Summary
Setting
South Africa is one of the world’s 22 high tuberculosis (TB) burden countries, with the second highest number of notified rifampicin-resistant TB (RR-TB) and multidrug-resistant TB (MDR-TB) cases.
Objective
To estimate patient costs associated with the diagnosis and treatment of RR-TB/MDR-TB in South Africa.
Design
Patients diagnosed with RR-TB/MDR-TB and accessing care at government health care facilities were surveyed using a structured questionnaire. Direct and indirect costs associated with accessing RR-TB/MDR-TB care were estimated at different treatment durations for each patient.
Results
A total of 134 patients were surveyed: 84 in the intensive phase and 50 in the continuation phase of treatment, 82 in-patients and 52 out-patients. The mean monthly patient costs associated with the diagnosis and treatment of RR-TB/MDR-TB were higher during the intensive phase than the continuation phase (US$235 vs. US$188) and among in-patients than among out-patients (US$269 vs. US$122). Patients in the continuation phase and those accessing care as out-patients reported higher out-of-pocket costs than other patients. Most patients did not access social protection for costs associated with RR-TB/MDR-TB illness.
Conclusion
Despite free health care, patients bear high costs when accessing diagnosis and treatment services for RR-TB/MDR-TB; appropriate social protection mechanisms should be provided to assist them in coping with these costs.
Keywords: treatment duration, out-of-pocket expenditure, poverty
HEALTH SERVICES for the diagnosis and treatment of tuberculosis (TB) are provided free of charge by public health services in most countries.1 However, many patients and their families still face high costs related to seeking and accessing care for their TB illness, and this puts them at an increased risk of financial difficulties and further impoverishment.2,3 The World Health Organization (WHO) has urged member countries to provide social protection mechanisms to support TB patients.4,5 The effectiveness of social protection interventions depends on their timely provision; for TB patients, this means at the time of diagnosis or treatment initiation.6
The synergistic relationship between poverty and TB illness has been documented in several studies.7,8 Not only does poverty increase the risk of contracting TB, TB also exacerbates poverty.7 Loss of income as a result of TB illness is the main reason for adverse economic circumstances among TB patients and their families.9 Time and income loss due to TB morbidity are estimated to represent approximately 3–4 months of work time and about 20–30% of annual household income for TB patients on treatment.10 In the case of premature death of the patient due to TB, families can lose up to 15 years worth of income or more.10,11
Extreme health care costs are commonly referred to as catastrophic.12 There is currently no consensus on the definition of catastrophic costs. However, some researchers define health care costs as catastrophic if they exceed a given proportion of total household income or total expenditure in a specified period.13 This proportion could be 10–25% of the annual household income13 or 40% of the household’s capacity to pay.14 Studies estimating patient costs associated with TB illness have consistently reported that total illness-related costs as a percentage of individual income are higher for patients with multidrug-resistant TB (MDR-TB) than drug-susceptible TB (DS-TB).12,15,16 In one of these studies, MDR-TB was independently associated with the likelihood of incurring catastrophic costs.12
South Africa has the second highest number of patients diagnosed with either MDR-TB or rifampicin-resistant TB (RR-TB) globally.1 As a result of the high patient burden, national policy in South Africa has moved towards models of RR-TB/MDR-TB treatment requiring less hospitalisation,17 which can potentially reduce the poverty impact on RR-TB/MDR-TB patients and their families. Data on patient costs associated with RR-TB/MDR-TB illness in South Africa are currently lacking. Knowledge about RR-TB/MDR-TB-related patient costs can help programme managers design models of care that minimise the impact of poverty.
The primary aim of the present study was to estimate patient costs associated with RR-TB/MDR-TB.
