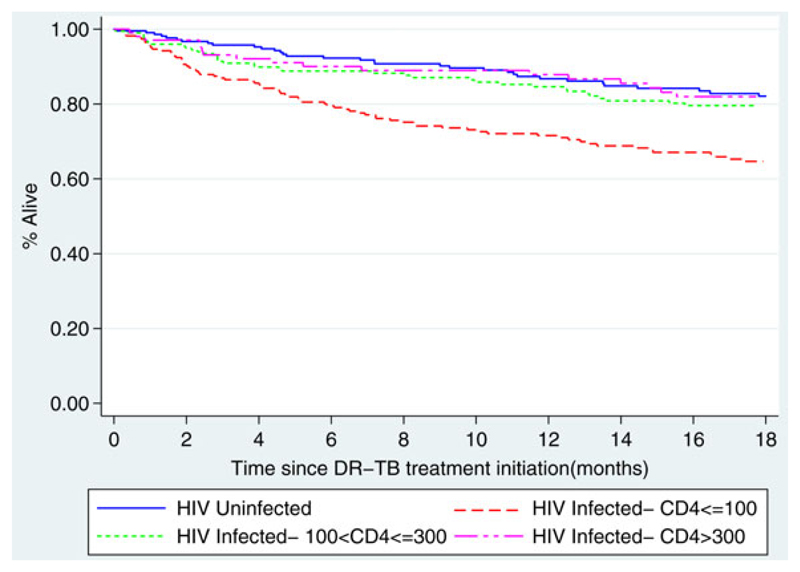
Overall mortality was calculated as any death during or post DR-TB treatment, thus deaths following LFT or treatment failure were included. Kaplan Meier curves were used to show the relationship between HIV status and CD4 count if HIV infected and overall mortality. Contrary to the significant difference in the outcome of death during treatment, there was no significant difference in overall mortality following treatment initiation between HIV-infected and uninfected patients (p=0.096). However, when CD4 count was considered, HIV-infected patients with CD4 values of ≤100 cells/ml had an increased hazard of death compared to both HIV- negative patients and HIV-infected patients with higher CD4 counts (p=0.0001; Figure 3). When CD4 count was investigated further for HIV-infected patients, those with CD4 counts ≤100 cells/ml initiating DR-TB treatment in 2008/09 versus 2010–12 had a similar hazard of death (p=0.12).
Figure 3.
Kaplan Meier curve for overall 18 month survival for DR-TB patients initiated on treatment by HIV status and CD4 count if HIV-infected (p=0.0001). This figure is available in black and white in print and in color at Transactions online.
Discussion
In the context of decentralised treatment for DR-TB, there was no difference in the proportion of treatment success among patients with and without HIV infection. Rather, factors associated with treatment success included being female, age over 25 years, no previous TB treatment and being infected with a DR-TB strain without second-line drug resistance. There was also no difference in the response to treatment with HIV infection as assessed with sputum culture conversion; similar results have been found in other studies.26 These data suggest that in a decentralised programmatic setting with access to ART, HIV-infected DR-TB patients respond similarly to current second-line treatment as do HIV-uninfected patients.
As shown in other settings6,8,9 mortality during treatment was higher among HIV-infected patients, with close to 20% of HIV-infected patients dying during treatment. However, when death after LFT and treatment failure were also considered, the difference in mortality over 18 months, between HIV-infected and uninfected patients lessened and was not significant. Not surprisingly, the remaining difference was due primarily to HIV-infected patients with CD4 counts less than 100 cells/ml at DR-TB treatment initiation, which is a predictor of mortality in HIV-infected patients.27 More than 40% of HIV-infected patients had CD4 counts less than 100 cells/ml at DR-TB treatment initiation, despite widespread access to ART in Khayelitsha.20 Over the same time period, the proportion of all HIV-infected individuals, who had CD4 levels ≤100 cells/ml when they initiated ART in Khayelitsha, fell from approximately 37% to 20%.28 Patients with low CD4 counts exhibited significantly higher mortality than both HIV-uninfected and HIV-infected patients with higher CD4 counts; the latter two groups died at a similar rate after treatment initiation. HIV-infected patients are immunocompromised and therefore, immune status, particularly at low CD4 levels, could have contributed to progression from infection to TB and DR-TB disease and an increased rate of mortality.29,30 Additional co-morbidities that could have impacted mortality and cause of death were not investigated in this analysis.
