Abstract
Objective
We assess associations of general and central adiposity in middle-age, and of young adulthood adiposity, with incident diabetes in adult Chinese, and estimate the associated population burden of diabetes.
Research Design and Methods
The prospective China Kadoorie Biobank enrolled 512,891 adults aged 30-79 years from 10 localities across China during 2004-08. During 9.2 years follow-up, 13,416 cases of diabetes were recorded among 482,589 participants without diabetes at baseline. Cox regression yielded adjusted hazard ratios (HRs) for incident diabetes associated with measures of general (eg, BMI, BMI at 25y) and central (eg, waist circumference [WC]) adiposity.
Results
Mean (SD) BMI was 23.6 (3.4) kg/m2 and 3.8% had BMI ≥30 kg/m2. Throughout the range examined (19-32 kg/m2), BMI showed a positive log-linear relationship with diabetes, with adjusted HRs per SD higher usual BMI greater in men (1.98, 95% CI 1.93-2.04) than women (1.77, 1.73-1.81) (p for heterogeneity <0.001). For WC, HRs per SD were 2.13 (2.07-2.19) in men and 1.91 (1.87-1.95) in women (p for heterogeneity <0.001). Mutual adjustment attenuated these associations, especially those of BMI. BMI at age 25 was weakly positively associated with diabetes (men 1.09 [1.05-1.12]; women 1.04 [1.02-1.07] per SD), which was reversed after adjustment for baseline BMI. In China, increase in adiposity accounted for ~50% of the increase in diabetes burden since 1980.
Conclusions
Among relatively lean Chinese adults, higher adiposity—general and central—was strongly positively associated with risk of incident diabetes. The predicted continuing increase in adiposity in China foreshadows escalating rates of diabetes.
China has the largest number of adults with diabetes of any country worldwide, following a rapid increase in prevalence (1). Between 1980 and 2010, diabetes prevalence increased 2.2-fold in the USA and 1.2-fold worldwide (2), but increased almost 10-fold in China (3; 4). The reasons for escalating rates of diabetes in China are still not properly characterised, but likely reflect a combination of demographic changes (eg, population aging) and increasing levels of adiposity, due to lifestyle changes (eg, increased consumption of energy-dense foods and declining physical activity) associated with rapid economic development and urbanisation (5).
Adiposity is an established causal risk factor for type 2 diabetes (6; 7). However, much existing evidence comes from Western populations, and focusses mainly on body mass index (BMI), a measure of general adiposity. Questions remain about the relative importance of general versus central (eg, waist circumference [WC]) adiposity for type 2 diabetes, and about the relevance of adiposity at different points during the life course. Although mean population BMI in China has increased by almost 1 kg/m2 per decade since the mid-1980s, it remains much lower than in the West (8). However, given BMI, Chinese adults are reported to have a higher proportion of body fat and greater propensity to central adiposity than their Western counterparts (9). Previous studies of adiposity and type 2 diabetes in China have been limited by small sample size, cross-sectional design, examination of later adulthood BMI only, or restriction to occupational or urban cohorts (10–14). We assess associations of general and central adiposity in middle-age, and of young adulthood adiposity, with incident diabetes in the prospective China Kadoorie Biobank of 0.5 million adult men and women and estimate the associated burden of diabetes in the general population.
Research Design and Methods
Study population
Details of the CKB design, methods, and population have been reported previously (http://www.ckbiobank.org/) (15; 16). Briefly, study participants were recruited from 10 diverse areas (five urban and five rural) of China, selected to ensure diversity in exposure and disease patterns, whilst taking account of population stability, and death and disease registry quality. Permanent, non-disabled residents of 100-150 rural villages or urban committees in each study area, aged 35 to 74 years, were invited to participate. A response rate of ~30% was achieved (16), and 512,891 men and women were enrolled (including ~10,000 slightly outside the target 35–74 year age range).
Local, national and international ethical approval was obtained prior to commencement of the study. All participants provided written informed consent.
Data collection
The baseline survey took place between June 2004 and July 2008. Trained health workers administered laptop-based questionnaires and undertook physical examinations. Data were collected on socio-demographic status, lifestyle factors, including smoking, alcohol consumption, diet and physical activity (leisure, household, occupational and commuting), personal and family medical history, and, using calibrated instruments with standard protocols, anthropometric measures, lung function, blood pressure and heart rate. A non-fasting venous blood sample was collected, and the time passed since participants last ate recorded. Immediate, on-site testing of plasma glucose level was undertaken using the Johnson and Johnson SureStep Plus meter (LifeScan, Milipitas, CA, USA). Participants with a glucose level ≥140 mg/dL and <200 mg/dL were invited to return the following day for fasting plasma glucose testing. Resurveys of 5% randomly selected samples of surviving participants were undertaken in 2008 and 2013-14, collating the same data as at baseline, including anthropometric data.
Anthropometric measurements
Standing height was measured to the nearest 0.1 cm using a portable stadiometer. Weight was measured to the nearest 0.1 kg using the scale function of the TBF-300 body composition analyser (Tanita Inc, Tokyo, Japan) and the estimated weight of clothing subtracted (summer 0.5 kg; spring/autumn 1.0 kg; winter 2.0-2.5 kg). Height and weight were measured without shoes. The TBF-300 body composition analyser used foot-to-foot bioelectrical impedance analysis to measure body fat percentage (BF%) using its in-built proprietary algorithm. Waist and hip circumferences were measured to the nearest 0.1 cm with a non-stretchable tape measure: WC was measured at the midpoint between the lowest rib margin and the iliac crest; hip circumference (HC) was measured around the maximum circumference of the buttocks. WC and HC were measured unclothed or 1-2 cm was subtracted from the WC reading to account for undergarments, and 1 cm and 2.5 cm were subtracted from the HC reading to account for skirt and trousers, respectively. BMI was calculated as weight in kilograms divided by height in meters squared. BMI at 25 years (BMI25) used self-reported weight at age 25 and measured height at baseline. BMI change was calculated by subtracting BMI25 from BMI. Proportional change in BMI was BMI change as a percentage of BMI25. Waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) were calculated as WC divided by HC and standing height, respectively.