Study Population and Methods
Study setting
The study was conducted in two municipalities located in two different South African provinces: the Alfred Nzo District Municipality and the City of Cape Town Metropolitan Municipality. The Alfred Nzo District Municipality (Eastern Cape Province) is a predominantly rural district, with approximately 800 000 inhabitants,18 and one of the highest unemployment rates in the province (43.6%).19 The City of Cape Town (Western Cape Province) has an estimated population of 3.6 million,18 with an estimated unemployment rate in the Cape Town metro of 24% in 2011.19
One subdistrict with high RR-TB or MDR-TB prevalence was purposively sampled in each municipality: Matatiele (Alfred Nzo) and Khayelitsha (Cape Town). At subdistrict level, health facilities were selected via purposive sampling to ensure representativeness according to level of care and size of facility. In Matatiele, the two facilities that provided treatment for RR-TB/MDR-TB were selected: a community health centre and a TB hospital (serving the entire Alfred Nzo municipality). In Khayelitsha, four of 10 facilities that provide RR-TB/MDR-TB treatment were included: two primary health care clinics, a community health centre and a sub-acute care facility. Patients were also recruited from a central TB hospital, which serves the entire Cape Town metro and surrounding areas.
In South Africa, services for the diagnosis and treatment of RR-TB/MDR-TB are provided at no cost to the patients, in accordance with the national policy guidelines on the management of drug-resistant TB.17 In Matatiele, all patients diagnosed with MDR-TB or RR-TB are routinely hospitalised as part of their treatment. In Khayelitsha, these patients can initiate and continue treatment at their primary health care clinics as out-patients, and hospitalisation is reserved only for clinically unstable patients.20 Patients on RR-TB/MDR-TB treatment are eligible for support in the form of nutritional supplementation or monthly disability grant.21 Disability grants (US$125/month)22 are offered to patients who are declared ‘physically incapacitated’ by a medical doctor.23
Sampling
All RR-TB/MDR-TB patients who were receiving treatment at participating facilities during February–June 2013 and were available at the facility during data collection times were invited to participate in the study. Inclusion criteria included a diagnosis of either RR-TB or MDR-TB, and age ⩾18 years. Patients who were too sick to participate based on the advice of the medical staff and those diagnosed with extensively drug-resistant TB (XDR-TB) were excluded. There was no predetermined sample. However, patients were selected to be inclusive of the following demographic profile: similar proportions of males and females, different MDR-TB treatment durations and similar numbers of out-patients and in-patients.
Measuring patient costs
A cross-sectional cost-of-illness survey was conducted between 13 February and 6 June 2013 using a structured questionnaire that had been successfully used to collect cost information for TB patients in previous studies.24 Minor adaptations were made to the questionnaire by replacing all references to ‘TB’ by ‘MDR-TB’, and rephrasing some questions to allow for a distinction between pre- and post-hospital admission periods. All interviews were conducted by a trained research assistant at the health facility during the patient’s regular facility visit or at the hospital (in-patients). Patients at different points of MDR-TB treatment were asked about their health services utilisation and RR-TB/MDR-TB illness-related costs. To minimise recall bias, patients were only asked about their illness-related costs over the past 2 months prior to the day of the interview. Patients were also asked about their individual income from any form of employment, government grants, pensions and non-monetary payments.
Patient costs were estimated according to TB costing guidelines.25 Two broad categories of costs were estimated: direct (out of pocket) costs and indirect costs for both patients and their care givers. Direct medical costs included non-prescribed medications bought by patients, consultation fees and diagnostic tests from private practitioners. Direct non-medical costs included transportation, nutritional diet supplements, miscellaneous costs (e.g., snacks/meals purchased during facility visits) and coping costs (e.g., loans taken to pay for RR-TB/MDR-TB illness-related expenses). Indirect costs included productivity losses resulting from the inability to work due to RR-TB/MDR-TB illness as well as the cost of illness-related lost home productivity for both the patients and their care givers.26
Before their MDR-TB illness, the majority of the study participants were either unemployed or were in jobs that paid close to the minimum wage rate. Income lost due to MDR-TB illness was therefore estimated by multiplying the estimated number of lost production hours due to MDR-TB illness by the official minimum wage (US$182/month) for un-skilled (domestic) workers in South Africa during the period from 1 December 2012 to 30 November 2013 (http://www.mywage.co.za/main/salary/minimum-wages). We assumed an average workday of 8 h/day and 22 working days/month. Care givers’ lost home productivity was also estimated by multiplying the total number of hours spent by care givers caring for the patient by the official minimum wage rate. Total costs for the entire RR-TB/MDR-TB treatment episode were estimated by adding the mean monthly costs for each phase of treatment multiplied by the duration of that phase of treatment.