The provision of ART and the regimens used were standard for everyone in this cohort.28 There was no difference in treatment success for patients who were on ART at the time of DR-TB treatment initiation when compared to those who were not on ART at treatment initiation (data not shown). Previously, the lack of ART has been demonstrated as a significant predictor of poor outcome during both TB and DR-TB treatment.15 In this setting, integration of DR-TB treatment into primary care services already providing ART, has afforded rapid ART provision, based on clinical need for all DR-TB patients. However, both higher mortality among DR-TB patients with low CD4 counts at DR-TB initiation and a high proportion of such patients suggests that further efforts are required to increase regular HIV testing uptake and improve linkage to care to support earlier ART initiation and greater ART coverage in Khayelitsha.
The rate of LFT observed in this study was high at 30%, consistent with other South African and global studies.6,31,32 The high rate of LFT is problematic as patients who are LFT often have poor post treatment outcomes.33 In the earlier years of the programme, during incremental implementation of decentralised care, HIV-uninfected patients had a higher rate of loss from treatment than did HIV-infected patients. However, from 2010 onwards, this difference was not apparent. In the earlier years of the programme, DR-TB counselling was less structured and focused primarily on treatment initiation. In this context, it is possible that the additional counselling on treatment adherence provided through ART initiation, and continued ART provision, might have contributed to reduced LFT among HIV-infected patients. As patient support has improved with programme implementation, LFT has reduced among HIV-uninfected patients and remained at a similarly high level in HIV-infected patients. Although there is likely still room for improvement in patient support and counselling strategies, in the absence of shorter, less toxic and more tolerable treatment regimens, dramatic reductions would seem unlikely. More efficacious, more tolerable and shorter regimens are likely to not only reduce LFT and therefore improve outcomes, but are also likely to increase access to treatment.34
This cohort study is subject to the limitations of routinely collected clinical data. We excluded a small number of patients with missing HIV status. These were predominantly patients who refused HIV testing. We also have no data on the previous HIV/ART treatment history of patients. Additionally, we relied on passive surveillance of mortality, particularly after loss from treatment. We have, however, previously demonstrated that passive mortality surveillance in this setting identifies a large majority of deaths as reported through the national deaths registry.17 GeneXpert MTB/RIF (GXP) was rolled out in Khayelitsha in the beginning of 2012, however since the GXP algorithms were not used consistently throughout 2012 we don’t think it was likely to have a large impact on the results observed in this study.
In conclusion, a similar response to DR-TB treatment regardless of HIV status in programmatic settings with access to ART is encouraging. While outcomes were poor overall, this was primarily due to high rates of LFT. With HIV-infected patients with low CD4 counts more vulnerable to DR-TB disease, and a concerning proportion of patients continuing to only access ART at low CD4 counts, improved HIV testing coverage and linkage to care are urgently needed to achieve earlier ART initiation. Integrated decentralised ART and DR-TB care, with improved DR-TB case detection and reduced delays to DR-TB treatment initiation, have likely contributed to improved outcomes for HIV infected DR-TB patients in this setting.
Acknowledgements
We would like to thank the City of Cape Town Health Department, the Western Cape Province, the National Health Laboratory Service, Khayelitsha clinic staff, the DR-TB counsellors who assist in the collection of outcomes data, and importantly people suffering from DR-TB and HIV in Khayelitsha, South Africa.
Funding: MSF provided funding for the pilot programme for the decentralisation of care for DR-TB in Khayelitsha as well as for this analysis. HC is supported by a Wellcome Trust fellowship [www.wellcome.ac.uk, 085215].
Footnotes
Authors’ contributions: SM and HC conceived the research question, designed the study, conducted the initial analysis, and contributed to and provided critical review of manuscript drafts; JD and OM collected and cleaned the data and were involved in the revision of the manuscript drafts; EM conducted the final analysis, wrote the manuscript and contributed to critical review or the manuscript drafts with the assistance of HC; LW, JH and VC contributed to and provided critical review of the manuscript drafts towards finalisation. All authors read and approved the final manuscript. EM is the guarantor of the paper.
Competing interests: None declared.
Ethical approval: Ethical approval for the ‘Evaluation of a pilot programme for decentralising care and treatment for drug-resistant tuberculosis in Khayelitsha’ has been granted to MSF by the Human Research Ethics Committee at the University of Cape Town [540/2010].