Follow-up for morbidity and mortality
Information on non-fatal disease outcomes was collected through linkage with established disease surveillance systems for certain diseases (diabetes, cancer, ischaemic heart disease, stroke), and, via unique national ID, with the national health insurance system, which includes details of ICD-10 coded diagnoses resulting in, or during, hospitalisation. Vital status of participants was monitored through death registries, checked annually against local residential and health insurance records, and by active confirmation. Deaths were ICD-10 coded by trained staff blinded to baseline information. Incident diabetes cases were identified through the disease surveillance system for diabetes, and through diabetes diagnoses (ICD-10 E10-E14) recorded in the health insurance databases or as underlying or contributing to death on death certificates. By January 1 2016, 37,289 (7.3%) participants had died and 4098 (0.8%) were lost to follow-up.
Statistical analysis
The present study excluded participants with missing BMI (n=2) data, or with previously diagnosed or screen-detected diabetes (17) at baseline (n=30 300), leaving 482,589 (198,574 men, 284,015 women) participants for inclusion in the main analyses.
All analyses were done separately for men and women. The prevalence and mean values of baseline characteristics were calculated across BMI categories (9), standardised by 5-year age groups and study area. Cox proportional hazards models were used to estimate hazard ratios (HRs) for the associations of baseline general (BMI, BF%) and central (WC, WHR, WHtR) adiposity measures, and BMI25 and BMI change, with incident diabetes, stratified by age-at-risk (5 year groups) and study area, and adjusted for education, income, occupation, smoking, alcohol consumption, physical activity and family history of diabetes. Further analyses additionally adjusted for selected adiposity measures to examine independent effects. Adiposity measures were categorised (cutpoints: 20th, 40th, 60th, 80th, 90th, 95th percentiles) to examine the full distribution, whilst ensuring adequate cases in each category. If the shape of the association was log-linear, adiposity measures were also investigated as continuous variables. The associations of BMI and WC with incident diabetes were examined across international and Asia-specific categories (9; 18). The ‘floating absolute risk’ method was used when examining adiposity measures as categorical variables (19); this does not alter the value of the HRs, but provides a 95% CI for each HR based on the amount of data in that category.
Single adiposity measurements may not accurately reflect an individual’s usual level due to random measurement error, including within-person variation or change over time (20). Repeat adiposity measurements available for 18,750 participants who attended resurvey three years after baseline were used to estimate regression dilution ratios, calculated as the slope of the regression line between baseline and resurvey measurements adjusted for age and study area (21). Log HR estimates for baseline BMI and WC (and other adiposity measures), examined as continuous variables, were multiplied by the reciprocal of the regression dilution ratio to estimate associations of usual BMI and WC with incident diabetes risk (20). Comparison of HRs for the first four and subsequent years of follow-up revealed no clear deviation from the proportional hazards assumption. Adjusted HRs were calculated across strata of other covariates, and chi square tests for trend and heterogeneity were applied to log HRs and their standard errors (22).
All analyses used SAS version 9.4. Figures were produced using R version 3.3.2.
Results
Among the 482,589 participants, the mean (SD) BMI was 23.6 (3.3) kg/m2, 4.5% were underweight (<18.5 kg/m2), 28.1% overweight (25.0-29.9 kg/m2) and 3.8% obese (≥30.0 kg/m2) (Table 1). Men with higher BMI were more likely to have higher socioeconomic status and to be alcohol drinkers, and less likely to be regular smokers. These associations were not evident in women. In both sexes, BMI was strongly positively associated with random plasma glucose level and family history of diabetes, and measures of both general and central adiposity tended to correlate strongly with each other (Supplemental Table S1, Supplemental Figure S1). BMI25 was positively associated with BMI at baseline, but was only weakly correlated with baseline adiposity measures. Mean BMI and WC were higher in urban than in rural areas. Between the baseline survey and 2013 resurvey (ie, ~8 years after baseline) there were modest increases in mean BMI (men: 23.4 kg/m2 to 24.0 kg/m2; women: 23.8 kg/m2 to 24.2 kg/m2) and WC (men: 82.0 cm to 86.3 cm; women: 79.1 cm to 83.4 cm), more marked in rural than urban areas (Supplemental Table S2).
Table 1. Baseline characteristics of participants by BMI.