Patients diagnosed with RR-TB are managed essentially in the same way as MDR-TB patients until confirmation results show rifampicin (RMP) susceptible TB, upon which MDR-TB treatment is stopped.17 Illness-related costs were therefore estimated the same way for these two groups of patients.
Data management and analysis
The data from paper-based questionnaires were double-entered into a database created using Epi-Info 3.4.1 (Centers for Disease Control and Prevention, Atlanta, GA, USA) by two different individuals. The two data sets were compared to eliminate data entry errors. Data analysis was performed using STATA (StataCorp. Stata Statistical Software: Release 12. College Station, TX, USA). Frequency counts and percentages were used to describe pertinent demographic and socio-economic and treatment variables.Mean, median and interquartile ranges for direct, indirect and total costs were also calculated. Cost information (in South African currency) was converted to $US using the following exchange rate: US$1 = ZAR9.62 (average exchange rate, January–December 2013; Currency Converter/Foreign Exchange Rates/OANDA. http://www.oanda.com/currency/converter/).
Ethics
The study was approved by the University of Health Sciences Faculty of Health Sciences Human Research Ethics Committee (HREC REF: 430/2012), Provincial Governments of the Western Cape, Eastern Cape and the City of Cape Town, Cape Town, South Africa.
Results
Participants description and health services utilisation
A total of 134 patients undergoing RR-TB/MDR-TB treatment participated in the study (Table 1): 35/42 at the two Alfred Nzo facilities, and 99/160 at the five Cape Town Metro facilities. Three patients (Cape Town) declined to participate, while the rest were not interviewed, either because they were not available during the interview days (1 in Alfred Nzo and 46 in Cape Town), or they were too sick to participate (6 in Alfred Nzo and 12 in Cape Town). Interviewed patients were divided into two groups: those in the intensive phase (first 6 months of treatment) and those in the continuation phase (time following intensive phase of at least 18 months’ duration).22
Table 1. Participants’ demographic information.
| Intensive phase (n = 84) n (%) |
Continuation phase (n = 50) n (%) |
Total (n = 134) n (%) |
|
|---|---|---|---|
| Sex | |||
| Male | 38 (45) | 24 (48) | 62 (46) |
| Female | 46 (55) | 26 (52) | 72 (54) |
| Age, years | |||
| 18–24 | 10 (12) | 8 (16) | 18 (13) |
| 25–44 | 63 (75) | 33 (66) | 96 (72) |
| ⩾45 | 11 (13) | 9 (18) | 20 (15) |
| Education level | |||
| Primary | 69 (82) | 39 (78) | 108 (81) |
| Secondary | 12 (14) | 9 (18) | 21 (16) |
| Tertiary | 1 (1) | 1 (2) | 2 (1) |
| Other | 2 (2) | 1 (2) | 3 (2) |
| Average monthly income | |||
| prior to MDR-TB illness, $US | |||
| ⩽60 | 5 (6) | 4 (8) | 9 (7) |
| 61–100 | 6 (7) | 0 (0) | 6 (4) |
| 101–200 | 11 (13) | 10 (20) | 21 (16) |
| 201–400 | 9 (11) | 7 (14) | 17 (12) |
| >400 | 4 (5) | 3 (6) | 7 (5) |
| No income | 49 (58) | 26 (52) | 75 (56) |
| Patient status | |||
| In-patient | 71 (85) | 11 (22) | 82 (61) |
| Out-patient | 13 (15) | 39 (78) | 52 (39) |
| Place of treatment initiation | |||
| Primary health care clinic | 29 (35) | 21 (42) | 50 (37) |
| Community health centre | 1 (1) | 4 (8) | 5 (4) |
| District hospital | 18 (21) | 16 (32) | 34 (25) |
| Central TB hospital | 22 (26) | 8 (16) | 30 (22) |
| Tertiary hospital | 8 (10) | 0 (0) | 8 (6) |
| Unknown | 6 (7) | 1 (2) | 7 (5) |
| Municipality | |||
| Alfred Nzo | 29 (35) | 6 (12) | 35 (26) |
| Cape Town | 55 (65) | 44 (88) | 99 (74) |
MDR-TB = multidrug-resistant tuberculosis.