References
- 1.WHO. Global Tuberculosis Report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.WHO. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2014. [PubMed] [Google Scholar]
- 3.Abdool SSK, Churchyard GJ, Karim QA, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2010;374:921–33. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Department of Health. Multi-drug resistant tuberculosis: a policy framework on decentralised and deinstitutionalised management for South Africa. Pretoria: Department of Health; 2011. [Google Scholar]
- 5.O’Donnell MR, Padayatchi N, Kvasnovsky C, et al. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19:416–24. doi: 10.3201/eid1903.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farley JE, Ram M, Pan W, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a cohort of South African patients with high HIV prevalence. PLoS One. 2011;6:e20436. doi: 10.1371/journal.pone.0020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–80. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 9.Brust JCM, Gandhi NR, Carrara H, et al. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14:413–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Shean K, Streicher E, Pieterson E, et al. Drug-associated adverse events and their relationship with outcomes in patients receiving treatment for extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8:e63057. doi: 10.1371/journal.pone.0063057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isaakidis P, Varghese B, Mansoor H, et al. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PLoS One. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007;196(Suppl 1):S63–75. doi: 10.1086/518655. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4:e6914. doi: 10.1371/journal.pone.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M, Pavlinac P, Kimerling ME, et al. Use of anti-retroviral therapy in tuberculosis patients on second-line anti-TB regimens: a systematic review. PLoS One. 2012;7:e47370. doi: 10.1371/journal.pone.0047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi NR, Andrews JR, Brust JCM, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2012;16:90–7. doi: 10.5588/ijtld.11.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis. 2014;18:441–8. doi: 10.5588/ijtld.13.0742. [DOI] [PubMed] [Google Scholar]
- 18.Daniels J, Khogali M, Mohr E, et al. Time to ART initiation among patients treated for RR-TB in Khayelitsha, South Africa: impact on mortality. Int J Tuberc Lung Dis. 2015 doi: 10.1371/journal.pone.0142873. [Forthcoming] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cape Town City Health. 2011 Census - Khayelitsha Health District. City of Cape Town: 2013. [Google Scholar]
- 20.MSF. Khayelitsha 2001–2011. Activity report 10 Years of HIV/TB care at primary health care level. Cape Town: Médecins Sans Frontières; 2011. [Google Scholar]
- 21.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 22.MSF. Scaling up diagnosis and treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Cape Town: Médecins Sans Frontières; 2011. [Google Scholar]
- 23.National Department of Health. The South African Anriretroviral Treatment Guidelines, 2013. Pretoria: Department of Health; 2013. [Google Scholar]
- 24.National Department of Health. National Tuberculosis Management Guidelines, 2014. Pretoria: Department of Health; 2014. [Google Scholar]
- 25.Cox HS, Daniels JF, Muller O, et al. Impact of decentralized care and the Xpert MTB / RIF test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infect Dis. 2015 doi: 10.1093/ofid/ofv014. [Forthcoming] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hafkin J, Modongo C, Newcomb C, et al. Impact of the human immunodeficiency virus on early multidrug-resistant tuberculosis treatment outcomes in Botswana. Int J Tuberc Lung Dis. 2013;17:348–53. doi: 10.5588/ijtld.12.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otwombe KN, Petzold M, Modisenyane T, et al. Factors associated with mortality in HIV-infected people in rural and urban South Africa. Glob Helath Action. 2014;7:1–10. doi: 10.3402/gha.v7.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patten GE, Cox V, Stinson K, et al. Advanced HIV disease at antiretroviral therapy (ART) initiation despite implementation of expanded ART eligibility guidelines during 2007–2012 in Khayelitsha, South Africa. Clin Infect Dis. 2014;59:456–7. doi: 10.1093/cid/ciu288. [DOI] [PubMed] [Google Scholar]
- 29.Maher D, Watt CJ, Williams BG, et al. Tuberculosis deaths in countries with high HIV prevalence: what is their use as an indicator in tuberculosis programme monitoring and epidemiological surveillance? Int J Tuberc Lung Dis. 2005;9:123–7. [PubMed] [Google Scholar]
- 30.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 31.Shean KP, Willcox PA, Siwendu SN, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–'2002. Int J Tuberc Lung Dis. 2008;12:1182–9. [PubMed] [Google Scholar]
- 32.Kendall E, Theron D, Franke MF, et al. Alcohol, hospital discharge, and socioeconomic risk factors for default from multidrug resistant tuberculosis treatment in rural South Africa: a retrospective cohort study. PLoS One. 2013;8:e83480. doi: 10.1371/journal.pone.0083480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korenromp EL, Bierrenbach AL, Williams BG, Dye C. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis. 2009;13:283–303. [PubMed] [Google Scholar]
- 34.Brigden G, Nyang B, du Cros P, et al. Principles for designing future regimens for multidrug- resistant tuberculosis. Bull World Health Organ. 2014;92:68–74. doi: 10.2471/BLT.13.122028. [DOI] [PMC free article] [PubMed] [Google Scholar]