| Characteristic* | Men |
Women |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Total | BMI (kg/m2) | Total | |||||||
| <18.5 | 18.5-24.9 | 25.0-29.9 | ≥30 | <18.5 | 18.5-24.9 | 25.0-29.9 | ≥30 | |||
| No. of participants | 9108 | 129855 | 54161 | 5450 | 198574 | 12528 | 177202 | 81426 | 12859 | 284015 |
| Age and socioeconomic factors | ||||||||||
| Mean age (SD), y | 56.4 (14.4) | 52.2 (11.0) | 50.9 (11.0) | 49.7 (13.1) | 52.1 (10.9) | 53.1 (14.7) | 49.7 (10.4) | 51.2 (9.7) | 52.1 (10.9) | 50.5 (10.4) |
| ≥6 y education, % | 56.9 | 58.5 | 62.5 | 62.9 | 57.5 | 42.1 | 43.5 | 41.0 | 38.3 | 43.7 |
| Living in urban area, % | 29.0 | 36.2 | 56.4 | 66.0 | 42.2 | 33.6 | 41.8 | 48.5 | 51.5 | 43.7 |
| Occupation, % | ||||||||||
| Agriculture and related | 47.4 | 45.8 | 38.0 | 34.5 | 44.7 | 44.2 | 41.9 | 39.3 | 37.1 | 41.6 |
| Other | 32.0 | 36.5 | 41.8 | 42.7 | 36.4 | 21.7 | 24.1 | 23.1 | 20.8 | 24.1 |
| Retired/Housewife/Unemployed | 20.6 | 17.6 | 20.2 | 22.8 | 18.9 | 34.0 | 34.0 | 37.6 | 42.1 | 34.3 |
| Annual household income, % | ||||||||||
| <10,000 yuan | 33.5 | 27.4 | 22.3 | 19.8 | 26.4 | 34.5 | 30.0 | 29.4 | 30.2 | 29.9 |
| 10,000-19,999 yuan | 29.3 | 28.9 | 27.7 | 28.6 | 28.3 | 29.6 | 29.0 | 29.8 | 30.1 | 29.4 |
| >19,999 yuan | 37.2 | 43.6 | 50.1 | 51.6 | 45.3 | 35.9 | 41.0 | 40.8 | 39.7 | 40.6 |
| Lifestyle factors | ||||||||||
| Ever-regular smoker, % | 79.9 | 75.4 | 70.7 | 70.4 | 74.5 | 5.1 | 3.3 | 2.8 | 3.3 | 3.1 |
| Ever-regular alcohol drinker, % | 36.6 | 41.3 | 43.0 | 44.1 | 41.8 | 2.7 | 3.0 | 3.2 | 2.8 | 3.0 |
| Mean physical activity (SD), MET-h/d | 22.1 (18.7) | 23.4 (13.6) | 21.5 (14.0) | 19.6 (16.1) | 22.4 (15.3) | 20.4 (13.7) | 20.7 (10.5) | 20.1 (11.0) | 19.1 (12.7) | 20.8 (12.8) |
| Anthropometry, blood pressure and plasma glucose, mean (SD) | ||||||||||
| BMI, kg/m2 | 17.7 (0.9) | 22.0 (1.7) | 26.7 (1.4) | 31.5 (1.9) | 23.3 (3.2) | 17.5 (1.0) | 22.2 (1.7) | 26.8 (1.4) | 31.9 (2.3) | 23.7 (3.4) |
| Waist circumference, cm | 67.6 (6.0) | 78.3 (6.5) | 90.7 (6.3) | 102.1 (8.8) | 81.7 (9.7) | 64.3 (6.4) | 75.3 (6.3) | 86.0 (6.2) | 96.5 (8.4) | 78.7 (9.4) |
| Hip circumference, cm | 81.9 (5.7) | 88.5 (4.4) | 95.7 (4.5) | 102.9 (6.7) | 90.5 (6.8) | 81.6 (4.7) | 88.7 (4.4) | 95.6 (4.6) | 103.5 (6.5) | 91.0 (6.8) |
| Waist-to-hip ratio | 0.83 (0.08) | 0.88 (0.06) | 0.95 (0.05) | 0.99 (0.07) | 0.90 (0.06) | 0.79 (0.08) | 0.85 (0.06) | 0.90 (0.06) | 0.93 (0.07) | 0.86 (0.07) |
| Waist-to-height ratio | 0.41 (0.04) | 0.47 (0.04) | 0.55 (0.04) | 0.62(0.05) | 0.49 (0.06) | 0.42 (0.04) | 0.49 (0.04) | 0.56 (0.04) | 0.63 (0.05) | 0.51 (0.06) |
| Body fat percentage† | 12.8 (4.1) | 20.0 (4.4) | 27.3 (4.7) | 32.5 (7.0) | 21.9 (6.2) | 19.2 (3.3) | 29.2 (4.5) | 37.8 (4.5) | 45.3 (6.4) | 31.9 (7.1) |
| BMI at 25 y‡, kg/m2 | 20.0 (2.5) | 21.6 (2.1) | 22.5 (2.5) | 23.8 (3.5) | 21.9 (2.3) | 20.0 (2.8) | 21.5 (2.6) | 22.7 (2.9) | 23.9 (3.8) | 21.8 (2.7) |
| BMI change‡, kg/m2 | -2.3 (2.5) | 0.6 (2.5) | 4.3 (2.7) | 7.7 (3.7) | 1.6 (3.4) | -2.4 (2.8) | 0.7 (2.8) | 4.1 (3.0) | 8.0 (4.2) | 2.0 (3.6) |
| Proportional change in BMI (%) | -10.7 (11.4) | 3.5 (12.2) | 20.2 (13.8) | 34.1 (20.2) | 8.0 (16.3) | -11.1 (12.6) | 4.6 (13.6) | 19.7 (15.8) | 35.7 (23.4) | 10.1 (17.0) |
| SBP, mmHg | 121.7 (23.0) | 129.5 (18.1) | 137.5 (19.8) | 144.8 (27.7) | 132.4 (19.9) | 120.5 (25.3) | 127.3 (19.8) | 134.6 (20.8) | 141.3 (24.7) | 129.0 (21.6) |
| RPG§, mg/dL | 99.0 (27.0) | 99.0 (19.8) | 102.6 (23.4) | 106.2 (32.4) | 100.8 (21.6) | 100.8 (27.0) | 102.6 (19.8) | 106.2 (21.6) | 108.0 (27.0) | 102.6 (19.8) |
| Family history of diabetes, % | 4.6 | 5.5 | 7.4 | 8.2 | 6.0 | 4.2 | 6.2 | 7.4 | 8.0 | 6.7 |
Standardised to age and study area structure of the study population
Data missing for 226 participants
Data missing for 77 745 participants
Data missing for 7975 participants. BMI, body mass index; MET-h/d, metabolic equivalent of task hours per day; RPG, random plasma glucose; SBP, systolic blood pressure. SI conversion factor: to convert glucose to mmol/L, multiply by 18.