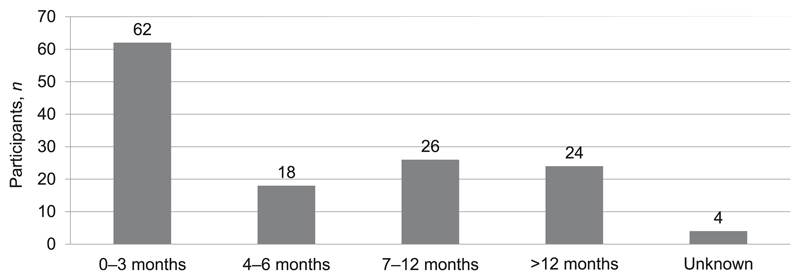
The majority (63%) of the patients were in the intensive phase of treatment (Figure 1). Among this selected (non-representative) patient sample, 88 (66%) were hospitalised at some point during treatment. The mean duration of hospitalisation among these patients at the time of interview was 39 ± 40 nights (intensive phase) and 19 ± 49 nights (continuation phase).
Figure 1. Distribution of participants according to duration of treatment.
Patient costs
The mean monthly RR-TB/MDR-TB illness-related costs were greater for in-patients than out-patients; production time lost due to hospitalisation represented the largest cost component for in-patients, while nutritional diet supplements were the largest cost component for out-patients (Table 2). The mean monthly RR-TB/MDR-TB illness-related costs were higher during the intensive phase than during the continuation phase of treatment, and production time lost due to hospitalisation was the largest cost component during both phases of treatment (Table 3).
Table 2. Mean monthly costs according to type of patient in 2013 $US.
| In-patients (n = 82) |
Out-patients (n = 52) |
|||||
|---|---|---|---|---|---|---|
| Patient status | mean ± SD | median [IQR] | Proportion of minimum monthly wage % | mean ± SD | median [IQR] | Proportion of minimum monthly wage % |
| Direct costs | ||||||
| Private health care | 1.4 ± 8.0 | 0 | 0.8 | 1.1 ± 4.4 | 0 | 0.6 |
| Transport | 5.3 ± 26.1 | 0 | 2.9 | 3.6 ± 8.6 | 0 [0-2.4] | 2.0 |
| Miscellaneous | 6.8 ± 14.5 | 0 [0–5.2] | 3.7 | 2.1 ± 8.4 | 0 | 1.2 |
| Nutritional diet supplements | 12.6 ± 35.3 | 1.5 [0–9.4] | 6.9 | 49.2 ± 44 | 42 [20-62] | 27.1 |
| Subtotal (direct) | 26.0 | 14.3 | 56.0 | 30.9 | ||
| Indirect costs | ||||||
| Productivity loss: seeking care | 10.9 ± 22.6 | 0.9 [0.1–11] | 6.0 | 8.3 ± 11 | 2.9 [0.6–14] | 4.6 |
| Productivity loss (hospitalisation) | 225.2 ± 248 | 149 [45–355] | 124.1 | 26.7 ± 80 | 0 | 14.7 |
| Carer costs | 6.7 ± 19.3 | 2.1 [0–4.6] | 3.7 | 28.2 ± 70.2 | 0 [0–6.2] | 15.5 |
| Subtotal (indirect) | 242.9 | 133.8 | 63.2 | 34.8 | ||
| Coping costs | ||||||
| Interest on loans | 0.3 ± 4.4 | 0 [0–0] | 0.2 | 2.9 ± 8.4 | 0 [0–2.6] | 1.6 |
| Mean monthly costs, $US | 269.2 | 148.3 | 122.1 | 67.3 | ||
SD = standard deviation; IQR = interquartile range.