During ~4.3 million person-years (mean 9 years) of follow-up, 13,416 (2.8%) participants were newly diagnosed with diabetes (13,198 non-fatal, 218 fatal) at age-at-risk 35 to 79 years. The overall diabetes incidence was 314 per 100,000 person-years, similar in urban and rural areas (320 vs 312), in contrast to higher diabetes prevalence in urban areas at baseline (Supplemental Figure S2). Among those who developed incident diabetes during follow-up, mean baseline BMI (25.5 [3.6] vs 23.5 [3.3] kg/m2) and WC (85.2 [10.1] vs 79.8 [9.6] cm) were higher than among those who did not develop diabetes.
Compared to men with so-called ‘normal’ weight, overweight or obese men had adjusted HRs of 2.87 (95% CI 2.76-2.98) and 6.10 (5.54-6.72), respectively, for incident diabetes (Table 2). Among women, the corresponding HRs were 2.35 (2.28-2.42) and 4.36 (4.09-4.65). Individuals who were underweight had significantly lower risk (men 0.64 [0.52-0.77]; women 0.61 [0.53-0.71]). When Asia-specific BMI cut-points (9) were applied, the magnitude of HRs associated with overweight and obesity were similar (Supplemental Table S4). There was a 4- to 5-fold higher risk associated with grade 2 abdominal obesity (18) compared with a ‘normal’ WC (Table 2).
Table 2. Number of cases of diabetes, standardised diabetes incidence rates and adjusted hazard ratios by BMI and waist circumference at baseline.
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| No. of cases | Standardised rate per 100 000 (95% CI)* |
Hazard ratio (95% CI)† |
No. of cases | Standardised rate per 100 000 (95% CI)* |
Hazard ratio (95% CI)† |
|
| BMI (kg/m2) | ||||||
| Underweight (<18.5) | 104 | 112.4 (84.7-140.2) | 0.64 (0.52-0.77) | 182 | 117.9 (89.9-145.9) | 0.61 (0.53-0.71) |
| Normal weight (18.5-24.9) | 2183 | 195.4 (188.6-202.2) | 1.00 (0.96-1.05) | 3492 | 200.1 (193.2-206.9) | 1.00 (0.97-1.04) |
| Overweight (25.0-29.9) | 2366 | 537.3 (519.4-555.1) | 2.87 (2.76-2.98) | 3680 | 528.8 (511.2-546.3) | 2.35 (2.28-2.42) |
| Obese (≥30.0) | 431 | 1055.9 (1023.7-1088.1) | 6.10 (5.54-6.72) | 978 | 1047.6 (1015.2-1080.0) | 4.36 (4.09-4.65) |
| Waist circumference (cm) | ||||||
| Normal‡ | 3781 | 178.6 (169.7-187.5) | 1.00 (0.96-1.04) | 2914 | 116.7 (109.9-123.5) | 1.00 (0.96-1.04) |
| Abdominal obesity grade 1§ | 947 | 713.8 (663.8-763.8) | 3.06 (2.87-3.26) | 2690 | 408.2 (392.4-423.9) | 2.19 (2.11-2.27) |
| Abdominal obesity grade 2∥ | 356 | 1379.9 (1309.3-1450.6) | 5.16 (4.65-5.73) | 2728 | 843.7 (829.8-857.6) | 3.94 (3.78-4.10) |
Standardised to age and study area structure of China Kadoorie Biobank population
Stratified by age and study area and adjusted for education, income, occupation, smoking, alcohol consumption, physical activity, family history of diabetes
Men: ≤94.0 cm, women: ≤80.0 cm
Men: 94.1-102.0 cm, women: 80.1-88.0 cm
Men: >102.0 cm, women >88.0 cm. Analyses restricted to participants who developed diabetes between 35 and 70 years- excludes 7 incident diabetes cases at ages <35 years and 236 at ages ≥80 years. BMI, body mass index.
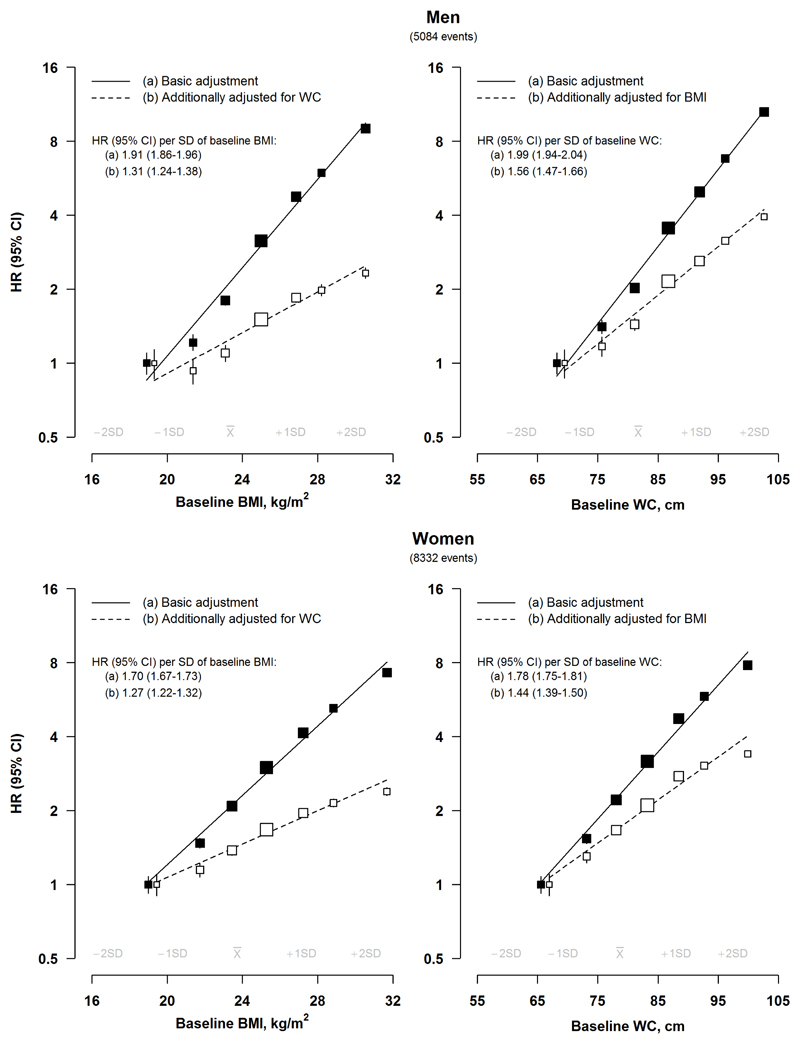
There were positive log-linear associations of baseline BMI and WC with risk of incident diabetes (p for trend <0.001) (Figure 1). For BMI, each 1 SD increment was associated with HRs of 1.91 (95% CI 1.86-1.96) in men and 1.70 (1.67-1.73) in women (p for heterogeneity <0.001), while for WC, they were 1.99 (1.94-2.04) and 1.78 (1.75-1.81) (p for heterogeneity <0.001), respectively. After mutual adjustment, the HRs for BMI were attenuated by more than 60% (men 66%, women 61%) and for WC by ~40% (men 43%, women 44%). For both BMI and WC, the HRs per SD increment were greater in men than in women at all ages (overall p for heterogeneity <0.001) (Supplemental Figure S3), and at younger than older ages in both sexes (p for trend <0.001) (Supplemental Figure S4, Supplemental Figure S5).
Figure 1. Adjusted hazard ratios (95% CI) for diabetes by BMI and waist circumference.
Basic adjustment results are stratified by age and study area and adjusted for education, household income, occupation, smoking, alcohol consumption, physical activity and family history of diabetes. Squares represent the HR with area inversely proportional to the variance of the log HR and error bars indicate the 95% CI. Adjusted HRs are plotted against mean BMI and waist circumference (WC) levels in each category. To avoid overlap of 95% CI lines, the boxes and their 95% CIs for the reference groups were moved apart slightly from the actual positions. Continuous associations reflect sex-specific BMI and waist circumference SDs.
The strength of associations of BMI and WC with diabetes were consistent between study areas (Supplemental Figure S6), but overall were stronger in rural, than urban, areas (p for heterogeneity <0.001), and in individuals without a family history of diabetes, although the difference reached significance only in men (p for heterogeneity=0.01) (Supplemental Figure S4, Supplemental Figure S5). Among men, there was evidence of a stronger association of WC with diabetes in ever-regular, than never-regular, smokers (p for heterogeneity=0.02) (Supplemental Figure S4).
After applying regression dilution ratios (men 0.95; women 0.93), each 1 SD higher usual BMI was associated with adjusted HRs of 1.98 (95% CI 1.93-2.04) and 1.77 (1.73-1.81) in men and women, respectively (Supplemental Table S5), while for usual WC the corresponding HRs were 2.13 (2.07-2.19) and 1.91 (1.87-1.95) (regression dilution ratios: men 0.91; women 0.89).
There were strong, positive log-linear associations of other measures of adiposity with risk of diabetes (Supplemental Figure S7, Supplemental Figure S8, Supplemental Table S5). HC was positively associated with incident diabetes after basic adjustment (p for trend <0.001), with a greater HR in men than in women (1.76 [1.71-1.82] vs. 1.49 [1.46-1.53] per 1 SD) (p for heterogeneity <0.001). After additional adjustment for WC, this association was reversed, and the sex difference attenuated (men 0.87 [0.83-0.92]; women 0.86 [0.83-0.89]) (p for heterogeneity=0.7). For WHR, each 1 SD increment was associated with adjusted HRs of 1.33 (1.31-1.35) in men and 1.27 (1.26-1.29) in women (p for heterogeneity <0.001), which were moderately attenuated by additional adjustment for BMI.
Further adjustment for blood pressure or dietary factors (fresh fruit, fresh vegetable, meat, rice, soybean product and wheat consumption), or, in women, for menopausal status did not materially alter the associations, nor did exclusion of individuals who developed cancer (which could cause significant weight change) prior to diagnosis of diabetes (Supplemental Table S6). Sensitivity analyses based on the resurvey population (n=14,881) revealed no significant differences in the strength of associations of BMI (p for heterogeneity: men 0.4, women 0.5) or WC (p for heterogeneity: men 0.3, women 0.4) with incident diagnosed diabetes and undiagnosed diabetes (Supplemental Figure S9).
There was a weak, positive log-linear association between BMI25 and risk of diabetes in later adulthood (men 1.09 [95% CI 1.05-1.12] and women 1.04 [1.02-1.07] per 1 SD higher) (p for trend <0.001) (Supplemental Figure S10). However, after additional adjustment for BMI at baseline, higher BMI25 was associated with a lower risk of incident diabetes (p for trend <0.001). Likewise, there was no clear association of BMI25 with incident diabetes within each baseline BMI category, while the converse was true for baseline BMI levels within each BMI25 category (Supplemental Table S7). Absolute (Supplemental Figure S10) and proportional (Supplemental Figure S11) changes in BMI between 25 years of age and baseline (mean 26.1 years) were strongly positively associated with risk of diabetes, but these associations were markedly attenuated after additional adjustment for BMI at baseline.