Table 3. Mean monthly costs according to phase of treatment in 2013 $US.
| Intensive phase (0–6 months) (n = 84) | Continuation phase (7–24 months) (n = 50) | |||||
|---|---|---|---|---|---|---|
| Duration on treatment | mean ± SD | median [IQR] | Proportion of minimum monthly wage % | mean ± SD | median [IQR] | Proportion of minimum monthly wage % |
| Direct costs | ||||||
| Private health care | 1.7 ± 7.3 | 0 | 1.1 | 1.4 ± 8.1 | 0 | 0.8 |
| Transport | 2.1 ± 7.4 | 0 | 0.9 | 10.5 ± 35 | 0 [0–3.1] | 5.8 |
| Miscellaneous | 9.2 ± 31.7 | 0 [0–5.7] | 5.1 | 2.9 ± 9.9 | 0 | 1.6 |
| Nutritional diet supplements | 16.3 ± 37 | 4.2 [0–14.3] | 9.0 | 44 ± 46 | 35 [10–62] | 24.1 |
| Subtotal (direct) | 29.2 | 16.1 | 58.5 | 32.3 | ||
| Indirect costs | ||||||
| Productivity loss: seeking care | 7.7 ± 14.2 | 0 [0–10.3] | 4.2 | 4.1 ± 6.5 | 1.3 [0.3–4.9] | 2.3 |
| Productivity loss: hospitalisation | 173.2 ± 190 | 115.6 [10–227] | 95.4 | 90 ± 229 | 0 [0–8.3] | 49.5 |
| Carer costs | 12.6 ± 40.8 | 1.8 [0–4.6] | 6.9 | 32.9 ± 102 | 0 [0–5.1] | 18.1 |
| Subtotal (indirect) | 193.5 | 106.6 | 127.0 | 70.0 | ||
| Coping costs | ||||||
| Interest on loans | 0.6 ± 4 | 0 | 0.3 | 2.6 ± 9 | 0 [0–2.6] | 1.5 |
| Mean monthly costs, US$ | 223.3 | 123.0 | 188.1 | 103.7 | ||
| Total costs, $US* | 4725.6 | |||||
Total costs for one MDR-TB treatment episode.
SD = standard deviation; IQR = interquartile range.
Impact of RR-TB/MDR-TB illness on patient income
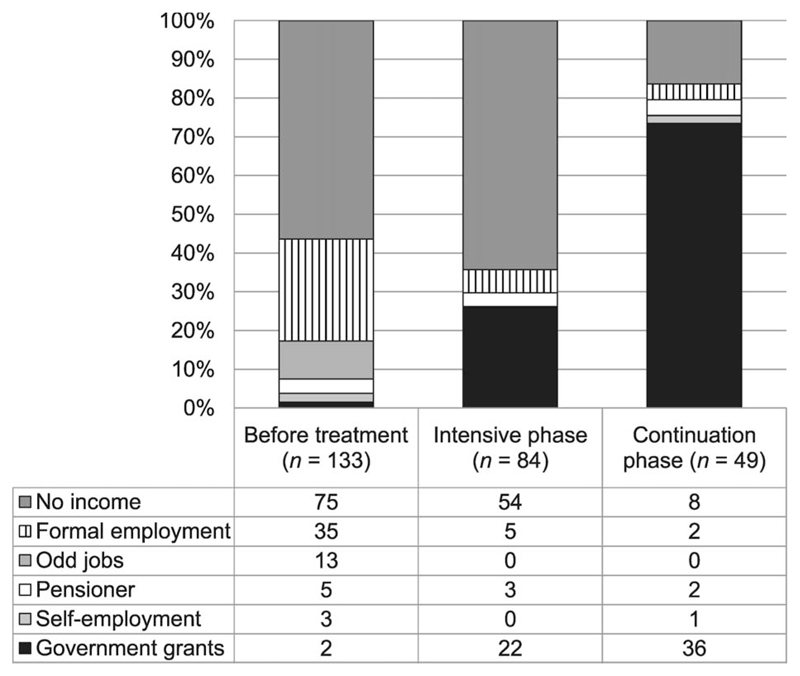
Prior to treatment initiation, 56% of the patients reported no source of income, while the rest reported a variety of income sources. The proportion of those who reported no source of income fell to 47% post-treatment initiation (Figure 2). Of the 72 patients who reported having a source of income post-treatment initiation, 58 (81%) reported that MDR-TB disability grants were their primary source of income. The proportion of patients who reported some form of paid employment dropped from 37% before illness to 3% during treatment. Out-of-pocket costs as a proportion of the monthly official minimum wage ranged from 14% to 32% (Tables 2 and 3). About 40% of patients in the continuation phase and 26% in the intensive phase reported contracting loans to pay for RR-TB/MDR-TB illness-related expenses; 44% of the loans had to be repaid with interest, while the remainder were interest free.
Figure 2. Source of income before initiation of treatment and during treatment.
Discussion
The study showed that patients on RR-TB/MDR-TB treatment generally bear a substantial financial burden as a result of their illness, particularly during the intensive phase of treatment. Out-patients and those in the continuation phase of treatment reported higher out-of-pocket costs than other categories of patients. Post treatment initiation only 3% of the patients were still employed and disability grants were the primary source of income for 44% of the patients.
Patients in the intensive phase of treatment generally reported longer periods of hospitalisation than those in the continuation phase. The intensive phase of treatment is the period when the patient is likely to be very sick and may need closer medical attention. Alternatively, this could have also been due to policy-driven admissions for treatment initiation, as was the case in Alfred Nzo municipality, which could lead to longer periods of hospitalisation. As productivity loss due to hospitalisation was one of the main contributors to indirect patient costs in this study, MDR-TB treatment models that require no or shorter hospitalisation may potentially reduce patient costs, in addition to reducing health system costs.26
Out-of-pocket costs as a proportion of the monthly minimum wage (14–32%) were also prohibitively high for the majority of the patients, particularly for those on continuation phase treatment and for out-patients. As many patients reported having no source of income before (56%) and during (47%) treatment, it is therefore highly likely that these costs were catastrophic for many patients.12,13 Furthermore, for patients without a source of income, any additional costs due to RR-TB/MDR-TB illness is likely to impoverish them further. Such an impact could be mitigated to some extent by providing socio-economic support to these patients.6 It was therefore a matter of concern to find that despite the existence of various forms of support for these patients,21 46% of them were still not accessing support mechanisms post treatment initiation. The fact that more patients reported accessing disability grants during the continuation (27%) than the intensive phase (16%) of treatment may also suggest that there are bottlenecks in the distribution of these grants. An efficient system is needed to ensure that those who are eligible for this support access it in a timely fashion.
The main study strengths are the fact that the patient sample was varied with respect to type of patient, treatment duration and geographical settings, and the use of validated tools and guidelines.24,25 However, the study has a number of limitations. The use of an official minimum wage to estimate productivity for all patients could have potentially overestimated indirect costs among patients who were unemployed prior to their illness or underestimated indirect costs among those who were employed.25 Future studies should explore alternative approaches of estimating patient costs to overcome this methodological limitation.27 Costs were also estimated only from the patients’ perspective and did not include the health service providers’ costs for providing RR-TB/MDR-TB treatment to these patients.