Conclusions
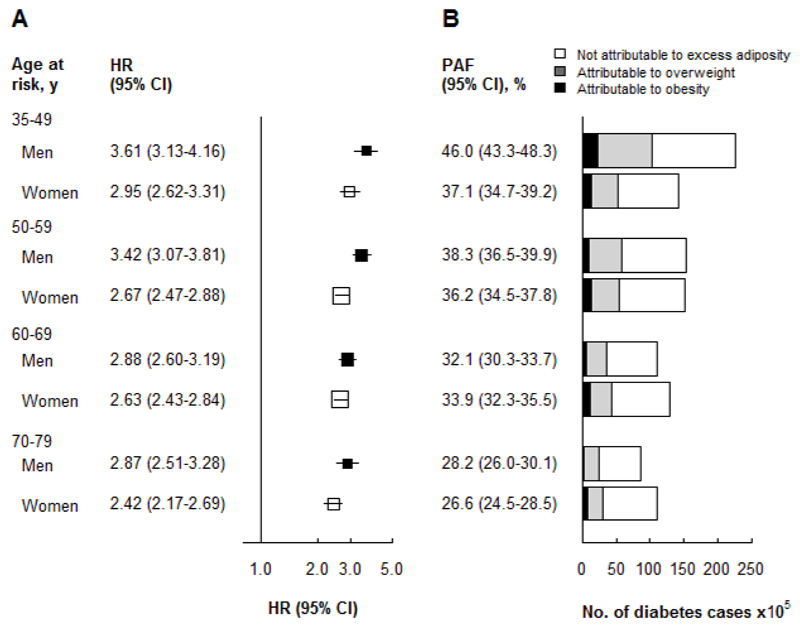
This is the largest ever prospective cohort study in China examining the relationship between adiposity and incident diabetes. In this relatively lean adult population there were strong, positive, apparently log-linear, associations between measures of central and general adiposity and incident diabetes, and general overweight and obesity were associated with 2- to 6-fold greater risks of diabetes. Relative risk estimates appeared to be somewhat greater among men than women, and for measures of central than general adiposity. Assuming a causal association and an increase in mean population BMI of 1 kg/m2 per decade, as has occurred in China (8), and applying our relative risk estimates to national diabetes prevalence, it was estimated that adiposity accounted for almost 50% of the increase in diabetes burden since 1980, and that 40 million (men 21.5 million, women 18.5 million) (36%) prevalent cases of diabetes could be attributed to excess general adiposity (BMI ≥25 kg/m2) in China in 2010 (Figure 2).
Figure 2. Adjusted hazard ratios for diabetes and diabetes cases due to excess adiposity (BMI 25+ kg/m2).
(A) Adjusted HR for diabetes associated with excess adiposity (BMI ≥25 kg/m2) by age and sex. Hazard ratios (HRs) are calculated for overweight or obese (BMI ≥25 kg/m2) participants vs absence of overweight or obesity (BMI <25 kg/m2) participants. HRs are stratified by age and study area and adjusted for education, household income, occupation, smoking, alcohol consumption, physical activity and family history of diabetes. Squares represent the HR with area inversely proportional to the variance of the log HR and error bars indicate the 95%CI.
(B) Diabetes cases attributable to excess adiposity (BMI ≥25 kg/m2) in 2010. Age- and sex-specific prevalence of overweight and obesity in the CKB are comparable with contemporaneous nationally representative surveys (32), and the population attributable fraction was calculated as P(HR-1) divided by HR, where P is the prevalence of excess adiposity among those who developed incident diabetes. By applying age- and sex-specific HRs to nationally representative, age- and sex-specific diabetes prevalence (4), we estimated the number of diabetes cases attributable to high adiposity.
Previous large prospective studies, or meta-analyses of such studies, have demonstrated clearly that adiposity is the strongest modifiable risk factor for type 2 diabetes. In a large pooling project of Western prospective cohorts, including 5,500 diabetes cases, each 5 kg/m2 increment in BMI was associated with a 2.7-fold higher risk of diabetes (23). This is comparable with findings from the EPIC-Interact nested case-control study of over 12,000 type 2 diabetes cases, which showed a doubling of the risk of type 2 diabetes per SD (~4 kg/m2) higher BMI (24). Our study shows reliably that risk estimates for BMI in Chinese adults are largely comparable with those from previous Western studies, thus further extending the positive log-linear association with diabetes to well below the range of BMI typically seen in Western populations (9). Central adiposity measures, indicating the degree of visceral adiposity, are proposed to be more important indicators of cardiometabolic risk than general adiposity measures (25). However, previous large studies in Western populations, and limited data from East Asia (24; 26), have found largely comparable strengths of association of WC (eg, 2-2.5-fold higher risk per SD higher after minimal adjustment for confounding) and BMI with risk of diabetes (24). Importantly, however, these studies did not take account of regression dilution bias, which would be expected to be somewhat greater for WC than for BMI. In CKB, more marked attenuation of the association of baseline BMI with incident diabetes after adjustment for baseline WC, than vice versa, and the stronger association of usual WC, than usual BMI, after accounting for measurement error, suggest a somewhat stronger association of WC with risk of diabetes.
Previous findings on sex-differences in the strength of association between adiposity and type 2 diabetes are inconsistent (13; 23; 27; 28). One prospective study in China showed a stronger association of BMI and WC in women, but the number of women included was small (~20%, n=16,680) (13). Several Western population studies have shown a stronger association of WC, but less clearly BMI, among women than men (24; 29). The CKB provides robust evidence of a stronger association of BMI among men, which was attenuated by additional adjustment for WC. This is consistent with the apparently stronger association of WC among men observed in the CKB, likely reflecting a greater propensity of men, than women, to visceral fat accumulation (18) and to higher levels of insulin resistance (30). Explanations for the discrepancy with previous findings in Western population studies, in particular for WC, are unclear, but may reflect ethnic differences in the sexual dimorphism in body composition (31) (eg, predisposition to visceral versus subcutaneous adiposity), but further investigation is required to understand this fully (31).