Furthermore, despite efforts to obtain a more representative sample, in-patients were over-represented (61%) in the study sample. This was mainly because patients were also recruited from TB hospitals, which had large numbers of hospitalised MDR-TB patients who were easily accessible participants. Finally, due to the cross-sectional study design, patients were not followed throughout their RR-TB/MDR-TB treatment. However, we interviewed patients who were at different points in their treatment to get a better sense of illness-related costs as treatment progressed, and used that information to estimate the total costs for a single RR-TB/MDR-TB treatment episode (Table 3). Despite these limitations, the study represents the first attempt to estimate patient costs associated with RR-TB/MDR-TB illness in South Africa, and can potentially inform models of RR-TB/MDR-TB care in the country.
Conclusion
Despite access to free health care, the majority of study patients reported incurring substantial costs as a result of RR-TB/MDR-TB, and for many of these patients these costs were potentially catastrophic. Access to government social protection mechanisms seemed to be a challenge for most patients, particularly during the intensive phase of treatment, which means that a high proportion of RR-TB/MDR-TB patients are still at risk of bearing undue financial burden as a result of their illness.
Acknowledgements
The study was funded in part by a grant from the Bill & Melinda Gates Foundation (BMGF), Seattle, WA, USA, and in part by Médecins Sans Frontiéres (MSF), Paris, France. BMGF had no role in study design, data analysis, decision to publish or preparation of manuscript. An author from MSF was involved in the study design, data collection and analysis; however, the final preparation of the manuscript and the decision to publish rests with the first, second and last authors.
Footnotes
Conflicts of interest: no other conflicts declared.
References
- 1.World Health Organization. Global tuberculosis report, 2014. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.08. [Google Scholar]
- 2.Mauch V, Melgen R, Mercelino B, Acosta I, Klinkenberg E, Suarez P. Tuberculosis patients in the Dominican Republic face severe direct and indirect costs and need social protection. Rev Panam Salud Publica. 2013;33:332–339. doi: 10.1590/s1020-49892013000500004. [DOI] [PubMed] [Google Scholar]
- 3.Mauch V, Bonsu F, Gyapong M, et al. Free tuberculosis and treatment are not enough: patient cost evidence from three continents. Int J Tuberc Lung Dis. 2013;17:381–387. doi: 10.5588/ijtld.12.0368. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. The World Health Report. Health system financing: path to universal coverage. Geneva, Switzerland: WHO; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. 67th World Health Assembly: Agenda Documents A67/11 and EB134/2014/REC/1, resolution EB134.R4. Geneva, Switzerland: WHO; 2014. [Accessed August 2015]. http://apps.who.int/gb/ebwha/pdf_files/WHA67/A67_1Rev1-en.pdf. [Google Scholar]
- 6.Tadayuki T, Ernesto J, Weil D, Raviglione M, Lönnroth K. Financial burden for tuberculosis patients in low-and middle-income countries: a systematic review. Eur Respir J. 2014;43:1763–1775. doi: 10.1183/09031936.00193413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Addressing poverty in TB control: options for National TB Control Programmes. WHO/HTM/TB2005.352. Geneva, Switzerland: WHO; 2005. [Google Scholar]
- 8.Hansel NN, Wu AW, Chang B, Diette GB. Quality of life in tuberculosis: patient and provider perspective. Qual Life Res. 2004;13:639–652. doi: 10.1023/B:QURE.0000021317.12945.f0. [DOI] [PubMed] [Google Scholar]
- 9.Barter DM, Agboola SO, Murray MB, Barnighausen T. Tuberculosis and poverty: the contribution of patient costs in sub-Saharan Africa: a systematic review. BMC Public Health. 2012;12:980. doi: 10.1186/1471-2458-12-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawert H, Kongsin S, Payanandana V, Akarasewi P, Nunn PP, Raviglione C. Costs and benefits of improving tuberculosis control: the case of Thailand. Soc Sci Med. 1997;44:1805–1816. doi: 10.1016/s0277-9536(96)00289-4. [DOI] [PubMed] [Google Scholar]