The diabetes incidence rates observed in the present study were similar in urban and rural areas, in contrast with a higher prevalence of diabetes seen in urban areas at baseline (17). A higher proportion of undiagnosed diabetes in urban areas could explain this pattern; however, evidence from a subset of study participants who attended baseline and two subsequent surveys suggests the rate of undiagnosed diabetes after baseline was higher in rural (44%) than urban (35%) areas. This would be expected to result in smaller relative risk estimates in rural areas, but the adiposity-associated diabetes risks, if anything, were somewhat greater in rural than urban areas, especially among men. The converging urban-rural diabetes incidence trends may in part reflect marked increases in adiposity in rural areas over recent years (32), as well as adverse lifestyle changes beyond adiposity. In combination with poorer diabetes-associated outcomes in rural areas (33), this highlights the need for focused attention on diabetes prevention and management in China’s rural areas.
Although some studies have suggested early adulthood adiposity is an independent risk factor for type 2 diabetes during later adulthood (34; 35), this is not supported by larger studies in which the association was attenuated (36; 37) or reversed (38) after accounting for later adulthood adiposity. One previous Chinese population study, including ~120,000 adults with a diabetes prevalence of 21%, found increasing odds of prevalent diabetes with increasing BMI at age 20 years after controlling for weight change between 20 and 40 years (35). However, the cross-sectional study design increases susceptibility of these analyses to biases. With more incident diabetes cases than previous studies combined (n=11,400 vs 4,500), we show clearly that later adulthood adiposity is critical in determining type 2 diabetes risk, consistent with the more functional—rather than anatomical—abnormalities underlying type 2 diabetes (36), and with observed type 2 diabetes remission (6). Although we used self-reported weight at 25 years, resurvey data demonstrated good correlation between repeated self-reports (Pearson correlation coefficients 0.81 and 0.77, comparing baseline with first and second resurveys, respectively), consistent with previous studies showing accurate recall of past body weight (39). These findings highlight the importance of weight management throughout adulthood, although their generalisability to current and future generations of young adults is unclear, given the low average BMI25 in CKB participants.
Apart from the large population, our study has several strengths. Exclusion of diagnosed and undiagnosed diabetes at baseline reduced potential for reverse causality, and the relatively lean study population enabled investigation of a uniquely wide adiposity range. Use of standardised protocols and extensive training across study centres ensured reliability of exposure measurements. Medical record review for almost 1000 incident diabetes cases confirmed the validity of the diagnosis (positive predictive value 97% based on American Diabetes Association diagnostic criteria (40) and medication use), and estimated diabetes prevalence based on the CKB resurvey population (to enable estimation of undiagnosed diabetes prevalence), was reasonably consistent with nationally representative surveys (4; 41) (eTable 8). Furthermore, mean BMI and prevalence of overweight and obesity in the CKB were comparable to contemporaneous nationally representative surveys in China (32). Extremely low loss to follow-up and the diversity of the study population limit potential for biased risk estimates and ensure generalisability of the findings. Furthermore, adjustment of estimates for regression dilution bias, using repeat adiposity measures during follow-up, ensures accurate estimates of the association of usual adiposity levels and risk of diabetes. Although repeat adiposity measurements used for adjustment were slightly earlier than the ideal midpoint of follow-up (intra-individual variation in exposures increases with longer periods of follow-up) (20), any resulting underestimation of intra-individual variation would be expected to be minimal for adiposity measures. However, our study has certain limitations. Incident diabetes was restricted to diagnosed cases, but presented sensitivity analyses suggest this would not impact significantly on risk estimates. Conversely, although the proportion of diabetes cases remaining undiagnosed may be lower in individuals with the highest BMI levels (given the known association of obesity with type 2 diabetes), leading to overestimation of adiposity-associated risks, this would not explain the observed association among individuals without overweight or obesity. Given the age of the cohort, all incident diabetes cases were assumed to be type 2 diabetes; a small proportion may have been type 1, and their inclusion would likely underestimate adiposity-associated risks.
In conclusion, the present study provides the first large-scale prospective evidence of strong, positive, independent relationships of general and central adiposity with incident diabetes risk in Chinese adults. Adiposity is the strongest modifiable, and causal (7), risk factor for type 2 diabetes, and projected further increases in adiposity levels in the population will foreshadow still higher diabetes prevalence in coming decades in China, perhaps particularly among rural populations. In the absence of known modifiable causal mediators of the association with type 2 diabetes, tackling adiposity at a whole-population level is imperative for control of China’s diabetes epidemic.
Supplementary Material
Acknowledgments
The chief acknowledgment is to the participants, the project staff, and the China National Centre for Disease Control and Prevention (CDC) and its regional offices for access to death and disease registries. The Chinese National Health Insurance scheme provides electronic linkage to all hospital admission data.
Funding The baseline survey and the first re-survey were supported by a research grant from the Kadoorie Charitable Foundation in Hong Kong. The long-term continuation of the project is supported by program grants from the UK Wellcome Trust (088158/Z/09/Z, 104085/Z/14/Z), the Chinese Ministry of Science and Technology (2011BAI09B01, 2012-14), the Chinese National Natural Science Foundation (81390540, 81390541, 81390544) and the National Key Research and Development Program of China (2016YFC0900500, 2016YFC0900501, 2016YFC0900504, 2016YFC1303904). The British Heart Foundation, Medical Research Council and Cancer Research UK provide core funding to the Oxford CTSU. Fiona Bragg acknowledges support from the BHF Centre of Research Excellence, Oxford.