- 11.Collins D, Hafidz F, Suraratdecha C. The economic burden of tuberculosis in Indonesia. TB CARE I. Cambridge, MA, USA: Management Sciences for Health; 2013. [Accessed August 2015]. http://www.msh.org/sites/msh.org/files/the_economic_burden_of_tuberculosis_in_indonesia.pdf. [Google Scholar]
- 12.Wingfield T, Boccia D, Tovar M, et al. Defining catastrophic costs and comparing their importance for adverse tuberculosis outcome with multi-drug resistance: a prospective cohort study, Peru. PLOS MED. 2014;11:e1001675. doi: 10.1371/journal.pmed.1001675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell S. The economic burden of illness for households in developing countries: a review of studies focusing on malaria, tuberculosis, and human immunodeficiency virus/acquired immunodeficiency syndrome. Am J Trop Med Hyg. 2004;71:147–155. [PubMed] [Google Scholar]
- 14.Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJL. Household catastrophic health expenditure: a multicountry analysis. Lancet. 2003;362:111–117. doi: 10.1016/S0140-6736(03)13861-5. [DOI] [PubMed] [Google Scholar]
- 15.Rouzier VA, Oxlade O, Verduga R, Gresely L, Menzies D. Patient and Family costs associated with tuberculosis, including multidrug-resistant tuberculosis, in Ecuador. Int J Tuberc Lung Dis. 2010;14:1316–1322. [PubMed] [Google Scholar]
- 16.Pichenda K, Nakamura K, Morita A, Kazuti M, Seino K, Takano T. Non-hospital DOT and early diagnosis of tuberculosis reduce costs while achieving treatment success. Int J Tuberc Lung Dis. 2012;16:828–834. doi: 10.5588/ijtld.11.0688. [DOI] [PubMed] [Google Scholar]
- 17.South African Department of Health. Management of drug-resistant tuberculosis: policy guidelines. Pretoria, South Africa: DoH; 2011. [Google Scholar]
- 18.Statistics South Africa. Census 2011 Statistical Release. Pretoria, South Africa: Statistics South Africa; 2011. [Google Scholar]
- 19.Massyn N, Day C, Dombo M, Barron P, English R, Padarath A. District Health Barometer 2012/13. Durban, South Africa: Health System Trust; 2013. [Google Scholar]
- 20.Médecins Sans Frontières. Scaling up diagnosis and treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Cape Town, South Africa: MSF; 2011. [Google Scholar]
- 21.Lutge E, Ndlela Z, Friedman I. Assessment of current support strategies for patients with TB in KwaZulu-Natal. Durban, South Africa: Health Systems Trust; 2009. [Google Scholar]
- 22.National Treasury, Republic of South Africa. Budget Review 2013: social security and the social wage. Pretoria, South Africa: National Treasury; 2013. [Accessed August 2015]. http://www.treasury.gov.za/documents/ [Google Scholar]
- 23.Western Cape Government. Multidrug-resistant TB Fact Sheet, 2014. Cape Town, South Africa: Western Cape Government; 2014. [Accessed August 2015]. http://www.westerncape.gov.za/general-publication/multi-drug-resistant-tb-fact-sheet. [Google Scholar]
- 24.Mauch V, Woods N, Kirubi B, Kipruto H, Sitienei J, Klinkenberg E. Assessing access barriers to tuberculosis care with the tool to estimate patients’ costs: pilot results from two districts in Kenya. BMC Public Health. 2011;11:43. doi: 10.1186/1471-2458-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Guidelines for cost and cost-effectiveness analysis of tuberculosis control. WHO/CDS/TB/2002/305b. Geneva, Switzerland: WHO; 2002. [Google Scholar]
- 26.Sinanovic E, Ramma L, Vassall A, et al. Impact of reduced hospitalisation on the cost of treatment for drug-resistant tuberculosis in South Africa. Int J Tuberc Lung Dis. 2015;19:172–178. doi: 10.5588/ijtld.14.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malaney P. CID Working Paper No. 99. Cambridge, MA, USA: Center for International Development, Harvard University; 2003. Micro-economic approaches to evaluating the burden of Malaria. [Google Scholar]