Footnotes
Declaration of interests All authors declare no competing interests
Author contributions FB and KT contributed equally to this study. FB, HD, ZC, MVH, KT contributed to the concept and design of the study. ZB, JC, YC, ZC, CD, YG, LL, QS, LY were involved in the acquisition of data. FB, AI, CK, KT conducted the statistical analyses. FB, ZC drafted the manuscript. All authors provided critical revision of the manuscript for important intellectual content. JC, RC, YC, ZC, LL, RP obtained funding. FB, ZC and LL had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.International Diabetes Federation. Diabetes Atlas, 7th Edition. 7th Edition. Brussels: 2015. [Google Scholar]
- 2.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 3.National Collaborative Group of Diabetes Study. [A mass survey of diabetes mellitus in a population of 300,000 in 14 provinces and municipalities in China] Zhonghua Nei Ke Za Zhi. 1981;20:678–683. [PubMed] [Google Scholar]
- 4.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 5.Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2:980–991. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Stoll CT, Song J, Varela J, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003-2012. JAMA Surgery. 2014;149:275–287. doi: 10.1001/jamasurg.2013.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale CE, Fatemifar G, Palmer TM, et al. Causal Associations of Adiposity and Body Fat Distribution With Coronary Heart Disease, Stroke Subtypes, and Type 2 Diabetes Mellitus: A Mendelian Randomization Analysis. Circulation. 2017;135:2373–2388. doi: 10.1161/CIRCULATIONAHA.116.026560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization expert consulation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang SL, Pan WH, Hwu CM, et al. Incidence of NIDDM and the effects of gender, obesity and hyperinsulinaemia in Taiwan. Diabetologia. 1997;40:1431–1438. doi: 10.1007/s001250050846. [DOI] [PubMed] [Google Scholar]
- 11.Li W-D, Fu K-F, Li G-M, et al. Comparison of effects of obesity and non-alcoholic fatty liver disease on incidence of type 2 diabetes mellitus. World J Gastroenterol. 2015;21:9607–9613. doi: 10.3748/wjg.v21.i32.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XY, Zhang M, Luo XP, et al. [Body mass index, waist circumference and waist-to-height ratio associated with the incidence of type 2 diabetes mellitus: a cohort study] Zhonghua Yu Fang Yi Xue Za Zhi. 2016;50:328–333. doi: 10.3760/cma.j.issn.0253-9624.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya A, Cui L, Sun L, et al. A prospective study of impaired fasting glucose and type 2 diabetes in China: The Kailuan study. Medicine. 2016;95:e5350. doi: 10.1097/MD.0000000000005350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CMY, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Lee L, Chen J, et al. Cohort profile: the Kadoorie Study of Chronic Disease in China (KSCDC) Int J Epidemiol. 2005;34:1243–1249. doi: 10.1093/ije/dyi174. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Chen J, Collins R, et al. China Kadoorie Biobank of 0.5 million people: survey methods, baseline characteristics and long-term follow-up. Int J Epidemiol. 2011;40:1652–1666. doi: 10.1093/ije/dyr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragg F, Li L, Smith M, et al. Associations of blood glucose and prevalent diabetes with risk of cardiovascular disease in 500 000 adult Chinese: the China Kadoorie Biobank. Diabet Med. 2014;31:540–551. doi: 10.1111/dme.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Waist circumference and waist-hip ratio Report of a WHO Expert Consultation. Geneva: World Health Organization; 2008. [Google Scholar]
- 19.Easton DF, Peto J, Babiker AG. Floating absolute risk: an alternative to relative risk in survival and case-control analysis avoiding an arbitrary reference group. Stat Med. 1991;10:1025–1035. doi: 10.1002/sim.4780100703. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 21.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–1069. doi: 10.1002/sim.4780080905. [DOI] [PubMed] [Google Scholar]
- 22.Early Breast Cancer Trialists’ Collaborative Group. Treatment of early breast cancer. Vol. 1: worldwide evidence 1985–1990—a systematic overview of all available randomized trials of adjuvant endocrine and cytotoxic therapy. Oxford: Oxford University Press; 1990. [Google Scholar]
- 23.Kivimäki M, Kuosma E, Ferrie JE, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2:e277–e285. doi: 10.1016/S2468-2667(17)30074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langenberg C, Sharp SJ, Schulze MB, et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS Med. 2012;9:e1001230. doi: 10.1371/journal.pmed.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2:367–373. doi: 10.2174/1573399810602040367. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 27.Xu L, Lam TH, Jiang CQ, et al. Adiposity and incident diabetes within 4 years of follow-up: the Guangzhou Biobank Cohort Study. Diabetic Med. 2017 doi: 10.1111/dme.13378. [DOI] [PubMed] [Google Scholar]
- 28.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meisinger C, Doring A, Thorand B, Heier M, Lowel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. 2006;84:483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- 30.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wells JCK. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab. 2007;21:415–430. doi: 10.1016/j.beem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Tian Y, Jiang C, Wang M, et al. BMI, leisure-time physical activity, and physical fitness in adults in China: results from a series of national surveys, 2000-14. Lancet Diabetes Endocrinol. 2016;4:487–497. doi: 10.1016/S2213-8587(16)00081-4. [DOI] [PubMed] [Google Scholar]
- 33.Bragg F, Holmes MV, Iona A, et al. Association between diabetes and cause-specific mortality in rural and urban areas of china. JAMA. 2017;317:280–289. doi: 10.1001/jama.2016.19720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Mutsert R, Sun Q, Willett WC, Hu FB, van Dam RM. Overweight in early adulthood, adult weight change, and risk of type 2 diabetes, cardiovascular diseases, and certain cancers in men: a cohort study. Am J Epidemiol. 2014;179:1353–1365. doi: 10.1093/aje/kwu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun W, Shi L, Ye Z, et al. Association between the change in body mass index from early adulthood to midlife and subsequent type 2 diabetes mellitus. Obesity. 2016;24:703–709. doi: 10.1002/oby.21336. [DOI] [PubMed] [Google Scholar]
- 36.Tirosh A, Shai I, Afek A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol. 1990;132:501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 38.de Lauzon-Guillain B, Balkau B, Charles MA, Romieu I, Boutron-Ruault MC, Clavel-Chapelon F. Birth weight, body silhouette over the life course, and incident diabetes in 91,453 middle-aged women from the French Etude Epidemiologique de Femmes de la Mutuelle Generale de l'Education Nationale (E3N) Cohort. Diabetes Care. 2010;33:298–303. doi: 10.2337/dc09-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2017;40:S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Gao P, Zhang M, et al. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.